Abstract
Background
Paclitaxel is a potent antitumor alkaloid widely used for the treatment of several cancer types. This valuable secondary metabolite naturally exists in the inner bark of Taxus species in very low amounts. The small-scale production of paclitaxel in Taxus cell cultures requires utilization of several elicitors.
Objective
The main objective of this work was to identify key genes that encode rate-limiting enzymes in paclitaxel biosynthesis pathway by investigating the possible relationship between paclitaxel production and a set of 13 involved genes’ relative expression in Taxus baccata L. cell suspension cultures affected by coronatine and methyl-β-cyclodextrin.
Methods
In the present research, the most important key genes were identified using gene expression profiling evaluation and paclitaxel production assessment in Taxus baccata L. cell cultures affected by mentioned elicitors.
Results and Conclusion
Gene expression levels were variably increased using methyl-β-cyclodextrin, and in some cases, a synergistic effect on transcript accumulation was observed when culture medium was supplemented with both elicitors. It was revealed that DBAT, BAPT, and DBTNBT are the most important rate-limiting enzymes in paclitaxel biosynthesis pathway in Taxus baccata L. cell suspension cultures under coronatine and methyl-β-cyclodextrin elicitation condition. Moreover, PAM was identified as one of the important key genes especially in the absence of β-phenylalanine. In cell cultures affected by these elicitors, paclitaxel was found largely in the culture media (more than 90%). The secretion of this secondary metabolite suggests a limited feedback inhibition and reduced paclitaxel toxicity for producer cells. It is the result of the ABC gene relative expression level increment under methyl-β-cyclodextrin elicitation and highly depends on methyl-β-cyclodextrin’s special property (complex formation with hydrophobic compounds). Paclitaxel biosynthesis was obviously increased due to the effect of coronatine and methyl-β-cyclodextrin elicitation, leading to the production level of 5.62 times higher than that of the untreated cultures.
Graphical abstract.
Rate Limiting Enzymes in Paclitaxel Biosynthesis Pathway: DBAT, BAPT, DBTNBT and PAM.
Keywords: Taxus baccata L., Cell suspension culture, Paclitaxel, Coronatine, Methyl-β-cyclodextrin, Transcription profiling
Introduction
Paclitaxel (Px) is a powerful antitumor diterpene alkaloid that is broadly used for the treatment of several cancer types such as head and neck tumors, melanoma, lung, breast, and ovarian cancers, and AIDS-related Kaposi’s sarcoma. Px exists in the inner bark of Taxus species in very low amounts (0.01% referred to dry weight) [1]. The genus Taxus belongs to the Class Pinopsida, the Order Taxales and the Family Taxaceae. As the species are highly similar, they are often easier to separate geographically than morphologically. Typically, eight species are recognized: T. baccata (European or English yew), T. brevifolia (Pacific yew or Western yew), T. canadensis (Canadian yew), T. chinensis (Chinese yew), T. cuspidata (Japanese yew), T. floridana (Florida yew), T. globosa (Mexican yew) and T. wallichiana (Himalayan yew). There are also two recognized hybrids: Taxus x media = T. baccata × T. cuspidata and Taxus x hunnewelliana = T. cuspidata × T. Canadensis [2].
Considering very low concentrations of Px and other taxanes, scarcity and very slow growth rate of yew trees, the presence of about 400 similar compounds together with increasing the world demand for these anticancer drugs, extraction procedure from the inner bark of Taxus species is not a cost-effective method and would destroy these valuable natural resources in the long time [3–6].
Taxus spp. cell culture has been proved as a biotechnological approach for production of Px and related taxanes in large scales [7, 8].
Nevertheless, the small-scale production of Px in Taxus cell cultures requires using elicitors that severely increase Px and related taxanes’ concentrations in Taxus cell cultures [9].
Coronatine (Cor) is a bacterial blight phytotoxin produced by several pathovars of Pseudomonas syringae, which stimulates chlorosis, hypertrophy, secondary metabolite production, accumulation of proteinase inhibitors, apoptotic cell death, ethylene emission, and accelerated senescence occurrence associated with some diseases [10].
It seems that Cor is the structural and functional analog of jasmonic acid (JA) and 12-oxo-phytodienoic acid (OPDA), the C18 precursor of jasmonic acid and methyl jasmonate (MeJA). It consists of polyketide biosynthesis product, polyketide coronafacic acid (CFA), and an isoleucine cyclised derivative, coronamic acid (CMA). It has been proved that CFA imitates MeJA’s action, conjugates to other amino acids such as threonine, isoleucine, or serine, and possesses phytotoxic activity. CMA, which is an isoleucine derivative, also improves the CFA toxicity [11]. JA and jasmonic acid-isoleucine (JA–Ile) have shown a key role in plant defense responses [12].
Cor has received much attention because of its potential role in plant growth regulation and acting as a potent elicitor by triggering the jasmonate signaling pathway [13].
Hu et al. [14] demonstrated that the induction level of secondary metabolism in plant cell cultures affected by Cor is higher than JAs. Although natural and synthetic JAs’ effects on secondary metabolite production in plant cell culture have been widely studied, relatively few reports are available in the case of Cor action on secondary metabolite biosynthesis.
Tamogami, Kodama [15] illustrated that Cor is able to induce the accumulation of flavonoid phytoalexins, sakuranetin, and momilactone A in rice leaves. They also demonstrated that the effect of Cor on flavonoid production is greater than JA or 12-oxo-phytodienoic acid.
According to Haider et al. [16], Cor and some of its related structural analogs have positive effects on benzoic phenanthridine production in Eschscholzia californica cell cultures, but lower than MeJA and some analogs.
Lauchli et al. [17] and Fliegmann et al. [18] showed the accumulation of glyceollin, the phytoalexin of Glycine max in soybean cell cultures, by adding some elicitors such as JA and MeJA. According to this study, JA and MeJA have had a weaker phytoalexin-inducing activity in comparison to an early jasmonate precursor, OPDA, and certain 6-substituted indanoyl-l-isoleucine methyl esters or the bacterial phytotoxin, Cor.
Onrubia. et al. [9] reported that Cor enhances both taxane production and expression of Px related genes in Taxus x media Rehder cell suspension cultures.
Furthermore, methyl-β-cyclodextrin (CD) is an elicitor that is chemically similar to alkyl-derived pectic oligosaccharides naturally released from cell walls attacked by fungal pathogens [19].
In recent years, CD has received much attention as a potent agent for inducing defense responses and acting as a true elicitor in plant cell cultures [19–21].
Moreover, the formation of inclusion complexes with hydrophobic compounds and facilitation their excretion from cells to the culture media, are two of CD’s most important features.
Belchi-Navarro et al. [22] stimulated the biosynthesis and extracellular accumulation of silymarin, a pharmacologically active flavolignan in Silybum marianum cell suspension cultures, by CD.
Sabater-Jara et al. [23] reported that in a selected Taxus x media Rehder cell line cultured in a two-step medium, gene expression levels were not considerably improved by the presence of CD but were variably induced by MeJA. They also reported a synergistic effect when the culture medium was supplemented with CD and MeJA.
Almagro et al. [24] demonstrated the synergistic effects of CD and Cor on both trans-resveratrol production and gene expression levels related to the stilbene biosynthesis pathway in Vitis vinifera L. cv Monastrell cell suspension cultures.
To sum up, the complicated Px biosynthesis pathway is not still thoroughly understood and studies have revealed that different Taxus species under different elicitation conditions and various substrates supplementations may have different rate-limiting enzymes [25–27]. Despite the positive correlation between related gene expression levels and Px amounts explained by several authors [9, 23, 28, 29], the key genes that produce related limiting enzymes and control Px bottleneck steps under Cor and CD elicitations, especially in Taxus baccata L. cell cultures, are still unknown.
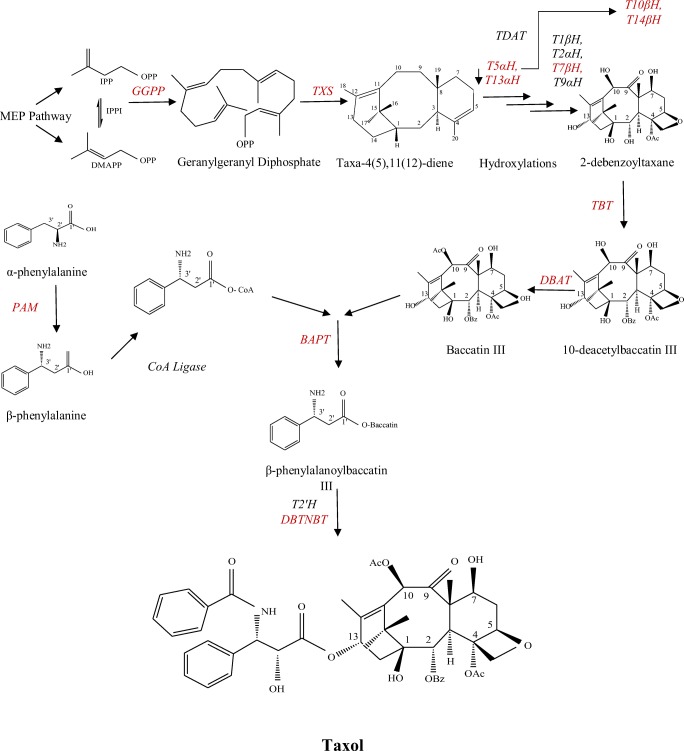
It has been assumed that 19 genes are involved in Px biosynthesis pathway after geranylgeranyl diphosphate (GGPP) formation. GGPP cyclizes and converts to taxadiene, the first committed step in Px biosynthesis pathway. Px obtains after eight oxidative steps, adding five acyl/ aroyl groups, one epoxidation reaction, one aminomutase enzyme effect, two CoA esterifications, and one N-benzoylation process [23] (Fig. 1).
Fig. 1.
A summarized paclitaxel biosynthesis pathway. IPPI; isopentenyl diphosphate isomerase, GGPP; geranylgeranyl diphosphate synthase, TXS; taxadiene synthase, T1βH; taxane 1β-hydroxylase, T2αH; taxane 2α-hydroxylase, T5αH; taxane 5α-hydroxylase, T7βH; taxane 7β-hydroxylase, T9αH; taxane 9α-hydroxylase, T10βH; taxane 10β-hydroxylase, T13αH; taxane 13α-hydroxylase, T14βOH; taxane 14β-hydroxylase, TDAT; taxa-4(20),11(12)-diene-5α-ol-O-acetyltransferase, TBT; taxane-2α-O-benzoyl transferase, DBAT; 10-deacetyl baccatin III-10-O-acetyltransferase, BAPT; baccatin III-3-amino 13-phenylpropanoyl- CoA transferase, T2´H; taxane 2´-hydroxylase, DBTNBT; de-benzoyltaxol N-benzoyl transferase, PAM; phenylalanine amino mutase and CoA Ligase. Adopted from [25] and [30]. Colored names point out to the genes that have been investigated in this research
The main aim of this study was to determine some possible rate-limiting enzymes in Taxus baccata L. cell suspension cultures through assessing the relative expression level of 13 genes (even side routes like T14βH and PAM) and evaluation of Px production. The results of this study would be applicable in the near future especially in the case of metabolic engineering. Facilitating the secretion of Px into the culture media due to CD special property (complex formation with hydrophobic compounds) was one of the important highlights in our research. Transcription profiling of ABC gene, as the only known transporter involved in paclitaxel excretion, was also investigated under Cor and CD elicitation for the first time to shed light on the molecular events in elicited T. baccata L. cell cultures. The main goal for studying this issue is the fact that the relative expression level increments in terms of this gene can facilitate paclitaxel excretion into the culture media and lead to feedback inhibition limitation.
It was determined that different species of Taxus under various elicitation conditions might have different rate-limiting enzymes [23, 27]. To the best of our knowledge, at this moment, there is no information about the identification of key genes involved in Taxus baccata L. cell suspension cultures under Cor and CD elicitation condition using transcription profiling evaluation and Px production assessment.
Methods
Plant materials, culture conditions, and elicitation procedure
Materials used for this purpose were prepared from Merck Company (Germany). To produce sterile calli, all explants (Juvenile stems, 1 cm in length) were taken from a Taxus baccata L. tree located in Tehran University botanic garden. Taxus baccata L. calli were established in our laboratory from disinfected explants in B5 solid growth medium based on a protocol described by Gamborg et al. [31]. Some modifications were applied in this medium as follows.
B5 salts (1 X), B5 vitamins (2 X), KNO3 2.5 g/L, myo-inositol 100 mg/L, sucrose 5 g/L, glucose 5 g/L, fructose 10 g/L, ascorbic acid 100 mg/L, casein hydrolysate 1 g/L, naphthalene acetic acid 2 mg/L, kinetin 0.1 mg/L, and plant agar 6 g/L. The pH of this medium was adjusted to 5.8. Then, calli were maintained at 25 °C in darkness. Taxus baccata L. calli were subcultured on solid growth medium every 3 weeks.
Homogeneous parental cell suspension cultures were initiated by inoculating 10 g of friable callus pieces (10 g fresh weight (FW) of 1 month-old calli) in 250 mL Erlenmeyer flasks containing 90 mL of B5 liquid growth medium [31], with some modifications as follows.
B5 salts (1 X), B5 vitamins (3 X), KNO3 2.5 g/L, myo-inositol 100 mg/L, sucrose 5 g/L, glucose 5 g/L, fructose 10 g/L, ascorbic acid 100 mg/L, casein hydrolysate 1 g/L, naphthalene acetic acid 2 mg/L, kinetin 0.1 mg/L, glutamine 250 mg/L, and polyvinylpyrrolidone (PVP) 5 g/L. The pH of this medium was adjusted to 5.8. These flasks were maintained in a rotary shaker (100 rpm) at 25 °C in darkness. After 5 days, parental cultures were mixed to produce homogenous inocula and reduce the flask-to-flask variation. These homogenous parental cultures were distributed into progeny ones. Progeny cultures were produced by inoculation of growth medium (20 mL) with 5-day-old homogeneous parental cell suspension culture (10 mL) in 100 mL Erlenmeyer flasks.
Based on biomass measurement results, elicitors were added to the production medium at two different times of culture. Cell suspension cultures were elicited by CD (final concentration: 50 mM) on day 13, at the start point of growth exponential phase, after removing the entire growth media and resuspending the remaining cells in the production medium (final volume: 30 mL). Production medium was prepared based on previously mentioned growth medium with some modifications in carbohydrate resources as follows: Glucose 10 g/L and fructose 5 g/L.
Since the start point of the stationary phase was on day 18 in Taxus baccata’s growth cycle, cell suspension cultures were also elicited by Cor (final concentration: 1 μM) on day 17. Samples were harvested over the periods of 1, 5, 24, 48, 72, and 96 h after Cor elicitation in dual elicited cell cultures and CD elicited ones for investigating the changes occurring in gene transcription profiles. Their Px contents were monitored on day 17 right after Cor elicitation, days 23, 30, 34, and 36 of cell culture initiation.
Biomass measurement and viability assay
Biomass accumulation was determined by measuring the dry weight’s increment. Cell viability was evaluated by fluorescein diacetate (FDA) staining procedure as described by Widholm et al. [32]. FDA is a cell-permeant esterase substrate that, once introduced inside a metabolically active cell, is hydrolyzed by intracellular esterases and yields a fluorescence-emitting product, fluorescein [32]. Viable cells were observed with a fluorescent microscope (Ceti 3100.5000-Triton II, UK).
Paclitaxel extraction and identification
Materials used for this purpose were prepared from Merck (Germany) and DaeJung (Korea). Px was extracted from the culture media as described previously [9] with some modifications. To perform this procedure, 15 mL of cell suspension culture medium was mixed with an equal volume of dichloromethane (DCM) and then shaken for 2 h followed by a lower phase separation. The solvent was eliminated from the organic phase by transferring to a vacuum oven, yielding the organic extract suspended in 0.5 mL of HPLC grade methanol. Then, this resuspended organic extract was filtered by passing through the 0.22 μm Millipore filter, before being injected into the HPLC instrument.
Cell extraction method for Px was in accordance with Wu, Lin [33] with slight modifications [34]. In this method, cells and medium were separated by micropipette then wet cells were dried in a freeze dryer. The freeze-dried cells were weighted, pulverized, and suspended in 4 mL of HPLC grade methanol, followed by ultrasonication for 30 min, and centrifugation at 1340 ×g for 15 min. Thereafter, the upper phase was separated and transferred to a vacuum oven for the elimination of solvent. The extract was resuspended in dichloromethane: water (1: 1, v/v) followed by centrifugation for 15 min at 1340 ×g. After centrifugation, the extract was divided into three phases: water, fatty acids, and dichloromethane, which was the lower phase. Dichloromethane was isolated by micropipette and then vacuum evaporated. Afterward, the residual material at the bottom of the container was resuspended in 500 μl of HPLC grade methanol and filtered passing through the 0.22 μm Millipore filter, before being injected into the HPLC instrument. The Px content of the extracts was evaluated by an HPLC system (Waters, USA) equipped with a C-18 column (NUCLEODUR 100–5 C18 ec, 250 × 4.6, China). Px was eluted with methanol/water (80/20) at a flow rate of 1 mL/min. It was detected at 230 nm using a UV detector (PDA, Germany). Identification of Px was carried out by comparing the retention times with an authentic standard.
Quantitative Real-Time PCR (polymerase chain reaction) analysis
Gene expression analyses were accomplished by T. baccata cell cultures induced by CD (final concentration: 50 mM) individually or in combination with Cor (final concentration: 1 μM). Samples were harvested at different times: 1, 5, 24, 48, 72, and 96 h after Cor elicitation in dual-treated cells, and at the same times in CD-treated ones. Total RNA was isolated from frozen cells according to the method described by Channuntapipat et al. [35].
The concentration of each extracted RNA sample was calculated by means of a NanoDrop spectrophotometer (WPA Biowave II+). Only RNA samples with a 260:280 ratio between 1.8 and 2.2 were used for the subsequent analyses. RNA samples’ integrity was evaluated by agarose gel electrophoresis (0.8%) in tris/ borate/ ethylene diamine tetra acetic acid (TBE) running buffer. Reverse transcription reaction was performed using 1 μg of total RNA from each sample and first-strand cDNA synthesis kit using MMLV-RT (Thermo Fisher Scientific, USA). Quantitative Real-Time PCR analyses were accomplished by means of Eva Green Real-Time PCR master mix (Solis BioDyne, Estonia), diluted cDNA and 300 nM of forward and reverse primers in a 96-well platform of BioRad (USA) instrument with the following parameters: 95 °C for 15 min and 40 cycles in 95 °C for 15 s, 60 °C for 20 s and 72 °C for 20 s. Gene-specific primers were designed by Oligo 7 software (Molecular Biology Insights, USA) (Table 1) and amplification efficiency of each primer pair was calculated using cDNA’s serial dilutions (Data not shown). Only those primer pairs with the efficiency range of 90–110% were used and the data were also processed using the BioRad CFX Manager Software Ver. 1.6 (BioRad, USA) and (1 + Efficiency)-ΔΔCt formula. The amplicon size of each primer pair was also evaluated by agarose gel electrophoresis compared to an appropriate DNA ladder to confirm their specific identity. We found that they were consistent with the expected fragment sizes. Moreover, the relative expression level for each gene was double normalized with respect to the Ct value of the reference gene and the same sample in the time-zero (reference value = 1). The selected reference gene in this experiment was glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which acts as an enzyme that catalyzes the sixth step of glycolysis.
Table 1.
Primer sequences used for gene amplification by qRT-PCR
| Gene Name | Accession No/ Ref | Primer Sequence 5′ to 3′ | Amplicon Size (bp) |
|---|---|---|---|
| GAPDH | L26922.1 | F: TTCCGAGTACCCACCCAAGATG R: CAGCTGTCTCCGATGAAATCTGT |
176 |
| GGPP | AY544994.1 | F: GGATGCTAATGTGGACCTGAAGAC R: TCTCGCAATCTCGTCCTCTGT |
124 |
| TXS | AY424738.1 | F: GAGCTTCCGACCTTGCATTTCC R: GTCCTTGCAAGTGCGTCTCTA |
91 |
| T5αH | JQ618880.1 | F: ACCACTTCGCCAATGGCTTTG R: GCATTTTGGACTGCGGATGAACTG |
159 |
| T7βH | JQ029683.1 [23] | F:GGTCCGCCCAAATTGCCAGAA R:CCCTGCAGAGCCCAAAAAACC |
110 |
| T10βH | JQ029684.1 | F: TTCCCTTACCCTCGCACCTA R: GTGGTGTTTCTGATCGGAGTGTC |
155 |
| T13αH | AY056019.1 | F: GGCTCTTCTTCCGCTCTAAACG R: CCACATCCCCAAATTTGCTCATTC |
159 |
| T14βH | AY188177.1 | F: GCATGAACGGGAACGACTGT R: TGAGCTTCGACCGTGCTTGA |
119 |
| TBT | JQ618928.1 | F: AGGCATGAAAATGTAGTCGGATG R: TTTGTTGCTGCATGGGGCTTAC |
106 |
| DBAT | KC571283.1 | F: TGCTGACACCCCGTTCTGGA R: TGCATGTCCCCACCCAAAGTC |
113 |
| BAPT | FJ717392.1 | F: CACTGCCGATATGGACAGAGTC R: CATGGCTTCCAGAAACAGAACAC |
169 |
| PAM | AY866411 [9] | F: GAACAGCACAACCAGGACATCA R: AGCCTCGCCTTTGTGTCGTTAG |
99 |
| DBTNBT | AY563629.1 [23] | F: CGGGGGGTTTGTTGTGGGATTA R: TTAGCCTCTCCCCTCGCCAT |
105 |
| ABC | DQ660357.1 | F: TGGCTTCATTGTGGCACCTAC R:GGCATTGTCATCAAACCAGCTAATC |
130 |
Statistical analysis
Statistical analyses were performed using SPSS Ver. 16.0.0 (SPSS Inc., Chicago, Illinois, USA). All data were the average of two biological × two technical determinations± SE. The one-way ANOVA analyses followed by Duncan’s multiple range tests used for statistical assessments at p value < 0.01. GraphPad Prism software Ver. 7:00 (USA) was used for bar chart drawing.
Results
Biomass measurement and viability assay
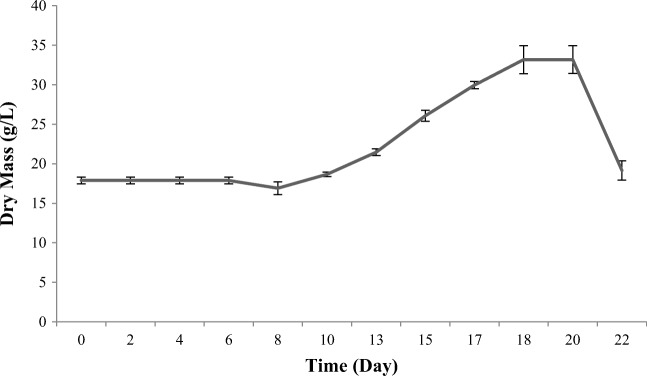
The biomass of T. baccata cell suspension cultures grown in the mentioned two-step medium under control conditions was evaluated by measuring the dry weight. Biomass measurement showed a drastic increase (up to 2 folds) until day 18, remained unchanged on days 18–20, and showed a gradual drop from day 20 to the end of the culture on day 22 (Fig. 2). In Taxus baccata L. cell suspension cultures, biomass hit its peak on day 18 of growth in optimized two-step medium. Viability assay using FDA staining also showed a viability value greater than 85–90% in each case.
Fig. 2.
Biomass production evaluated by measuring T. baccata cells’ dry mass (g/L) in optimized two-step medium over the period of 22 days. Each value is the average of three biological × three technical replicates ± SE
Paclitaxel contents isolated from cells and culture media
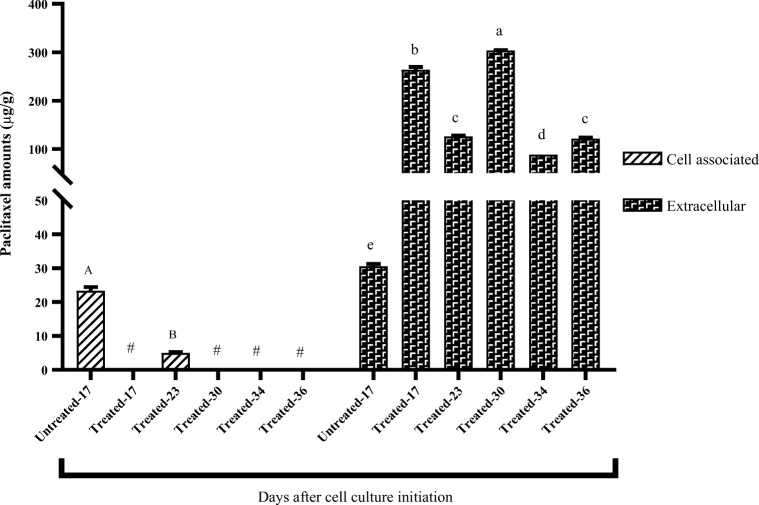
Cell-associated and extracellular Px contents isolated from untreated and dual elicited samples were measured on day 17 of experiment right after the Cor elicitation, days 23, 30, 34, and 36 of cell culture initiation (Fig. 3).
Fig. 3.
Cell-associated and extracellular paclitaxel amounts in CD + Cor treated and untreated samples. Each value is the average of two replicates ± SE. Y-axis: paclitaxel amounts (μg/g); X-axis: days after cell culture initiation in untreated and treated cultures. Uppercase and lowercase letters show the results of mean comparative test accomplished on cell-associated and extracellular paclitaxel contents, respectively. Dissimilar letters showsignificant difference between compared treatments. # shows non-detectable paclitaxel amounts
The results showed that elicitation has had a statistically significant effect on cell-associated and extracellular Px contents at a p-value of 0.01. Although an equal volume of cell-associated and extracellular Px contents in untreated conditions was observed, the extracellular Px amounts obviously increased by synergistic effects of Cor and CD, achieving the production level of 303.75 μg/g on day 30 while it was 5.62 times higher than that of the untreated cultures. In all cell cultures affected by mentioned elicitors, Px was greatly found in the culture media (more than 90%), showing the reduced paclitaxel toxicity and limited feedback inhibition for producer cells.
Quantitative Real-Time PCR analysis
The amplification efficiency of each primer pair was calculated with cDNA serial dilutions using this formula: E = 10-1/slope-1. Only those primer pairs with the efficiency range of 90–110% were used in this experiment.
Quantitative Real-Time PCR data were analyzed with BioRad CFX Manager Software Ver. 1.6 (BioRad, USA), using the formula (1 + E)-ΔΔCt.
Genes with highly increased relative expression level
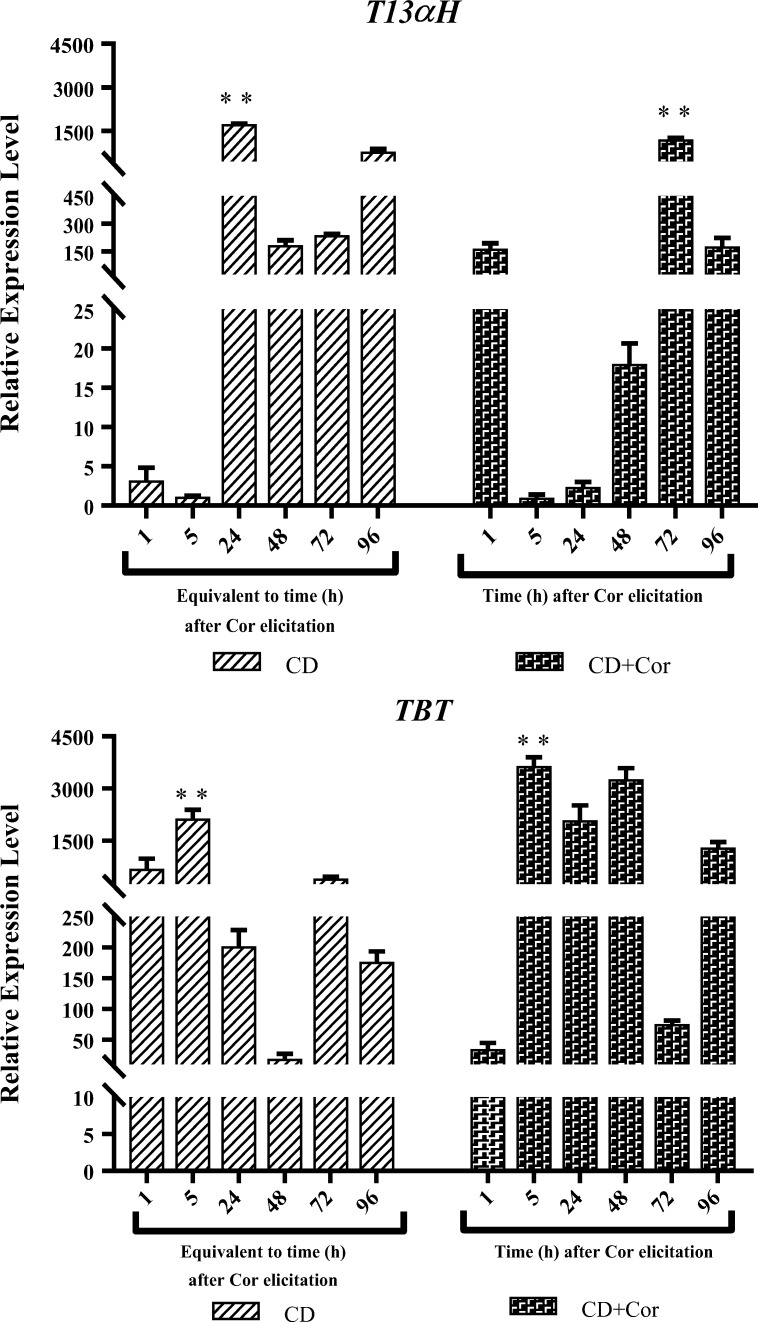
According to statistical analysis (ANOVA), CD and dual elicitation showed statistically significant effects on taxane 13α-hydroxylase (T13αH) and taxane-2α-O-benzoyl transferase (TBT) genes’ relative expression level at p value < 0.01.
T13αH and TBT genes were more induced by CD and dual elicitation, gaining the maximum transcription fold change of 1699.34 and 3622.80 after 24 and 5 h, respectively.
T13αH and TBT genes reached a peak of expression after 72 and 5 h, when the cell suspension cultures were induced by dual and CD elicitation, respectively (Fig. 4).
Fig. 4.
Relative expression level of genes with highly increased expression (T13αH and TBT) in Taxus baccata L. cell suspension cultures during 5 days in production medium (from day 4 to 8) supplemented with CD (50 mM) individually or in combination with Cor (1 μM). Y-axis: gene expression relative to that of the expression level in cell suspension cultures maintained for 13 days in the growth medium, prior to 4 days in the production medium; X-axis: equivalent time or time after Cor elicitation in cell suspension cultures affected by CD and CD + Cor, respectively. Each value is the average of two biological × two technical replicates ± SE. **; significant difference between compared treatments at p-value < 0.01, CD; Methyl-β-Cyclodextrin and CD+Cor; Methyl-β-Cyclodextrin and Coronatine
Genes with moderately increased relative expression level
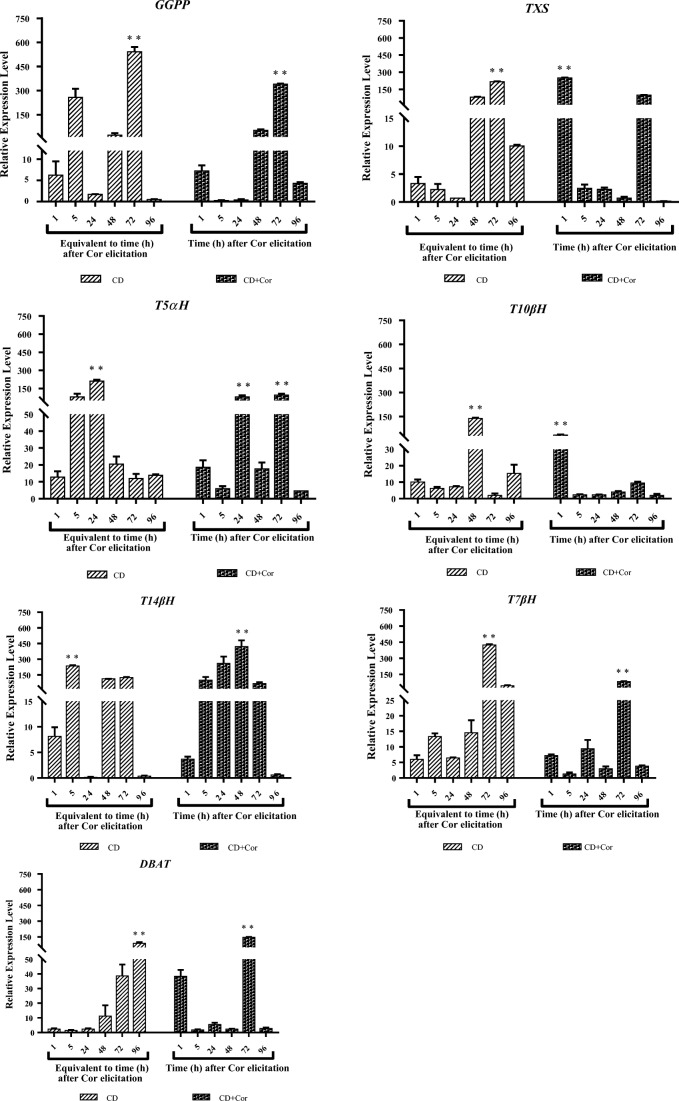
According to statistical analysis (ANOVA), CD and dual elicitation showed significant effects on geranylgeranyl diphosphate synthase (GGPP), taxadiene synthase (TXS), taxane 5α-hydroxylase (T5αH), taxane 10β-hydroxylase (T10βH), taxane 14β-hydroxylase (T14βH), taxane 7β-hydroxylase (T7βH) and 10-deacetylbaccatin III-10-O-acetyltransferase (DBAT) genes’ relative expression level at p-value < 0.01.
According to relative expression level evaluation, GGPP, T5αH, T10βH, and T7βH genes were more induced by CD elicitation, reaching their maximum fold change at the mRNA level as 541.5, 211.4, 138.5 and 424.1 after 72, 24, 48, and 72 h, respectively. These genes also reached their relative expression peak over the periods of 72, 72, 1, and 72 h, respectively, when the cell cultures were induced by dual elicitation.
TXS, T14βH, and DBAT genes were more induced by dual elicitation, achieving their maximum relative expression levels of 248.9, 419.8 and 144.8 after 1, 48, and 72 h, respectively. These genes also reached their transcript accumulation peak after 72, 5, and 96 h, respectively, while the cell cultures were induced by CD (Fig. 5).
Fig. 5.
Relative expression level of genes with moderately increased expression (GGPP, TXS, T5αH, T10βH, T14βH, T7βH and DBAT) in Taxus baccata L. cell suspension cultures during 5 days in production medium (from day 4 to 8) supplemented with CD (50 mM) individually or in combination with Cor (1 μM). Y-axis: gene expression relative to that of the expression level in cell suspension cultures maintained for 13 days in the growth medium, prior to 4 days in the production medium; X-axis: equivalent time or time after Cor elicitation in cell suspension cultures affected by CD and CD + Cor, respectively. Each value is the average of two biological × two technical replicates ± SE. **; significant difference between compared treatments at p-value < 0.01, CD; Methyl-β-Cyclodextrin and CD+Cor; Methyl-β-Cyclodextrin and Coronatine
Genes with slightly increased relative expression level
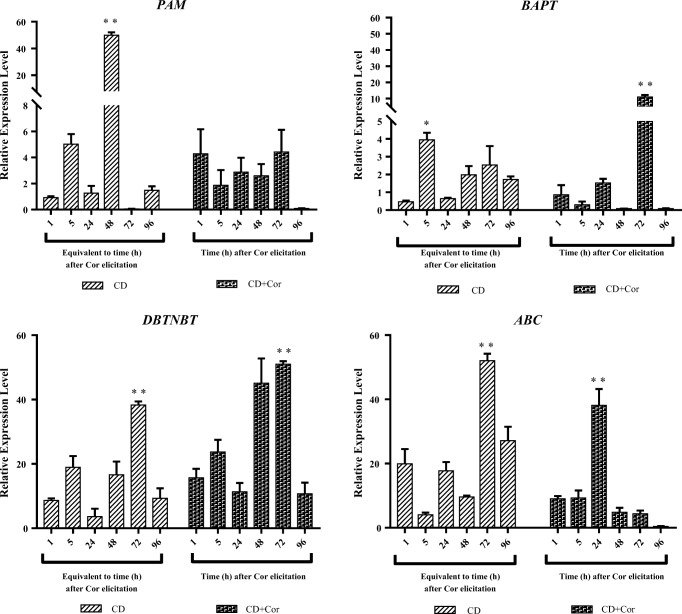
In accordance with statistical analysis (ANOVA), CD elicitation showed a significant effect on phenylalanine amino mutase (PAM) gene relative expression level at p-value < 0.01, but dual elicitation had no significant effect in this case.
Based on accomplished statistical analysis (ANOVA), dual and CD elicitation have shown significant effects on baccatin III-3-amino 13-phenylpropanoyl- CoA transferase (BAPT) gene relative expression level at p-value < 0.01 and < 0.05, respectively.
According to statistical analysis (ANOVA), dual and CD elicitation have had significant effects on debenzoyltaxol N-benzoyl transferase (DBTNBT) and ABC (ATP-binding cassette) genes’ relative expression level at p-value < 0.01.
PAM and ABC genes were more induced by CD elicitation, reaching the maximum level of gene expression as 49.9 and 52.01 over the periods of 48 and 72 h, respectively. These genes also reached the highest transcript accumulation after 72 and 24 h, respectively, when the cell cultures were induced by dual elicitation.
BAPT and DBTNBT genes were more induced by dual elicitation, achieving the maximum relative expression of 11.04 and 50.9 after 72 h in both cases. These genes also reached the highest transcript accumulation over the periods of 5 and 72 h, respectively, while the cell suspension cultures were induced by CD (Fig. 6).
Fig. 6.
Relative expression level of genes with slightly increased expression (PAM, BAPT, DBTNBT, and ABC) in Taxus baccata L. cell suspension cultures during 5 days in production medium (from day 4 to 8) supplemented with CD (50 mM) individually or in combination with Cor (1 μM). Y-axis: gene expression relative to that of the expression level in cell suspension cultures maintained for 13 days in the growth medium, prior to 4 days in the production medium; X-axis: equivalent time or time after Cor elicitation in cell suspension cultures affected by CD and CD + Cor, respectively. Each value is the average of two biological × two technical replicates ± SE. * and **;significant difference between compared treatments at p-value < 0.05 and p-value < 0.01, respectively, CD; Methyl-β-Cyclodextrin and CD+Cor; Methyl-β-Cyclodextrin and Coronatine.
Discussion
To the best of our knowledge, elicitation mechanisms have not been fully recognized yet. The increased production of secondary metabolites in plant cell cultures through elicitation methods has opened up a new area of research, which could have important economic benefits for bioindustries. Elicitors, which can be used as plant secondary metabolite inducers, may play an important role in biosynthesis pathways for enhanced production of commercially important compounds. Several mechanisms including Ca+2 signaling, factors affecting cell wall permeability, inhibition and activation of intracellular pathways, rapid changes in proteins through phosphorylation, activation of protein kinases, and changes in osmotic stress are known as effective factors in metabolic pathways. However, not all kinds of elicitors traverse such a continuous path. Various factors such as elicitor source, its specificity, concentration, physicochemical conditions, plant growth stages, callus conditions, and so forth can influence Px biosynthesis pathway. Furthermore, the effects of AP2 (activating enhancer binding protein 2) and WRKY transcription factors on the expression of genes involved in Px biosynthesis pathway has been demonstrated in previous studies [36].
Overproduction of some substrates may lead to increased production of their related hydrolytic enzymes to prevent excessive accumulation of these substrates. This issue is debatable considering the expression-induction profile of PAM after CD and Cor elicitation. As can be seen, this gene is out of the expression-induction cascade that joins to this pathway. The relative stability of PAM’s expression could lead us to this hypothesis that the excessive production of a substrate, which did not happen in this case, leads to increased production of related hydrolytic enzyme in order to prevent its unnecessary accumulation.
Evidently, GGPP had a high excitability and its transcripts showed short half-lives. Moreover, its overexpression could result in overproduction of all diterpenoids including Px and other taxanes.
Several studies have mentioned that TXS is not a rate-limiting enzyme in Px biosynthesis pathway because this gene indicated a very high fold change and its transcripts also showed short half-lives. The highest expression level of TXS affected by dual elicitation occurred over the period of 1 h after using the second elicitor (Cor). This issue cannot be attributed to the increase in substrate concentration because a period of 1 h was not a sufficient time for the significant overproduction in the case of its precursor “geranylgeranyl diphosphate”. Therefore, it can be concluded that this overexpression was probably due to the effects of transcription factors. In addition, the half-life of TXS’s transcripts was short so at the time period of 72 h, the second peak was considerably shorter than the first one, which was most likely due to the increased amount of its substrate.
In fact, neither taxadiene nor any primary intermediates of Px pathway accumulate in a significant level in the cell cultures, suggesting the rapid conversion and consumption of these metabolites in downstream reactions. Thus, it seems that the bottlenecks are located in further downstream regions of Px biosynthesis pathway. Gene expression profile assessments of all hydroxylases in Px pathway showed that T10βH was one of hydroxylases in this cascade that did not increase significantly by dual elicitation. Since the increased amounts of T10βH transcripts most likely lead to metabolic pathway deviation toward increased T14βH gene expression, small changes in T10βH expression seems favorable.
Apparently, none of the hydroxylases in this pathway are rate-limiting enzymes, because, in addition to having very high fold changes, their hydroxylated intermediates were used as TBT (the gene showing the greatest increased amount of expression among the investigated ones) substrate. Moreover, according to Ramirez-Estrada et al. [30], the amounts of 10-deacetylbaccatin III (DABIII) and baccatin III metabolites are very higher than Px. As a result, not only the amount of increment in terms of TBT gene expression was very high, but it also produced an active enzyme that led to an increased production of intermediate secondary metabolites such as DABIII or baccatin III. With respect to TBT gene, it seems that the simultaneous effect of increment in substrate amounts and gene expression induction by transcription factors were effective on its increased expression level.
Seemingly, if the whole reservoir of cell in terms of secondary metabolites like DABIII or baccatin III was converted to Px, the amount of this valuable secondary metabolite would be much higher.
Overall, concerning the hydroxylases of this pathway including T5αH, T10βH, T13αH, T14βH and T7βH, the simultaneous effect of upward trends in the substrate and gene expression induction by transcription factors were probably effective on their overexpression.
In case of DBAT gene, it is notable that its maximum fold change was 144.8, which was lower than the maximum one in the case of TXS gene (248.9). In this regard, there are two different perspectives that are mostly associated with the species of Taxus. The results of investigations conducted on Taxus x media Rehder have shown that although the amount of increase in DBAT gene expression was relatively limited, the content of baccatin III was significantly higher than Px [30]. These results indicate that DABIII is actively converted to baccatin III. In other words, DBAT gene produces an active enzyme and hence this gene is not the bottleneck of this pathway in Taxus x media Rehder. However, some studies conducted on Taxus baccata L. have resulted in the greater accumulation of DABIII compared to baccatin III, suggesting that this gene is one of the possible bottlenecks of this pathway in Taxus baccata L. [27]. Our results in this case were also in accordance with Onrubia et al. (2011). Regarding the DBAT gene, it seemed that the simultaneous effects of the increased amounts in its substrate and gene expression induction by transcription factors were effective on its relative expression level increment.
PAM is one of the side route genes in Px pathway that relatively remained unchanged and showed a low expression level over the period of 72 h after induction by Cor and CD. According to our results, PAM is the other rate-limiting enzyme in this pathway especially in β-phenylalanine absence. Since this gene is not directly involved in Px biosynthesis pathway, β-phenylalanine (PAM’s product) can be added as a precursor to the culture medium.
According to the results of current study, BAPT gene is apparently one of the possible rate-limiting enzymes in this pathway that its relative expression level increment was related to the influence of an increase in its substrate amount. It is notable that this gene showed a very low-fold change under elicitation condition in Taxus baccata L. cell suspension culture, as it reached its maximum value (11.04) over the period of 72 h after induction.
DBTNBT is the gene responsible for the benzoylation of Px side chain. Due to its low-fold change, it can probably be another rate-limiting enzyme in this pathway. Perhaps, the limited expression of this gene is the result of Px damaging effect on Taxus cells. Based on our results, DBTNBT’s expression level increased after relative exporting of Px by the product of ABC gene or by the special action of CD (complex formation with hydrophobic compounds). In the case of BAPT and DBTNBT genes, our results are in agreement with Onrubia. et al. [9] and Sabater-Jara et al. [23].
ABC gene illustrated a low-fold change under elicitation circumstances but a significant increase in its expression level was observed when culture medium supplemented with CD. In this case, almost 90% of Px was secreted into the culture medium, suggesting that this gene encodes another important rate-limiting enzyme related to Px pathway that its overexpression can lead to Px overproduction due to the limited feedback inhibition and the reduced paclitaxel toxicity for producer cells.
Overall, according to bar charts of gene expression studies, the downstream genes usually show lower relative expression level increases, suggesting the natural intelligence in converting the downstream genes to the rate-limiting enzymes. Thus, Px is produced but to the extent that does not harm the cell viability.
Conclusion
Deacetylbaccatin III-10-O-acetyltransferase- DBAT, baccatin III-3-amino 13- phenylpropanoyl-CoA transferase- BAPT, and debenzoyltaxol N-benzoyl transferase - DBTNBT seem to be key genes involved in paclitaxel biosynthesis pathway affected by Cor and CD in Taxus baccata L. cell suspension cultures. The overexpression of these genes can lead to overproduction of Px and related taxanes. Phenylalanine amino mutase - PAM is also known as another rate-limiting enzyme especially in β-phenylalanine absence. Moreover, ABC gene (the only known gene involved in Px secretion) is a gene that its relative expression level incredibly increases by CD elicitation and its overexpression can lead to secretion of Px and related taxanes to the culture media facilitating their extraction.
Acknowledgements
All authors gratefully acknowledge the support of Tarbiat Modares University.
Abbreviations
- Px
Paclitaxel
- Cor
Coronatine
- JA
Jasmonic Acid
- MeJA
Methyl Jasmonate
- CFA
Polyketide Coronafacic Acid
- CMA
Coronamic Acid
- JA–Ile
Jasmonic Acid-Isoleucine
- OPDA
12-Oxo-Phytodienoic Acid
- CD
Methyl-β-Cyclodextrin
- FW
Fresh Weight
- PVP
Polyvinylpyrrolidone
- FDA
Fluorescein Diacetate
- PCR
Polymerase Chain Reaction
- TBE
Tris/ Borate/ Ethylene Diamine Tetra Acetic Acid
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- T13αH
taxane 13α-hydroxylase
- TBT
taxane-2α-O-benzoyl transferase
- GGPP
geranylgeranyl diphosphate synthase
- TXS
taxadiene synthase
- T5αH
taxane 5α-hydroxylase
- T10βH
taxane 10β-hydroxylase
- T14βH
taxane 14β-hydroxylase
- T7βH
taxane 7β-hydroxylase
- DBAT
10-deacetylbaccatin III-10-O-acetyltransferase
- PAM
phenylalanine amino mutase
- BAPT
baccatin III-3-amino 13-phenylpropanoyl- CoA transferase
- DBTNBT
debenzoyltaxol N-benzoyl transferase
- ABC
(ATP-binding cassette)
- AP2
Activating enhancer binding Protein 2
- DAB III
10-deacetylbaccatin III
- MEP
Methylerythritol Phosphate
- IPP
Isopentenyl Diphosphate
- IPPI
isopentenyl diphosphate isomerase)
- DMAPP
Dimethylallyl Diphosphate
- GGPP
geranylgeranyl diphosphate synthase
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interests.
Contributor Information
Mokhtar Jalali Javaran, Phone: +982148292104, Email: m_jalali@modares.ac.ir.
Mohammad Sadegh Sabet, Phone: +982148292345, Email: ms.sabet@modares.ac.ir.
References
- 1.Cragg GM, Schepartz SA, Suffness M, Grever MR. The taxol supply crisis. New NCI policies for handling the large-scale production of novel natural product anticancer and anti-HIV agents. J Nat Prod. 1993;56(10):1657–1668. doi: 10.1021/np50100a001. [DOI] [PubMed] [Google Scholar]
- 2.Cope EA. Taxaceae: the genera and cultivated species. Bot Rev. 1998;64(4):291–322. doi: 10.1007/BF02857621. [DOI] [Google Scholar]
- 3.Jaziri M, Zhiri A, Guo Y-W, Dupont J-P, Shimomura K, Hamada H, Vanhaelen M, Homès J. Taxus sp. cell, tissue and organ cultures as alternative sources for taxoids production: a literature survey. Plant Cell Tissue Organ Cult. 1996;46(1):59–75. doi: 10.1007/BF00039697. [DOI] [Google Scholar]
- 4.Sabater-Jara AB, Tudela LR, López-Pérez AJ. In vitro culture of Taxus sp.: strategies to increase cell growth and taxoid production. Phytochem Rev. 2010;9(2):343–356. doi: 10.1007/s11101-010-9167-z. [DOI] [Google Scholar]
- 5.Zhang CH, Xu HB. Improved paclitaxel production by in situ extraction and elicitation in cell suspension cultures of Taxus chinensis. Biotechnol Lett. 2001;23(3):189–193. doi: 10.1023/A:1005655219649. [DOI] [Google Scholar]
- 6.Zhong JJ. Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng. 2002;94(6):591–599. doi: 10.1016/S1389-1723(02)80200-6. [DOI] [PubMed] [Google Scholar]
- 7.Fett-Neto AG, Melanson SJ, Sakata K, DiCosmo F. Improved growth and taxol yield in developing calli of Taxus cuspidata by medium composition modification. Nat Biotechnol. 1993;11(6):731–734. doi: 10.1038/nbt0693-731. [DOI] [PubMed] [Google Scholar]
- 8.Wickremesinhe ER, Arteea RN. Taxus callus cultures: initiation, growth optimization, characterization and taxol production. Plant Cell Tissue Organ Cult. 1993;35(2):181–193. doi: 10.1007/BF00032968. [DOI] [Google Scholar]
- 9.Onrubia., Moyano E, Bonfill M, Cusido RM, Goossens A, Palazon J Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J Plant Physiol. 2013;170(2):211–219. doi: 10.1016/j.jplph.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Bender CL, Alarcón-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol R. 1999;63(2):266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauchli R, Boland W. Indanoyl amino acid conjugates: tunable elicitors of plant secondary metabolism. Chem Rec. 2003;3(1):12–21. doi: 10.1002/tcr.10043. [DOI] [PubMed] [Google Scholar]
- 12.Svoboda J, Boland W. Plant defense elicitors: analogues of jasmonoyl–isoleucine conjugate. Phytochemistry. 2010;71(13):1445–1449. doi: 10.1016/j.phytochem.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA. Virulence systems of Pseudomonas syringae pv. Tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 2003;36(4):485–499. doi: 10.1046/j.1365-313X.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu F, Huang J, Xu Y, Qian X, Zhong JJ. Responses of defense signals, biosynthetic gene transcription and taxoid biosynthesis to elicitation by a novel synthetic jasmonate in cell cultures of Taxus chinensis. Biotechnol Bioeng. 2006;94(6):1064–1071. doi: 10.1002/bit.20921. [DOI] [PubMed] [Google Scholar]
- 15.Tamogami S, Kodama O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry. 2000;54(7):689–694. doi: 10.1016/S0031-9422(00)00190-4. [DOI] [PubMed] [Google Scholar]
- 16.Haider G. Schrader Tv, Füßlein M, Blechert S, Kutchan TM. Structure-activity relationships of synthetic analogs of Jasmonic acid and Coronatine on induction of Benzophenanthridine alkaloid accumulation in Eschscholzia californica cell cultures. Biol Chem. 2000;381(8):741–748. doi: 10.1515/BC.2000.094. [DOI] [PubMed] [Google Scholar]
- 17.Lauchli R, Schüler G, Boland W. Selective induction of secondary metabolism in Phaseolus lunatus by 6-substituted indanoyl isoleucine conjugates. Phytochemistry. 2002;61(7):807–817. doi: 10.1016/S0031-9422(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 18.Fliegmann J. SCHüLER G, Boland W, Ebel J, Mithöfer a. the role of octadecanoids and functional mimics in soybean defense responses. Biol Chem. 2003;384(3):437–446. doi: 10.1515/BC.2003.049. [DOI] [PubMed] [Google Scholar]
- 19.Bru R, Sellés S, Casado-Vela J, Belchí-Navarro S, Pedreño MA. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J Agr Food Chem. 2006;54(1):65–71. doi: 10.1021/jf051485j. [DOI] [PubMed] [Google Scholar]
- 20.Lijavetzky D, Almagro L, Belchi-Navarro S, Martínez-Zapater JM, Bru R, Pedreño MA. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC research notes. 2008;1(1):132. doi: 10.1186/1756-0500-1-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamboni A, Gatto P, Cestaro A, Pilati S, Viola R, Mattivi F, Moser C, Velasco R. Grapevine cell early activation of specific responses to DIMEB, a resveratrol elicitor. BMC Genomics. 2009;10(1):363. doi: 10.1186/1471-2164-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belchi-Navarro S, Pedreño MA, Corchete P. Methyl jasmonate increases silymarin production in Silybum marianum (L.) Gaernt cell cultures treated with β-cyclodextrins. Biotechnol Lett. 2011;33(1):179–184. doi: 10.1007/s10529-010-0406-6. [DOI] [PubMed] [Google Scholar]
- 23.Sabater-Jara AB, Onrubia M, Moyano E, Bonfill M, Palazón J, Pedreño MA, Cusidó RM. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol J. 2014;12(8):1075–1084. doi: 10.1111/pbi.12214. [DOI] [PubMed] [Google Scholar]
- 24.Almagro L, Belchí-Navarro S, Martínez-Márquez A, Bru R, Pedreño MA. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and coronatine. Plant Physiolog Bioch. 2015;97:361–367. doi: 10.1016/j.plaphy.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Croteau R, Ketchum RB, Long R, Kaspera R, Wildung M. Taxol biosynthesis and molecular genetics. Phytochem Rev. 2006;5(1):75–97. doi: 10.1007/s11101-005-3748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onrubia C. R, Ramirez K, Hernandez-Vazquez L, Moyano E, Bonfill M et al. bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: paclitaxel and its derivatives. Curr Med Chem. 2013;20(7):880–891. [PubMed] [Google Scholar]
- 27.Onrubia M., Moyano E., Bonfill M., Palazón J., Goossens A., Cusidó R.M. The relationship between TXS, DBAT, BAPT and DBTNBT gene expression and taxane production during the development of Taxus baccata plantlets. Plant Science. 2011;181(3):282–287. doi: 10.1016/j.plantsci.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Brunˇáková K, Košuth J. Gene expression profiling in Taxus baccata L. seedlings and cell cultures. Methods Mol Biol. 2009:249–62. [DOI] [PubMed]
- 29.Bruňáková K, Košuth J, Katkovčinová Z, Lázárová M, Čellárová E. Expression of two genes of paclitaxel biosynthetic pathway during germination of Taxus baccata zygotic embryos. Biol Plant. 2010;54(3):515–519. doi: 10.1007/s10535-010-0090-3. [DOI] [Google Scholar]
- 30.Ramirez-Estrada K, Osuna L, Moyano E, Bonfill M, Tapia N, Cusido RM, Palazon J. Changes in gene transcription and taxane production in elicited cell cultures of Taxus× media and Taxus globosa. Phytochemistry. 2015;117:174–184. doi: 10.1016/j.phytochem.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Gamborg OLc, Miller RA, Ojima K Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 32.Widholm JM. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Lin L. Enhancement of taxol production and release in Taxus chinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Appl Microbiol Biotechnol. 2003;62(2–3):151–155. doi: 10.1007/s00253-003-1275-x. [DOI] [PubMed] [Google Scholar]
- 34.Rahpeyma SA, Moieni A, Jalali JM. Paclitaxel production is enhanced in suspension-cultured hazel (Corylus avellana L.) cells by using a combination of sugar, precursor, and elicitor. Eng Life Sci. 2015;15(2):234–242. doi: 10.1002/elsc.201400115. [DOI] [Google Scholar]
- 35.Channuntapipat C, Sedgley M, Collins G. Sequences of the cDNAs and genomic DNAs encoding the S1, S7, S8, and Sf alleles from almond. Theor Appl Genet. 2001;103(6–7):1115–1122. doi: 10.1007/s001220100629. [DOI] [Google Scholar]
- 36.Li S, Zhang P, Zhang M, Fu C, Yu L. Functional analysis of a WRKY transcription factor involved in transcriptional activation of the DBAT gene in Taxus chinensis. Plant Biol. 2013;15(1):19–26. doi: 10.1111/j.1438-8677.2012.00611.x. [DOI] [PubMed] [Google Scholar]