Abstract
Background
Acute kidney injury complicating high-dose methotrexate therapy increases the risk for severe mucositis, myelosuppression, and death. It is unclear whether high-dose leucovorin and supportive therapy without the use of glucarpidase can reduce toxicity from high-dose methotrexate.
Study Design
The charts of all patients at Memorial Sloan Kettering Cancer Center whose methotrexate drug levels at 48 or 72 hours after administration were ≥10 times the toxic level were reviewed between January 2000 and December 2011.
Results
Eighty-eight patients (median age 51 years, range 9–90 years) who received 100 courses of high-dose methotrexate were identified. Serum creatinine increased by 2-fold from baseline (median, range 1- to 10-fold), but all patients recovered kidney function. Serum levels of methotrexate were 69 μmol/L (median, range 2.2–400), 6.9 μmol/L (1.3–64), and 2.0 μmol/L (0.05–26) at 24, 48, and 72 hours, respectively, after administration. There was a statistically significant correlation between methotrexate levels at 48, 72, 96, and 120 hours after administration, but not between 24 and 72 hours or subsequent time points. High-dose leucovorin was given in 81% of courses in accordance to institutional protocols in most cases. Myelosuppression was present in 42%; Grade ≥III neutropenia in 29% and thrombocytopenia in 25%. Infectious complications, oral mucositis, and diarrhea occurred in 21%, 17%, and 6% of patients, respectively. There were 5 deaths, none directly attributed to complications from methotrexate administration. Seven additional patients received glucarpidase at the discretion of a treating physician during the study period, and results are reported separately.
Conclusion
Patients who had 100 episodes of high-dose methotrexate-associated acute kidney injury were treated with a strategy that only included usual supportive measures and high-dose leucovorin. There were no deaths directly attributed to complications related to high-dose methotrexate therapy. Glucarpidase, an expensive drug, may not be necessary for a significant number of patients.
Keywords: Acute kidney injury, high-dose methotrexate, kidney toxicity, glucarpidase, leucovorin
INTRODUCTION
High-dose methotrexate (HDMTX) with leucovorin rescue is a component of systemic therapy for osteosarcoma, lymphoma, and leukemia (1). Administration of HDMTX is associated with the risk of acute kidney injury (AKI), leading to delayed clearance of methotrexate (MTX). Prolonged exposure to elevated MTX levels creates risk for severe mucositis, hematopoietic suppression, and death. Treatment of HDMTX associated renal dysfunction and delayed MTX excretion is a medical emergency.
Several interventions have been employed to treat this life-threatening complication including high-dose leucovorin (HDLV), glucarpidase, and thymidine. Because of the concern that HDLV alone cannot prevent tissue toxicity (2), glucarpidase (an enzyme that converts MTX into inactive metabolites) has been introduced as an agent to prevent or ameliorate MTX toxicity in patients with AKI and delayed MTX clearance.(3) Widemann and colleagues reported a large series of patients treated with glucarpidase, and have advocated that glucarpidase use should be a standard approach to the management of HDMTX associated renal dysfunction and delayed MTX excretion.(4–6) Small series of patients treated successfully with HDLV without glucarpidase has been previously reported.(7) More recent experience with the management of HDMTX–associated renal dysfunction and delayed MTX excretion in a larger group of patients treated in a similar fashion was reviewed and reported in this paper. The results were also compared with other studies that used glucarpidase.(5, 8–12) The relationship between the degree of AKI and serial MTX levels, the lack of correlation between MTX levels at 24 hours and subsequent time points, and the influence of additional cytotoxic chemotherapy in the development of severe (grade ≥III) myelosuppression were also analyzed in this manuscript.
METHODS
To help identify patients at high risk for developing severe MTX toxicity, various cutoff points have been established (1). At Memorial Sloan-Kettering Cancer Center, patients with serum MTX levels ≥10 μmol/L at 24 hours, ≥1 μmol/L at 48 hours, and ≥0.1μmol/L at 72 hours are considered to be at high risk of impending toxicity. The institution’s database was reviewed and all patients treated between January 2000 and December 2011 who had delayed MTX clearance with MTX levels ≥10 times of toxic levels at 48 or 72 hours (≥10 μmol/L at 48-hours and/or ≥1 μmol/L at 72-hours) were considered for inclusion. Because some patients received more than one course of HDMTX, each course of HDMTX was considered as an individual patient for statistical analysis and tabulation in the results.
A retrospective chart analysis at Memorial Sloan-Kettering Cancer Center was performed after approval by the Institutional Review Board. Patient discharge summaries were used to review the cases and extract data related to myelosuppression, infectious complications, incidence of gastrointestinal toxicity, and mortality. Analysis of laboratory data included serial evaluation of serum creatinine and MTX levels as well as hematologic parameters. MTX was measured in serum using a dihydrofolate reductase inhibition assay. For the purpose of this study, HDLV was defined as 0.4 g/day or more given intravenously. Treatment-related toxicities were graded according to the National Cancer Institute Terminology Criteria for Adverse Events (CTCAE) version 4.0.
RESULTS
There were 88 patients who received 100 courses of HDMTX and who were treated with the usual supportive measures of hydration and HDLV as the sole mode of therapy. In addition, between January 2000 and December 2011, there were 7 patients who also received glucarpidase who will be analyzed separately.
The study included children and adults. Median age was 51 years old with a range of 9–90 years (Table 1). Seventy five percent of the patients were 19 years old or older. The underlying malignancy included osteosarcoma in 36 courses, central nervous system lymphoma (CNSL) in 30, non-Hodgkin’s lymphoma (NHL) in 21, and other malignancies in 13. Baseline serum creatinine was defined as the closest creatinine level to the time of HDMTX administration and ranged between 0.2 to 1.8 mg/dL. Eight patients had underlying abnormal kidney function prior to administration of HDMTX. The median dose of MTX was 3.5 g/m2 with a range from 1.0 to 13 g/m2. Sixty-four courses included MTX as a single agent while 36 of the courses included additional cytotoxic chemotherapy within 2 weeks prior-to or at the time of HDMTX. The chemotherapy agents used in these 36 courses included procarbazine, cyclophosphamide, cytarabine, and doxorubicin, given either as a single agent or in combination. Four patients had received a prior dose of MTX 2 weeks or less before the present course of HDMTX and 1 received pegylated asparaginase. Eighty one percent of the courses included HDLV, with a median dose of 1 g/day (range: 0.4–2.3 g/day) intravenously. In 83% of the courses HDLV was administered within the first 48 hours.
Table 1.
Characteristics of HDMTX courses
| Variable | N (%) |
|---|---|
|
| |
| Number of courses | 100 |
| Age, years, median (IQR) | 51 (19–69) |
| Underlying disease | |
| Osteosarcoma | 36 (36) |
| Primary central nervous system lymphoma | 30 (30) |
| Non-Hodgkin’s lymphoma | 21 (21) |
| Breast cancer | 7 (7) |
| Chronic lymphocytic leukemia | 3 (3) |
| Other | 3 (3) |
| Baseline kidney function | |
| Serum creatinine, mg/dL, median (IQR) | 0.85 (0.70–1.00) |
| Chemotherapy | |
| MTX dose, g/m2, median (IQR) | 3.5 (3.5–12) |
| Received MTX as a single agent | 64 (64) |
| Received MTX with other cytotoxic chemotherapy | 36 (36) |
| High-dose leucovorin rescue | |
| Received | 81 (81) |
| Did not receive | 19 (19) |
| Dose, g/day, median (IQR) | 1.0 (1.0–1.5) |
| Within 48 hours of MTX therapy | 67 (83) |
| Beyond 48 hours of MTX therapy | 14 (17) |
| Nephrotoxicity | |
| Serum creatinine, peak, mg/dL, median (IQR) | 1.80 (1.20–2.53) |
| Fold change increase from baseline, median (IQR) | 2.0 (1.44–3.05) |
| MTX levels, μg/L, median (IQR) | |
| 24 hours after MTX dose | 69 (39.5–100) |
| 48 hours after MTX dose | 6.9 (4.05–16.45) |
| 72 hours after MTX dose | 2.04 (1.3–5.0) |
| Myelosuppression | |
| MTX alone | 64 (64) |
| Neutropenia, grade III–IV | 16 (25) |
| Thrombocytopenia, grade III–IV | 15 (23) |
| Neutropenia or thrombocytopenia | 24 (38) |
| MTX with other cytotoxic chemotherapy | 36 (36) |
| Neutropenia, grade III–IV | 13 (36) |
| Thrombocytopenia, grade III–IV | 10 (28) |
| Neutropenia or thrombocytopenia | 18 (50) |
| Infection | 21 (21) |
| Mucositis, grade I–III | 17 (1) |
| Diarrhea | 6 (6) |
| Death (attributed to MTX) | 0 (0) |
HDMTX=high-dose methotrexate; IQR=interquartile range; MTX=methotrexate.
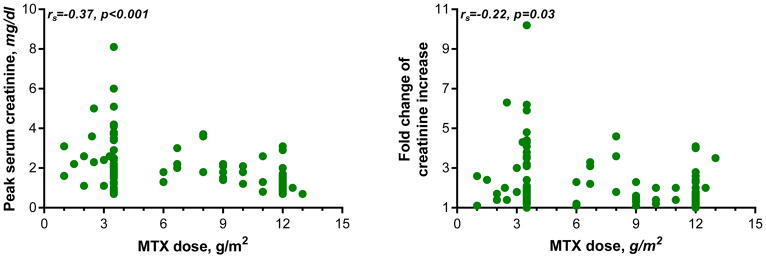
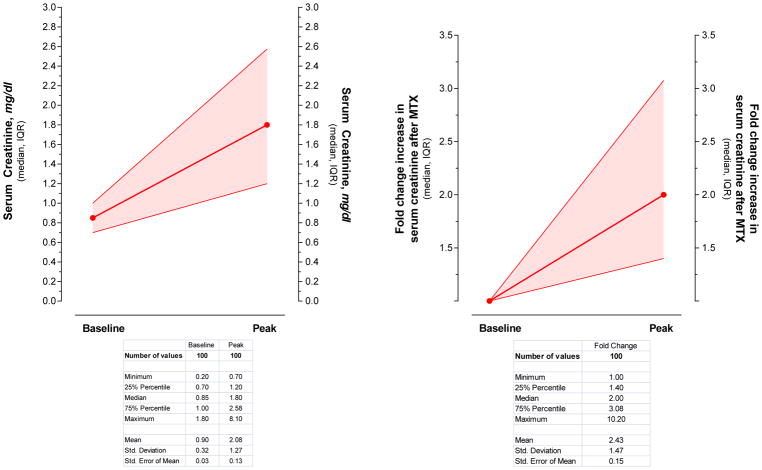
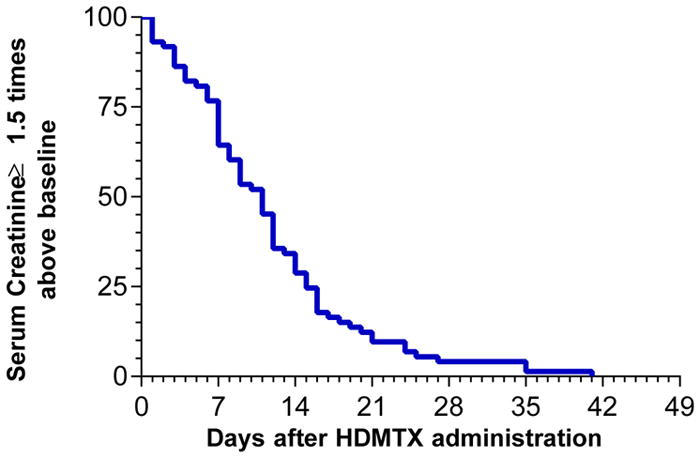
The changes in serum creatinine levels following HDMTX are shown in Figure 1. Because of the wide range of baseline serum creatinine levels associated with different ages, weight, sex, and race, the severity of renal dysfunction was expressed as the percent or fold change from baseline creatinine levels. Serum creatinine increased from a median baseline of 0.85 mg/dL (range 0.2–1.8 mg/dL) to a median peak level of 1.8 (range 0.7–8.1 mg/dL) with 25% of courses having a peak serum creatinine of ≥2.7 mg/dL. The serum creatinine level increased by a median of two-fold (range 1- to 10-fold) with 25% of courses having an increase of 3-fold or more than the baseline level. In 27% of courses the increase in serum creatinine was <1.5 of baseline, with 8 of these 27 courses having an abnormal creatinine before HDMTX administration. There was an inverse relationship between the dose of MTX and either the peak serum creatinine or the fold change in creatinine levels. Paradoxically, lower doses tended to produce more nephrotoxicity (Figure 2). The duration of renal dysfunction, as assessed by the number of days that the creatinine levels remained equal or above 1.5 times baseline, is shown in Figure 3. From those patients who had an increase in creatinine level ≥1.5 times baseline, the levels remained above this value for a median time of 11 days, but all patients eventually recovered renal function. One patient required transient hemodialysis.
Figure 1. Serum creatinine change from baseline after MTX administration.
Figure depicts the serum creatinine change from baseline after 100 courses of HDMTX administration in 88 patients. The left panel is the serum creatinine levels in mg/dL and the right panel is the fold change increase in serum creatinine after HDMTX administration. The red line is the median value and the colored band is the interquartile interval.
HDMTX=high-dose methotrexate; IQR=interquartile range; MTX=methotrexate
Figure 2. Relation between MTX dose and kidney function.
Figure depicts the relationship between HDMTX dose (x-axis) and peak serum creatinine (y-axis, left panel) or fold change of serum creatinine increase (right panel). By Spearman rank order correlation, the inverse relation between HDMTX dose and peak serum creatinine or fold change of serum creatinine increase was statistically significant.
HDMTX=high-dose methotrexate; MTX=methotrexate
Figure 3. Number of days serum creatinine remained above baseline after HDMTX administration.
Among the 100 courses of HDMTX, 73 courses resulted in serum creatinine levels >150% of the baseline value. Figure depicts the number of days after the administration of HDMTX that serum creatinine levels remained ≥1.5 times above the baseline value.
HDMTX=high-dose methotrexate; S. Creatinine=serum creatinine
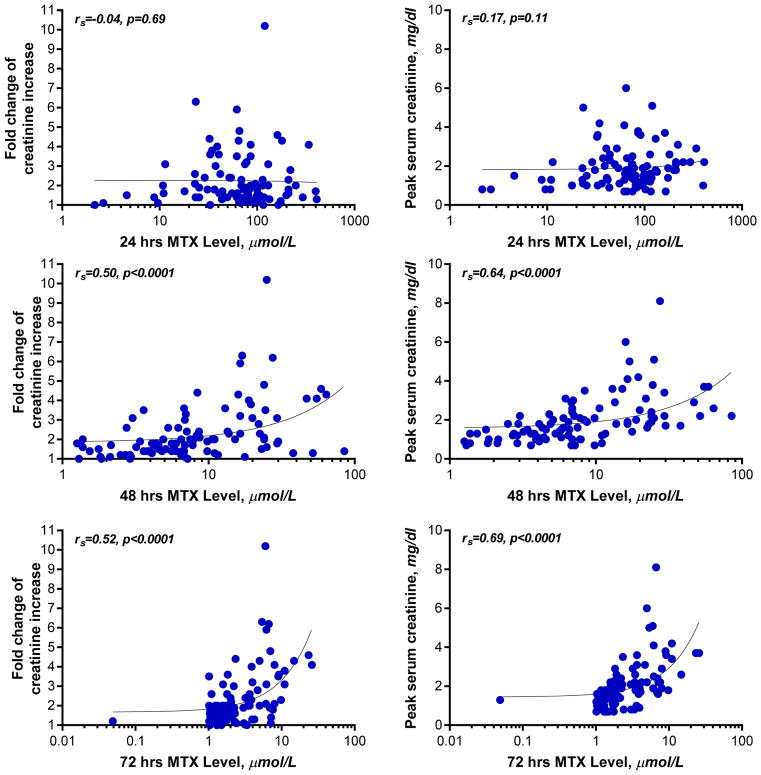
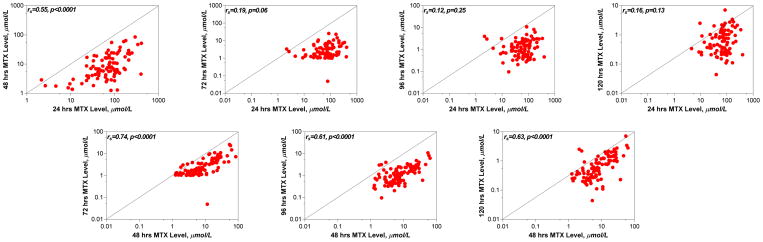
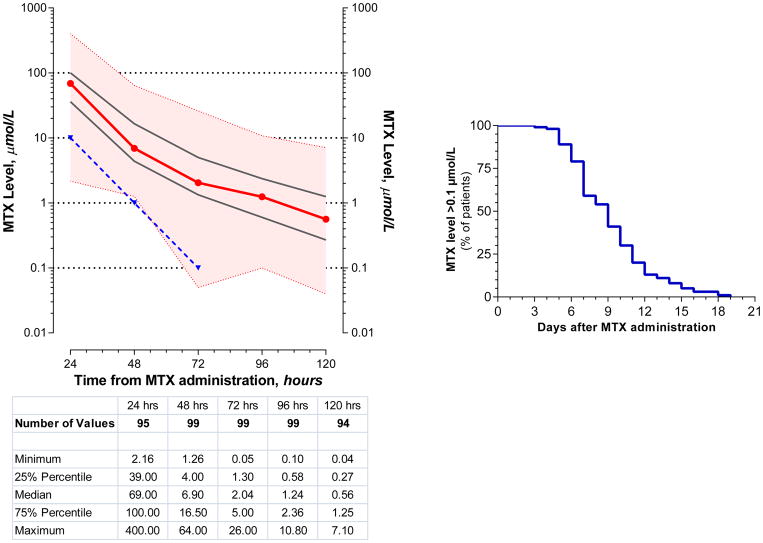
Methotrexate levels during the first 5 days are shown in Figure 4. The median (range) MTX levels at 24, 48, and 72 hours after administration of HDMTX was 69 (2.2–400) μmol/L, 6.9 (1.3–64) μmol/L, and 2.0 (0.05–26) μmol/L, respectively. Twenty five percent of courses had MTX levels ≥100 μmol/L, 17 μmol/L, and 5 μmol/L at 24, 48, and 72 hours, respectively. Levels of MTX remained above 0.1 μmol/L for a median of 9 days (Figure 4). There was no relationship between the MTX dose and MTX levels at 24 hours (p=0.96) but there was an inverse correlation at 48 and 72 hours (p=0.04 and p=0.03, respectively). There was a high correlation between the degree of AKI, as reflected by the peak serum creatinine levels or the fold change, and the MTX levels at 48 and 72 hours (Figure 5), but not between changes in renal function and MTX levels at 24 hours. There was a high correlation between the MTX levels at 24 and 48 hours, and between 48 and 72, 96, and 120 hours, but not between 24 and 72, 96, or 120 hours (Figure 6).
Figure 4. Serum MTX levels after administration of HDMTX.
Left panel: Figure depicts the serum methotrexate levels at 24, 48, 72, 96, and 120 hours after the administration of 100 courses of HDMTX. The x-axis is time in hours after HDMTX administration. The y-axis is MTX level, in μmol/L, shown in logarithmic scale. The red line is the median value and the black lines represent the 25th and 75th percentile values. The colored band is the range of values from minimum to maximum. The blue line is the designated limit of the toxic level at 24, 48, and 72 hours after HDMTX administration.
Right panel: Figure depicts the number of days after administration of HDMTX that >0.01 μmol/L of serum MTX remained.
HDMTX=high-dose methotrexate; MTX=methotrexate
Figure 5. Relation between serum MTX level and kidney function.
Figure depicts the relation between fold change of serum creatinine increase (y-axis, left panel) or peak serum creatinine (y-axis, right panel) and HDMTX levels. X-axis depicts the serum MTX levels at 24 hours (top panel), 48 hours (middle panel), and 72 hours (bottom panel) after HDMTX administration. By Spearman rank order correlation, the relation between fold change of serum creatinine increase or peak serum creatinine and HDMTX levels at 48 hours and 72 hours (but not 24 hours) after HDMTX administration were statistically significant.
HDMTX=high-dose methotrexate; MTX=methotrexate
Figure 6. Relation between serum MTX levels at different time points after HDMTX administration.
Figure depicts the relation between serum MTX level at 24 hours after HDMTX administration (x-axis, top panel) or 48 hours after HDMTX administration (x-axis, bottom panel) and drug levels at subsequent time points. By Spearman rank order correlation, the relation between 24 hours MTX level and 48 hours MTX (but not subsequent time points) level was statistically significant. The relation between 48 hours MTX level and subsequent time points (72, 96, and 120 hours) were statistically significant.
HDMTX=high-dose methotrexate; MTX=methotrexate
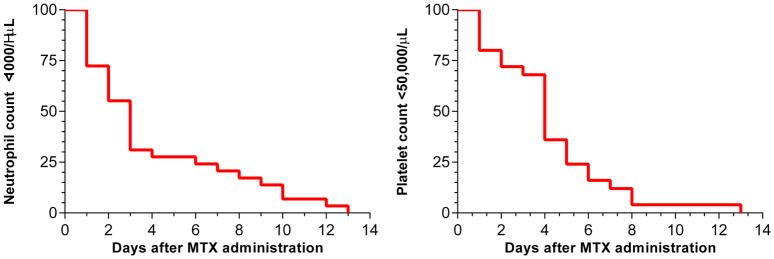
In 6 patients, neutropenia or thrombocytopenia was already present prior to HDMTX administration and these patients were not included in the analysis. Grade III or higher neutropenia (absolute neutrophil count <1000/μL) developed in 29% of the courses; grade III or higher thrombocytopenia (platelet count <50,000/μL) developed in 25% of the courses. Thus, evidence of myelosuppression was present in 42% of the courses (Table 1). The duration of neutropenia and thrombocytopenia is shown in Figure 7.
Figure 7. Myelosuppression after MTX administration.
Figure depicts the number of days after HDMTX administration the neutrophil counts remained below 1000/μL (left panel) and the platelet count remained below 50,000/μL (right panel). Among the 100 courses of HDMTX, 29 courses resulted in neutrophil counts below 1000/μL and 25 courses resulted in platelet counts below 50,000/μL.
HDMTX=high-dose methotrexate; MTX=methotrexate
Grade III or higher neutropenia or thrombocytopenia occurred in 18 of 36 (50%) courses that included additional cytotoxic chemotherapy but only in 24 of 64 (38%) courses of MTX alone. The influence of additional cytotoxic chemotherapy on myelosuppression can be further appreciated by the fact that of 42 courses associated with neutropenia or thrombocytopenia, 18 (43%) had received other chemotherapy while of 58 courses not associated with myelosuppression only 18 (31%) had received other chemotherapy (Table 1). Furthermore, the incidence of myelosuppression was affected by the type of concomitant chemotherapy used: 51% with procarbazine versus 85% when cyclophosphamide, cytarabine, or doxorubicin was used alone or in combination.
Twenty one percent of the courses had infectious complications: 11 instances of catheter-related blood stream infection, 1 of which included concomitant cellulitis at the site of insertion of a central catheter; 3 episodes of neutropenic fever; 2 documented cases of clostridia difficile colitis; 2 urinary tract infections; 1 case each of oral genital herpes simplex virus infection and herpes zoster virus infection, and 1 episode of pneumonia. Oral mucositis developed in 17% courses (grade I during 3 courses and grade II and III during 7 courses each). Diarrhea occurred in 6% of the courses. Three patients were transferred to hospice care with progression of disease. Five patients died during hospitalization but the cause of death was attributed to progression of disease in all. Care was withdrawn in 1 patient with anaplastic lymphoma due to ongoing gastrointestinal bleed and urinary obstruction with post mortem examination revealing widely disseminated lymphoma. Care was withdrawn in 2 patients with CNSL and 1 with breast cancer associated brain metastases due to progressive deterioration of mental status and seizures. One patient with chronic lymphocytic leukemia admitted with fever and pulmonary infiltrates developed adult respiratory distress syndrome leading to withdrawal of care.
Between January 2000 and December 2011, 7 patients were treated with glucarpidase at the discretion of a treating physician and the results are shown in Table 2. Two patients required hemodialysis. Patient 2 was anuric with multisystem organ failure (which was present before administration of HDMTX) and hemodialysis was started when the serum creatinine was only 1.5 mg/dL because of fluid overload and respiratory failure. The patient expired from complications related to sepsis and multiorgan failure. In patient 6, hemodialysis was started because of worsening renal function. In 5 patients (patients 1, 3, 4, 5, and 7) glucarpidase was administered at MTX levels that were within the range and the timeframe observed in the group of patients who were treated with HDLCV and supported measures alone. Patient 1 received glucarpidase 32 hours after HDMTX because the initial 24 hours MTX level was erroneously reported as 500 μmol/L at 24 hours instead of the correct level of 50 μmol/L. Grade III or higher neutropenia developed in 3 patients and thrombocytopenia in 2 patients. In patient 2, pancytopenia was present before MTX administration.
Table 2.
Characteristics of patients who received glucarpidase after HDMTX administration
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Diagnosis | OS | NHL | OS | OS | OS | CNSL | OS |
| Age, years | 27 | 55 | 20 | 13 | 29 | 61 | 17 |
| MTX dose, g/m2 | 12 | 3 | 12 | 12 | 12 | 3.5 | 12 |
| Serum Creatinine, mg/dL, initial | 1.2 | 0.9 | 0.7 | 0.6 | 1.3 | 0.5 | 0.7 |
| Serum Creatinine, mg/dL, peak | 1.3 | 1.5 (dialysis) | 3.5 | 2.8 | 1.8 | 6.3 (dialysis) | 4.0 |
| MTX level, μmol/L at time of glucarpidase administration | 8.2 | 56.5 | 58 | 376 | 2.1 | 8.2 | 4.5 |
| Time after MTX administration | 32 | 72 | 36 | 28 | 75 | 144 | 72 |
| Days MTX level >0.01 μmol/L | 1 | 10 | 9 | 8 | 15 | 20 | 6 |
| HDLCV dose, g/day | 1.8 | 2.4 | 1.0 | 1.4 | 1.0 | 2.2 | 1.8 |
| Grade ≥III neutropenia, days | No | Present prior to MTX | No | 1 | No | 5 | 1 |
| Grade ≥III thrombocytopenia, days | No | Present prior to MTX | No | No | No | 2 | 4 |
| Complications | none | Sepsis, pneumonia, ARDS prior to MTX | none | Severe mucositis | Catheter-related bacteremia | Encephalopathy and seizures attributed to uremia and MTX toxicity | none |
ARDS=acute respiratory distress syndrome; CNSL=central nervous system lymphoma; HDLCV=high-dose leucovorin; HDMTX=high-dose methotrexate; MTX=methotrexate; NHL=non-Hodgkin’s lymphoma; OS=osteosarcoma.
DISCUSSION
In this study, 100 episodes of HDMTX associated renal dysfunction were treated with a strategy that included HDLV and aggressive hydration with alkaline solutions, without the use of glucarpidase. No mortality was directly attributed to MTX toxicity. All patients successfully cleared MTX to a level that allowed safe discontinuation of leucovorin. All patients recovered kidney function. This study compares well with recent reports that used glucarpidase to treat similar patients(5, 8–12). The demographic characteristics of the patients studied, including range of MTX dose, range of MTX levels, and degree of AKI, were similar to that in recent publications (Table 3).
Table 3.
Published series of HDMTX and acute kidney failure
| First author | Flombaum | Krause | Buchen | Schwartz | Widemann | Christensen | Scott | Present study |
|---|---|---|---|---|---|---|---|---|
| Year published | 1999 | 2002 | 2005 | 2007 | 2010 | 2012 | 2015 | |
| PMID | 10334548 | 12533039 | 15668713 | 18055849 | 20679598 | 22252903 | 25631103 | - |
| Patients studied, n (# of courses) | 13 | 8 | 65 | 43 | 100 | 20 | 26 | 88 (100) |
| Age, years, median (range) | 18 (8–80) | 55 (27–61) | 15 (1–72) | 54 (18–78) | 17 (1–82) | 12 (4–20) | 12 (4–20) | 51 (9–90) |
| Entry criteria | MTX level at 24 h ≥100 and 48 h ≥10 and 72 h ≥1 | MTX level at 36 h ≥10 or 42 h ≥5 or 48 h ≥3 or SCr >1.5 ULN or oliguria | MTX level at 36h ≥10 or 42h ≥5 or 48h ≥3 and s.cr >1.5 ULN or oliguria | MTX level at 42 h >5 or SCr >1.5 ULN and/or oliguria at 42 h and MTX level at 42h ≥1 or 48h >0.4 | MTX level at 42 h ≥10 or SCr ≥1.5 ULN or CrCl ≤60 and MTX level at 12 h ≥2 SD above mean | MTX level at 24 h ≥50 or 42 h ≥10 and SCr ≥1.5 baseline | MTX level at 24 h ≥50 and 42 h ≥10 and SCr ≥1.5 baseline | MTX level at 48 h ≥10 and/or 72 h ≥1 |
| Patients who received >1 dose of glucarpidase, n (%) | glucarpidase not used | 2 (25) | 9 (14) | 3 (7) | 38 (38) | 4 (20) | - | glucarpidase not used† |
| MTX dose, g/m2, median (range) | 10 (3.5–12) | (3–12) | (1–12) | (0.9–12) | 7.7 (0.4–12) | 5.7 (3.3–12) | 7.4 (2.5–12.4) | 3.5 (1–13) |
| MTX blood level, μmol/L, median (range) | 24 h: 164 (102–940) 48 h: 16 (10.5–190) 72 h: 6 (1.4–39) |
11.8 (6.4–138) | 12 (0.5–901) | 5.1 (0.4–166) | 17 (0.4–849) | 29 (1.3–591) | 39 (1.3–591) | 24 h: 69 (2.2–40) 48 h: 6.9 (1.3–64) 72 h: 2.0 (0.05–26) |
| Time from MTX infusion to glucarpidase use, hours, median (range) | glucarpidase not used | 29 (20–59) | 52 (25–178) | 56 (27–176) | thymidine rescue +: 96 (22–294) thymidine rescue−: 66 (22–192) |
46 (26–96) | 44 (26–95) | glucarpidase not used |
| SCr, mg/dL, median (range) | 2.0 (0.9–7.8) | 2.2 (1.2–4.6) | 2.0 (0.6–7.4) | 2.6 | 4.0 (0.8–12.7) | 2.2 (0.8–9.6) | 1.8 (0.7–4.8) | 1.8 (0.7–8.1) |
| Time to recovery of kidney function, days, median (range) | (0–25) | - | - | (12–36) | 22 (5–77) | 21 (7–56) | 18 (2–54) | Number of days SCr ≥1.5 baseline: 7 (0–43) |
| Received other cytotoxic chemotherapy | 15% | 100% | - | 55% | 19% | - | - | 36% |
| Grade III/IV myelosuppression | Neutropenia: 62% | 63% | - | 60% | 43% | 15% | - | 42% |
| Infection | - | - | 33% | 16% | 19% | 20% | - | 21% |
| Grade III/IV mucositis | 46% | 13% | 15% | 35% | 22% | 5% | - | 14% |
| Deaths related to MTX | 0% | 0% | 6% | 23% | 6% | 0% | - | 0% |
CrCl=creatinine clearance; HDMTX=high-dose methotrexate; MTX=methotrexate; SCr=serum creatinine; SD=standard deviation; ULN=upper limit of normal.
Comment:
Additional seven patients who received glucarpidase in the current study are described in the text and in the Table 2.
Regarding nephrotoxicity, there was a significant and inverse relationship between lower MTX dose and the severity of HDMTX-induced AKI. This could have been related to the fact that 50% of the patients were 51 years old or older, and sicker and older patients tend to receive lower doses. Elderly individuals may be more prone to AKI from HDMTX as reflected by an incidence of grade ≥III nephrotoxicity of 4% to 5% in elderly patients with CNSL versus 0.6% in young patients with osteosarcoma.(13–15)
The MTX in the renal collecting system likely is unaffected by the administration of glucarpidase as it only acts on extracellular MTX.(16) This fact is reflected in another study where a similar rate of renal recovery was observed, as in other reports.(5, 10–12) Although in 3 other studies the median MTX levels at 48–96 hours were higher, the range of MTX levels were similar to the levels in this study.(5, 11, 12)
No correlation between the MTX levels at 24 hours and subsequent evidence of delayed MTX clearance was observed, as reflected by the MTX levels at 72, 96, and 120 hours (Figure 6). Similarly, there was no correlation between MTX levels at 24 hours and increases in creatinine levels, although the MTX levels at 48 and 72 hours correlated with the severity of AKI (Figure 5). These findings suggest that MTX levels obtained during the first 24 hours after MTX administration are not predictive of subsequent high MTX levels and should not be used as the basis for glucarpidase administration. Furthermore, high MTX levels during the first 24 hours prior to HDLV have been reported to produce minimal toxicity (1). Of note, in the reports where glucarpidase was used, this agent was often administered during the first 24 hours. Even after the first dose, additional doses of glucarpidase were given to some patients, although the MTX levels fell well within the range reported here.
The incidence of myelosuppression, mucositis, diarrhea, and infections was comparable to the studies where glucarpidase was used. Furthermore, 36% of the courses received concomitant cytotoxic chemotherapy, which by itself can be responsible for or exacerbate myelosuppression and mucositis. In the studies that reported statistics regarding the use of additional chemotherapy, the incidence of infection, grade ≥III mucositis, and myelosuppression were similar or higher than in the present study. Despite the older age of the patients, no deaths were directly attributed to MTX toxicity, although even when glucarpidase is used, death rates of up to 23% have been reported in the literature.(5, 9, 10)
The timely administration of adequate doses of HDLV is essential, no matter what other modalities are employed. In the published experience with glucarpidase, elderly patients, patients who received concomitant cytotoxic chemotherapy, and those who received delayed and/or inadequate dosing of HDLV had higher rates of systemic toxicity and death (Table 3). In the study by Widemann and colleagues, (5) the correlation between inadequate dosing of HDLV and death was one, and for this reason, HDLV dosing was omitted from the multivariable analysis assessing the factors predicting success for the use of glucarpidase. Administration of glucarpidase results in a rapid decrease in plasma MTX levels, but there are no data on its effect on the intracellular compartment. Administration of glucarpidase requires interruption of HDLV administration, increasing the risk of MTX toxicity during that interruption, and makes measurement of plasma MTX levels unreliable, increasing the complexity of management. In addition, glucarpidase is an extremely expensive drug with an estimated cost of approximately $108,000 per dose in a 70 kg individual.(3)
CONCLUSION
These results suggest that glucarpidase use in the setting of HDMTX-associated renal failure and delayed MTX excretion should be limited for patients with MTX levels above the range observed in this study or for patients who have oliguric renal failure, where the strategy of HDLV and aggressive alkaline hydration cannot succeed. For patients with nonoliguric renal dysfunction and with MTX levels similar to those in this study, avoidance of glucarpidase appears to be safe in a significant number of patients as long as HDLV is initiated within the first 48 hours and according to published guidelines (1, 17).
Acknowledgments
FUNDING SUPPORT
This research was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Presented as a Poster: American Society of Nephrology Annual Meeting, Chicago, IL, November 15–20, 2016
References
- 1.Ackland SP, Schilsky RL. High-dose methotrexate: a critical reappraisal. J Clin Oncol. 1987;5(12):2017–31. doi: 10.1200/JCO.1987.5.12.2017. [DOI] [PubMed] [Google Scholar]
- 2.Pinedo HM, Zaharko DS, Bull JM, Chabner BA. The reversal of methotrexate cytotoxicity to mouse bone marrow cells by leucovorin and nucleosides. Cancer Res. 1976;36(12):4418–24. [PubMed] [Google Scholar]
- 3.Cavone JL, Yang D, Wang A. Glucarpidase intervention for delayed methotrexate clearance. Ann Pharmacother. 2014;48(7):897–907. doi: 10.1177/1060028014526159. [DOI] [PubMed] [Google Scholar]
- 4.Widemann BC. Practical considerations for the administration of glucarpidase in high-dose methotrexate (HDMTX) induced renal dysfunction. Pediatr Blood Cancer. 2015;62(9):1512–3. doi: 10.1002/pbc.25577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widemann BC, Balis FM, Kim A, Boron M, Jayaprakash N, Shalabi A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28(25):3979–86. doi: 10.1200/JCO.2009.25.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widemann BC, Schwartz S, Jayaprakash N, Christensen R, Pui CH, Chauhan N, et al. Efficacy of glucarpidase (carboxypeptidase g2) in patients with acute kidney injury after high-dose methotrexate therapy. Pharmacotherapy. 2014;34(5):427–39. doi: 10.1002/phar.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flombaum CD, Meyers PA. High-dose leucovorin as sole therapy for methotrexate toxicity. J Clin Oncol. 1999;17(5):1589–94. doi: 10.1200/JCO.1999.17.5.1589. [DOI] [PubMed] [Google Scholar]
- 8.Krause AS, Weihrauch MR, Bode U, Fleischhack G, Elter T, Heuer T, et al. Carboxypeptidase-G2 rescue in cancer patients with delayed methotrexate elimination after high-dose methotrexate therapy. Leuk Lymphoma. 2002;43(11):2139–43. doi: 10.1080/1042819021000032953. [DOI] [PubMed] [Google Scholar]
- 9.Buchen S, Ngampolo D, Melton RG, Hasan C, Zoubek A, Henze G, et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92(3):480–7. doi: 10.1038/sj.bjc.6602337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz S, Borner K, Muller K, Martus P, Fischer L, Korfel A, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12(11):1299–308. doi: 10.1634/theoncologist.12-11-1299. [DOI] [PubMed] [Google Scholar]
- 11.Christensen AM, Pauley JL, Molinelli AR, Panetta JC, Ward DA, Stewart CF, et al. Resumption of high-dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients. Cancer. 2012;118(17):4321–30. doi: 10.1002/cncr.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott JR, Zhou Y, Cheng C, Ward DA, Swanson HD, Molinelli AR, et al. Comparable efficacy with varying dosages of glucarpidase in pediatric oncology patients. Pediatr Blood Cancer. 2015;62(9):1518–22. doi: 10.1002/pbc.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang-Xuan K, Taillandier L, Chinot O, Soubeyran P, Bogdhan U, Hildebrand J, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21(14):2726–31. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Jahnke K, Korfel A, Martus P, Weller M, Herrlinger U, Schmittel A, et al. High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol. 2005;16(3):445–9. doi: 10.1093/annonc/mdi075. [DOI] [PubMed] [Google Scholar]
- 15.Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222–32. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 16.Fermiano M, Bergsbaken J, Kolesar JM. Glucarpidase for the management of elevated methotrexate levels in patients with impaired renal function. AJHP. 2014;71(10):793–8. doi: 10.2146/ajhp130483. [DOI] [PubMed] [Google Scholar]
- 17.LaCasce AS. Therapeutic use and toxicity of high-dose methotrexate. In: Maki R, Freewman AS, Pappo AS, editors. UpToDate [Internet] Waltham, MA: UpToDate Inc; [cited March 8, 2018]. Available from: https://www.uptodate.com/contents/therapeutic-use-and-toxicity-of-high-dose-methotrexate?search=therapeutic%20use%20of%20MTX&source=search_result&selectedTitle=5~150&usage_type=default&display_rank=5. [Google Scholar]