Abstract
Background
Nicotinamide is considered to be effective in halting the Alzheimer’s disease progression. The body could absorb a limited amount of nicotinamide at a time, requiring multiple doses through a day. To overcome such an obstacle which reduces the patient compliance, a sustained/controlled delivery system could be useful.
Method
Nicotinamide loaded solid lipid nanoparticles (SLN) were prepared and functionalized with polysorbate 80 (S80), phosphatidylserine (PS) or phosphatidic acid (PA). The acquired particles were characterized and evaluated in respect of their cytotoxicity, biodistribution, and in vivo effectiveness through the different routes of administration.
Results
The optimum sizes of 112 ± 1.6 nm, 124 ± 0.8 nm, and 137 ± 1.05 nm were acquired for S80-, PS-, and PA-functionalized SLNs, respectively. The in vitro cytotoxicity on SH-SY5Y cell line showed the safety of formulations except for S80-functionalized SLNs. Biodistribution study of SLNs has proved the benefits of functionalization in improving the brain delivery. The results of spatial and memory test, i.e. Morris water maze, and also histopathology and biochemical tests demonstrated the effectiveness of i.p. injection of PS -functionalized SLNs in improving the cognition, preserving the neuronal cells and reducing tau hyperphosphorylation in a rat model of Alzheimer’s disease.
Conclusion
The acquired PS-functionalized SLN could be a potential brain delivery system. Loaded with nicotinamide, an HDAC inhibitor, it could ameliorate the cognition impairment of rats more effectively than the conventional administration of nicotinamide, i.e. oral, in the early stage of Alzheimer’s disease.
Graphical abstract.
ᅟ
Keywords: Nicotinamide, Alzheimer’s disease, Solid lipid nanoparticle (SLN), Tau protein, Phosphatidylserine
Background
Patients have gone the long way before finally diagnosed with Alzheimer’s disease (AD). It starts with occasionally memory loss, progresses to cognition impairment, leading to functional disabilities and death [1]. Among all the reasons, it is considered to be the foremost cause of dementia [2]. In 2010, it was estimated that over $172 billion per year was burdened to the patient’s families and society for the extensive care of such patients in the United States; categorized it as the 3rd most costly disease [1, 2]. The large prevalence of AD, about 33.9 million people diagnosed in 2010 and expected to reach 80 million people in 2040; brings myriad efforts to the scientists’ and researchers’ world to do something about it [3].
Although the researchers are still uncertain about the exact cause and pathogenesis of AD, however, several mechanisms such as amyloid-beta aggregation, neuroinflammation, tau protein hyperphosphorylation and etc.; have been proposed [4, 5].
Assembly and stabilization of neuronal cytoskeletons are highly structured with microtubule-associated proteins such as Tau proteins. Abnormal tau phosphorylation, phosphorylation of tau in more than 3 amino acid residues, interfere with its interaction with microtubules which affects its aforementioned role and impairs axonal integrity and transport. The correlation between cognition decline and the number of tau protein aggregation that formed intraneuronal tangles, introduce the importance of tau pathology in AD progression. [6–8].
On the other hand, the progression of Alzheimer’s disease is involved with neuronal cells death. Diverse mechanisms are attributed to this event among which glutamate over-excitation could be addressed. This over-excitation results in activation of unnecessary calcium-dependent processes and high production of free radicals from mitochondria which finally leads to DNA and protein damage, impairing cell integrity and triggering cell apoptosis [9, 10]. It is reported that neuronal cells could be protected from this glutamate excitation by inhibiting the histone deacetylase enzymes [11, 12].
Histone acetylation plays an important role in chromatin condensation and gene expression. Histone acetyltransferases (HATs) and Histone deacetylases (HDACs) are among the key responsible enzymes characterizing the cell fate. Some diseases such as cancer and neurodegenerative disorder considered to be the consequence of this activity deregulation [13–15]. In the neurophysiological field, this deregulation impaired learning and memory process [12, 16].
Nicotinamide, as an HDAC inhibitor, has shown some cognition improvement in preclinical studies. Accordingly, there is an ongoing clinical trial for evaluating its effectiveness in ameliorating the progression of Alzheimer’s disease [17, 18]. Nicotinamide, although readily penetrates through BBB, has relatively low brain concentration due to sink condition therefore high dose is needed to achieve the desired response (maximum dose of 1500 mg BID is under investigation in the aforementioned clinical trial) [17, 19]. Despite the reported high dose in the clinical trial, it had been determined that the body could at most absorb 250 mg of nicotinamide at a time and multiple doses would be essential to be effective [20]. Designing sustained and/or localized delivery systems could eliminate adverse effect such as hot flashing and hepatotoxicity by lowering the needed dose [21, 22]. It could also reduce the administration times that could always be beneficial in the medical field especially when there is a possibility that patients forget to take their medications.
Considering the biocompatibility and safety of physiological lipids composition, and also controlled release ability; solid lipid nanoparticles (SLNs) have been widely used as the promising controlled drug delivery systems [23–25].
Among the physiological lipids that could be used for SLN preparation, polysorbate 80 (S80), phosphatidic acid (PA) and phosphatidylserine (PS) have shown to be beneficial for brain delivery. In particular, PS seems to be essential for cell signaling and apoptosis [26, 27]. This phospholipid nutrient is mostly concentrated in brain although its concentration varies with age. It is involved in a variety of neuronal cell functions such as transmitter release and synaptic activity. Its translocation to the external cell membrane, which occurs at the onset of cell apoptosis, activate the phagocytes function for removing the soon to be apoptotic cells [28, 29]. Although this may make the carrier vulnerable to phagocytes removal, its ability in the delivery of carrier to affected cells and its suggested role in dementia treatment situation such as AD could not be denied [30, 31]. Therefore herein PS considered as a part of the designed carrier and not the base lipid.
The purpose of this project is to design functionalized systems for brain delivery of nicotinamide. The desired system would be the one that not only could sufficiently deliver nicotinamide into the brain, but it could also reduce its administration time and dose. In vitro cytotoxicity of prepared formulations were studied before they are evaluated in animals. General and behavioral tests, histopathology and biological studies were conducted for evaluating the efficacy of prepared formulations on ameliorating the cognition caused due to the Alzheimer’s disease in an animal model and then compared to the conventional oral administration of nicotinamide.
Methods
Materials
Stearic acid was provided from Merck, Germany. Phospholipon® 90G was purchased from Lipoid, Germany. All the others synthetic materials were procured in analytical grade from Sigma Aldrich, Germany. Required solvents were purchased locally. The SH-SY5Y cell line was provided from Pasteur Institute, Iran.
Preparation and functionalization of SLNs
SLNs were prepared using microemulsion method as described previously in fair detail [32]. Briefly, stearic acid and phospholipon® 90G (as the oil phase) and sodium taurocholate and ultrapure water (as the water phase) were separately heated up to 70 ± 2 °C. Nicotinamide was dispersed in the internal oil phase. The oil phase was added to the water phase producing primary microemulsion. The microemulsion was then dispersed in cold ultrapure water. The resulted aqueous dispersion of SLN was filtered, washed and lyophilized.
SLNs were functionalized using different amounts of polysorbate 80 (S80), phosphatidylserine (PS) or phosphatidic acid (PA), as described previously [33]. Briefly, to functionalize SLNs with S80, the aforementioned procedure was conducted while the primary microemulsion was dispersed in the cold ultrapure water containing 1%, 2% or 3% v/v of S80. To functionalize SLNs with PS or PA, different amount of phospholipon® 90G (2.5%, 5% or 10%) was substituted with either of these substances. The rest of the procedure remained unchanged.
The acquired particles were evaluated in respect of their size, size distribution and zeta potential (NANO-flex®, Microtrac, USA). The encapsulation efficiency of nicotinamide was also determined according to the previously reported method (HPLC, A20 Shimadzu, Japan) [32].
To evaluate the effect of plasma on SLNs surface charge, Human plasma, taken from “Iranian blood transfusion organization”, was used. The effect of corona 10% and 100% on the zeta potential of SLNs were evaluated. Particles were incubated for an hour at 37 °C in freshly prepared phosphate buffer containing 10% human plasma or in 100% human plasma, respectively. The samples were centrifuged, washed with phosphate buffer three times and their zeta potentials were measured.
In vitro release pattern of nicotinamide
The release profile of nicotinamide from SLNs was studied in phosphate buffer solution (PBS, pH 7.4) and also in phosphate buffer media containing 10% plasma. Dialysis bag (MWCO 12 kDa) diffusion technique was used. Samples were withdrawn at different time intervals; i.e. 0, 5, 15, 30, 60, 120, 240, and 360 min; and analyzed by HPLC. All measurements were done in triplicates.
In vitro cytotoxicity evaluation
The SH-SY5Y human neuroblastoma cells, an in vitro model of neurodegenerative disorders, was used for cytotoxicity assay [32]. The Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin was used for cell cultivation.
Cells were seeded in 96-well plates at a concentration of 1 × 104 cells per well and incubated for 24 h prior to investigation. The solution of nicotinamide (NA), and the suspension of blank non-functionalized SLN (NF-SLN), NA-loaded Polysorbate80 functionalized SLN (S80-SLN), NA-loaded Phosphatidylserine functionalized SLN (PS-SLN), and NA-loaded phosphatidic acid functionalized SLN (PA-SLN) were prepared in culture medium and 150 μl of each solution or suspension were transferred to each well. All experiments were done in triplicate. Plates were then incubated for 24 h. Afterward, the content of each well was replaced with the same volume of media containing MTT (5 mg/ml), then plates were incubated for another 4 h period. After that MTT was removed and replaced with 150 μl of DMSO to lyse cells. A microplate reader (Anthos 2020, USA) was used to measure absorption at 570 nm. Control cells, assumed to have 100% viability, were remained untreated.
Animal studies
Animals were obtained from Center of Comparative and Experimental Medicine, Animal Breeding Department, Shiraz University of Medical Sciences (Shiraz, Iran). Adult male Sprague-Dawley rats, weighing 250-300 g, were acclimatized to laboratory condition a week prior to the experiment. The animals were kept under 12:12 h of light-dark cycle and temperature of 25 ± 2 °C. Food and water were available ad libitum throughout the experiment. Experiments were carried out based on Ethical Guidelines for the Care and Use of Animals in Medical Research (SUMS protocol#7409).
Biodistribution study
The biodistribution of SLNs was studied by incorporating the fluorescent marker, DiR, into the SLNs. The animals received either i.p. or i.v. injection of a DiR-saline solution, DiR-loaded NF-SLN, DiR-loaded PS-SLN, or DiR-loaded PA-SLN. Animals were sacrificed after specific times and their brains were harvested and washed with PBS. The tissues were minced and DiR was extracted with DMSO. The extraction quantified using fluorimetric plate reader (Tecan, Infinite M200) [34, 35].
Induction of Alzheimer’s disease
The animal was anesthetized by i.p. injection of xylazine (8 mg/kg) and ketamine hydrochloride (70 mg/kg). The head was fixed in a stereotaxic apparatus, the skull was exposed and coordinates of the intracerebroventricular (ICV) injection, according to Paxinos and Watson stereotaxic atlas, was drilled. The coordination was defined as 0.8 mm posterior to bregma (AP); 1.4 mm lateral to the sagittal suture (L); 3.6 mm beneath the surface, ventral (V).
Streptozotocin (STZ) was dissolved in artificial CSF containing 147 mM NaCl, 2.9 mM KCl, 1.6 mM MgCl2, 1.7 mM CaCl2 and 2.2 mM dextrose. The solution of STZ (3 mg/kg) was injected into each lateral cerebral ventricle by injection rate of 1 μl/min. The injection repeated after 48 h [36, 37].
The control group was similarly treated except for the absent of STZ in the injected artificial CSF.
Antibiotic was applied on the sutures and each animal was injected with 1 ml saline subcutaneously to prevent dehydration.
Animal treatment
The treatment of animals was started a week after the second injection through the rest of the investigation. Animals were randomly divided into the nine groups as described in Table 1.
Table 1.
Animals classifications
| Group | ICV injection | Treatment | Route of administration | Administration time | |
|---|---|---|---|---|---|
| I | Sham | CSF | Saline | i.p. | Every other day |
| II | Negative control | STZ | Saline | i.p. | Every other day |
| III | Positive control | STZ | NA solution | oral | Daily |
| IV | STZ | NA solution | i.p. | Every other day | |
| V | STZ | NA solution | i.v. | Every other day | |
| VI | STZ | PS-SLN | i.p. | Every other day | |
| VII | STZ | PS-SLN | i.v. | Every other day | |
| VIII | STZ | PA-SLN | i.p. | Every other day | |
| IX | STZ | PA-SLN | i.v. | Every other day |
ICV intracerebroventricular, STZ Streptozotocin, NA Nicotinamide, PS-SLN Phosphatidylserine functionalized SLN, PA-SLN Phosphatidic acid functionalized SLN
General behavior characterization
Rats were subjected to some experiments. The animals were weighted in one-week intervals. Their blood glucose was measured before and a week after the ICV injection using the glucose oxidase method. The locomotors activity of animals were measured a day before the surgery and a day before the Morris water maze test.
Spatial learning and memory test
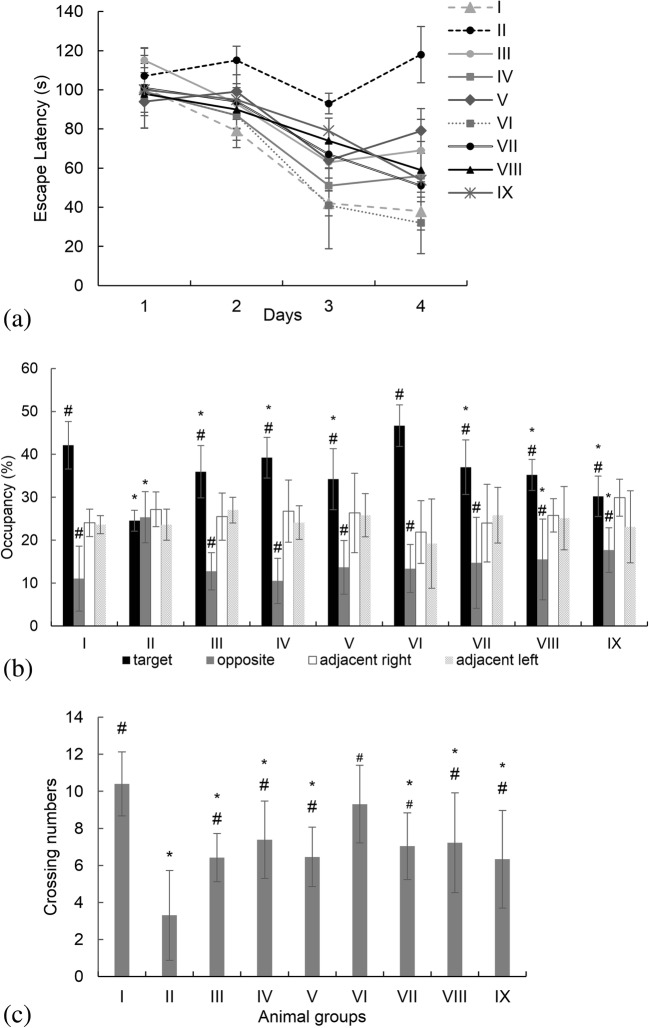
Spatial learning and memory of experimented animals were evaluated using the Morris water maze test [38]. The test was performed in a 150 cm diameter circular pool filled with tap water and maintained at room temperature of 25 ± 2 °C. The animals were trained to find the platform placed 1 cm below the water surface. Each rat was given four trials a day for four consecutive days. In each trial, rat gently placed at a semi-random starting point facing the pool wall and allowed to find the hidden platform for 120 s. If the rat could not reach the platform within the maximum time, it was guided by hand. Once sitting on the platform, the rat was allowed to remain there for 30 s, dried and returned to its cage. To analyze the performance, escape latency was measured.
The day after the last training trial, the platform was removed and rats were subjected to the so-called probe trial. Animals were allowed to explore the pool for 120 s. The crossing number and percentage of time that each rat spent in the previously located platform quadrant were recorded.
Histopathology and biochemical studies
After the behavioral tests, the animals were sacrificed and their brain was excised carefully and weighted. Along the coronal line, two hemispheres were dissected; hippocampus was micro-dissected from one hemisphere, and the other hemisphere was fixed in paraformaldehyde.
The paraformaldehyde-fixed hemisphere was sectioned, randomly stained with cresyl violet (CV) acetate, and the neuronal cells were counted.
The hippocampus tissue was treated as instructed by manufactures. Briefly, the tissue was homogenized in PBS, ultrasonicated and then centrifuged. The supernatant was used for evaluating by commercially available ELISA kit, Rat tau protein ELISA kit (Cat.no. MBS009429), and Rat phosphorylated tau231 ELISA kit (Cat.no. MBS729788).
Data analysis and statistics
The data are reported as mean ± SD. For statistical analysis, ANOVA test performed by SigmaPlot 12.0. Statistical significant considered to be p value <0.05.
Results
Preparation and functionalization of SLNs
SLNs were prepared and functionalized with S80, PS or PA as described elsewhere. Table 2 shows the size, size distribution, and zeta potential of prepared particles.
Table 2.
Characterization of SLNs
| Functionalizing agent amount | Size (nm) | Size distribution (SPAN) | Zeta potential (mV) | %Encapsulation efficiency | |||
|---|---|---|---|---|---|---|---|
| In PBS | In 10% plasma | In 100% plasma | |||||
| NF-SLN | – | 107 ± 0.5 | 0.957 | −40.9 ± 0.4 | −22.8 ± 0.63 | −5.61 ± 0.48 | %35.6 ± 0.4 |
| S80-SLN | 1% | 112 ± 1.6 | 1.114 | −29.3 ± 1.07 | −10.6 ± 0.9 | −8.1 ± 1.2 | %18.94 ± 0.5 |
| 2% | 149 ± 2.4 | 2.13 | −14.3 ± 1.25 | – | – | – | |
| 3% | 177 ± 2.05 | 1.96 | −25.6 ± 1.87 | – | – | – | |
| PS-SLN | 2.5% | 132 ± 1.3 | 1.43 | −29.3 ± 1.07 | – | – | – |
| 5% | 124 ± 0.8 | 0.831 | −46.1 ± 0.65 | −17.31 ± 1.4 | −12.5 ± 0.7 | %41.3 ± 0.41 | |
| 10% | 169 ± 1.57 | 2.11 | −41.6 ± 0.87 | – | – | – | |
| PA-SLN | 2.5% | 132 ± 2.17 | 2.17 | −35.2 ± 1.68 | – | – | – |
| 5% | 146 ± 1.26 | 1.85 | −29.1 ± 1.34 | – | – | – | |
| 10% | 137 ± 1.05 | 1.03 | −50.6 ± 0.78 | −30.2 ± 0.5 | −11.4 ± 0.92 | %36.9 ± 0.6 | |
NF-SLN Non-functionalized SLNs, S80-SLN Polysorbate80 functionalized SLNs, PS-SLN Phosphatidylserine functionalized SLNs, PA-SLN Phosphatidic acid functionalized SLNs
The NF-SLNs with the size of 107 ± 0.5 nm, the size distribution of 0.95 ± 0.2 and zeta potential of −40.9 ± 0.4 were obtained.
Functionalizing SLNs with S80 increased the particles’ average diameter that was accordingly related to polysorbate amount (Table 2). Considering the acquired results, the smallest particle size and narrowest size distribution were achieved for SLNs that were functionalization with 1% S80. The particles of 112 ± 1.6 nm size produced. Zeta potential of −29.3 ± 1.07 suggested the coverage of anionic moiety on SLNs surface by S80.
The SLNs that were functionalized with 5% PS showed smaller particle size and narrower size distribution comparing to the 2.5% or 10% PS. Substituting 5% of phospholipon® 90G with PS increased the average diameter of particles (i.e. 124 ± 0.8 nm) and more negative zeta potential (i.e. -46.1 ± 0.6) obtained comparing to NF-SLNs. The more negative surface charge suggested the incorporation of an anionic moiety of PS on SLN’s surface.
The same results were achieved by PA. SLNs functionalized with 10% of PA showed higher average diameter (i.e. 137 ± 1.05 nm) and more negative zeta potential (i.e. -50.6 ± 0.8) comparing to NF-SLNs. The more negative zeta potential may be due to the incorporation of an anionic moiety of PA on SLN’s surface.
As reported in Table 2, the zeta potential of SLNs was positively changed in media containing 10% and 100% plasma, leading to the idea of possible interaction between the SLNs and plasma components.
The selected formulations were evaluated in respect of their encapsulation efficiency (Table 2). The encapsulation efficiency of %35.6 ± 0.4, %18.94 ± 0.5, %41.3 ± 0.41, and %36.9 ± 0.6 were calculated for non-functionalized SLN (NF-SLN), 1% Polysorbate80 functionalized SLN (S80-SLN), 5% Phosphatidylserine functionalized SLN (PS-SLN), and 10% phosphatidic acid functionalized SLN (PA-SLN), respectively.
In vitro release pattern of nicotinamide
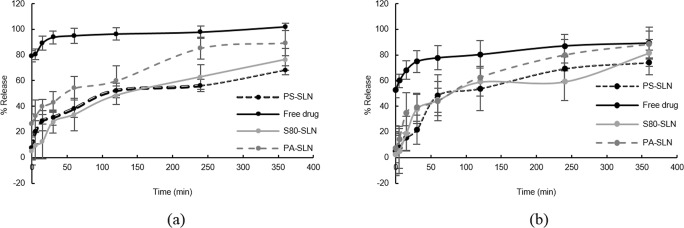
The in vitro release pattern of loaded nicotinamide from SLNs is shown in Fig. 1. S80-SLNs and PS-SLNs have shown slower release pattern than PA-SLNs. In almost 2 h, S80-SLNs and PS-SLNs have released half of their cargo but PA-SLNs released the same amount in almost an hour. The lower initial release of NA from SLNs had observed in a plasma containing release media. By the end of the investigation, i.e. 6 h, S80-SLNs and PS-SLNs have released more amount of their loaded drug in a plasma containing media in comparison to the PBS releasing media; however, PA-SLNs have released almost the same amount of drug in both releasing media by this time.
Fig. 1.
Release pattern of nicotinamide from Polysorbate80 functionalized SLN (S80-SLN), Phosphatidylserine functionalized SLN (PS-SLN), and phosphatidic acid functionalized SLN (PA-SLN) in (a) PBS, and (b) plasma containing PBS
In vitro cytotoxicity evaluation
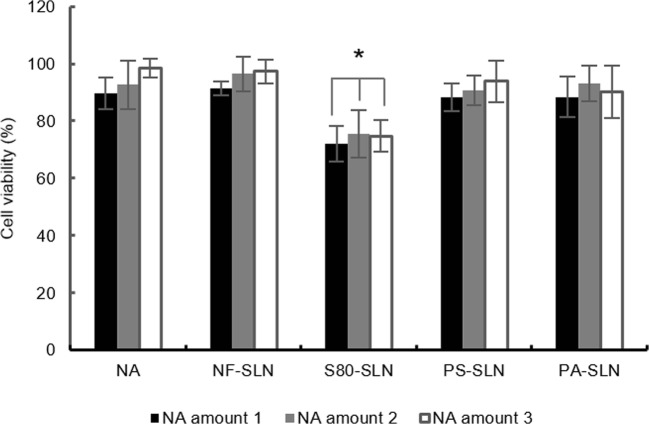
The cytotoxicity of NA solution, suspension of NF-SLNs, S80-SLNs, PS-SLN, and PA-SLNs were investigated. Results are shown in Fig. 2. The results show that neither the NA nor the SLNs has considerable toxicity in the studied concentrations. However, S80-SLNs demonstrated more toxicity than the other formulations which could be attributed to the presence of S80 as a functionalizing agent.
Fig. 2.
Cytotoxicity evaluation. Cell viability in exposure to nicotinamide solution (NA), suspension of non-functionalized SLN (NF-SLN), Polysorbate80 functionalized SLN (S80-SLN), Phosphatidylserine functionalized SLN (PS-SLN), and phosphatidic acid functionalized SLN (PA-SLN). Nicotinamide amounts 1, 2 and 3 represent the equivalent amounts of 60, 30 and 15 mg NA (calculated based on the loading parameters of SLNs).*p value <0.05 comparing to the NA solution
The S80-SLNs were excluded from further investigations considering its higher toxicity besides its low encapsulation efficiency. The PS-SLNs and PA-SLNs were used for in vivo animal studies.
Animal studies
Biodistribution study
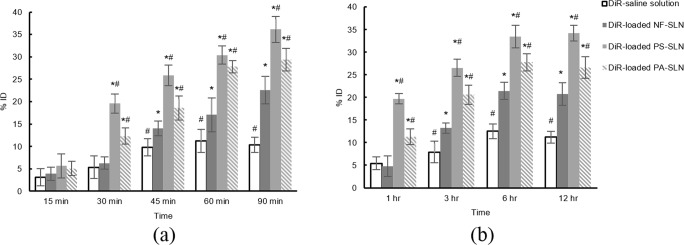
DiR used as the fluorescent marker to evaluate the biodistribution of prepared SLNs in the brain. Figure 3 shows the results. The brain concentration of all formulations increased after their i.v. injection. DiR-loaded PS-SLNs has significantly higher brain concentration after 30 min in comparison to the DiR-loaded PA-SLNs and NF-SLNs (p < 0.05). The brain concentration of PA-SLNs was significantly lower than DiR-loaded PS-SLNs (p < 0.05) except in 15 min and 60 min (p > 0.05) after i.v. injection. Both DiR-loaded PS-SLNs and DiR-loaded PA-SLNs reached significantly higher brain concentration than the DiR-loaded NF-SLNs and DiR-saline solution (p < 0.05).
Fig. 3.
The distribution of DiR-loaded formulations in the brain. (a) i.v. injection and (b) i.p. injection. *p value <0.05 comparing to the DiR-saline solution. #p value <0.05 comparing to the DiR-loaded NF-SLN
The brain concentration of formulations showed almost the same pattern after i.p. injection with slower rate and also the lag time of about an hour. Anyway, the brain concentrations of both DiR-loaded PS-SLNs and DiR-loaded PA-SLNs were significantly higher than DiR-loaded NF-SLNs and DiR-saline solution (p < 0.05). These results could determine the efficiency of functionalization for brain delivery.
General behavior characterization
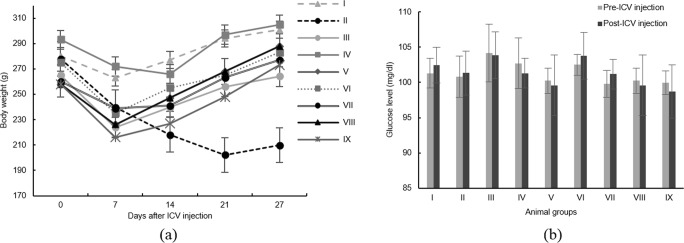
Rats’ body weight and blood glucose were measured. The results are presented in Fig. 4. All groups have shown a decline in their weight in one week after their surgery. The animals’ weight were recovered afterward except for the negative control group (group II). The animals’ glucose level was estimated using the glucose oxidase-peroxidase method. No statistically significant glucose change in blood was observed before and a week after STZ injections (p > 0.05).
Fig. 4.
General behavioral characterization. (a) Body weight and (b) Blood glucose level. Groups’ description is explained in Table 1
Locomotors activity of experimented animals were evaluated allowing the rat to freely explore in the activity monitor apparatus for 5 min. The number of ambulatory counts was counted. The animal was also observed for grooming, forward and backward walking, sniffing, rearing, immobility and ptosis. All animals showed almost the same locomotors activity level before and a week after the surgery, suggesting they don’t have any motor impairment interfering with Morris water maze test and that neither the surgery nor the disease induction affected their mobility (data not shown). In rare cases that the animal’s mobility was affected, the animal was excluded from the further investigation.
Spatial learning and memory test
To analyze the spatial learning and memory of experimented animals, Morris water maze test was conducted. The acquired data are presented in Fig. 5. Decreasing the escape latency in the training procedure, revealed the success of the learning program. However, the training was not much of a success in the negative control group (more training were conducted, data not shown). These results would suggest an impairment in spatial memory, disabling the rat to find the platform location. The group of rats that were treated with i.p. injection of PS-SLN (group VI) tended to learn the platform location more accurately than the other groups. No significant difference was observed in their escape latency time than the sham group (p > 0.05). In the probe trial, the memory test was analyzed by the number of the platform site crossings and the percentage of time spent in the target, opposite, adjacent right and adjacent left quadrants. Comparing to the sham group, the negative control group crossed all the four quadrants almost equally showing no special performance in the former platform quadrant. These results suggest the lack of spatial reference memory in this group. The i.p. injected PS-SLN treated rats were spent significantly more time in the goal area and cross the targeted quadrant more often than the other groups (p < 0.05).
Fig. 5.
Morris water maze study. (a) The animals’ escape latency, (b) Percentage of time spent in the target, opposite, adjacent right and adjacent left quadrants during probe trial, and (c) The number of the platform site crossings during the probe trial. Group classification is described in Table 1. *p value <0.05 comparing to the group I (sham group). #p value <0.05 comparing to the group II (negative control group)
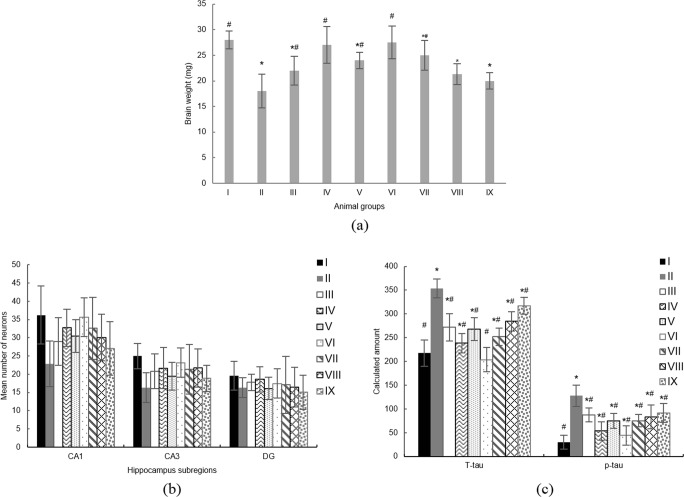
Histopathology and biochemical studies
The animals’ brains were weighed after they have been sacrificed at the end of the memory test. The average brain weight of each group was compared to the sham group (Fig. 6a). There was a significant decrease in brain weight in the negative control group, group II (p < 0.05). Comparing to the sham group, no significant differences in the brain weight of i.p. treated NA solution (group IV) or i.p. treated PS-SLNs (group VI) rats were detected. However, brains’ weight of i.v. treated NA solution (group V) or i.v. treated PS-SLNs (group VII) animals were significantly lower than the sham group. The animals receiving PA-SLN, whether i.p. or i.v., had significantly lower brain weight than the sham group (p < 0.05).
Fig. 6.
Histopathology and Biochemical Studies. (a) Animals’ brain weight, (b) Neuronal counts in hippocampus subregions, and (c) ELISA tests results. Group classification is described in Table 1. *p value <0.05 comparing to the group I (sham group). #p value <0.05 comparing to the group II (negative control group)
CV staining was used to evaluate the histopathology of animals’ brains. Neuronal cells were counted in hippocampus subregions (Fig. 6b). The typical pathological changes observed in Alzheimer’s induced group receiving no treatment; i.e. shrunk cytoplasm, darkly stained nuclei, and apoptotic neurons. In all the groups treated with any NA formulation, moderate pathological changes were observed. In PS-SLN treated groups, although some apoptotic neurons have seen, however, more neurons appeared normally than the negative control group. The neuron count in the CA1 hippocampus subregions of the negative control group was significantly less than the sham group (p < 0.05). In all the groups treated with NA, neuron count was more than the negative control group but less than the sham group. Although the neuron count in i.p. treated PS-SLN group was less than the sham group, however, it was not significantly different (p > 0.05).
ELISA tests were used to calculate the total tau protein amount (T-tau) and phosphorylated tau 231 amount (p-tau231). The data presented in Fig. 6c. Both T-tau and p-tau were significantly increased in negative control group comparing to the sham group (p < 0.05). In i.p. injected PS-SLN group the T-tau amount was not significantly different from the sham group (p > 0.05). Although the p-tau was increased in all the groups treated with any NA formulation comparing to the sham group, however, it was significantly less than the negative control group (p < 0.05).
Discussion
The controlled/ sustained delivery system has shown beneficial in the pharmaceutical field by overcoming the undesired characteristics of drugs and reducing the required dose and administration times, hence improving patients compliance. To our interest nicotinamide, although considered as a hydrophilic drug, has limited absorption. It has been reported that only 250 mg of the drug could be absorbed in once [20]. As an HDAC inhibitor, nicotinamide has shown its effect in improving the cognition impairment due to Alzheimer’s disease in preclinical studies. There is an ongoing clinical trial evaluating its effect [18].
Herein, solid lipid nanoparticles were considered for controlled delivery of nicotinamide to overcome such an obstacle. Using the previously reported optimized preparation method [32], SLNs have been prepared and then functionalized. The primary non-functionalized SLNs, NF-SLNs, demonstrated the particle size of about 107 ± 0.5 nm. Functionalizing SLNs increased their particle size. Considering the acquired size, size distribution and zeta potential, the suitable amount of functionalizing agents were demonstrated. Accordingly, 1% polysorbate 80, 5% phosphatidylserine, and 10% phosphatidic acid chose which was in accordance with the previously reported amounts [33]. The particle size of functionalized SLNs increased to 112 ± 1.6, 124 ± 0.8, and 137 ± 1.05 nm, respectively. The negative increase of zeta potential in PS and PA functionalized SLNs were attributed to the incorporation of these functionalizing agents into the surface of prepared SLNs [33]. However, polysorbate 80 seemed to cover the SLNs’ surface, changing the particles’ zeta potential positively.
In overall, PA-SLN shows the highest particle size. The PA-SLN has significantly larger particle size than S80-SLN (137 ± 1.05 nm comparing to 112 ± 1.6 nm, p < 0.05) and also larger than PS-SLN, though not statistically significant (137 ± 1.05 nm comparing to 124 ± 0.8 nm, p > 0.05). The PS-SLN has the narrowest size distribution (0.83 ± 0.12 comparing to 1.1 ± 0.3 and 1.03 ± 0.2 for S80-SLN and PA-SLN respectively). The zeta potential of S80-SLN was significantly less negative than the other formulations (−29.3 ± 1.07 comparing to −46.1 ± 0.6 and − 50.6 ± 0.8 for PS-SLN and PA-SLN respectively). The positive change in particles’ zeta potential in the presence of plasma, suggested the SLNs interactions with plasma components.
Among the formulations, S80-SLN has the lowest encapsulation efficiency (%18.94 ± 0.5 comparing to %41.3 ± 0.41, and %36.9 ± 0.6 for PS-SLN and PA-SLN respectively), which could be attributed to its preparation method in which the SLNs functionalized by dispersing and stirring in the S80 containing extraction media for about extra 30 min. This could lead to the NA release from the superficial layers of SLNs.
Considering the hydrophilicity of the nicotinamide, SLNs were able to control its release at some point. The initial fast drug release in the first hour could be attributed to the un-entrapped nicotinamide and/or the nicotinamide incorporated in the superficial lipid layer. In the PBS releasing media, nicotinamide released more rapidly from functionalized SLNs comparing to the previously reported non-functionalized SLNs [32]. However, the initial release of nicotinamide reduced in a plasma containing media. Since the results of the free drug study have shown the possible NA interaction with plasma component, it could be suggested that there must have been more amount of drug released from particles that could not be detected. Although the PA-SLNs released almost the same amount of its loaded drug, however, it demonstrated the different release pattern in each releasing media. In addition, neither the S80-SLN nor the PS-SLN demonstrated the same releasing patterns as they did in the PBS releasing media.
The desired carrier systems should not present cytotoxic effect. NF-SLNs along with all the three functionalized SLNs were evaluated for their cytotoxicity profile. To evaluate the cytotoxic effect of NA itself, its solution was also investigated. As reported previously, nicotinamide was effective orally in a dose of 200 mg/kg in mice [21]. Accordingly, about 60 mg NA for an average 300 g rat’s body weight is calculated to be the effective dose in the investigated rats. Therefore, herein the three amounts of NA; i.e. 60, 30 and 15 mg, were considered for the toxicity evaluation. The SLN amount was calculated based on their loading parameters. Although these particles did not reach their IC50 in the experimented concentration, S80-SLNs has shown the highest cytotoxicity of all.
In this regards, and also considering its low encapsulation efficiency, it had been omitted from further evaluations. Two other formulations; i.e. PS-SLN and PA-SLN, were chosen for the animal studies.
The distribution of prepared formulations was evaluated to determine the efficiency of functionalization. All formulations could somehow reach the brain, however, the significantly higher biodistribution of functionalized SLNs in the brain, in both routes of administration, comparing to the NF-SLNs, showed the effectiveness of functionalization for target delivery. Although the accumulation of PA-SLNs in the brain was significantly higher than the NF-SLNs, however, functionalization with phosphatidylserine seemed to be more effective than phosphatidic acid. It seemed that the PS-SLN could efficiently accumulate in the brain in 30 min after i.v. injection, when it releases only about %20 of its loaded drug. The i.p. administration of formulations delayed their brain accumulation. However, PS-SLNs could reach the brain in significantly higher amount than NF-SLNs and PA-SLN.
One method for Alzheimer induction in murine that has been widely studied is the intracerebroventricular (ICV) injection of streptozotocin (STZ). STZ would cause type 1 diabetes by destroying pancreatic β cells. It could not cause glucose deregulation in the brain when given systemically, as it is not able to pass the blood-brain barrier [39]. On the other hand, STZ reported increasing tau phosphorylation in animal models by inhibiting the phosphatase activities when it has been directly injected into the brain [40, 41]. Using this protocol, the Alzheimer’s disease was induced in rats. However, since the Amyloid-beta fibril did not form in one month after STZ injection, the effect of the designed system could only be evaluated on the tau protein hyperphosphorylation and neuroinflammation.
The surgery has always accompanied by animals weight loss. All animals lost their weight in a week after surgery. Body weight was evaluated to confirm the suitable housing for their recovery. All animals regained their weight afterward. Only the negative control group (group II) did not regain their weights (Fig. 4a). This weight reduction could be attributed to the progression of disease in this group of animals.
STZ transporter, GLUT2, have found heterogeneously inside the brain, but not in the BBB; and that’s the reason ICV injection of STZ can selectively decrease the level of insulin in the brain without disturbing systemic insulin and glucose levels [42, 43]. However, some experiments have reported blood glucose increase in animals receiving the phosphatidylserine [30]. The glucose level of experimented animals was evaluated to make sure no systemic insulin-glucose deregulation occurred. As expected, blood glucose did not significantly change a week after the STZ injections (p > 0.05, Fig. 4b).
The most commonly used paradigm for testing spatial learning ability of rats is the Morris water maze (MWM) [44]. A primary advantage of using the water maze over other common behavioral mazes to test memory is that there are no olfactory trails for animals to use scent tracking to find the target. In addition, considering the self-driven nature of the task, food deprivation is not required for motivational purposes. However, in this experiment the animals’ locomotors activity is the important factor for performing the task. As a part of surgery’s and/or ICV injections’ risks, animals’ locomotors activity could be impaired. The animals with almost the same level of activity as they had shown before the surgery were chosen for the MWM test.
As a positive control, the previously reported dose of 200 mg/kg was administered orally. Two different routes of administration were also evaluated; i.e. i.p. and i.v. injection. The disability of Alzheimer’s induced rats in learning the MWM task was obvious (group II, Fig. 5a). More training sections were performed in such animals (data not shown). The animals that were treated with any formulation of nicotinamide required significantly less time to learn the task comparing to the negative control group. The escape latency in NA solution treated (oral, i.p. or i.v.) and PA-SLN treated (i.p. or i.v.) groups showed no significant different (p > 0.05). However, the escape latency time of i.p. injected PS-SLN treated group was decreased significantly (p < 0.05), suggesting the better improvement in animals’ spatial memory. In the probe trial, the confirming results were observed. The i.p. injected PS-SLN treated group spent more time in the pre-located quadrant in comparison to the other treated groups. The negative control group explores all the four quadrants almost equally.
The brain shrinkage was previously reported in animals suffering from Alzheimer disease. In this study the observation of significant lighter brain weight along with the typical pathological changes in the negative control group than the sham group suggesting the disease progression. The presence of moderate pathological changes and also higher neuronal counts in hippocampus subregions of i.p. injected PS-SLN treated animal groups, suggests the ability of nicotinamide loaded PS-SLNs in preserving the neurons from apoptosis, hence halting the Alzheimer’s disease progression.
Biochemical ELISA tests confirmed that nicotinamide suppresses the Alzheimer progression by preventing the tau protein hyperphosphorylation as it has been reported previously [21].
In general, the study suggested the effectiveness of the acquired delivery systems especially the PS-SLNs in controlling the nicotinamide release, and its delivery to the brain, therefore, improving the memory behavioral of the animals. Treating rats with the i.p injection of PS-SLN resulted in the cognition improvement and preserved higher neuronal cell than treating the rats with NA solution administrated by anyother routes. Besides, using PS-SLN as a delivery system could reduce the drug administration time. Although, this could be more effective when the loaded drug could be released in a slow manner; i.e. being more hydrophobic.
Conclusions
Nicotinamide, as an HDAC inhibitor, had shown to be effective in halting the progression of Alzheimer’s disease. Preparation of the controlled release delivery system could be useful in overcoming its absorption’s limitation and multiple needed doses through a day. Therefore, nicotinamide loaded SLNs were prepared and functionalized using S80, PS or PA. The i.p. administration of nicotinamide loaded PS-SLN showed better memory improvement, preserved more neuronal cells and reduced the tau hyperphosphorylation in experimented animals comparing to its non-formulated conventional administration in the early stage of Alzheimer’s disease. The evidence indicates nicotinamide mostly can prevent hyperphosphorylation of tau protein. However, the effect of other factors involving in the disease process could not be denied. Although the designed formulations have ameliorated the cognition attributed to Alzheimer’s disease, it could only be beneficial in some aspects and its combination with other therapeutical substances affecting other pathologies of disease should be considered. Our designed delivery system is promising, although not sufficient, in improving the Alzheimer’s disease at early stages. Since the designed PS-SLN could deliver its cargo into the brain efficiently in somehow a sustained manner, it could be beneficial for sustain delivery of other effective substances.
Acknowledgments
Authors would like to acknowledge Dr. Ali-Mohammad Tamaddon from the School of Pharmacy and Research Center for Nanotechnology in Drug Delivery, Shiraz University of Medical Sciences for his consult and cooperation throughout the study.
Funding
This study is part of Ph.D. thesis supported by Tehran University of Medical Sciences (TUMS); Grant no. 94–02–33-29374.
Compliance with ethical standards
Declaration of interest
The authors report no declarations of interest.
References
- 1.Grabowski, T.J., Clinical features and diagnosis of Alzheimer disease. 2015: www.uptodate.com.
- 2.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks WA. Drug delivery to the brain in Alzheimer's disease: consideration of the blood–brain barrier. Adv Drug Deliv Rev. 2012;64:629–639. doi: 10.1016/j.addr.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teunissen, C.E. and T.J.M.V.D. Cammen, Alzheimer’s Disease, In Protein Misfolding in Neurodegenerative Diseases - Mechanisms and Therapeutic Strategies H.J. Smith, C. Simons, and R.D.E. Sewell, editors. 2008, CRC Press, Boca Raton.
- 5.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32(3):150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259(8):5301–5305. [PubMed] [Google Scholar]
- 8.Harrington CR. The aetiology of Alzheimer's disease: diverse routes into a common Tau PathwayI. Aluminium and Alzheimer's disease; The science that describes the link. In: Exley C, Editor. 2001. p. 97–132.
- 9.Durham B. Novel histone deacetylase (HDAC) inhibitors with improved selectivity for HDAC2 and 3 protect against neural cell death. Bioscience Horizons. 2012;5(0):hzs003–hzs003. [Google Scholar]
- 10.Bardai FH, D’Mello SR. Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3β. J Neurosci. 2011;31(5):1746–1751. doi: 10.1523/JNEUROSCI.5704-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha R, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006;13(4):539–550. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stilling RM, Fischer A. The role of histone acetylation in age-associated memory impairment and Alzheimer’s disease. Neurobiol Learn Mem. 2011;96(1):19–26. doi: 10.1016/j.nlm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Timmermann S, Lehrmann H, Polesskaya A, Harel-Bellan A. Histone acetylation and disease. Cell Mol Life Sci. 2001;58(5–6):728–736. doi: 10.1007/PL00000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth S, Denu J, Allis C. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38(1):62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grill J, Irvine UOC. Nicotinamide as an Early Alzheimer's Disease Treatment (NEAT). 2017, ClinicalTrials.gov - Identifier: NCT03061474.
- 18.Fillit H et al. Closing in on a cure - 2017 Alzheimer’s clinical trials report. Alzheimer drug Discovery Foundation, 2017. https://www.alzdiscovery.org/research-and-grants/clinical-trials-report/closing-in-on-a-cure-2017
- 19.Schreiber S, Irvine UOC. Safety study of nicotinamide to treat Alzheimer's disease. 2007, ClinicalTrials.gov - Identifier: NCT00580931.
- 20.Prousky JE. The use of Niacinamide and Solanaceae (nightshade) elimination in the treatment of osteoarthritis. J Orthomol Med. 2015;30(1):13–21. [Google Scholar]
- 21.Green K, et al. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving Sirtuin inhibition and selective reduction of Thr231-Phosphotau. J Neurosci. 2008;28(45):11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knip M, Douek IF, Moore WPT, Gillmor HA, McLean AEM, Bingley PJ, Gale EAM. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 23.Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 24.Blasi P, et al. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59(6):454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Mozzi R, Buratta S, Goracci G. Metabolism and functions of phosphatidylserine in mammalian brain. Neurochem Res. 2003;28(2):195–214. doi: 10.1023/A:1022412831330. [DOI] [PubMed] [Google Scholar]
- 27.Kim H-Y, Huang BX, Spector AA. Phosphatidylserine in the brain: metabolism and function. Prog Lipid Res. 2014;56:1–18. doi: 10.1016/j.plipres.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2012;29(3):691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010;15:1072–1082. doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai M, Yamatoya H, Kudo S. Pharmacological effects of phosphatidylserine enzymatically synthesized from soybean lecithin on brain functions in rodents. J Nutr Sci Vitaminol (Tokyo) 1996;42(1):47–54. doi: 10.3177/jnsv.42.47. [DOI] [PubMed] [Google Scholar]
- 31.Kidd PM. Phosphatidylserine; Membrane Nutrient for Memory. A clinical and mechanistic assessment. Altern Med Rev. 1996;1(2):70–84. [Google Scholar]
- 32.Vakilinezhad MA, Tanha S, Montaseri H, Dinarvand R, Azadi A, Akbari Javar H. Application of response surface method for preparation, optimization, and characterization of nicotinamide loaded solid lipid nanoparticles. Adv Pharm Bull. 2018;8(2):245–256. doi: 10.15171/apb.2018.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gobbi M, Re F, Canovi M, Beeg M, Gregori M, Sesana S, Sonnino S, Brogioli D, Musicanti C, Gasco P, Salmona M, Masserini ME. Lipid-based nanoparticles with high binding affinity for amyloid-b1-42 peptide. Biomaterials. 2010;31:6519–6529. doi: 10.1016/j.biomaterials.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, Singh J. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: biodistribution and transfection. J Control Release. 2013;167(1):1–10. doi: 10.1016/j.jconrel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Wen Z, Yan Z, He R, Pang Z, Guo L, Qian Y, Jiang X, Fang L. Brain targeting and toxicity study of odorranalectin-conjugated nanoparticles following intranasal administration. Drug Deliv. 2011;18(8):555–561. doi: 10.3109/10717544.2011.596583. [DOI] [PubMed] [Google Scholar]
- 36.Kosaraju J, Madhunapantula SRV, Chinni S, Khatwal RB, Dubala A, Muthureddy Nataraj SK, Basavan D. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer’s disease. Behav Brain Res. 2014;267:55–65. doi: 10.1016/j.bbr.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 37.Liu P, Zou LB, Wang LH, Jiao Q, Chi TY, Ji XF, Jin G. Xanthoceraside attenuates tau hyperphosphorylation and cognitive deficits in intracerebroventricular-streptozotocin injected rats. Psychopharmacology. 2014;231(2):345–356. doi: 10.1007/s00213-013-3240-4. [DOI] [PubMed] [Google Scholar]
- 38.Kamalinia G, Khodagholi F, Atyabi F, Amini M, Shaerzadeh F, Sharifzadeh M, Dinarvand R. Enhanced brain delivery of deferasirox-lactoferrin conjugates for iron chelation therapy in neurodegenerative disorders: in vitro and in vivo studies. Mol Pharm. 2013;10(12):4418–4431. doi: 10.1021/mp4002014. [DOI] [PubMed] [Google Scholar]
- 39.Grieb Paweł. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: in Search of a Relevant Mechanism. Molecular Neurobiology. 2015;53(3):1741–1752. doi: 10.1007/s12035-015-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazem A et al. Rodent models of neuroinflammation for Alzheimer’s disease. J Neuroinflamation. 2015;12(74). 10.1186/s12974-015-0291-y. [DOI] [PMC free article] [PubMed]
- 41.Lecanu L, Papadopoulos V. Modeling Alzheimer’s disease with non-transgenic rat models. Alzheimers Res Ther. 2013;5(3):17. doi: 10.1186/alzrt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamat PK. Streptozotocin induced Alzheimer’s disease like changes and the underlying neural degeneration and regeneration mechanism. Neural Regen Res. 2015;10(7):1050–1052. doi: 10.4103/1673-5374.160076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agrawal R, Tyagi E, Shukla R, Nath C. A study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology. 2009;56:779–787. doi: 10.1016/j.neuropharm.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 44.D’Hooge R, Deyn PPD. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]