Abstract
The advent of combination antiretroviral therapy (cART) has transformed HIV-1 infection into a controllable chronic disease, but these therapies are incapable of eradicating the virus to bring about an HIV cure. Multiple strategies have been proposed and investigated to eradicate latent viral reservoirs from various biological sanctuaries. However, due to the complexity of HIV infection and latency maintenance, a single drug is unlikely to eliminate all HIV reservoirs and novel strategies may be needed to achieve better efficacy while limiting systemic toxicity. In this review, we describe HIV latency in cellular and anatomical reservoirs, and present an overview of current strategies for HIV cure with a focus on their challenges for clinical translation. We then provide a summary of nanotechnology solutions that have been used to address challenges in HIV cure by delivering physicochemically diverse agents for combination therapy or targeting HIV reservoir sites. We also review nanocarrier-based gene delivery and immunotherapy used in cancer treatment but may have potential applications in HIV cure.
Keywords: HIV-1, HIV reservoirs, nanotechnology, antiretroviral drugs, latency reversing agents, gene therapy, immunotherapy, combination therapy, targeted nanocarriers
Graphical abstract
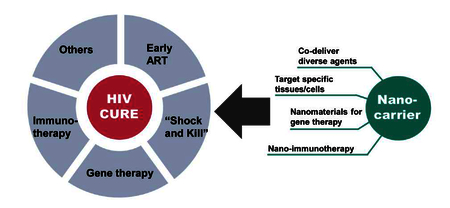
1. Introduction
At the end of 2016, the toll of the HIV/AIDS pandemic included 36.7 million infections and 1 million deaths [1]. The combination of antiretroviral drugs (ARVs) for HIV treatment, also referred to as highly active or combination antiretroviral therapy (HAART, cART), can successfully suppress viral replication in plasma to undetectable levels. These treatments have greatly extended the life span and improved the quality of life for people living with HIV-1 infection. However, none of these therapies are capable of eradicating the virus from long-lived cellular reservoirs, which leads to virus rebound once treatment is stopped. Moreover, a lifetime of cART is expensive and causes both short- and long-term side effects such as cardiovascular diseases, neurocognitive disorders, and liver injury [2–5].
Currently, there is no cure for HIV/AIDS, but a singular success has proven that a cure is possible and has galvanized the field [6]. The “Berlin Patient” describes the clinical case study of Timothy Ray Brown who underwent treatment for acute myeloid leukemia (AML), and was cured of both AML and HIV after transplantation with CCR5-deficient hematopoietic stem cells that are inherently resistant to HIV infection [7]. Chemotherapy and radiotherapy was used to eradicate all leukocytes including AML cells. Stem cell transplantation was used to reconstitute the immune system, which also effectively eliminated the need for cART to control plamsa viremia to undetectable levels. This remarkable case bolstered support for HIV cure research, but considerable and sustained efforts are needed to develop clinical approaches that can be applied safely and effectively with the lowest barriers to accessing healthcare.
Viral reservoirs are the primary obstacle that must be overcome to realize an HIV cure. These reservoirs can be defined as cellular sites (e.g. long-lived infected memory CD4+ T cells, macrophages) where viral replication in infected cells is arrested, and anatomical sites (e.g., gut-associated lymphoid tissue, lymph node, genital tract, central nervous system) that harb these latently infected cells [8]. These tissues exhibit limited access to ARVs which may contribute to viral persistence [9, 10]. Numerous approaches are aimed at finding and diminishing these HIV reservoirs. For example, a number of studies have suggested that early initiation of cART during acute infection could be a realistic approach to cure HIV at the population level as it may block the initial establishment of latent reservoirs [11, 12]. Another approach focuses on reactivation of HIV from latently infected cells by use of latency reversing agents (LRAs). Once these latent cells express viral antigen, they may become vulnerable to the immune system or other therapeutics that result in their elimination [13]. However, clinical studies of LRAs have failed to reduce the reservoir size, which might be due to insufficient potency of LRAs, off-target effects in uninfected cells leading to dose-limiting toxicities, or lack of effective “kill” strategies [14]. Cell and gene therapies have also been investigated for HIV cure, where immune cells have been engineered to be resistant to HIV, or HIV proviral DNA has been targeted for excision from latently-infected cells [15]. Other strategies such as broadly neutralizing HIV antibody (bnAb) [16–18], permanently silencing the HIV provirus [19], and anti-proliferative therapy have also been investigated [20].
Due to the complexity of HIV pathogenesis, more efficient therapeutic combinations and novel reservoir-targeted drug delivery approaches may be needed to optimize current approaches for eradicating latently infected cells. Nanocarrier drug delivery systems have unique attributes that are ideally suited to address challenges with targeting the HIV reservoir and eradicating the latent virus to realize an HIV cure. This technology has already shown enormous potential for use in the diagnosis and treatment of several diseases, with a major emphasis in cancer. Nanocarrier attributes that may be ideally suited for HIV cure strategies include: (1) improvement of bioavailability and pharmacokinetic (PK) profiles of cure agents [21]; (2) reduction of drug toxicity [22], and avoidance of surface efflux pump mediated drug resistance [23]; (3) enhanced or synergistic efficacy through combination of multiple drugs in a single particle [24]; (4) ability to penetrate into HIV reservoir sites such as lymphatic tissues or to target specific vulnerable cells such as CD4+ T cells [25–29]; (5) ability to release therapeutics in controlled rates for sustained drug delivery [30, 31].
In this review, we will describe HIV sanctuaries and the current strategies toward diminishing HIV reservoirs and provide an overview of current and future nanotechnology approaches to address HIV cure.
2. HIV reservoirs are the obstacle to a cure
The main obstacle to HIV eradication is the existence of reservoirs of latently infected cells. Two models have been proposed to explain the establishment of latency in long-lived memory cells. The pre-activation latency model hypothesizes that HIV can directly infect resting CD4+ T cells [32, 33], and the post-activation latency model relies on an idea that the activated CD4+ memory T cells revert to a resting state instead of cell death after being infected by HIV [34]. HIV viral DNA integrates at various locations into the genome of these long-lived host cells [35], and the integration at specific sites (e.g. MKL2 and BACH2) has been attributed to cell clonal expansion and therefore persistent infection [36, 37]. HIV latency is maintained under several molecular mechanisms including: (1) the recruitment of histone deacetylases (HDACs) and histone methyltransferases (HMTs) to HIV-1 long term repeat (LTR), leading to histone deacetylation and methylation on Nuc-0 and Nuc-1, which can restrict the initiation of transcription [38, 39]; (2) sequestration or inactivation of transcription and elongation factors such as nuclear factor κB (NF-κB) and NFAT, or positive transcription elongation factor b (P-TEFb) that are important for initiating or elongating transcription [40, 41]; and (3) other mechanisms such as HIV-1 DNA methylation [42], post-transcriptional blocks [43], and cellular microRNAs that inhibit HIV-1 production [44] Compared to virus-producing CD4+ T cells that have a half-life of only 0.7–1.1 days [45], latently-infected CD4+ T cells have a half-life ranging from 4.6 to 44.2 months based on different studies in patients receiving ART [46–48]. These long-lived infected CD4+ T cells with the ability of clonal expansion keep proliferate without releasing virus, leads to their high stability and persistence in sanctuary sites [49]. The variability of integration sites, complexity of latency maintenance, and clonal expansion of long-lived reservoirs are all challenges for the HIV cure field.
HIV latency can be found in different cell types and tissue compartments, which presents additional barriers to curative strategies (Figure 1) [8]. Cellular reservoirs include a wide-range of cell types that are susceptible to HIV infections, such as memory CD4+ T lymphocytes and macrophages. These cells are found in the peripheral blood, but also in anatomical reservoirs that include lymph nodes (LNs), spleen, gut-associated lymphoid tissue (GALT) and brain or central nervous system (CNS).
Figure 1.
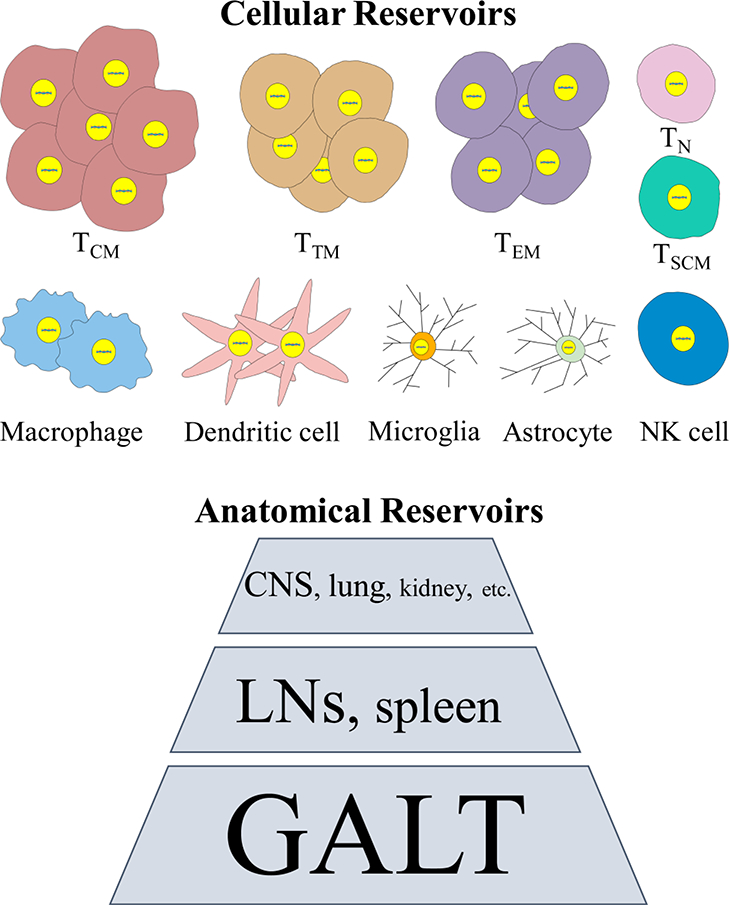
Schematic illustration on major HIV cellular and anatomical reservoirs. The relative size of the reservoir is represented by the size of the compartment as shown. TCM, central memory CD4+ T cells; TTM, transitional memory CD4+ T cells; TEM, effector memory CD4+ T cells; TN, naïve CD4+ T cells; TSCM, CD4+ T memory stem cells; NK cell, natural killer cell; CNS, central neural system; LNs, lymph nodes; GALT, gut-associated lymphoid tissue.
HIV proviral DNA is detected primarily in central memory (TCM), transitional memory T cells (TTM), and effector memory T cells (TEM) which maintain latency and persistence through clonal expansion [49–51]. In particular, a subset of TCM called peripheral T follicular helper cells (pTfh cells) are highly susceptible to HIV infection and contribute to HIV persistence [52]. CD4+ T memory stem cells (TSCM) also significantly contribute to reservoir expansion and viral persistence because these cells are long-lived and differentiate into mature memory T cells, although their proportion in circulating lymphocytes is only 2–4% [53, 54]. Macrophages are also a chronic and latent HIV reservoir in infected patients that contributes to disease progression [55]. Macrophages are antigen presenting cells (APCs) that have been shown to spread virus particle to bystander CD4+ T cells [56], as well as recruit and activate latently-infected CD4+ T cells through chemokine and virus antigen production [45] Macrophages are present in almost all organ systems, and can transmit virus to different anatomical sites including the brain (e.g., microglia as the resident macrophage in the brain and spinal cord) [58]. Infected macrophages have a much longer half-life (>30 days) and are less prone to cytopathic effects of the virus compared to CD4+ T cells [59, 60]. In particular, HIV-infected microglia show a strong correlation with HIV-associated neurological symptoms due to neurotoxin secretion and attraction of adaptive immune responses to the brain that cause neural damage [58, 61]., Dendritic cells (DCs) are another type of APC that can be infected directly by HIV-1, although less efficiently than CD4+ T cells, but can transmit virus to T cells [62, 63]. It has also been suggested that DC subtypes in the LNs, but not the peripheral blood, act as long-lived HIV reservoirs [64, 65].
The major anatomical sites that harbor infected cells include lymphatic tissues (LNs, spleen, and GALT), CNS, and lung (Figure 1) [66]. HIV-1 or cells producing viral RNA (vRNA+ cells) can still be detected in many of these tissues of patients whose viral loads in the peripheral blood are undetectable [10]. Estes et al. have reported that in an NHP model, ~98.6% vRNA+ cells reside in lymphatic tissues (LNs, spleen, and GALT) [66]. Ineffective viral suppression in these tissues may be due to poor immune surveillance, less efficient drug penetration, high CD4+ T cell density that favors cell-to-cell HIV transmission, and interactions with autologous B cells or dendritic cells [67–69]. A clear majority of cells that contain HIV proviral DNA (vDNA+ cells) are found in lymphoid tissues harboring significant numbers of memory T cells. A frequency on the order of ~105 vDNA+ cells/g was detected in both LN and GALT from patients under ART [66]. The GALT is one of the largest lymphoid organs infected by HIV-1 [70, 71] estimated to contain ~62.3% of vRNA+ cells before ART and ~98.0% after the therapy in an NHP model [66]. Distinct from other lymphatic tissues, the frequency of vDNA+ cells in GALT does not decline after ART, leaving it as the largest sanctuary for HIV latency. The gut mucosal CD4+ lymphocytes are uniquely susceptible to HIV infections due to higher expression of chemokine receptor CCR5 and high levels of activation [72]. In addition, HIV-1 infected CD4+ T cells from other parts of the body regardless of the primary infection route can traffic to the GALT mediated by α4β7 and CCR9 [73, 74]. It has also been reported that resting central memory α4β7+CD4+ cells are preferential targets of simian immunodeficiency virus (SIV) and contribute to reservoir establishment and expansion in mucosal sites [75]. Aside from these lymphatic tissues, the CNS is another key anatomical reservoir for HIV-1. The virus enters the brain at the early stage of infection and is difficult to eradicate, making the brain a lifelong virus pool Clinically, most ARVs have limited penetration into the CNS due to tight junctions of the blood-brain barrier (BBB) and transmembrane proteins that exist on the BBB to pump the drug out of the brain [77]. Other tissues such as lung and kidney have also been regarded as HIV reservoirs although they harbor much less proportions of latently infected cells [78, 79].
3. Current strategies toward diminishing HIV reservoirs
With the recognition that viral reservoirs are major barriers to an HIV cure, several therapeutic strategies toward finding and diminishing latently infected cells have become the focus of recent research efforts. These cure strategies include early cART, reactivation of latently infected HIV reservoirs, gene therapy, and immunotherapy.
3.1. Minimizing the size of HIV reservoirs by early cART
Many studies suggest that initiation of ART during the acute phase of infection could be an effective first step toward achieving sustained virologic remission. HIV latency is likely established early within days of acquiring infection although the specific timing in human is still uncertain [80, 81]. Studies in non-human primates (NHPs) have observed high levels of integrated SIV DNA in resting CD4+ T cells within 10 days post-infection, but the seeding of the reservoir is thought to occur as early as 3 days. [82–84]. Patient initiated early ART has resulted in observed faster decay of HIV reservoirs and better preserved immunity against HIV compared to those who delayed ART [11, 46, 48, 85–87]. For example, the VISCONTI study investigating 14 patients who received ART during the acute-early phase of infection found that plasma viremia remained controlled for prolonged periods of time after cessation of therapy [12]. In a clinical case study known as the “Mississippi baby”, an infant born to an HIV-positive mother had ART initiated at 30 hours from birth but discontinued therapy at 18 months of age and remained aviremic for more than 2 years [88]. Unfortunately, the child’s plasma viremia ultimately rebounded and ART had to be reinitiated [89]. Aside from impeding the initial seeding and development of reservoirs, it has been suggested that early ART could also reverse chronic mucosal and systemic immune activation, which is the hallmark of HIV infection, contributes to reservoir size, and viral distribution through preservation of mucosal Th17 cells [90]. While multiple studies have shown that early ART can successfully suppress HIV in peripheral blood to undetectable levels, it has shown limited effect in inhibiting viral replication in lymphatic tissues such as intestinal tissues and lymph nodes which may contribute to viral rebound [91, 92]. Collectively, the data suggests that early ART alone may not be sufficient to achieve virologic remission. The practicality of initiating ART at the population level during the early phase of HIV infection also remains a challenge. As such, future direction may need to focus on combination therapy with other cure strategies, such as latency-reversing agents, therapeutic vaccines, or immune-modulating agents, as well as increase drug concentration in anatomical reservoir sites [81].
3.2. Reactivating HIV reservoirs by LRAs
Viral rebound normally occurs within 2–3 weeks after the interruption of ART, mainly due to viral reactivation in latently infected resting CD4+ T cells as well as other cellular reservoirs [93]. Several strategies for reducing the reservoir size have focused on targeting these cells. A widely investigated approach that has reached the clinic known as “shock and kill” reactivates HIV-1 proviruses while maintaining ART in order to prevent the spread of new infections and the establishment of new cellular reservoirs. These reactivated cells are then eliminated by viral cytopathic effects, the host immune response, or by a combination of therapies involving therapeutic vaccines, neutralizing antibodies or immune checkpoint blockade agents [94]. Several classes of LRAs have been identified and developed to overcome obstacles to transcription of the HIV-1 provirus that leads to maintenance of latency (Table 1). HDAC inhibitors (HDACi) unleashes the repression of viral LTR that is maintained by histone deacetylase to allow transcription of provirus [95]. While these drugs are effective and do not lead to global T cell activation, they may affect expression of a large numbers of genes [96]. HMT inhibitors (HMTi) similarly activate viral LTR through inhibition of histone H3 methylation [39]. HMTi can enhance the potency of HDACi but most agents are still under preliminary studies [97]. Protein kinase C (PKC) agonist activates NF-κB which binds to LTR to increase proviral transcription [98, 99]. Many agents from this class are highly potent, but have major concerns such as nonspecific induction of many genes, toxicity, and tumor-promoting potential. P-TEFb activators, such as bromodomain extra-terminal (BET) inhibitor JQ1, enhance interaction of P-TEFb with LTR to activate transcription [100]. JQ1 is also advantageous as it suppresses T cell proliferation and activation, which may be beneficial for cure therapy. DNA methyltransferase inhibitors function based on the role of DNA methylation on HIV latency [101]. This role is still controversial, and latency reversal from such inhibitors is relatively weak but could be strengthened when combined with other agents. Some agents may act based on multiple mechanisms. For instance, SAHA as an HDACi also activate latency through the activation of PI3K/Akt pathways [102]. Also, some short chain fatty acids induce latency by activating P-TEFb as well as inducing multiple histone modifications [103].
Table 1.
Major latency reversing agent (LRA) categories and their representatives
| Categories | Representative Agents |
Clinical trial number* | Reference |
|---|---|---|---|
| HDAC inhibitor | Vorinostat (SAHA) |
NCT01365065; NCT03198559; NCT02707900; NCT01319383; NCT03212989; NCT02475915; NCT02336074 |
[102, 104–107] |
| Panobinostat | NCT01680094; NCT02471430 | [108] | |
| valproic acid (VPA) |
NCT00312546; NCT00614458; NCT00000629; NCT00289952 |
[109, 110] | |
| romidepsin |
NCT02616874; NCT02092116; NCT01933594; NCT03041012; NCT02850016 |
[111–113] | |
| chidamide | NCT02513901; NCT02902185 | [114] | |
| sodium butyrate | Not tested | [115] | |
| HMT inhibitor | BIX01294 | Not tested | [116] |
| Chaetocin | Not tested | [39] | |
| PKC agonist | prostratin | Not tested | [117, 118] |
| bryostatin-1 | NCT02269605 | [119, 120] | |
| ingenol derivatives |
Not tested | [99, 121, 122] |
|
| P-TEFb activator | hexamethylene bisacetamide |
Not tested | [123] |
| JQ1 | Not tested | [100] | |
| DNA methyltransferase inhibitors |
decitabine and its analog azacytidine |
Not tested | [124] |
| unclassified | disulfiram |
NCT00878306; NCT01944371; NCT03198559; NCT01286259; NCT00002065 |
[125–127] |
source: clinicaltrials.gov
HDAC, histone deacetylase; HMT, histone methyltransferase; PKC, protein kinase C; P-TEFb, positive transcription elongation factor b.
Several HDACi, (e.g., vorinostat [104–107], panobinostat [108], romidepsin [111], valproic acid [109] and chidamide [114]), disulfiram [126, 127], and bryostatin-1 [119] have already been tested in clinical trials (Table 1), and other agents are under ongoing clinical trials (reviewed in [14, 128]). Many clinical studies of LRAs have shown an increase in cell-associated or plasma HIV RNA in CD4+ T cells. This is with the exception of bryostatin-1, where no effect on PKC activity or HIV latency reversal was observed, which may due to low plasma concentrations after a single dose [119]. However, while all these LRAs have shown ability to reactivate HIV in patients, none have shown a reduction on the HIV reservoir size [14, 129]. This might be due to a lack of effective “kill” approaches used in these clinical trials, as only ART has been incorporated so far to suppress viral spreading. Only few “kill” strategies such as broadly neutralizing antibodies have been tested in combination with LRA in humanized mice [130]. It has been shown that some of these LRAs act synergistically to enhance potency when used in combination. The combination may also reduce the dose of single LRAs to minimize their toxicity. For example, HMTi can enhance proviral reactivation by HDAC inhibitors such as SAHA [97]. Recent studies from several groups have shown that protein kinase C activators such as prostratin, bryostatin 1, or ingenol-B synergistically and robustly induced HIV-1 transcription and virus production from in vitro or ex vivo models when combined with bromodomain inhibitor (also known as pTEFb-releasing agents as described above) or HDACi, [121, 131–134].
“Shock and Kill” is still a controversial strategy especially based on recent safety issues and unenthusiastic outcomes from clinical studies. As such, whether or not a single LRAs is able to significantly reduce the size of HIV reservoirs is still questionable. A stronger focus is now on the use of LRAs combinations and combination with other therapeutics, as well as novel drug delivery systems to enhance therapeutic effects, avoid global immune cell activation, and reduce off-target side effects.
3.3. Gene-editing approaches
Gene-editing technologies are also being investigated for HIV cure strategies [15]. Gene-editing enzymes such as homing endonucleases, evolved recombinases, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR/Cas9 have been used to directly excise HIV provirus from the host genome and have proven effective in culture with cell lines and primary cells [135–138]. Recent studies in humanized mouse models have shown successful proviral excision in most major organs after single intravenous administration of Staphylococcus aureus Cas9 (saCas9) and multiplex single-guide RNAs (sgRNAs) using adeno-associated virus (AAV) [139, 140]. However, due to various integration sites of latent virus and low percentage of latently infected cells, off-target effects and poor gene delivery efficiency remain big challenges towards clinical trials.
Gene-editing tools have also been used to knock out CCR5 in CD4+ T cells or hematopoietic stem/progenitor cells (HSPCs) in order to block virus entry [141], one of the mechanisms underlying the “Berlin Patient” case. ZFNs have been used to modify autologous CD4+ T cells and tested in HIV-infected patients [142]. However, patients that completed analytical treatment interruption (ATI) all had viral rebound after the therapy, even though the therapy resulted in a slower decay rate for the CCR5-modified CD4+ T cells compared to unmodified cells. A challenge for this approach is the low frequency of modified CD4+ T cells that are adoptively transferred, which likely limits its effect. CRISPP/Cas9 has been used successfully to disrupt CCR5 as well as CXCR4 on primary CD4+ T cells in vitro [143–145]. Gene-editing of primary CD4+ T cells in vivo is also likely to result in a low frequency of modified cells and would be associated with relatively high off-target effects. Disrupting CCR5 on HSPCs, which gives rise to all cell lineages that HIV-1 infects, has the advantages of producing longer-term effects than targeting CD4+ T cells. ZFNs or CRISPR/Cas9 have been used to modify HSPCs, showing successful CCR5 disruption and anti-HIV effect in reconstituted or transplanted mice [146–148]. Similarly, induced pluripotent stem cells (iPSCs) have been modified and can be differentiated into HIV-resistant monocytes/macrophages in vitro [149–151]. Aside from generating HIV-resistant immune cells, human hematopoietic stem cells (HSCs) have also been induced to HIV-specific cytotoxic T lymphocytes to inhibit viral replication in vivo [152].
Permanently silencing the non-expressing provirus in latently infected cells has been investigated using different strategies. RNA interference (RNAi) with small interfering RNA (siRNA) silences the expression of viral RNA or cellular mRNAs that are necessary for viral production, and has been shown to control viral replication (reviewed in [19]). HIV-1 encoded genes such as tat, rev, vif, gag, nef, pol, env, vpr that are important for viral replication and are targets for siRNA silencing.
siRNAs have potential to overcome the high rate of HIV mutation through targeting highly conserved sequences [153]. These agents may offer an effective and safe approach towards an HIV cure, but still have challenges for reaching the clinic such as low delivery efficiency and instability. Challenges with gene editing strategies have included delivering genes or editing enzymes specifically to latently infected resting CD4+ T cells, off-target effects, and insufficient activity of enzymes that reach target cells in vivo [154]. These issues will collectively impact the efficacy and safety of gene editing therapies for HIV cure.
3.4. Immunotherapy
HIV therapeutic vaccines have focused on inducing HIV-1 specific CD8+ T cells responses to selectively kill virus-producing cells [155, 156], and ultimately control the disease. Several therapeutic vaccines have been tested on HIV-1 infected individuals including vector-based vaccines that express HIV-1 antigens from harmless or attenuated viruses and plasmid DNA vaccines [157–160], but most have failed to show sufficient efficacy and some have raised safety concerns. Nonetheless, several promising vaccines have been tested in preclinical studies and will go into clinical trials in the near future. For example, a vaccine based on cytomegalovirus (CMV) has been investigated and showed induction of broad cellular immune responses against novel epitopes resulting in efficient control of pathogenic SIV infection [161, 162].
The use of broadly neutralizing antibodies (bnAbs) has also attracted attention as a therapeutic to prevent and treat HIV infection based on promising animal and human data. These bnAbs are also regarded as a potential cure strategy based on their antiviral activity as well as their ability to reduce reservoir size [163]. For the treatment of HIV, bnAbs have demonstrated remarkable efficacy in reducing viral load through clearance of free virus, elimination of infected T cells, as well as reduction of proviral DNA in the LN and GALT [16–18, 163, 164]. bnAbs can also act as vaccines by enhancing autologous neutralizing antibody responses [165], or boosting and broadening humoral immunity [166]. Another strategy combines bnAbs and LRAs showed reduced viral rebound from the reservoir in established infections in humanized HIV-infected mice [130]. Currently, bnAbs still have challenges such as limited accessibility to certain anatomical reservoirs, as well as possible hurdles for clinical application related to their bioavailability, PK profile and high cost.
Many regulatory pathways designed to blunt cell activation are turned on during chronic infection, and one of the well-characterized immune regulators is the programmed cell death protein-1 (PD-1). For example, HIV-specific CD8 T cells that express high levels of PD-1 show functional exhaustion with low proliferation and cytotoxic effects [167]. The ligands for PD-1 (e.g., PD-L1 and PD-L2) are widely expressed in tissues, and inhibitors or blockades of the PD-1 pathway result in restoration of T cell function [168]. Thus, the blockades may enhance immune responses to clear chronic viral infections [169]. It has been demonstrated that the size of the reservoirs is positively correlated with the frequency of PD-i expressing cells [49, 170]. PD-1 inhibitors have been administered into SIV-infected macaques, and induce a significant viral load reduction and prolong survival [171]. It has been further shown that PD-1 blockade reduces hyperimmune activation in the blood and colorectal tissue and decreases microbial translocation [172].
Chimeric antigen receptor (CAR) T cell therapy has shown great promise in treating multiple cancers such as leukemia [173], and is also being investigated for HIV cure. The immunotherapy uses gene-editing tools to engineer T cells to express receptor on their surface in order to recognize and bind to specific target cells and then mediate cell lysis [174]. Previously, this technology has been applied to engineer CD8+ cytotoxic T cells to bind to and lyse HIV-infected CD4+ T cells [175], but has shown limited efficacy in clinical studies A recent study induced CAR onto HSPCs to consistently generate CAR T cells against SHIV infection in an NHP model of suppressive SHIV that are relevant to HIV cure research. They observed long-term (> 2 years) and stable production of CAR T cells in multiple lymphatic tissues, as well as a lower viral rebound after cessation of cART [177].
3.5. Other strategies
While early cART, reactivation of latently infected HIV reservoirs, gene therapy, and immunotherapy represent the most active areas of HIV cure research, several other approaches are also being actively pursued. One strategy known as “block and lock” is proposed to permanently inhibit transcription to prevent viral reactivation from latency, which may provide a functional cure for HIV infection. For example, ruxolitinib and tofacitinib are FDA-approved for myelofibrosis and rheumatoid arthritis (RA), respectively, and inhibit the JAK-STAT pathway [178, 179]. Another agent, didehydro-cortistatin A (dCA) binds to the HIV regulatory protein Tat and suppresses transcription [180, 181]. While most of these agents are still under preclinical studies, the JAK inhibitor ruxolitinib (clinicaltrials.gov # NCT02475655) is currently being tested clinically in a phase 2 study. There is still concern about whether or not these agents alone are sufficient enough to permanently block viral reactivation, and they may need to be integrated with other strategies to realize an HIV cure in the future.
An innovative idea in cure research hypothesizes that reducing the proliferation of long-lived latently infected T cells could deplete the reservoir. Reeves et al. predicted that substantial reductions in the reservoir size may result from modest but continuous decreases in the proliferation rate of latently infected CD4 T cells with the use of mathematical modeling of the HIV reservoir under suppressive cART [20]. They identified that sustained anti-proliferation and ART treatment could potentially induce a functional cure within 2–10 years, which is much shorter than is predicted to occur with LRA and ART treatment. Mycophenolate mofetil (MMF) or its active metabolite, mycophenolic acid (MPA) have been tested in patients, and clinical trials for evaluating its effect on reducing reservoir size are currently enrolling patients [20] (clinicaltrials.gov # NCT03262441).
A recent study treated SIV+ macaques with a monoclonal antibody against the α4β7 integrin in combination with ART, and observed normal CD4+ T cell counts as well as undetectable viral loads in both plasma and GALT, even after cessation of ART [182, 183]. α4β7 integrin is involved in trafficking of CD4+ T cells to GITs, where there are high levels of viral replication and viral reservoirs can be rapidly established [70]. CD4+ T cells that express high levels of α4β7 are also preferential targets of HIV during acute infection [74, 75, 184]. While the mechanisms by which α4β7 mAb therapy promoted virologic control remains to be defined, it is hypothesized that the antibody can block α4β7 binding to mucosal vascular addressin cell adhesion molecular 1 (MAdCAM-1) leading to interrupted cell migration to the GALT. It is noteworthy that α4β7 integrin is also a target for the HIV-1 envelop protein gp120, and binding leads to rapid activation of LFA that contributes to forming of virological synapses and facilitating cell-to-cell virus spreading [185]. More research efforts are needed to investigate this promising therapy for use in HIV treatment towards eradicating viral reservoirs.
4. Nanocarriers for eradicating HIV reservoirs
Many HIV cure strategies still face hurdles that limit their clinical translation. For example, clinical studies of LRAs have failed to meaningfully reduce reservoir size [42]. Sufficient reduction of the latent pool to curative levels may require mechanistically distinct LRAs used in combination. Many LRAs differ in solubility, bioavailability, and toxicity which make dosing and formulation challenging [131, 186]. Gene-based therapies must overcome challenges associated with off-target effects, low delivery efficiency, as well as safety issues especially when using viral-based vectors in vivo [187]. The complexity of HIV infection and latency may also require complimentary strategies for curing HIV, making it important but challenging to deliver physicochemically diverse cure agents simultaneously. HIV persistence in some anatomical sites has been attributed to lower concentrations of ARV drugs [10, 188], which could be addressed by novel drug delivery system to improve the bioavailability of cure therapeutics. New approaches are required to address these multiple complex molecular mechanisms associated with latent reservoir dynamics in cells and tissue compartments that present physiological barriers to therapeutic delivery in vivo. Nanotechnology has emerged as a promising approach for HIV cure due to several key attributes including ability to encapsulate diverse agents, increase circulation or tissue retention time, sustain drug release, enhance solubility and bioavailability, reduce toxicity or side effects, and enhance drug potency [22, 189]. Some nanocarriers can also be modified to target specific cells or tissues, which have shown broad applications in cancer therapy [190]. Various types of nanocarriers including liposomes, polymeric nanoparticles, solid lipid nanoparticles, micelles, dendrimers, and inorganic nanoparticles have been reported for ARV drug delivery (reviewed in [191]). Some prodrugs or analogs of ARV drugs have also been developed and used to enhance drug loading or extend half-life when encapsulated in nanocarriers [192, 193]. LRAs have been incorporated into nanocarriers for HIV cure applications and demonstrate CD4+ T cell specific reactivation both in vitro and in vivo [29, 194]. Here, we summarize current nanocarrier-based anti-HIV therapeutics and focus on strategies using drug combination or targeted nanocarriers to enhance drug potency and reduce toxicity. We also review nanocarrier-based gene delivery and immunotherapy approaches that have attracted more attention in cancer therapy and may have potential applications for curing HIV.
4.1. Combination therapy using nanocarriers
Based on multiple cellular mechanisms that suppress viral reactivation in latent cells and from results of preclinical studies, LRA combinations have been proposed to achieve a clinical cure by maximizing potency and minimizing toxicity [131]. However, there are several challenges that may limit such combinations using conventional drug formulations [195]. Co-dissolving of multiple hydrophobic drugs for injection may result in precipitation or aggregation, inaccuracy of dosing and risk of embolisms. Also, different drugs have their own bioavailability and PK profiles that make it more difficult to reach sufficient and safe concentrations in target tissues when delivered simultaneously.
Several nanotechnology-based systems have been developed to deliver incompatible drug combinations simultaneously, which is especially important and beneficial for both ARV and LRA therapies being considered for HIV cure. Table 2 summarizes recent studies that use nanocarriers to co-deliver multiple anti-HIV drugs to enhance their therapeutic effects. ARV drugs are physicochemically diverse and are used clinically in combination, but have been incorporated in nanocarriers for the dominant purpose of enhancing potency and prolonging drug residence time to reduce dosing frequency [24, 196–202]. For example, the Ho group has developed a long-acting triple-ARV drug combination using lipid nanoparticles that have shown enhanced drug exposure in primate blood and lymph nodes, as well as persistent drug levels in peripheral blood mononuclear cells (PBMCs) and lymph node mononuclear cells (LNMCs) [196, 197, 203]. The synergistic enhancement of antiviral activity may be attributed to higher intracellular ARV drug concentrations, which has been observed by several studies (Table 2). Similar to ARV drugs, LRAs are mostly small molecule compounds and are physicochemically and mechanistically diverse. Several LRAs have been incorporated into nanocarriers individually. For example, Buehler et al. have reported a self-assembling vault nanoparticle incorporating a PKC agonist bryostatin-1. They demonstrated its ability to reactivate latent virus in a human cell line and induce CD69 expression in primary human PBMCs and mice after intravenous administration. However, whether or not the nanoparticle had any effect on the potency of free drug was not investigated. From studies investigating co-delivery of ARV drug combinations, nanocarriers also have the potential to deliver LRAs in combination. To date, single LRAs have been co-delivered with ARVs using nanocarriers [29, 30, 204]. For example, Kovochich et al. loaded bryostatin-2 and a protease inhibitor nelfinavir into lipid nanoparticles. They observed that the nanoparticle formulation enhanced latency reactivation both in human T cell lines and PBMCs from humanized mice with the ability to control viral spreading. In another study, Jayant et al. established ultrasmall magnetic nanoparticles (MNPs) used a layer-by-layer process to incorporate tenofovir (ARV) and vorinostat (LRA) for reactivating and suppressing HIV in a sustained manner for 5 days in vitro [30]. These promising preclinical studies will need to demonstrate PK, safety and efficacy in vivo before advancing into human clinical studies. Prioritizing drug combinations that are safe and effective for HIV cure is also challenging since there is a large pool of multiple combinations that are difficult to test in complex, expensive models such as patient blood or HIV- or SIV-infected animal models. These examples demonstrate that nanocarriers can overcome challenges with co-formulating physicochemically diverse drugs. Due to thehigher potency of some combination drug therapies, dose-reduction could reduce toxicity that may be associated with single drugs. The toxicity associated with LRAs arises primarily from nonspecific induction and systemic release of cytokines [132]. Using biodegradable and non-toxic nanoparticles may further protect drug from degradation, increase circulation half-life and exhibit improved PK profiles, and lowering toxicity [22]. Thus, nanotechnology could provide alternative strategies to optimize latency reversal therapies. Future studies are being directed towards using a single nanocarrier to combine delivery of mechanistically distinct LRAs alone or co-formulated with other small molecule cure agents.
Table 2.
Nanocarriers incorporating combination therapeutics
| Combination agents | Nanosystems | Test model | Observed effects | Ref. |
|---|---|---|---|---|
| ARVs (loponavir, ritonavir, and tenofovir) |
Lipid nanoparticles | Nonhuman primates | Higher intracellular ARV concentrations in lymph nodes (50-fold higher than free drugs), long-acting plasma and lymphatic PK profiles |
[197, 198, 204] |
| ARVs (loponavir/ritonavir and efavirenz) |
Biodegradable nanoparticles |
In Vitro cell lines | Higher intracellular ARV concentration | [199] |
| ARVs (binary or trinary combination of maraviroc, etravirine and raltegravir) |
PLGA nanoparticles |
In Vitro cell lines, and ex vivo macaque cervicoviginal tissue |
Higher intracellular ARV concentration, enhanced antiviral activities in relevant in vitro and ex vivo models |
[24] |
| ARVs (tenofovir, alafenamide, and elvitegravir) |
PLGA nanoparticles | Mice | Long residence time and exposure for both drugs |
[200] |
| ARVs (atazanavir, ritonavir, and efavirenz) | NanoART |
In Vitro cell lines, ex vivo human primary cells, and in vivo in humanized mice |
Attenuated viral replication and preserved CD4+ T cell numbers | [201, 202] |
| ARVs (abacavir and lamivudine) | Glucose-coated gold nanoparticles |
In Vitro cell lines | Co-deliver and pH-mediated release of the drug and similar antiretroviral activity compared to free drug |
[203] |
| ARVs (NNRTI: DAAN-14f, and HIV-1 fusion inhibitor: T1144) |
PEG-PLA nanoparticles |
In Vitro cell lines and in vivo pharmacokinetic studies in rats |
Enhanced in vitro antiviral activity against various HIV-1 strains and sustained controlled release both in vitro and in vivo |
[206] |
| ARVs (zidovudine, efavirenz, and lamivudine) |
Lactoferrin nanoparticles |
In Vitro cell lines and in vivo pharmacokinetic and safety studies in rats |
Enhanced in vitro antiviral activity and improved pharmacokinetic and biodistribution profiles in vivo |
[207] |
| LRA (bryostatin-2) + ARV (nelfinavir) |
Lipid nanoparticles |
In Vitro cell lines, and ex vivo cells from an HIV- infected humanized mouse model |
Reactivated latent virus and inhibited viral spreading simultaneously |
[29] |
| LRA (vorinostat) + ARV (tenofovir) |
Magnetic nanoparticles |
In Vitro HIV-infected human astrocytes |
Reactivated latent virus and suppressed the viral replication |
[30] |
| LRA (vorinostat) + ARV (nelfinavir) |
PLGA-PEG nanoparticles |
In Vitro cell lines | Reactivated latent virus and suppressed the viral replication |
[205] |
| ARV (nelfinavir) + sigma receptor antagonist (rimcazole) |
Magnetic nanoparticles | In Vitro cell lines | Mitigated co-effect of drug of abuse and inhibited HIV-1 infection |
[208] |
| siRNAs (TNPO3, CD4, tat,rev) | PAMAM dendrimer |
In Vitro cell lines, ex vivo human primary cells, and in vivo in humanized mice |
Suppressed HIV-1 infection and protected mice from CD4+ T cell loss |
[209] |
| siRNAs (p24, gag1, nef) | Carbosilane dendrimers |
In Vitro cell lines, ex vivo human primary cells |
Reduced HIV-1 replication | [210] |
| siRNAs (CCR5, vif, and tat) | Peptide carriers |
In Vitro cell lines and in vivo humanized mouse models |
Suppressed HIV-1 replication and prevented mice from CD4+ T cell loss |
[153] |
| siRNA (tat/rev) + RNA aptamer (gp120) |
RNA nanoparticles |
In Vitro cell lines and in vivo humanized mouse models |
Suppressed HIV-1 replication and prevented mice from CD4+ T cell loss |
[211, 212] |
ARV, antiretroviral drugs; dsiRNA, dicer substrate small interfering RNA; NNRTI, Non-nucleoside reverse-transcriptase inhibitors; PAMAM, poly(amidoamine); PEG-PLA, polyethylene glycol–polylactic acid; PLGA, poly(lactic-co-glycolic acid).
Many other HIV cure agents such as antiproliferative agents, bnAbs, and oligonucleotides drugs may also need combinations for achieving improved efficacy in eradicating HIV reservoirs. For example, antiproliferative agents are required to be dosed with ARVs in order to suppress both viral replication and reservoir proliferation, and show potential benefits when combined with LRAs [20]. Preclinical studies in humanized mice have demonstrated improved anti-HIV effects from treatment with a combination of multiple bnAbs [212, 213] as well as single bnAbs combined with ARV drugs [214]. Combination gene therapies that target multiple HIV transcriptional genes as well as expression of CCR5 are being investigated to address rapid viral mutations and the complexity of HIV infection and latency maintenance [215].
Such combinations of antibodies, or nucleic acids with small molecules can be even more challenging due to their incompatibility, requirement of different vectors and administration routes, various PK or pharmacodynamic (PD) properties, and site of action. Nanocarriers have demonstrated their utility for co-delivering combinations of antibodies with chemotherapeutics [216], different RNAi targets [217], or siRNA with miRNA [218]. Due to the complexity of HIV integration sites and rapid mutation, combination might also bolster efficacy even for single gene therapies such as delivery of multiple siRNA. For example, Zhou et al. demonstrated the ability of poly(amidoamine) (PAMAM) dendrimer to deliver a combination of siRNAs to suppress HIV infection [208]. These siRNAs target viral as well as cellular transcripts for minimizing viral escape mutants and resulted in prolonged HIV suppression in humanized mice. However, dendrimer-siRNA nanoparticles primarily accumulated in PBMCs and liver, and biodistribution data was not provided for other major lymphatic tissues such as lymph nodes and GALT. Further investigation on biodistribution and PK profiles of these nanoparticles are needed to develop nanocarriers that can deliver multiple agent more efficiently to different target sites relevant for HIV cure.
4.2. Targeting tissue and cellular HIV sanctuaries by nanocarriers
The compartments that harbor HIV are primarily the blood, lymphatic systems (e.g. lymph nodes, GALT, spleen, etc.), and CNS. Inconsistent ARV drug penetration and accumulation in some of these sites is a challenge for effective HIV treatment and can lead to HIV persistence [10]. Targeted nanocarriers have been investigated for delivering ARV drugs or HIV cure agents to specific cells or tissues that harbor latent virus (Table 3).
Table 3.
Nanocarriers targeting HIV sanctuaries
|
Cellular Reservoirs |
Target | Targeting ligands | Therapeutics | Nanosystems | Test model | Reference |
| Leukocytes | LFA-1 | anti-CCR5 siRNA | Liposome |
Ex vivo human lymphocytes and in vivo humanized mouse model |
[220] | |
| CD7-expressing T cells |
Anti-CD7 single- chain antibody |
siRNAs (CCR5, vif and tat) |
Peptide carrier |
In Vitro cell lines and in vivo humanized mouse model |
[153] | |
| CD4+ T cells | CD4-binding peptides |
ARV (indinavir) | Lipid nanoparticles | In Vitro cell lines | [221, 222] | |
| anti-CD4 antibody | LRA (bryostatin-2) + ARV (nelfinavir) |
Lipid nanoparticles |
In Vitro cell lines and ex vivo latently infected cells from humanized mouse model |
[29] | ||
| Resting memory T cells |
CD45RO | LRA (vorinostat) + ARV (nelfinavir) |
PEG-PLGA nanoparticles | In Vitro cell lines | [205] | |
| Macrophage | Folate | ARV (atazanavir) | Polymer coated ARV nanoformulations (NanoART) |
In Vitro cell lines, ex vivo human primary cells, and in vivo mice |
[223, 224] | |
| Astrocytes | bradykinin B2 antibody | siRNA | Chitosan nanoparticles | In Vitro cell lines | [225] | |
|
Anatomical Reservoirs |
Target |
Administration route |
Therapeutics | Nanosystems | Test model | Reference |
| Lymph nodes | SC | ARV (Indinavir) | Lipid-drug complexes | HIV-2287-infected macaques |
[226] | |
| SC | ARV (lopinavir, ritonavir, and tenofovir) |
Lipid nanoparticles | Pig-tailed macaques | [197, 198] | ||
| CNS | N/A | ARV (azidothymidine 5’- triphosphate, AZTTP) |
Magnetic liposomal nanoformulation |
In Vitro BBB model | [227] | |
| IN | ARV (efavirenz) | PEO-PPO micelles | Rats | [228] | ||
| IV | ARV (indinavir) | Transferrin coupled submicron lipid emulsions |
Mice | [229] | ||
| Oral or IV | ARV (saquinavir) | Nanoemulsion | Mice | [230] | ||
| IP | ARV (azidothymidine) |
Nanogel decorated with the peptide binding brain- specific apolipoprotein E receptor |
Mice | [231] | ||
| IV | ARV (nevirapine) | Surface modified nanosuspensions |
Rats | [232] | ||
| N/A | ARV (stavudine, delavirdine, or saquinavir) |
PBCA, MMA-SPM, and SLN |
In Vitro BBB model, human brain- microvascular endothelial cells (HBMEC) |
[233, 234] | ||
| IV | ARV (ritonavir) | TAT peptide conjugated PLA nanoparticles |
Mice | [235] | ||
| SC | ARV (atazanavir and ritonavir) |
macrophage-carriage system for nanoformulated crystalline ART (nanoART) |
In Vitro cell lines, ex vivo human primary cells, and in vivo mice |
[236] | ||
| Retro-orbital injection |
siRNA (nef) | carbosilane dendrimer |
In Vitro BBB model, human brain- microvascular endothelial cells (HBMEC), and in vivo mice |
[237, 238] |
ARV: antiretroviral drugs; BBB: blood brain barrier; CNS: central neural system; LRA: latency-reversing agents; MMA-SPM: methylmethacrylate-sulfopropylmethacrylate; GALT: gut-associated lymphoid tissue; IN: intranasal; IP: intraperitoneal; IV: intravenous; PBCA: Polybutylcyanoacrylate; PEG-PLGA: Poly(ethylene glycol) methyl ether-block-poly(lactide-co-glycolide); PEO-PPO: poly(ethylene oxide)-poly(propylene oxide); PLA: Poly(lactic acid); SLN: solid lipid nanoparticle; TAT: trans-activating transcriptor; SC: subcutaneous.
Cell-specific accumulation of nanocarriers is achieved by the use of targeting moieties. Due to their high specificity, antibodies have also been widely used for targeting nanocarriers to T cells in various applications [26–28, 219, 238]. It has been suggested that anti-CD4 monoclonal antibodies, or its fragments, are good candidates to direct negatively-charged nanoparticles to CD4+ T cells [239]. Kovochich et al. reported lipid nanoparticles decorated with an anti-CD4 antibody for targeting both LRAs and ARVs to primary human CD4+ T cells in order to activate latent virus and inhibit viral spread [29]. The CD4 antibody led to specific targeting of nanoparticles to CD4+ T cells in PBMC culture, as well as preferential activation on CD4+ T cells over CD8+ T cells. This system shows great potential in systemic T cell targeting, however, PK profiles and biodistributions in some key HIV anatomical reservoirs were not investigated but would be important for future applications. Among CD4+ T cells, latent HIV viruses typically reside in resting memory (CD45RO+) T cells. Tang et al. conjugated anti-CD45RO antibody to PLGA-PEG nanoparticles that were loaded with a HDACi and protease inhibitors for targeting and eliminating latent HIV reservoirs in vitro [204]. They demonstrated that CD45RO antibody enhanced nanoparticle binding to human memory T cells, but the binding specificity was not measured and it is unclear how targeting improved drug potency. Aside from CD4+ T cells, macrophages are another target for ARV drug delivery and have been found to store and transport ARV drugs to lymph nodes and the CNS. Puligujja et al. developed folic acid (FA)-linked polymer-coated nanoformulated antiretroviral therapy (FA-nanoART), that induced five-fold enhanced plasma and tissue drug levels in mice and enabled 2.5-fold improvement in treating compared to untargeted nanoART [222, 223]. These targeted nanocarriers demonstrate the potential for applications in HIV cure research, but most are still in early stages of development and need to be tested in relevant animal models. Also, there is a need of more options to select binding ligands aside from whole antibodies to overcome high costs, nonspecific conjugation to nanocarriers, and effect on reducing nanocarrier circulation half-life for in vivo cellular targeting applications [240].
Several studies have focused on developing nanocarriers that preferentially distribute to lymph nodes after subcutaneous administration to address insufficient drug concentrations in these tissue [241, 242]. Therapeutics administered subcutaneously are preferentially taken up by blood capillaries or lymphatic vessels depending on their physicochemical properties. Small molecular drugs, peptides, and proteins (<16 kDa) have been shown to be absorbed by blood capillaries while particulates between 10–100 nm diameter are transported through the interstitium and preferentially into the lymphatic system [243]. It has been reported that a lipid-drug or lipid nanoparticles (50–80 in diameter) enhanced delivery of ARV drugs to lymph nodes, increased intracellular drug concentration, acted as a long-acting dosage form, and led to significant virus load reduction after subcutaneous administration to NHP models [196, 197, 225]. Such nanoparticle systems can be potentially used to enhance accumulation of other HIV cure agents in the lymph nodes, or direct multiple drugs with different PK profiles to lymph nodes for eradicating latent virus.
The GALT is an important site for early HIV replication and reservoir establishment, and has also shown significantly lower ARV concentrations compared with peripheral blood in patients under ART that are considered fully suppressive [10]. Nanoparticles have been used to target the GALT for various applications. For example, a variety of biodegradable antigen delivery systems have been developed for oral vaccines. Some of these were decorated with microfold cells (or M cells)-specific ligands to actively target delivery of nanoparticles to these cells in the GALT that transport nanoparticles across the intestinal epithelium [244]. Some limitations for this M cell targeting strategy may be the low efficiencies for GALT delivery due to a small percentage of M cells in the follicle-associated epithelium [245]. Peers et al. used antibodies against the β7 integrin to target mutilamellar vesicles to specific leukocytes subsets involved in gut inflammation, showing more than 10-fold higher accumulation in the gut compared to an isotype antibody control group administered intravenously in mice [26]. These nanocarriers have been barely studied in the HIV field, but have potential to deliver HIV cure agents to the GALT.
Nanotechnology-based methods are also being developed to deliver ARVs into the brain, which shows limited accessibility of many drugs and is another key anatomical reservoir harboring the virus [76, 77, 246]. Most investigations focus on crossing the BBB by developing nanocarriers with increased permeability, uptake, or transcytosis by brain microvascular endothelial cells (BMVECs) [247]. Many types of nanocarriers have been applied for the management of HIV infection in the CNS such as liposomes [226], micelles [227], nanoemulsions [228, 229], nanogel [230], nanosuspensions [231], and polymeric nanoparticles [232–234]. Ligands such as transferrin and trans-acting transcriptional activator (TAT) peptide are widely used for targeting nanocarriers to the brain. For example, transferrin has been linked to indinavir lipid nanoemulsions and resulted in up to 5 times higher bioavailability in brain compared to free drug [228]. This has been attributed to the overexpression of transferrin receptors on brain cells that can mediate endocytosis and transcytosis of transferrin-coupled substances. The TAT-peptide has been shown to penetrate through cell membranes and enhance transport of nanocarriers across the BBB. Rao et al. demonstrated that TAT-conjugated poly (L-lactide) (PLA) nanoparticles efficiently enhance CNS bioavailability and maintain drug level of encapsulated ritonavir in the brain [234]. A peptide that binds to brain-specific apolipoprotein E receptor has also been decorated to cationic nanogels for delivering nucleoside reverse transcriptase inhibitors (NRTIs) against HIV infection in the brain [230]. The macrophage-targeting nanoART described above showed the ability to be transferred from macrophages to human brain microvascular endothelial cells (HBMECs), and led to four-fold higher ARV levels in the mice brain compared to untargeted nanoART [235]. Alternative delivery routes such as intranasal delivery also offer a non-invasive way to transport drugs through olfactory neurons to the brain [227]. These solutions have demonstrated significant increases of ARV drug concentrations in the brain compared to free drug administration, therefore showing unique promise as efficient tools to deliver HIV cure agents into the brain.
4.3. Future directions: nanocarrier-based gene- or immunotherapy
Gene therapy offers promising solutions in treating multiple diseases including HIV, and has greatly advanced since the discovery of CRISPR as an easy and robust gene-editing tool. While current gene therapy for HIV cure has mostly relied on viral vectors, the advances in nanocarrier-based gene delivery technology may enhance its impact on the HIV cure field due to its lower risk, potential targeting abilities, and lower off-target effects. Some big hurdles for applying gene therapy to HIV cure research, particularly for strategies proposing to knock-out HIV provirus in the host genome, are low efficiency and targeting specificity in vivo. Currently, viral vectors such as adenoviruses and AAVs are still the most widely used for in vivo gene delivery due to their relatively high transfection efficiency but have associated concerns with off-target effects, immunogenicity, and toxicity. Non-viral vectors such as nanocarriers have been investigated as an alternative and safe way to deliver genes (reviewed in [248–250]). Studies have used lipid-based or polymer-based nanocarriers, or dendrimers as non-viral vectors to deliver exogeneous nucleic acids such as DNA, mRNA, siRNA and miRNA [249]. Many reports use nanoparticles for anti-HIV siRNA delivery [153, 208–211, 219, 236, 237, 251–255]. For example, Weber et al. have used carbosilane dendrimers to deliver siRNAs targeting HIV p24, gag1, or nef genes [209]. The dendrimer-siRNA complex showed highest transfection efficiency in both cell line and HIV-infected PBMCs without causing cytotoxicity, and protected siRNA from rapid degradation in the presence of RNase. A similar dendrimer system was also tested in mice and efficient siRNA transport across BBB was observed [237]. More investigations on siRNA transfection in vivo, particularly anti-HIV effects in relevant animal models are needed for future clinical applications. There are relatively few examples of using non-viral vectors to deliver whole genome editing systems such as ZFNs and CRISPR-Cas9 systems because they are large and complex [250]. Recently, Lee at al. developed a polymer-coated gold nanoparticle that can co-deliver CRISPR components, including the Cas9 and gRNA ribonucleoprotein (RNP) complex and donor DNA, resulting in efficient correction on the mutated dystrophin gene with low off-target effects [256].
HIV therapeutic vaccines, bnAbs, PD-1 blockades, and CAR T cell therapy have all shown promise in HIV cure but also have limitations such as low delivery efficiency to specific tissues or cells. Over the past decades, nanocarrier-based delivery platforms such as liposomes, polymeric nanoparticles, lipid-polymer hybrid nanoparticles, and virus-like nanoparticles have been used as carriers for vaccine delivery in the field of cancer immunotherapy (reviewed in [257]). The advantages of nanocarriers for delivering vaccines include simultaneous delivery of multiple antigens and adjuvants, rapid endocytosis by immune cells (especially DCs and macrophages), and accumulation to lymphatic tissues based on their size [257]. These attributes could be employed for nanocarriers to deliver HIV immunotherapeutics to specific cells or tissues that are inaccessible with current dosage forms.
PD-1 blockades, when delivered systematically, have concerns with systemic immune stimulation that is associated with some immune-related adverse effects [258]. The use of a drug delivery system to enhance accumulation of these blockades to specific cells or tissues may reduce these side effects. For example, Kosmides et al. developed nanoparticles decorated with both anti-PD-L1 antibody and anti-4–1BB antibody (a costimulatory molecule), and observed increased targeting of CD8+ T cells, enhanced anti-tumor activities and low off-target toxicity in vivo [259]. Alternatively, Schmid et al. have used CD8 and PD-1 dual-targeting nanoparticles to deliver a TGFβR1 inhibitor, another immunostimulatory drug, to restore CD8+PD-1+ T cell functions for killing cancer cells [260]. These delivery systems may also be applied in delivering PD-1 blockade with other stimulators for targeting and activating HIV-specific CD8+ T cells that highly express PD-1. This targeted delivery system could achieve specific restoration of T cell function towards eradication of HIV infection.
Current CAR T cell-based immunotherapy that engineers T cells ex vivo may be too elaborate for widespread implementation for HIV and cancer treatment. This therapy involves complex procedures that requires expensive equipment and technical expertise for T cell isolation, modification, and expansion followed by infusion back into patients. Nanocarriers could offer an alternative and inexpensive solution by delivering modifying genes to program host T cells in vivo. Recently, Smith et al. have developed nanoparticles decorated with anti-CD3 antibody and loaded with leukemia-targeting CAR genes to target and engineer tumor-specific T cells directly in situ [261]. Such technology has potential to be applied to CAR T cell strategy to engineer CD8+ cytotoxic T cells in vivo to kill HIV-infected CD4+ T cells.
5. Conclusion
A variety of strategies have been developed to minimize HIV reservoirs, but several obstacles remain, such as insufficient potency and off-target effects in normal cells that may also result in dose-limiting toxicities [14]. Nanocarriers offer a promising treatment for HIV infection by enhancing drug potency using synergistic combinations, or targeting specific and hard-to-reach sites that harbor virus. Nanocarriers also offer advantages compared to traditional delivery systems that include encapsulation of physicochemically diverse agents, sustained drug release, lower drug dosing, better bioavailability, and fewer or less severe side effects. These promising properties of nanocarriers help address problems in current novel strategies toward HIV cure, such as LRA combination for reactivating latently infected cells. There are still many hurdles that remain in the translation of nanocarriers from the lab to the clinic. Interdisciplinary collaborations on nanocarrier development and evaluation, as well as appropriate in vitro, ex vivo and in vivo models are the best way to ensure rapid translation of nanomedicine for curing HIV.
Acknowledgements
This work was supported in part by an NIH/NIAID grant (AI 094412) to KAW, and amfAR grant for Bringing Bioengineers to Cure HIV (109541–61-RGRL). The authors thank Jamie Hernandez for her critical reading and editing of the manuscript.
1Abbreviations:
- AAV
adeno-associated virus
- AML
acute myeloid leukemia
- APC
antigen presenting cells
- ARVs
antiretroviral drugs
- ATI
analytical treatment interruption
- BBB
blood-brain barrier
- BET
bromodomain extra-terminal
- BMVECs
brain microvascular endothelial cells
- bnAb
broadly neutralizing HIV antibody
- cART
combination antiretroviral therapy
- CAR
chimeric antigen receptor
- CMV
cytomegalovirus
- CNS
central nervous system
- CRISPR
clustered regularly interspersed palindromic repeats
- DC
dendritic cell
- dCA
didehydro-cortistatin
- GALT
gut-associated lymphoid tissue
- HAART
highly active antiretroviral therapy
- HBMECs
human brain microvascular endothelial cells
- HDAC
histone deacetylase
- HMT
histone methyltransferase
- HSPC
hematopoietic stem/progenitor cell
- iPSC
induced pluripotent stem cell
- LN
lymph node
- LRA
latency reversing agent
- LTR
long term repeat
- MAdCAM-1
mucosal vascular addressin cell adhesion molecular 1
- M cell
microfold cell
- MMF
mycophenolate mofetil
- MPA
mycophenolic acid
- NF-κB
nuclear factor κB
- NHP
non-human primates
- NK cell
natural killer cell
- NRTIs
nucleoside reverse transcriptase inhibitors
- PAMAM
poly(amidoamine)
- PD
pharmacodynamic
- PD-1
programmed cell death protein-1
- PK
pharmacokinetic
- PKC
Protein kinase C
- P-TEFb
positive transcription elongation factor b
- RNAi
RNA interference
- RNP
ribonucleoprotein
- saCas9
Staphylococcus aureus Cas9
- SAHA
vorinostat or suberanilohydroxamic acid
- sgRNA
single-guide RNA
- siRNA
small interfering RNA
- SIV
simian immunodeficiency virus
- TALEN
transcription activator-like effector nuclease
- TAT
trans-acting transcriptor
- TCM
central memory CD4+ T cells
- TEM
effector memory CD4+ T cells
- TN
naïve CD4+ T cells
- TSCM
CD4+ T memory stem cells
- TTM
transitional memory CD4+ T cells
- ZFN
zinc finger nuclease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].UNAIDS, Fact sheet - Latest global and regional statistics on the status of the AIDS epidemic, (2017).
- [2].T.D.C.o.A.E.o.A.-H.D.S. Group, Combination Antiretroviral Therapy and the Risk of Myocardial Infarction, New England Journal of Medicine, 349 (2003) 1993–2003. [DOI] [PubMed] [Google Scholar]
- [3].Lai S, Bartlett J, Lai H, Moore R, Cofrancesco J, Pannu H, Tong W, Meng W, Sun H, Fishman EK, Long-Term Combination Antiretroviral Therapy Is Associated with the Risk of Coronary Plaques in African Americans with HIV Infection, AIDS Patient Care and STDs, 23 (2009) 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Treisman GJ, Kaplin AI, Neurologic and psychiatric complications of antiretroviral agents, AIDS, 16 (2002) 1201–1215. [DOI] [PubMed] [Google Scholar]
- [5].Kovari H, Weber R, Influence of antiretroviral therapy on liver disease, Current Opinion in HIV and AIDS, 6 (2011) 272–277. [DOI] [PubMed] [Google Scholar]
- [6].Hütter G, Nowak D, Mossner M, Ganepola S, Müßig A, Allers K, Schneider T, Hofmann J, Kücherer C, Blau O, Blau IW, Hofmann WK, Thiel E Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation, New England Journal of Medicine, 360 (2009) 692–698. [DOI] [PubMed] [Google Scholar]
- [7].Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T, Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation, Blood, 117 (2011) 2791–2799. [DOI] [PubMed] [Google Scholar]
- [8].Saksena NK, Wang B, Zhou L, Soedjono M, Ho YS, Conceicao V, HIV reservoirs in vivo and new strategies for possible eradication of HIV from the reservoir sites, HIV AIDS (Auckl), 2 (2010) 103–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ostrowski M, Benko E, Yue FY, Kim CJ, Huibner S, Lee T, Singer J, Pankovich J, Laeyendecker O, Kaul R, Kandel G, Kovacs C, Intensifying Antiretroviral Therapy With Raltegravir and Maraviroc During Early Human Immunodeficiency Virus (HIV) Infection Does Not Accelerate HIV Reservoir Reduction, Open Forum Infectious Diseases, 2 (2015) ofv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, Anderson J, Perkey K, Stevenson M, Perelson AS, Douek DC, Haase AT, Schacker TW, Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues, Proceedings of the National Academy of Sciences, 111 (2014) 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hecht FM, Wang L, Collier A, Little S, Markowitz M, Margolick J, Kilby JM, Daar E, Conway B, Network A, A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection, Journal of Infectious Diseases, 194 (2006) 725–733. [DOI] [PubMed] [Google Scholar]
- [12].Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study, PLoS Pathog, 9 (2013) e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Margolis DM, Garcia JV, Hazuda DJ, Haynes BF, Latency reversal and viral clearance to cure HIV-1, Science, 353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rasmussen TA, Lewin SR, Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents?, Current Opinion in HIV and AIDS, 11 (2016) 394–401. [DOI] [PubMed] [Google Scholar]
- [15].Wang CX, Cannon PM, The clinical applications of genome editing in HIV, Blood, 127 (2016) 2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Caskey M, Klein F, Lorenzi JCC, Seaman MS, West AP Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117, Nature, 522 (2015) 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O’Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun T-W, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE, Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection, Science Translational Medicine, 7 (2015) 319ra206.. [DOI] [PubMed] [Google Scholar]
- [18].Lu C-L, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK, Nussenzweig MC, Enhanced clearance of HIV-1–infected cells by broadly neutralizing antibodies against HIV-1 in vivo, Science, 352 (2016) 1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bobbin ML, Burnett JC, Rossi JJ, RNA interference approaches for treatment of HIV-1 infection, Genome Medicine, 7 (2015) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reeves DB, Duke ER, Hughes SM, Prlic M, Hladik F, Schiffer JT, Anti-proliferative therapy for HIV cure: a compound interest approach, Scientific Reports, 7 (2017) 4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sharma P, Garg S, Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs, Advanced Drug Delivery Reviews, 62 (2010) 491–502. [DOI] [PubMed] [Google Scholar]
- [22].Yildirimer L, Thanh NT, Loizidou M, Seifalian AM, Toxicology and clinical potential of nanoparticles, Nano today, 6 (2011) 585–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Davis ME, Chen Z, Shin DM, Nanoparticle therapeutics: an emerging treatment modality for cancer, Nat Rev Drug Discov, 7 (2008) 771–782. [DOI] [PubMed] [Google Scholar]
- [24].Jiang Y, Cao S, Bright DK, Bever AM, Blakney AK, Suydam IT, Woodrow KA, Nanoparticle-Based ARV Drug Combinations for Synergistic Inhibition of Cell-Free and Cell–Cell HIV Transmission, Molecular pharmaceutics, 12 (2015) 4363–4374. [DOI] [PubMed] [Google Scholar]
- [25].Freeling JP, Ho RJY, Anti-HIV drug particles may overcome lymphatic drug insufficiency and associated HIV persistence, Proceedings of the National Academy of Sciences of the United States of America, 111 (2014) E2512–E2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M, Systemic Leukocyte-Directed siRNA Delivery Revealing Cyclin D1 as an Anti-Inflammatory Target, Science, 319 (2008) 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee J, Yun K-S, Choi CS, Shin S-H, Ban H-S, Rhim T, Lee SK, Lee KY, T Cell-Specific siRNA Delivery Using Antibody-Conjugated Chitosan Nanoparticles, Bioconjugate Chemistry, 23 (2012) 1174–1180. [DOI] [PubMed] [Google Scholar]
- [28].Dinauer N, Balthasar S, Weber C, Kreuter J, Langer K, von Briesen H, Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes, Biomaterials, 26 (2005) 5898–5906. [DOI] [PubMed] [Google Scholar]
- [29].Kovochich M, Marsden MD, Zack JA, Activation of Latent HIV Using Drug-Loaded Nanoparticles, PLoS ONE, 6 (2011) e18270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jayant RD, Atluri VSR, Agudelo M, Sagar V, Kaushik A, Nair M, Sustained-release nanoART formulation for the treatment of neuroAIDS, International Journal of Nanomedicine, 10 (2015) 1077–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kamaly N, Yameen B, Wu J, Farokhzad OC, Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release, Chemical Reviews, 116 (2016) 2602–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R, Theodosopoulos T, O’Doherty U, Human Immunodeficiency Virus Type 1 Can Establish Latent Infection in Resting CD4+ T Cells in the Absence of Activating Stimuli, Journal of Virology, 79 (2005) 14179–14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, Boucher G, Haddad EK, Sekaly R-P, Harman AN, Anderson JL, Jones KL, Mak J, Cunningham AL, Jaworowski A, Lewin SR, Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton, Proceedings of the National Academy of Sciences, 107 (2010) 16934–16939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bosque A, Planelles V, Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells, Blood, 113 (2009) 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Craigie R, Bushman FD, HIV DNA Integration, Cold Spring Harbor Perspectives in Medicine, 2 (2012) a006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wagner TA, McLaughlin S, Garg K, Cheung CYK, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, Frenkel LM, Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection, Science (New York, N.Y.), 345 (2014) 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH, Specific HIV integration sites are linked to clonal expansion and persistence of infected cells, Science (New York, N.Y.), 345 (2014) 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ylisastigui L, Coull JJ, Rucker VC, Melander C, Bosch RJ, Brodie SJ, Corey L, Sodora DL, Dervan PB, Margolis DM, Polyamides Reveal a Role for Repression in Latency within Resting T Cells of HIV-Infected Donors, Journal of Infectious Diseases, 190 (2004) 1429–1437. [DOI] [PubMed] [Google Scholar]
- [39].Chéné I.d., Basyuk E, Lin Y-L, Triboulet R, Knezevich A, Chable-Bessia C, Mettling C, Baillat V, Reynes J, Corbeau P, Bertrand E, Marcello A, Emiliani S, Kiernan R, Benkirane M, Suv39H1 and HP1γ are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency, The EMBO Journal, 26 (2007) 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barboric M, Kohoutek J, Price JP, Blazek D, Price DH, Peterlin BM, Interplay between 7SK snRNA and oppositely charged regions in HEXIM1 direct the inhibition of P-TEFb, The EMBO Journal, 24 (2005) 4291–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yik JHN, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q, Inhibition of P-TEFb (CDK9/Cyclin T) Kinase and RNA Polymerase II Transcription by the Coordinated Actions of HEXIM1 and 7SK snRNA, Molecular Cell, 12 (2003) 971–982. [DOI] [PubMed] [Google Scholar]
- [42].Xing S, Siliciano RF, Targeting HIV latency: pharmacologic strategies toward eradication, Drug Discovery Today, 18 (2013) 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF, Nuclear Retention of Multiply Spliced HIV-1 RNA in Resting CD4(+) T Cells, PLoS Pathogens, 2 (2006) e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, Huang W, Squires K, Verlinghieri G, Zhang H, Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes, Nature Medicine, 13 (2007) 1241. [DOI] [PubMed] [Google Scholar]
- [45].Markowitz M, Louie M, Hurley A, Sun E, Di Mascio M, Perelson AS, Ho DD, A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo, Journal of virology, 77 (2003) 5037–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson AS, Korber BT, Markowitz M, Guo Y, Duran M, Hurley A, Tsay J, Huang Y-C, Wang C-C, Ho DD Quantifying Residual HIV-1 Replication in Patients Receiving Combination Antiretroviral Therapy, New England Journal of Medicine, 340 (1999) 1605–1613. [DOI] [PubMed] [Google Scholar]
- [47].Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF, Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells, Nature Medicine, 9 (2003) 727. [DOI] [PubMed] [Google Scholar]
- [48].Chun T-W, Justement JS, Moir S, Hallahan CW, Maenza J, Mullins JI, Collier AC, Corey L, Fauci AS, Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus, Journal of Infectious Diseases, 195 (2007) 1762–1764. [DOI] [PubMed] [Google Scholar]
- [49].Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy J-P, Haddad EK, Sekaly R-P, HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation, Nat Med, 15 (2009) 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lorenzo-Redondo R, Fryer HR, Bedford T, Kim E-Y, Archer J, Kosakovsky Pond SL, Chung Y-S, Penugonda S, Chipman JG, Fletcher CV, Schacker TW, Malim MH, Rambaut A, Haase AT, McLean AR, Wolinsky SM, Persistent HIV-1 replication maintains the tissue reservoir during therapy, Nature, 530 (2016) 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bui JK, Sobolewski MD, Keele BF, Spindler J, Musick A, Wiegand A, Luke BT, Shao W, Hughes SH, Coffin JM, Kearney MF, Mellors JW, Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir, PLOS Pathogens, 13 (2017) e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pallikkuth S, Sharkey M, Babic DZ, Gupta S, Stone GW, Fischl MA, Stevenson M, Pahwa S, Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy, Journal of Virology, 90 (2016) 2718–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M, HIV-1 persistence in CD4(+) T cells with stem cell-like properties, Nature medicine, 20 (2014) 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP, A human memory T-cell subset with stem cell-like properties, Nature medicine, 17 (2011) 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kumar A, Herbein G, The macrophage: a therapeutic target in HIV-1 infection, Molecular and Cellular Therapies, 2 (2014) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Groot F, Welsch S, Sattentau QJ, Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses, Blood, 111 (2008) 4660–4663. [DOI] [PubMed] [Google Scholar]
- 57.[] Swingler S, Mann A, Jacque JM, Brichacek B, Sasseville VG, Williams K, Lackner AA, Janoff EN, Wang R, Fisher D, Stevenson M, HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages, Nat Med, 5 (1999) 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK, Role of Microglia in Central Nervous System Infections, Clinical Microbiology Reviews, 17 (2004) 942–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, Wu Y, Human macrophages support persistent transcription from unintegrated HIV-1 DNA, Virology, 372 (2008) 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Carter CA, Ehrlich LS, Cell Biology of HIV-1 Infection of Macrophages, Annual Review of Microbiology, 62 (2008) 425–443. [DOI] [PubMed] [Google Scholar]
- [61].Ghorpade A, Persidskaia R, Suryadevara R, Che M, Liu XJ, Persidsky Y, Gendelman HE, Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia, Journal of virology, 75 (2001) 6572–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Loré K, Smed-Sörensen A, Vasudevan J, Mascola JR, Koup RA, Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4(+) T cells, The Journal of Experimental Medicine, 201 (2005) 2023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wu L, KewalRamani VN, Dendritic-cell interactions with HIV: infection and viral dissemination, Nature reviews. Immunology, 6 (2006) 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Otero M, Nunnari G, Leto D, Sullivan J, Wang F-X, Frank I, Xu Y, Patel C, Dornadula G, Kulkosky J, Peripheral blood dendritic cells are not a major reservoir for HIV type 1 in infected individuals on virally suppressive HAART, AIDS research and human retroviruses, 19 (2003) 1097–1103. [DOI] [PubMed] [Google Scholar]
- [65].Spiegel H, Herbst H, Niedobitek G, Foss HD, Stein H, Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells, The American Journal of Pathology, 140 (1992) 15–22. [PMC free article] [PubMed] [Google Scholar]
- [66].Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, Deeks SG, Luciw PA, Chipman JG, Beilman GJ, Hoskuldsson T, Khoruts A, Anderson J, Deleage C, Jasurda J, Schmidt TE, Hafertepe M, Callisto SP, Pearson H, Reimann T, Schuster J, Schoephoerster J, Southern P, Perkey K, Shang L, Wietgrefe SW, Fletcher CV, Lifson JD, Douek DC, McCune JM, Haase AT, Schacker TW, Defining total-body AIDS-virus burden with implications for curative strategies, Nature Medicine, 23 (2017) 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D, Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy, Nature, 477 (2011) 95–98. [DOI] [PubMed] [Google Scholar]
- [68].Döpper S, Wilflingseder D, Prodinger WM, Stiegler G, Speth C, Dierich MP, Stoiber H, Mechanism(s) promoting HIV-1 infection of primary unstimulated T lymphocytes in autologous B cell/T cell co-cultures, European Journal of Immunology, 33 (2003) 2098–2107. [DOI] [PubMed] [Google Scholar]
- [69].Arrighi J-F, Pion M, Garcia E, Escola J-M, van Kooyk Y, Geijtenbeek TB, Piguet V, DC-SIGN–mediated Infectious Synapse Formation Enhances X4 HIV-1 Transmission from Dendritic Cells to T Cells, The Journal of Experimental Medicine, 200 (2004) 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA, Gastrointestinal Tract as a Major Site of CD4+ T Cell Depletion and Viral Replication in SIV Infection, Science, 280 (1998) 427–431. [DOI] [PubMed] [Google Scholar]
- [71].Ling B, Mohan M, Lackner AA, Green LC, Marx PA, Doyle LA, Veazey RS, The Large Intestine as a Major Reservoir for Simian Immunodeficiency Virus in Macaques with Long-Term, Nonprogressing Infection, Journal of Infectious Diseases, 202 (2010) 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Poles MA, Elliott J, Taing P, Anton PA, Chen IS, A preponderance of CCR5+ CXCR4+ mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection, Journal of virology, 75 (2001) 8390–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shacklett BL, Anton PA, HIV Infection and Gut Mucosal Immune Function: Updates on Pathogenesis with Implications for Management and Intervention, Current Infectious Disease Reports, 12 (2010) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O’Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J, The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1, Proceedings of the National Academy of Sciences, 106 (2009) 20877–20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, Mattapallil JJ, α4+β7hiCD4+ memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection, Mucosal Immunol, 2 (2009) 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kanmogne GD, Kennedy RC, Grammas P, HIV-1 gp120 Proteins and gp160 Peptides Are Toxic to Brain Endothelial Cells and Neurons: Possible Pathway for HIV Entry into the Brain and HIV-Associated Dementia, Journal of Neuropathology & Experimental Neurology, 61 (2002) 992–1000. [DOI] [PubMed] [Google Scholar]
- [77].McGee B, Smith N, Aweeka F, HIV Pharmacology: Barriers to the Eradication of HIV from the CNS, HIV Clinical Trials, 7 (2006) 142–153. [DOI] [PubMed] [Google Scholar]
- [78].Costiniuk CT, Jenabian M-A, The lungs as anatomical reservoirs of HIV infection, Reviews in Medical Virology, 24 (2014) 35–54. [DOI] [PubMed] [Google Scholar]
- [79].Fierer DS, Klotman ME, Kidney and central nervous system as reservoirs of HIV infection, Current opinion in HIV and AIDS, 1 (2006) 115–120. [DOI] [PubMed] [Google Scholar]
- [80].Chun T-W, Engel D, Berrey MM, Shea T, Corey L, Fauci AS, Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection, Proceedings of the National Academy of Sciences, 95 (1998) 8869–8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chun T-W, Moir S, Fauci AS, HIV reservoirs as obstacles and opportunities for an HIV cure, Nat Immunol, 16 (2015) 584–589. [DOI] [PubMed] [Google Scholar]
- [82].Nishimura Y, Sadjadpour R, Mattapallil JJ, Igarashi T, Lee W, Buckler-White A, Roederer M, Chun T-W, Martin MA, High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections, Proceedings of the National Academy of Sciences, 106 (2009) 8015–8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DIS, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH, Rapid Seeding of the Viral Reservoir Prior to SIV Viremia in Rhesus Monkeys, Nature, 512 (2014) 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ananworanich J, Dubé K, Chomont N, How does the timing of antiretroviral therapy initiation in acute infection affect HIV reservoirs?, Current opinion in HIV and AIDS, 10 (2015) 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, Epling L, Lee T-H, Busch MP, McCune JM, Pilcher CD, Hecht FM, Deeks SG, Antiretroviral Therapy Initiated Within 6 Months of HIV Infection Is Associated With Lower T-Cell Activation and Smaller HIV Reservoir Size, The Journal of Infectious Diseases, 208 (2013) 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hill AL, Rosenbloom DIS, Goldstein E, Hanhauser E, Kuritzkes DR, Siliciano RF, Henrich TJ, Real-Time Predictions of Reservoir Size and Rebound Time during Antiretroviral Therapy Interruption Trials for HIV, PLoS Pathogens, 12 (2016) e1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, Kuritzkes DR, Lederman MM, Para M, Gandhi RT, The Size of the Expressed HIV Reservoir Predicts Timing of Viral Rebound after Treatment Interruption, AIDS (London, England), 30 (2016) 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Persaud D, Gay H, Ziemniak C, Chen YH, Piatak MJ, Chun T-W, Strain M, Richman D, Luzuriaga K Absence of Detectable HIV-1 Viremia after Treatment Cessation in an Infant, New England Journal of Medicine, 369 (2013) 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, Mellors JW, Rosenbloom D, Persaud D Viremic Relapse after HIV-1 Remission in a Perinatally Infected Child, New England Journal of Medicine, 372 (2015) 786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, Estes JD, Sandler NG, Sukhumvittaya S, Marovich M, Jongrakthaitae S, Akapirat S, Fletscher JLK, Kroon E, Dewar R, Trichavaroj R, Chomchey N, Douek DC, Connell RJO′, Ngauy V, Robb ML, Phanuphak P, Michael NL, Excler J-L, Kim JH, de Souza MS, Ananworanich J, R.S. on behalf of the, R.S.S. Groups, Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation, PLOS Pathogens, 10 (2014) e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hong JJ, Silveira E.L.d.V., Amancha PK, Byrareddy SN, Gumber S, Chang K-T, Ansari AA, Villinger F, Early initiation of antiretroviral treatment postSIV infection does not resolve lymphoid tissue activation, AIDS, 31 (2017) 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Malzahn J, Shen C, Caruso L, Ghosh P, Sankapal SR, Barratt-Boyes S, Gupta P, Chen Y, Effect of early anti-retroviral therapy on the pathogenic changes in mucosal tissues of SIV infected rhesus macaques, Virology Journal, 9 (2012) 269–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Davey RT, Bhat N, Yoder C, Chun T-W, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC, HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression, Proceedings of the National Academy of Sciences of the United States of America, 96 (1999) 15109–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Barouch DH, Deeks SG, Immunologic strategies for HIV-1 remission and eradication, Science, 345 (2014) 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Barton KM, Archin NM, Keedy KS, Espeseth AS, Zhang Y.-l., Gale J, Wagner FF, Holson EB, Margolis DM, Selective HDAC Inhibition for the Disruption of Latent HIV-1 Infection, PLOS ONE, 9 (2014) e102684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bannister AJ, Kouzarides T, Regulation of chromatin by histone modifications, Cell Research, 21 (2011) 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bernhard W, Barreto K, Saunders A, Dahabieh MS, Johnson P, Sadowski I, The Suv39H1 methyltransferase inhibitor chaetocin causes induction of integrated HIV-1 without producing a T cell response, FEBS Letters, 585 (2011) 3549–3554. [DOI] [PubMed] [Google Scholar]
- [98].McKernan LN, Momjian D, Kulkosky J, Protein Kinase C: One Pathway towards the Eradication of Latent HIV-1 Reservoirs, Adv Virol, 2012 (2012) 805347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jiang G, Mendes EA, Kaiser P, Sankaran-Walters S, Tang Y, Weber MG, Melcher GP, Thompson GR, Tanuri A, Pianowski LF, Wong JK, Dandekar S, Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cδ–NF-κB signaling, AIDS (London, England), 28 (2014) 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, Sebastiani P, Margolis DM, Montano M, BET bromodomain inhibition as a novel strategy for reactivation of HIV-1, Journal of leukocyte biology, 92 (2012) 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E, Epigenetic Regulation of HIV-1 Latency by Cytosine Methylation, PLOS Pathogens, 5 (2009) e1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Contreras X, Schweneker M, Chen C-S, McCune JM, Deeks SG, Martin J, Peterlin BM, Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells, The Journal of Biological Chemistry, 284 (2009) 6782–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Das B, Dobrowolski C, Shahir A-M, Feng Z, Yu X, Sha J, Bissada NF, Weinberg A, Karn J, Ye F, Short chain fatty acids potently induce latent HIV-1 in T-cells by activating P-TEFb and multiple histone modifications, Virology, 474 (2015) 65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Archin NM, Liberty A, Kashuba AD, Choudhary SK, Kuruc J, Crooks A, Parker D, Anderson E, Kearney M, Strain M, Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy, Nature, 487 (2012) 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Archin NM, Bateson R, Tripathy M, Crooks AM, Yang K-H, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, HIV-1 expression within resting CD4 T-cells following multiple doses of vorinostat, Journal of Infectious Diseases, (2014) jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy, PLoS Pathog, 10 (2014) e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Archin NM, Kirchherr JL, Sung JAM, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, Eron J, Gay CL, Goonetilleke N, Margolis DM, Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency, The Journal of Clinical Investigation, 127 (2017) 3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial, The Lancet HIV, 1 (2014) e13–e21. [DOI] [PubMed] [Google Scholar]
- [109].Archin NM, Cheema M, Parker D, Wiegand A, Bosch RJ, Coffin JM, Eron J, Cohen M, Margolis DM, Antiretroviral Intensification and Valproic Acid Lack Sustained Effect on Residual HIV-1 Viremia or Resting CD4+ Cell Infection, PLoS ONE, 5 (2010) e9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Routy JP, Tremblay CL, Angel JB, Trottier B, Rouleau D, Baril JG, Harris M, Trottier S, Singer J, Chomont N, Sékaly RP, Boulassel MR, Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study, HIV Medicine, 13 (2012) 291–296. [DOI] [PubMed] [Google Scholar]
- [111].Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, The depsipeptide romidepsin reverses HIV-1 latency in vivo, PLoS Pathog, 11 (2015) e1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Policicchio BB, Xu C, Brocca-Cofano E, Raehtz KD, He T, Ma D, Li H, Sivanandham R, Haret-Richter GS, Dunsmore T, Trichel A, Mellors JW, Hahn BH, Shaw GM, Ribeiro RM, Pandrea I, Apetrei C, Multi-dose Romidepsin Reactivates Replication Competent SIV in Post-antiretroviral Rhesus Macaque Controllers, PLoS Pathog, 12 (2016) e1005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Del Prete GQ, Oswald K, Lara A, Shoemaker R, Smedley J, Macallister R, Coalter V, Wiles A, Wiles R, Li Y, Fast R, Kiser R, Lu B, Zheng J, Alvord WG, Trubey CM, Piatak M, Deleage C, Keele BF, Estes JD, Hesselgesser J, Geleziunas R, Lifson JD, Elevated Plasma Viral Loads in Romidepsin-Treated Simian Immunodeficiency Virus-Infected Rhesus Macaques on Suppressive Combination Antiretroviral Therapy, Antimicrobial Agents and Chemotherapy, 60 (2016) 1560–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yang W, Sun Z, Hua C, Wang Q, Xu W, Deng Q, Pan Y, Lu L, Jiang S, Chidamide, a histone deacetylase inhibitor-based anticancer drug, effectively reactivates latent HIV-1 provirus, Microbes and Infection, (2017). [DOI] [PubMed] [Google Scholar]
- [115].Kashanchi F, Melpolder JC, Epstein JS, Sadaie MR, Rapid and sensitive detection of cell‐associated HIV‐1 in latently infected cell lines and in patient cells using sodium‐ n‐butyrate induction and RT‐PCR, Journal of medical virology, 52 (1997) 179–189. [PubMed] [Google Scholar]
- [116].Imai K, Togami H, Okamoto T, Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294, Journal of Biological Chemistry, 285 (2010) 16538–16545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Williams SA, Chen L-F, Kwon H, Fenard D, Bisgrove D, Verdin E, Greene WC, Prostratin antagonizes HIV latency by activating NF-κB, Journal of Biological Chemistry, 279 (2004) 42008–42017. [DOI] [PubMed] [Google Scholar]
- [118].Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, Boyd MR, Pomerantz RJ, Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART, Blood, 98 (2001) 3006–3015. [DOI] [PubMed] [Google Scholar]
- [119].Gutiérrez C, Serrano-Villar S, Madrid-Elena N, Pérez-Elías MJ, Martín ME, Barbas C, Ruipérez J, Muñoz E, Muñoz-Fernández MA, Castor T, Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy, AIDS, 30 (2016) 1385–1392. [DOI] [PubMed] [Google Scholar]
- [120].Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, Nagarkatti P, Nagarkatti M, Chauhan A, Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner, PloS one, 5 (2010) e11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jiang G, Mendes EA, Kaiser P, Wong DP, Tang Y, Cai I, Fenton A, Melcher GP, Hildreth JEK, Thompson GR, Wong JK, Dandekar S, Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation, PLOS Pathogens, 11 (2015) e1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang P, Lu P, Qu X, Shen Y, Zeng H, Zhu X, Zhu Y, Li X, Wu H, Xu J, Lu H, Ma Z, Zhu H, Reactivation of HIV-1 from Latency by an Ingenol Derivative from Euphorbia Kansui, Scientific Reports, 7 (2017) 9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Contreras X, Barboric M, Lenasi T, Peterlin BM, HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription, PLoS Pathog, 3 (2007) e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Fenaux P, Inhibitors of DNA methylation: beyond myelodysplastic syndromes, Nature Clinical Practice Oncology, 2 (2005) S36–S44. [DOI] [PubMed] [Google Scholar]
- [125].Xing S, Bullen CK, Shroff NS, Shan L, Yang H-C, Manucci JL, Bhat S, Zhang H, Margolick JB, Quinn TC, Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation, Journal of virology, 85 (2011) 6060–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, Emad F, Buckheit R, McCance-Katz EF, Lai J, A Pilot Study Assessing the Safety and Latency Reversing Activity of Disulfiram in HIV-1-Infected Adults on Antiretroviral Therapy, Clinical infectious diseases, (2013) cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, Savic R, Roney J, Hoh R, Solomon A, Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study, The Lancet HIV, 2 (2015) e520–e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Delagrèverie HM, Delaugerre C, Lewin SR, Deeks SG, Li JZ, Ongoing Clinical Trials of Human Immunodeficiency Virus Latency-Reversing and Immunomodulatory Agents, Open Forum Infectious Diseases, 3 (2016) ofw189–ofw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Rasmussen TA, Tolstrup M, Søgaard OS, Reversal of Latency as Part of a Cure for HIV-1, Trends in Microbiology, 24 (2016) 90–97. [DOI] [PubMed] [Google Scholar]
- [130].Halper-Stromberg A, Lu C-L, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC, Broadly Neutralizing Antibodies and Viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice, Cell, 158 (2014) 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF, Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations, J Clin Invest, 125 (2015) 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Darcis G, Kula A, Bouchat S, Fujinaga K, Corazza F, Ait-Ammar A, Delacourt N, Melard A, Kabeya K, Vanhulle C, Van Driessche B, Gatot J-S, Cherrier T, Pianowski LF, Gama L, Schwartz C, Vila J, Burny A, Clumeck N, Moutschen M, De Wit S, Peterlin BM, Rouzioux C, Rohr O, Van Lint C, An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression, PLoS Pathogens, 11 (2015) e1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Pérez M, de Vinuesa AG, Sanchez-Duffhues G, Marquez N, Bellido ML, Munoz-Fernandez M, Moreno S, Castor TP, Calzado MA, Muñoz E, Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency, Current HIV research, 8 (2010) 418–429. [DOI] [PubMed] [Google Scholar]
- [134].Reuse S, Calao M, Kabeya K, Guiguen A, Gatot J-S, Quivy V, Vanhulle C, Lamine A, Vaira D, Demonte D, Martinelli V, Veithen E, Cherrier T, Avettand V, Poutrel S, Piette J, de Launoit Y, Moutschen M, Burny A, Rouzioux C, De Wit S, Herbein G, Rohr O, Collette Y, Lambotte O, Clumeck N, Van Lint C, Synergistic Activation of HIV-1 Expression by Deacetylase Inhibitors and Prostratin: Implications for Treatment of Latent Infection, PLOS ONE, 4 (2009) e6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sarkar I, Hauber I, Hauber J, Buchholz F, HIV-1 Proviral DNA Excision Using an Evolved Recombinase, Science, 316 (2007) 1912–1915. [DOI] [PubMed] [Google Scholar]
- [136].Qu X, Wang P, Ding D, Li L, Wang H, Ma L, Zhou X, Liu S, Lin S, Wang X, Zhang G, Liu S, Liu L, Wang J, Zhang F, Lu D, Zhu H, Zinc-finger-nucleases mediate specific and efficient excision of HIV-1 proviral DNA from infected and latently infected human T cells, Nucleic Acids Research, 41 (2013) 7771–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ebina H, Kanemura Y, Misawa N, Sakuma T, Kobayashi T, Yamamoto T, Koyanagi Y, A High Excision Potential of TALENs for Integrated DNA of HIV-Based Lentiviral Vector, PLOS ONE, 10 (2015) e0120047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ebina H, Misawa N, Kanemura Y, Koyanagi Y, Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus, Scientific Reports, 3 (2013) 2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kaminski R, Bella R, Yin C, Otte J, Ferrante P, Gendelman HE, Li H, Booze R, Gordon J, Hu W, Khalili K, Excision of HIV-1 DNA by gene editing: a proof-of-concept in vivo study, Gene Therapy, 23 (2016) 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yin C, Zhang T, Qu X, Zhang Y, Putatunda R, Xiao X, Li F, Xiao W, Zhao H, Dai S, Qin X, Mo X, Young W-B, Khalili K, Hu W, In Vivo Excision of HIV-1 Provirus by saCas9 and Multiplex Single-Guide RNAs in Animal Models, Molecular Therapy, 25 (2017) 1168–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Cannon P, June C, CCR5 Knockout Strategies, Current opinion in HIV and AIDS, 6 (2011) 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang W-T, Levine BL, June CH Gene Editing of CCR5 in Autologous CD4 T Cells of Persons Infected with HIV, New England Journal of Medicine, 370 (2014) 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Li C, Guan X, Du T, Jin W, Wu B, Liu Y, Wang P, Hu B, Griffin GE, Shattock RJ, Hu Q, Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9, Journal of General Virology, 96 (2015) 2381–2393. [DOI] [PubMed] [Google Scholar]
- [144].Hou P, Chen S, Wang S, Yu X, Chen Y, Jiang M, Zhuang K, Ho W, Hou W, Huang J, Guo D, Genome editing of CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection, Scientific Reports, 5 (2015) 15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Liu Z, Chen S, Jin X, Wang Q, Yang K, Li C, Xiao Q, Hou P, Liu S, Wu S, Hou W, Xiong Y, Kong C, Zhao X, Wu L, Li C, Sun G, Guo D, Genome editing of the HIV co-receptors CCR5 and CXCR4 by CRISPR-Cas9 protects CD4(+) T cells from HIV-1 infection, Cell & Bioscience, 7 (2017) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC, Cannon PM, Zinc finger nuclease-mediated CCR5 knockout hematopoietic stem cell transplantation controls HIV-1 in vivo, Nature biotechnology, 28 (2010) 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Xu L, Yang H, Gao Y, Chen Z, Xie L, Liu Y, Liu Y, Wang X, Li H, Lai W, He Y, Yao A, Ma L, Shao Y, Zhang B, Wang C, Chen H, Deng H, CRISPR/Cas9-Mediated CCR5 Ablation in Human Hematopoietic Stem/Progenitor Cells Confers HIV-1 Resistance In Vivo, Molecular Therapy, 25 1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].DiGiusto DL, Cannon PM, Holmes MC, Li L, Rao A, Wang J, Lee G, Gregory PD, Kim KA, Hayward SB, Meyer K, Exline C, Lopez E, Henley J, Gonzalez N, Bedell V, Stan R, Zaia JA, Preclinical development and qualification of ZFN-mediated CCR5 disruption in human hematopoietic stem/progenitor cells, Molecular Therapy. Methods & Clinical Development, 3 (2016) 16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ye L, Wang J, Beyer AI, Teque F, Cradick TJ, Qi Z, Chang JC, Bao G, Muench MO, Yu J, Levy JA, Kan YW, Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Δ32 mutation confers resistance to HIV infection, Proceedings of the National Academy of Sciences, 111 (2014) 9591–9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kang H, Minder P, Park MA, Mesquitta W-T, Torbett BE, Slukvin II, CCR5 disruption in induced pluripotent stem cells using CRISPR/Cas9 provides selective resistance of immune cells to CCR5-tropic HIV-1 Virus, Mol. Ther. – Nucl. Acids, 4 (2015) e268. [DOI] [PubMed] [Google Scholar]
- [151].Kambal A, Mitchell G, Cary W, Gruenloh W, Jung Y, Kalomoiris S, Nacey C, McGee J, Lindsey M, Fury B, Bauer G, Nolta JA, Anderson JS, Generation of HIV-1 Resistant and Functional Macrophages From Hematopoietic Stem Cell–derived Induced Pluripotent Stem Cells, Molecular Therapy, 19 (2011) 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kitchen SG, Levin BR, Bristol G, Rezek V, Kim S, Aguilera-Sandoval C, Balamurugan A, Yang OO, Zack JA, In Vivo Suppression of HIV by Antigen Specific T Cells Derived from Engineered Hematopoietic Stem Cells, PLoS Pathogens, 8 (2012) e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kumar P, Ban H-S, Kim S-S, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang Y-G, Jeong J-H, Lee K-Y, Kim Y-H, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee S-K, Shankar P, T Cell-Specific siRNA Delivery Suppresses HIV-1 Infection in Humanized Mice, Cell, 134 (2008) 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Marsden MD, Zack JA, Experimental Approaches for Eliminating Latent HIV, Forum on immunopathological diseases and therapeutics, 6 (2015) 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP, Barriers to a Cure: New concepts in targeting and eradicating HIV-1 reservoirs, Lancet, 381 (2013) 10.1016/S0140–6736(1013)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Shan L, Deng K, Shroff NS, Durand C, Rabi SA, Yang H-C, Zhang H, Margolick JB, Blankson JN, Siliciano RF, Stimulation of HIV-1-specific cytolytic T-lymphocytes facilitates elimination of latent viral reservoir after virus reactivation, Immunity, 36 (2012) 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Schooley RT, Spritzler J, Wang H, Lederman MM, Havlir D, Kuritzkes DR, Pollard R, Battaglia C, Robertson M, Mehrotra D, Casimiro D, Cox K, Schock B, Team AS, ACTG 5197: A Placebo Controlled Trial of Immunization of HIV-1 Infected Persons with a Replication Deficient Ad5 Vaccine Expressing the HIV-1 Core Protein, The Journal of infectious diseases, 202 (2010) 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Gandhi RT, O’Neill D, Bosch RJ, Chan ES, Bucy RP, Shopis J, Baglyos L, Adams E, Fox L, Purdue L, Marshak A, Flynn T, Masih R, Schock B, Mildvan D, Schlesinger SJ, Marovich MA, Bhardwaj N, Jacobson JM, A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy, Vaccine, 27 (2009) 6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Angel JB, Routy J-P, Tremblay C, Ayers D, Woods R, Singer J, Bernard N, Kovacs C, Smaill F, Gurunathan S, Sekaly R-P, A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune, AIDS, 25 (2011) 731–739. [DOI] [PubMed] [Google Scholar]
- [160].Autran B, Murphy RL, Costagliola D, Tubiana R, Clotet B, Gatell J, Staszewski S, Wincker N, Assoumou L, El-Habib R, Calvez V, Walker B, Katlama C, a.t.O.S. Group, Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452), AIDS, 22 (2008) 1313–1322. [DOI] [PubMed] [Google Scholar]
- [161].Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Lifson JD, Picker LJ, Profound early control of highly pathogenic SIV by an effector-memory T cell vaccine, Nature, 473 (2011) 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hansen SG, Piatak M, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Früh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ, Immune clearance of highly pathogenic SIV infection, Nature, 502 (2013) 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang H-W, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR, Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys, Nature, 503 (2013) 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Williams LD, Ofek G, Schätzle S, McDaniel JR, Lu X, Nicely NI, Wu L, Lougheed CS, Bradley T, Louder MK, McKee K, Bailer RT, O’Dell S, Georgiev IS, Seaman MS, Parks RJ, Marshall DJ, Anasti K, Yang G, Nie X, Tumba NL, Wiehe K, Wagh K, Korber B, Kepler TB, Munir Alam S, Morris L, Kamanga G, Cohen MS, Bonsignori M, Xia S-M, Montefiori DC, Kelsoe G, Gao F, Mascola JR, Moody MA, Saunders KO, Liao H-X, Tomaras GD, Georgiou G, Haynes BF, Potent and broad HIV-neutralizing antibodies in memory B cells and plasma, Science Immunology, 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, Oliveira T, Lorenzi JCC, Parrish EH, Learn GH, West AP, Bjorkman PJ, Schlesinger SJ, Seaman MS, Czartoski J, McElrath MJ, Pfeifer N, Hahn BH, Caskey M, Nussenzweig MC, HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1, Science, 352 (2016) 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Liao H-X, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia S-M, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BTM, Kwong PD, Mascola JR, Haynes BF, Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus, Nature, 496 (2013) 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Velu V, Shetty RD, Larsson M, Shankar EM, Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options, Retrovirology, 12 (2015) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Safety, activity, and immune correlates of anti–PD-1 antibody in cancer, New England Journal of Medicine, 366 (2012) 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang K-M, Sulkowski M, Marro SO, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I, A Randomized, Double-Blind, Placebo-Controlled Assessment of BMS-936558, a Fully Human Monoclonal Antibody to Programmed Death-1 (PD-1), in Patients with Chronic Hepatitis C Virus Infection, PLoS ONE, 8 (2013) e63818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Hatano H, Jain V, Hunt PW, Lee T-H, Sinclair E, Do TD, Hoh R, Martin JN, McCune JM, Hecht F, Busch MP, Deeks SG, Cell-Based Measures of Viral Persistence Are Associated With Immune Activation and Programmed Cell Death Protein 1 (PD-1)– Expressing CD4(+) T cells, The Journal of Infectious Diseases, 208 (2013) 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR, Enhancing SIV-Specific Immunity In Vivo by PD-1 Blockade, Nature, 458 (2009) 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, Amara RR, PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques, The Journal of Clinical Investigation, 122 (2012) 1712–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia, New England Journal of Medicine, 371 (2014) 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X, Engineering chimeric antigen receptor-T cells for cancer treatment, Molecular Cancer, 17 (2018) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Yang OO, Tran A-C, Kalams SA, Johnson RP, Roberts MR, Walker BD, Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells, Proceedings of the National Academy of Sciences of the United States of America, 94 (1997) 11478–11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.[] Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, Macken C, Richman DD, Christopherson C, June CH, Lazar R, Broad DF, Jalali S, Hege KM, A Phase II Randomized Study of HIV-Specific T-Cell Gene Therapy in Subjects with Undetectable Plasma Viremia on Combination Antiretroviral Therapy, Molecular Therapy, 5 (2002) 788–797. [DOI] [PubMed] [Google Scholar]
- [177].Zhen A, Peterson CW, Carrillo MA, Reddy SS, Youn CS, Lam BB, Chang NY, Martin HA, Rick JW, Kim J, Neel NC, Rezek VK, Kamata M, Chen ISY, Zack JA, Kiem H-P, Kitchen SG, Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS, PLOS Pathogens, 13 (2017) e1006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Gavegnano C, Detorio M, Montero C, Bosque A, Planelles V, Schinazi RF, Ruxolitinib and Tofacitinib Are Potent and Selective Inhibitors of HIV-1 Replication and Virus Reactivation In Vitro, Antimicrobial Agents and Chemotherapy, 58 (2014) 1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Gavegnano C, Brehm JH, Dupuy FP, Talla A, Ribeiro SP, Kulpa DA, Cameron C, Santos S, Hurwitz SJ, Marconi VC, Routy J-P, Sabbagh L, Schinazi RF, Sékaly RP, Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors, PLOS Pathogens, 13 (2017) e1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente ST, The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency, mBio, 6 (2015) e00465–00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Kessing CF, Nixon CC, Li C, Tsai P, Takata H, Mousseau G, Ho PT, Honeycutt JB, Fallahi M, Trautmann L, Garcia JV, Valente ST, In vivo suppression of HIV rebound by Didehydro-Cortistatin A, a “Block-and-Lock” strategy for HIV-1 treatment, Cell Rep, 21 (2017) 600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, Brameier M, Walter L, Rogers K, Mayne AE, Dunbar P, Villinger T, Little D, Parslow TG, Santangelo PJ, Villinger F, Fauci AS, Ansari AA, Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection, Nat Med 20 (2014) 1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, Sidell N, Kane MA, Yu J, Jones JW, Santangelo PJ, Zurla C, McKinnon LR, Arnold KB, Woody CE, Walter L, Roos C, Noll A, Van Ryk D, Jelicic K, Cimbro R, Gumber S, Reid MD, Adsay V, Amancha PK, Mayne AE, Parslow TG, Fauci AS, Ansari AA, Virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy, Science, 354 (2016) 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lu X, Li Z, Li Q, Jiao Y, Ji Y, Zhang H, Liu Z, Li W, Wu H, Preferential loss of gut-homing α4β7 CD4(+) T cells and their circulating functional subsets in acute HIV-1 infection, Cellular and Molecular Immunology, 13 (2016) 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS, HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells, Nat Immunol, 9 (2008) 301–309. [DOI] [PubMed] [Google Scholar]
- [186].Hill AL, Rosenbloom DIS, Fu F, Nowak MA, Siliciano RF, Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1, Proceedings of the National Academy of Sciences, 111 (2014) 13475–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Mout R, Ray M, Lee Y-W, Scaletti F, Rotello VM, In Vivo Delivery of CRISPR/Cas9 for Therapeutic Gene Editing: Progress and Challenges, Bioconjugate Chemistry, 28 (2017) 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Martinez-Picado J, Deeks SG, Persistent HIV-1 replication during antiretroviral therapy, Current Opinion in HIV and AIDS, 11 (2016) 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].das Neves J, Amiji MM, Bahia MF, Sarmento B, Nanotechnology-based systems for the treatment and prevention of HIV/AIDS, Advanced Drug Delivery Reviews, 62 (2010) 458–477. [DOI] [PubMed] [Google Scholar]
- [190].Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, Langer RS, Farokhzad OC, Targeted nanoparticles for cancer therapy, Nano Today, 2 (2007) 14–21. [Google Scholar]
- [191].Shao J, Kraft JC, Li B, Yu J, Freeling J, Koehn J, Ho RJY, Nanodrug formulations to enhance HIV drug exposure in lymphoid tissues and cells: clinical significance and potential impact on treatment and eradication of HIV/AIDS, Nanomedicine, 11 (2016) 545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Zhou T, Su H, Dash P, Lin Z, Dyavar Shetty BL, Kocher T, Szlachetka A, Lamberty B, Fox HS, Poluektova L, Gorantla S, McMillan J, Gautam N, Mosley RL, Alnouti Y, Edagwa B, Gendelman HE, Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles, Biomaterials, 151 (2018) 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Wen L, Qian W, Yuan L, Fei Y, Qi L, Bingjie Q, Lan X, Lu L, Shibo J, A Nanoparticle-Encapsulated Non-Nucleoside Reverse-Transcriptase Inhibitor with Enhanced Anti-HIV-1 Activity and Prolonged Circulation Time in Plasma, Current Pharmaceutical Design, 21 (2015) 925–935. [DOI] [PubMed] [Google Scholar]
- [194].Buehler DC, Marsden MD, Shen S, Toso DB, Wu X, Loo JA, Zhou ZH, Kickhoefer VA, Wender PA, Zack JA, Rome LH, Bioengineered Vaults: Self-Assembling Protein Shell–Lipophilic Core Nanoparticles for Drug Delivery, ACS Nano, 8 (2014) 7723–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Bae Y, Diezi TA, Zhao A, Kwon GS, Mixed polymeric micelles for combination cancer chemotherapy through the concurrent delivery of multiple chemotherapeutic agents, Journal of Controlled Release, 122 (2007) 324–330. [DOI] [PubMed] [Google Scholar]
- [196].Freeling JP, Koehn J, Shu C, Sun J, Ho RJY, Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood, AIDS, 28 (2014) 2625–2627. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Freeling JP, Koehn J, Shu C, Sun J, Ho RJ, Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates, AIDS research and human retroviruses, 31 (2015) 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Shibata A, McMullen E, Pham A, Belshan M, Sanford B, Zhou Y, Goede M, Date AA, Destache CJ, Polymeric Nanoparticles Containing Combination Antiretroviral Drugs for HIV Type 1 Treatment, AIDS Research and Human Retroviruses, 29 (2013) 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Prathipati PK, Mandal S, Pon G, Vivekanandan R, Destache CJ, Pharmacokinetic and Tissue Distribution Profile of Long Acting Tenofovir Alafenamide and Elvitegravir Loaded Nanoparticles in Humanized Mice Model, Pharm Res, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Nowacek AS, Miller RL, McMillan J, Kanmogne G, Kanmogne M, Mosley RL, Ma Z, Graham S, Chaubal M, Werling J, NanoART synthesis, characterization, uptake, release and toxicology for human monocyte–macrophage drug delivery, Nanomedicine, 4 (2009) 903–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Roy U, McMillan J, Alnouti Y, Gautum N, Smith N, Balkundi S, Dash P, Gorantla S, Martinez-Skinner A, Meza J, Pharmacodynamic and antiretroviral activities of combination nanoformulated antiretrovirals in HIV-1–infected human peripheral blood lymphocyte–reconstituted mice, The Journal of infectious diseases, 206 (2012) 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Chiodo F, Marradi M, Calvo J, Yuste E, Penadés S, Glycosystems in nanotechnology: Gold glyconanoparticles as carrier for anti-HIV prodrugs, Beilstein Journal of Organic Chemistry, 10 (2014) 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Kraft JC, McConnachie LA, Koehn J, Kinman L, Sun J, Collier AC, Collins C, Shen DD, Ho RJY, Mechanism-based pharmacokinetic (MBPK) models describe the complex plasma kinetics of three antiretrovirals delivered by a long-acting anti-HIV drug combination nanoparticle formulation, Journal of Controlled Release, 275 (2018) 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Tang X, Liang Y, Liu X, Zhou S, Liu L, Zhang F, Xie C, Cai S, Wei J, Zhu Y, Hou W, PLGA-PEG Nanoparticles Coated with Anti-CD45RO and Loaded with HDAC Plus Protease Inhibitors Activate Latent HIV and Inhibit Viral Spread, Nanoscale Research Letters, 10 (2015) 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Li W, Yu F, Wang Q, Qi Q, Su S, Xie L, Lu L, Jiang S, Co-delivery of HIV-1 entry inhibitor and nonnucleoside reverse transcriptase inhibitor shuttled by nanoparticles: cocktail therapeutic strategy for antiviral therapy, AIDS, 30 (2016) 827–838. [DOI] [PubMed] [Google Scholar]
- [206].Kumar P, Lakshmi YS, Kondapi AK, Triple Drug Combination of Zidovudine, Efavirenz and Lamivudine Loaded Lactoferrin Nanoparticles: an Effective Nano First-Line Regimen for HIV Therapy, Pharm Res, 34 (2017) 257–268. [DOI] [PubMed] [Google Scholar]
- [207].Jayant RD, Atluri VSR, Tiwari S, Pilakka-Kanthikeel S, Kaushik A, Yndart A, Nair M, Novel nanoformulation to mitigate co-effects of drugs of abuse and HIV-1 infection: towards the treatment of NeuroAIDS, J. Neurovirol, 23 (2017) 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Zhou J, Neff CP, Liu X, Zhang J, Li H, Smith DD, Swiderski P, Aboellail T, Huang Y, Du Q, Liang Z, Peng L, Akkina R, Rossi JJ, Systemic Administration of Combinatorial dsiRNAs via Nanoparticles Efficiently Suppresses HIV-1 Infection in Humanized Mice, Molecular Therapy, 19 (2011) 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Weber N, Ortega P, Clemente MI, Shcharbin D, Bryszewska M, de la Mata FJ, Gómez R, Muñoz-Fernández MA, Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes, Journal of Controlled Release, 132 (2008) 55–64. [DOI] [PubMed] [Google Scholar]
- [210].Zhou J, Shu Y, Guo P, Smith DD, Rossi JJ, Dual functional RNA nanoparticles containing phi29 motor pRNA and anti-gp120 aptamer for cell-type specific delivery and HIV-1 Inhibition, Methods, 54 (2011) 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, Smith DD, Swiderski P, Rossi JJ, Akkina R, An Aptamer-siRNA Chimera Suppresses HIV-1 Viral Loads and Protects from Helper CD4+T Cell Decline in Humanized Mice, Science Translational Medicine, 3 (2011) 66ra66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio P, Incesu R-B, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC, HIV therapy by a combination of broadly neutralizing antibodies in humanized mice, Nature, 492 (2012) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Freund NT, Wang H, Scharf L, Nogueira L, Horwitz JA, Bar-On Y, Golijanin J, Sievers SA, Sok D, Cai H, Cesar Lorenzi JC, Halper-Stromberg A, Toth I, Piechocka-Trocha A, Gristick HB, van Gils MJ, Sanders RW, Wang L-X, Seaman MS, Burton DR, Gazumyan A, Walker BD, West AP, Bjorkman PJ, Nussenzweig MC, Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller, Science Translational Medicine, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Büning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC, HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) 16538–16543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Li M, Li H, Rossi JJ, RNAi in Combination with a Ribozyme and TAR Decoy for Treatment of HIV Infection in Hematopoietic Cell Gene Therapy, Annals of the New York Academy of Sciences, 1082 (2006) 172–179. [DOI] [PubMed] [Google Scholar]
- [216].Lee ALZ, Wang Y, Cheng HY, Pervaiz S, Yang YY, The co-delivery of paclitaxel and Herceptin using cationic micellar nanoparticles, Biomaterials, 30 (2009) 919–927. [DOI] [PubMed] [Google Scholar]
- [217].Jang M, Han HD, Ahn HJ, A RNA nanotechnology platform for a simultaneous two-in-one siRNA delivery and its application in synergistic RNAi therapy, Scientific Reports, 6 (2016) 32363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Chen Y, Zhu X, Zhang X, Liu B, Huang L, nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy, Mol. Ther, 18 (2010) 1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Kim S-S, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, Habiro K, Yang Y-G, Manjunath N, Shimaoka M, Shankar P, RNAi-mediated CCR5 Silencing by LFA-1-targeted Nanoparticles Prevents HIV Infection in BLT Mice, Mol Ther, 18 (2009) 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Endsley AN, Ho RJY, Design and Characterization of Novel Peptide-Coated Lipid Nanoparticles for Targeting Anti-HIV Drug to CD4 Expressing Cells, The AAPS Journal, 14 (2012) 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Endsley AN, Ho RJY, Enhanced anti-HIV efficacy of Indinavir after inclusion in CD4 targeted lipid nanoparticles, Journal of acquired immune deficiency syndromes (1999), 61 (2012) 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T, Gendelman HE, Liu X-M, Macrophage Folate Receptor-Targeted Antiretroviral Therapy Facilitates Drug Entry, Retention, Antiretroviral Activities and Biodistribution for Reduction of Human Immunodeficiency Virus Infections, Nanomedicine : nanotechnology, biology, and medicine, 9 (2013) 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Puligujja P, Araínga M, Dash P, Palandri D, Mosley RL, Gorantla S, Poluektova L, McMillan J, Gendelman HE, Pharmacodynamics of folic acid-receptor targeted antiretroviral nanotherapy in HIV-1-infected humanized mice, Antiviral research, 120 (2015) 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Gu J, Al-Bayati K, Ho EA, Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes, Drug Delivery and Translational Research, 7 (2017) 497–506. [DOI] [PubMed] [Google Scholar]
- [225].Kinman L, Brodie SJ, Tsai CC, Bui T, Larsen K, Schmidt A, Anderson D, Morton WR, Hu S-L, Ho RJY, Lipid–Drug Association Enhanced HIV-1 Protease Inhibitor Indinavir Localization in Lymphoid Tissues and Viral Load Reduction: A Proof of Concept Study in HIV-2287-Infected Macaques, JAIDS Journal of Acquired Immune Deficiency Syndromes, 34 (2003) 387–397. [DOI] [PubMed] [Google Scholar]
- [226].Saiyed ZM, Gandhi NH, Nair M, Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood-brain barrier, Int J Nanomedicine, 5 (2010) 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Chiappetta DA, Hocht C, Opezzo JAW, Sosnik A, Intranasal administration of antiretroviral-loaded micelles for anatomical targeting to the brain in HIV, Nanomedicine, 8 (2013) 223–237. [DOI] [PubMed] [Google Scholar]
- [228].Prabhakar K, Afzal SM, Kumar PU, Rajanna A, Kishan V, Brain delivery of transferrin coupled indinavir submicron lipid emulsions—Pharmacokinetics and tissue distribution, Colloids and Surfaces B: Biointerfaces, 86 (2011) 305–313. [DOI] [PubMed] [Google Scholar]
- [229].Vyas TK, Shahiwala A, Amiji MM, Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations, International Journal of Pharmaceutics, 347 (2008) 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV, Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain, Nanomedicine: Nanotechnology, Biology and Medicine, 10 (2014) 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Shegokar R, Singh KK, Surface modified nevirapine nanosuspensions for viral reservoir targeting: In Vitro and in vivo evaluation, International journal of pharmaceutics, 421 (2011) 341–352. [DOI] [PubMed] [Google Scholar]
- [232].Kuo Y-C, Kuo C-Y, Electromagnetic interference in the permeability of saquinavir across the blood–brain barrier using nanoparticulate carriers, International journal of pharmaceutics, 351 (2008) 271–281. [DOI] [PubMed] [Google Scholar]
- [233].Kuo Y-C, Su F-L, Transport of stavudine, delavirdine, and saquinavir across the blood–brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles, International journal of pharmaceutics, 340 (2007) 143–152. [DOI] [PubMed] [Google Scholar]
- [234].Rao KS, Reddy MK, Horning JL, Labhasetwar V, TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs, Biomaterials, 29 (2008) 4429–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, Balkundi S, Smith N, Alnouti Y, Gautam N, Zhou Y, Poluektova L, Kabanov A, Bronich T, Gendelman HE, Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells, International Journal of Nanomedicine, 7 (2012) 2373–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Jiménez JL, Clemente MI, Weber ND, Sanchez J, Ortega P, de la Mata FJ, Gómez R, García D, López-Fernández LA, Muñoz-Fernández MÁ, Carbosilane Dendrimers to Transfect Human Astrocytes with Small Interfering RNA Targeting Human Immunodeficiency Virus, BioDrugs, 24 (2010) 331–343. [DOI] [PubMed] [Google Scholar]
- [237].Serramía MJ, Álvarez S, Fuentes-Paniagua E, Clemente MI, Sánchez-Nieves J, Gómez R, de la Mata J, Muñoz-Fernández MÁ, In vivo delivery of siRNA to the brain by carbosilane dendrimer, Journal of Controlled Release, 200 (2015) 60–70. [DOI] [PubMed] [Google Scholar]
- [238].Ramishetti S, Kedmi R, Goldsmith M, Leonard F, Sprague AG, Godin B, Gozin M, Cullis PR, Dykxhoorn DM, Peer D, Systemic Gene Silencing in Primary T Lymphocytes Using Targeted Lipid Nanoparticles, ACS Nano, (2015). [DOI] [PubMed] [Google Scholar]
- [239].Cao S, Jiang Y, Levy Claire N, Hughes Sean M, Zhang H, Hladik F, Woodrow Kim A, Optimization and comparison of CD4‐targeting lipid–polymer hybrid nanoparticles using different binding ligands, Journal of Biomedical Materials Research Part A, 106 (2017) 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Hu C-MJ, Kaushal S, Cao HST, Aryal S, Sartor M, Esener S, Bouvet M, Zhang L, Half-Antibody Functionalized Lipid−Polymer Hybrid Nanoparticles for Targeted Drug Delivery to Carcinoembryonic Antigen Presenting Pancreatic Cancer Cells, Molecular Pharmaceutics, 7 (2010) 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Kaur CD, Nahar M, Jain NK, Lymphatic targeting of zidovudine using surface-engineered liposomes, Journal of drug targeting, 16 (2008) 798–805. [DOI] [PubMed] [Google Scholar]
- [242].Kaminskas LM, Kota J, McLeod VM, Kelly BD, Karellas P, Porter CJH, PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats, Journal of Controlled Release, 140 (2009) 108–116. [DOI] [PubMed] [Google Scholar]
- [243].Richter WF, Bhansali SG, Morris ME, Mechanistic Determinants of Biotherapeutics Absorption Following SC Administration, The AAPS Journal, 14 (2012) 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Kunisawa J, Kurashima Y, Kiyono H, Gut-associated lymphoid tissues for the development of oral vaccines, Advanced Drug Delivery Reviews, 64 (2012) 523–530. [DOI] [PubMed] [Google Scholar]
- [245].Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR, Human Intestinal M Cells Display the Sialyl Lewis A Antigen, Infection and Immunity, 67 (1999) 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Aungst BJ, P-glycoprotein, secretory transport, and other barriers to the oral delivery of anti-HIV drugs, Advanced Drug Delivery Reviews, 39 (1999) 105–116. [DOI] [PubMed] [Google Scholar]
- [247].Nowacek A, Gendelman HE, NanoART, neuroAIDS and CNS drug delivery, Nanomedicine, 4 (2009) 557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Yin H, Kauffman KJ, Anderson DG, Delivery technologies for genome editing, Nature Reviews Drug Discovery, 16 (2017) 387. [DOI] [PubMed] [Google Scholar]
- [249].Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, Non-viral vectors for gene-based therapy, Nature Reviews Genetics, 15 (2014) 541. [DOI] [PubMed] [Google Scholar]
- [250].LaFountaine JS, Fathe K, Smyth HDC, Delivery and therapeutic applications of gene editing technologies ZFNs, TALENs, and CRISPR/Cas9, International Journal of Pharmaceutics, 494 (2015) 180–194. [DOI] [PubMed] [Google Scholar]
- [251].Mishra V, Kesharwani P, Jain NK, siRNA nanotherapeutics: a Trojan horse approach against HIV, Drug Discovery Today, 19 (2014) 1913–1920. [DOI] [PubMed] [Google Scholar]
- [252].Adesina SK, Akala EO, Nanotechnology approaches for the delivery of exogenous siRNA for HIV therapy, Molecular pharmaceutics, 12 (2015) 4175–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Perisé-Barrios AJ, Jiménez JL, Domínguez-Soto A, de la Mata FJ, Corbí AL, Gomez R, Muñoz-Fernandez MÁ, Carbosilane dendrimers as gene delivery agents for the treatment of HIV infection, Journal of Controlled Release, 184 (2014) 51–57. [DOI] [PubMed] [Google Scholar]
- [254].Shcharbin D, Pedziwiatr E, Nowacka O, Kumar M, Zaborski M, Ortega P, Javier de la Mata F, Gómez R, Muñoz-Fernandez MA, Bryszewska M, Carbosilane dendrimers NN8 and NN16 form a stable complex with siGAG1, Colloids and Surfaces B: Biointerfaces, 83 (2011) 388–391. [DOI] [PubMed] [Google Scholar]
- [255].Briz V, Serramia MJ, Madrid R, Hameau A, Anne-Marie C, Majoral JP, Munoz-Fernandez MA, Validation of a Generation 4 Phosphorus-Containing Polycationic Dendrimer for Gene Delivery Against HIV-1, Current Medicinal Chemistry, 19 (2012) 5044–5051. [DOI] [PubMed] [Google Scholar]
- [256].Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, Mackley VA, Chang K, Rao A, Skinner C, Shobha T, Mehdipour M, Liu H, Huang W.-c., Lan F, Bray NL, Li S, Corn JE, Kataoka K, Doudna JA, Conboy I, Murthy N, Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair, Nature Biomedical Engineering, 1 (2017) 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Park Y-M, Lee SJ, Kim YS, Lee MH, Cha GS, Jung ID, Kang TH, Han HD, Nanoparticle-Based Vaccine Delivery for Cancer Immunotherapy, Immune Network, 13 (2013) 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Friedman CF, Proverbs-Singh TA, Postow MA, Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review, JAMA Oncology, 2 (2016) 1346–1353. [DOI] [PubMed] [Google Scholar]
- [259].Kosmides AK, Sidhom J-W, Fraser A, Bessell CA, Schneck JP, Dual Targeting Nanoparticle Stimulates the Immune System To Inhibit Tumor Growth, ACS Nano, 11 (2017) 5417–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS, T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity, Nature Communications, 8 (2017) 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS, Stephan MT, In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers, Nat Nano, 12 (2017) 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]


