Abstract
This is a case of a 48-year-old man who presented with a pulmonary embolism and was found to have left ventricular non-compaction cardiomyopathy. Initial echocardiograms demonstrated prominent apical trabeculations with reduced biventricular function. These findings were further confirmed and characterized by cardiac magnetic resonance imaging. He met all major criteria used to identify left ventricular non-compaction cardiomyopathy. He underwent medical management for heart failure and during follow-up was noted to have significant improvement in left ventricular systolic function and symptoms. While most management attention is focused on rhythm disturbances or embolic risk, particular attention should also be exercised to ensure that heart failure medical therapy is optimized. While many with left ventricular non-compaction cardiomyopathy have irreversible dysfunction, this case highlights that there may be some who will respond well to aggressive medical therapy. The diagnosis and medical management of left ventricular non-compaction cardiomyopathy are reviewed in light of our patient and his clinical course.
<Learning objective: Historically, left ventricular non-compaction cardiomyopathy (LVNC) has been associated with significant morbidity and mortality. Discussion often focuses on sudden cardiac death and prevention of embolisms. Many of the initial reports and case series were written in an era when standard medical therapy for congestive heart failure was not yet defined. While many do not respond as this case did, this case emphasizes that optimal medical therapy can make a substantial difference, even for LVNC.>
Keywords: Heart failure, Cardiomyopathy, Non-compaction, Cardiac magnetic resonance imaging, Echocardiography
Introduction
Isolated left ventricular non-compaction cardiomyopathy (LVNC) is an uncommon myocardial disorder characterized by prominent trabeculae and deep intertrabecular recesses forming thickened myocardium consisting of a thin compacted epicardial layer and a thickened non-compacted endocardial layer [1]. Initial case series reported significant morbidity and mortality from heart failure, ventricular arrhythmias, thromboembolism, and sudden cardiac death among individuals affected with LVNC 2, 3. Given this association with adverse outcomes, several imaging criteria have been proposed to identify those with LVNC. As cardiac imaging techniques have improved, it has become more common for cardiac imaging to identify trabeculations, and at times, it is more difficult to separate the normal from the pathological. More difficult still is the management of those with LVNC.
Case report
The patient was a 48-year-old Caucasian male with a history of hyperlipidemia and peripheral vascular disease who presented to the emergency department with a 4-day history of shortness of breath, cough, hemoptysis, and bilateral swelling of the legs. He was found to have a pulmonary embolism via computed tomography angiography imaging. He also complained of intermittent shortness of breath and symptoms of orthopnea for the past year. He was still able to carry out his job as a construction worker, albeit with reduced workload tolerance. His vital signs on presentation showed a heart rate of 78 beats per minute with a blood pressure of 148/102 mmHg. His oxygen saturation was 94%. On physical examination, heart sounds were normal. There was mild tachypnea, but the lungs were clear to auscultation. Symmetric bilateral lower extremity edema and tenderness to right lower extremity were also present. His 12-lead electrocardiogram showed non-specific anterior T-wave abnormalities.
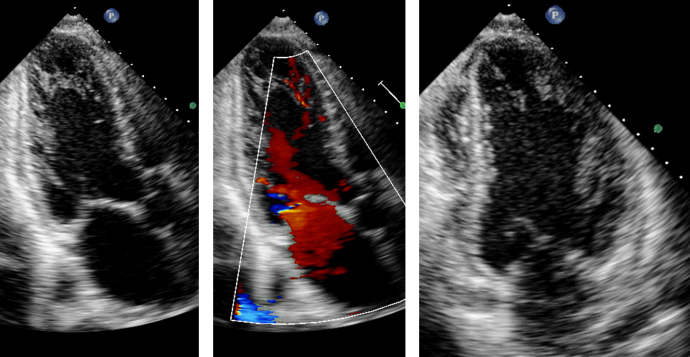
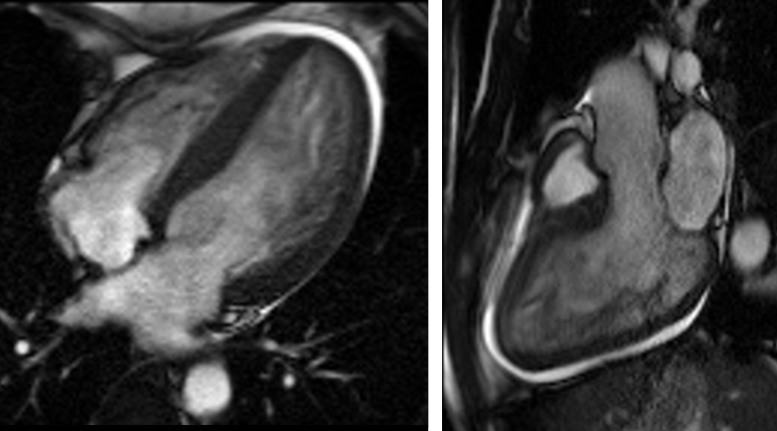
The initial transthoracic echocardiogram showed prominent trabeculations in both the left and right ventricular apex with severely reduced left ventricular ejection fraction (LVEF) of 15–20% as well as color flow Doppler in the trabecular recesses (see Fig. 1 and Movie 1). The ratio of non-compacted to compacted myocardium by echocardiography was 2.4 at end-diastole and 2.1 (normal: <2) at end-systole from the short-axis apical slices. Cardiac magnetic resonance imaging (MRI) demonstrated a moderately dilated left ventricle with a severely reduced LVEF of 11% (see Fig. 2 and Movie 2). Trabecular mass to total mass ratio was 50% (normal: <20%), the ratio of non-compacted to compacted myocardial thickness was 2.6 at end-diastole from the apical short axis view (normal: <2.3), and the ratio of non-compacted to compacted myocardial thickness was also 2.3 at end-systole from the same view (normal: <2). In reviewing the long-axis views, the average of the end-diastolic non-compacted to compacted ratios from the 3 long-axis views was 3.3 (normal: <2.3). These measurements confirmed the diagnosis of LVNC. There was no late gadolinium enhancement seen during the MRI study, nor was there any increase in the T2 signal of the compacted myocardium to suggest a stress-related response. Of note, the right ventricular apical trabecular thickness was 45 mm [4].
Fig. 1.
Initial echocardiogram with apical 3-chamber view presented with (center) and without (left) color flow Doppler to demonstrate blood flow within the trabecular recesses. Apical 2-chamber view shown to demonstrate trabeculations (right).
Fig. 2.
Cardiac magnetic resonance imaging of 4-chamber view (left) and 3-chamber view (right) to demonstrate trabeculations.
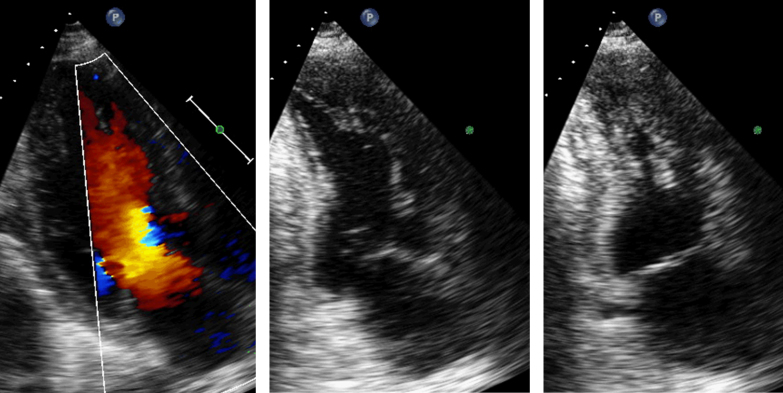
The patient was referred to the cardiology service and was treated with furosemide, carvedilol, and lisinopril for the management of heart failure. One year later, the patient was able to work with little restriction and was classified as having New York Heart Association Class I heart failure. Echocardiogram demonstrated a normal left ventricular size and an improved LVEF of 55%. Trabeculations were still present and unchanged in the left ventricular apex (see Fig. 3 and Movie 3). Interestingly, the initial echocardiogram showed a biplane left ventricular end-diastolic volume of 210 ml, and the follow-up echocardiogram 1 year later showed a left ventricular end-diastolic volume of 145 ml, which shows that the patient did have reverse remodeling during his clinical follow-up. The patient was also noted during his presentation to have dysmorphic features suggestive of an underlying genetic abnormality. He was offered genetic counseling and testing but declined.
Fig. 3.
Echocardiogram 1 year after presentation with continued flow by color Doppler of the trabeculated recesses (left). Apical 2-chamber view of end-diastole (center) and end-systole (right) to demonstrate improved left ventricular systolic function.
Discussion
There are several inherent challenges in interpreting the images of those with potential LVNC. First, there are several ways to quantify trabeculations and identify those with LVNC. During the 1980s, multiple case reports and series identified reduced left ventricular systolic function with increased trabeculations as a new etiology of heart failure. Chin et al. [2] presented the first set of criteria which sought to use the x:y ratio, which compares the thickness of the non-compacted layer with its deepest recess. Due to technical issues present both then and now, it continues to be difficult to consistently measure this ratio. As such, Jenni's group proposed echocardiographic criteria which sought, among other things, to describe a thickened left ventricular wall presenting with a bi-layered structure that consisted of a non-compacted to compacted wall thickness ratio of >2:1 at end-systole [3]. In addition, it was paramount to demonstrate Doppler flow in the trabecular recesses, as well as to exclude the presence of other congenital anomalies. These criteria were subsequently validated in a separate population [5]. With the increasing use of cardiac MRI, Petersen et al. [6] translated the measurements from the Jenni criteria to MRI by using an end-diastolic non-compacted to compacted ratio >2.3. Further still, Jacquier et al. [7] proposed the trabeculated mass-to-total left ventricular mass ratio, which had a sensitivity of 93% and a specificity of 94%. While many have noted that the right ventricle is more trabeculated than the left ventricle, it makes identifying those with pathological trabeculations more difficult. Previously, the thickness of the trabeculations present in the right ventricular apex was found to be a more specific finding in those with significant left ventricular trabeculations [4].
Second, it is unclear if these different criteria are identifying the same patients. From the Multi-Ethnic Study of Atherosclerosis, it was found that the end-diastolic ratio from cardiac MRI was >2.3 in 43% of participants [8]. Using the short-axis measurements, Dawson et al. [9] demonstrated that 3% of normal volunteers had an end-diastolic ratio >2. However, none of the participants had an end-systolic ratio >2. Comparing the end-diastolic and end-systolic ratio in a trabeculated population, we demonstrated that the end-systolic ratio was more strongly associated with clinical events that were historically associated with LVNC [10]. This finding suggests that different criteria may capture different levels of information. Our patient meets end-diastolic criteria both at initial presentation as well as at follow-up, but he meets end-systolic criteria only at initial presentation, not at follow-up. These observations invite several questions: Does the presence of increased trabeculations at end-diastole indicate risk of future decline in systolic dysfunction? Do increased trabeculations further increase the risk and complications in those with left ventricular systolic dysfunction? With no deep venous thrombosis to account for the embolism, could the right ventricular trabeculations have served as the origin? Some of the initial case reports involved younger patients who presented with pulmonary embolism in absence of deep venous thrombosis, which suggested in some cases that right ventricular trabeculations may serve as a limited nidus for clot formation, much as trabeculations have been implicated in embolic phenomenon from trabeculations located within the left ventricle [3].
Third, there are no overt guidelines for the medical management of patients with LVNC. Symptomatic patients with heart failure should be treated based on clinical presentation with established medical management, including β-blockers, angiotensin-converting enzyme inhibitors, and diuretics, according to consensus guidelines [11]. Our patient demonstrated a rather robust response to standard heart failure therapy, which is often not encountered in those with documented LVNC. Nevertheless, those with LVNC should be consistently optimized with standard medical therapy, since some cases may respond well. While medical therapy may account for some of the improvement in left ventricular systolic function, it is difficult to know for sure the exact reasons why our case had such a dramatic improvement. Conversely, the initial left ventricular functional decline may have represented an abnormal stress-related response.
Treatment is also concentrated on preventing the development or recurrence of complications with prognostic relevance, such as heart failure, ventricular arrhythmias, or cardioembolism [12]. Routine use of anticoagulation to prevent thromboembolism in patients with LVNC remains controversial. While there are no robust data to support it, most centers recommend anticoagulation in those with atrial fibrillation, LVEF <40%, previous history of embolic events, or those with known ventricular thrombi 12, 13, 14. Given our patient's pulmonary embolism, he was placed on anti-coagulation. Our discussion concerning a potential implantable cardiac defibrillator was deferred given the results of his follow-up imaging and improvement in symptoms as well as absence of significant ventricular ectopy. In general, patients who are asymptomatic and have normal left ventricular systolic function usually have good prognosis and would only require follow-up every 2–3 years with clinical assessment and echocardiography [12]. Asymptomatic patients with echocardiographic evidence of systolic dysfunction are recommended to have evidence-based medical heart failure therapy and follow-up every 1–2 years [12]. Since LVNC is associated with potential genetic underpinnings and familial occurrences, comprehensive clinical screening is recommended in all first-degree relatives of patients diagnosed with LVNC [13].
Conclusion
While many with LVNC do not improve, even with aggressive medical therapy, there may be a select subpopulation of patients with LVNC who are able to respond to intervention.
Funding
None declared.
Conflict of interest
None declared.
Footnotes
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2014.08.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Initial 2-chamber view from echocardiogram to demonstrate decreased systolic function with increased trabeculations.
Cardiac magnetic resonance imaging of 4-chamber view to demonstrate decreased systolic function with increased trabeculations.
Follow-up 2-chamber view from echocardiogram that continues to have increased trabeculations, but has marked left ventricular systolic improvement.
References
- 1.Ritter M., Oechslin E., Sutsch G., Attenhofer C., Schneider J., Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 2.Chin T.K., Perloff J.K., Williams R.G., Jue K., Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 3.Oechslin E.N., Attenhofer Jost C.H., Rojas J.R., Kaufmann P.A., Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36:493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 4.Stacey R.B., Andersen M., Haag J., Hall M.E., McLeod G., Upadhya B., Hundley W.G., Thohan V. Right ventricular morphology and systolic function in left ventricular noncompaction cardiomyopathy. Am J Cardiol. 2014;113:1018–1023. doi: 10.1016/j.amjcard.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frischknecht B.S., Attenhofer Jost C.H., Oechslin E.N., Seifert B., Hoigne P., Roos M., Jenni R. Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J Am Soc Echocardiogr. 2005;18:865–872. doi: 10.1016/j.echo.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Petersen S.E., Selvanayagam J.B., Wiesmann F., Robson M.D., Francis J.M., Anderson R.H., Watkins H., Neubauer S. Left ventricular non-compaction: insights from cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Jacquier A., Thuny F., Jop B., Giorgi R., Cohen F., Gaubert J.Y., Vidal V., Bartoli J.M., Habib G., Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J. 2010;31:1098–1104. doi: 10.1093/eurheartj/ehp595. [DOI] [PubMed] [Google Scholar]
- 8.Kawel N., Nacif M., Arai A.E., Gomes A.S., Hundley W.G., Johnson W.C., Prince M.R., Stacey R.B., Lima J.A., Bluemke D.A. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012;5:357–366. doi: 10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson D.K., Maceira A.M., Raj V.J., Graham C., Pennell D.J., Kilner P.J. Regional thicknesses and thickening of compacted and trabeculated myocardial. Circ Cardiovasc Imaging. 2011;4:139–146. doi: 10.1161/CIRCIMAGING.110.960229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacey R.B., Andersen M.M., St. Clair S.M., Hundley W.G., Thohan V. Comparison of systolic and diastolic criteria for isolated left ventricular noncompaction in cardiac MRI. JACC Cardiovasc Imaging. 2013;6:931–940. doi: 10.1016/j.jcmg.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Hunt S.A., Abraham W.T., Chin M.H., Feldman A.M., Francis G.S., Ganiats T.G., Jessup M., Konstam M.A., Mancini D.M., Michl K., Oates J.A., Rahko P.S., Silver M.A., Stevenson L.W., Yancy C.W. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 12.Finsterer J. Left ventricular non-compaction and its cardiac and neurologic implications. Heart Fail Rev. 2010;15:589–603. doi: 10.1007/s10741-010-9175-5. [DOI] [PubMed] [Google Scholar]
- 13.Thavendiranathan P., Dahiya A., Phelan D., Desai M.Y., Tang W.H. Isolated left ventricular non-compaction controversies in diagnostic criteria, adverse outcomes and management. Heart. 2013;99:681–689. doi: 10.1136/heartjnl-2012-302816. [DOI] [PubMed] [Google Scholar]
- 14.Sarma R.J., Chana A., Elkayam U. Left ventricular noncompaction. Prog Cardiovasc Dis. 2010;52:264–273. doi: 10.1016/j.pcad.2009.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Initial 2-chamber view from echocardiogram to demonstrate decreased systolic function with increased trabeculations.
Cardiac magnetic resonance imaging of 4-chamber view to demonstrate decreased systolic function with increased trabeculations.
Follow-up 2-chamber view from echocardiogram that continues to have increased trabeculations, but has marked left ventricular systolic improvement.