Abstract
We present two cases in which takotsubo cardiomyopathy (TC) developed immediately after a diagnosis of microvascular angina had been established. One patient who had been diagnosed as having endothelium-dependent microvascular angina (microvascular spasm) developed TC three weeks after the initial admission. The other patient was diagnosed as having endothelium-independent microvascular angina (decreased coronary flow reserve) and subsequently developed TC after the discontinuation of nicorandil treatment. These cases may provide insight into the possible mechanisms underlying the pathophysiological findings of TC.
<Learning objective: Impaired coronary microcirculation has been recently reported in many cases during the acute phase of takotsubo cardiomyopathy. However, the exact mechanism responsible for the coronary microvascular dysfunction associated with this entity remains unclear. This report highlights the importance of microvascular angina, which may play a role in the development of this cardiomyopathy.>
Keywords: Stress cardiomyopathy, Apical ballooning, Microcirculation, Coronary vasospasm
Introduction
Takotsubo cardiomyopathy (TC) was first described in 1991; since then, several possible mechanisms, such as multivessel epicardial spasm, catecholamine toxicity, plaque rupture, midseptal hypertrophy, and microvascular dysfunction, have been proposed to explain its occurrence [1]. Despite being widely recognized for more than two decades, its precise pathophysiology remains unclear. We present two cases of TC occurring in patients with microvascular angina, which suggest the possible underlying mechanisms of this cardiomyopathy.
Case 1
A 76-year-old woman with a history of diabetes mellitus presented to the emergency room with chest pain and palpitation. Although her symptoms disappeared shortly after arrival, she was admitted to the hospital because of suspected angina pectoris. She had been taking glimepiride (1 mg/day) and diazepam (2 mg, as needed). Routine laboratory studies on admission produced normal results, with the exception of a higher hemoglobin A1c level of 6.6%. An electrocardiogram (ECG) revealed normal findings, and an echocardiography showed normal left ventricular ejection fraction (LVEF, 69%). She underwent coronary angiography, which revealed no signs of significant atherosclerosis; an acetylcholine (ACH) provocation test was subsequently performed to assess coronary vasoconstriction. During the administration of ACH at a dose of 50 μg into the left coronary artery, the patient reported chest pain, and an ECG showed ST-segment elevation in the inferior leads, which gradually extended to the precordial leads (Fig. 1A). A coronary angiography revealed no evidence of epicardial spasm, but “to-and-fro” phenomenon was observed distal to the left anterior descending coronary artery (LAD), which resolved after the intracoronary injection of nitroglycerin. These findings were consistent with microvascular spasm, as previously described by Mohri et al. [2]; consequently, diltiazem (200 mg/day) was started to relieve the microvascular constriction. The patient recovered with a partial improvement of her angina during a 5-day hospital stay.
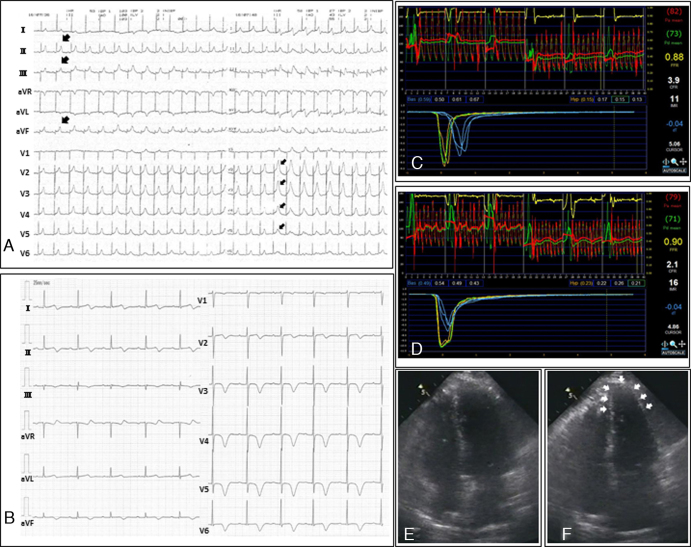
Fig. 1.
(A) During the administration of acetylcholine into the left coronary artery, an electrocardiogram (ECG) showed ST-segment elevation in the inferior leads (large arrows), which gradually extended to the precordial leads (small arrows). (B) An ECG performed at the second admission showed T wave inversions with prolonged QT intervals in leads I, II, III, aVF, and V2 to V6. (C) The coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) were 3.9 and 11, respectively, at the time of the initial admission. (D) During apical ballooning, the CFR and IMR values were poorer than those observed during the previous catheterization (i.e. the CFR and IMR values were 2.1 and 16, respectively). (E and F) An echocardiography showed apical hypokinesis with preserved basal function (E, end-diastole; F, end-systole).
Three weeks later, she suffered from worsening chest pain without any accompanying emotional or physical stress and visited our hospital once again. A laboratory workup revealed mildly increased troponin-I level (0.82 ng/mL), and an ECG showed T wave inversions with prolonged QT intervals in leads I, II, III, aVF, and V2 to V6 (Fig. 1B). A coronary angiography revealed normal findings, but the coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) in the LAD, which represent the coronary microcirculatory state, were poorer than at the time of the previous catheterization (i.e. CFR decreased from 3.9 to 2.1, and IMR increased from 11 to 16) (Fig. 1C and D). An echocardiography showed apical hypokinesis with preserved basal function, consistent with TC (Fig. 1E and F). Nicorandil (15 mg/day) was administered to further suppress the microvascular constriction. The abnormal wall motion and ECG results resolved completely within three days and six months, respectively.
Case 2
An 83-year-old woman with a history of well-controlled hypertension and depression was referred to our hospital because of chest pressure occurring during times of exercise and at rest. She was admitted to the hospital because a 99mTc-tetrofosmin myocardial single photon emission computed tomography demonstrated transient ischemic dilatation and reduced uptake in the LAD territory. She had been taking metoprolol (60 mg/day) for hypertension and ethyl loflazepate (1 mg/day) for depression. On admission, an ECG showed sinus rhythm with left bundle branch block. The laboratory data were normal, and an echocardiography demonstrated normal left ventricular function (LVEF, 67%). She underwent coronary angiography, which showed no apparent epicardial obstruction. An ACH provocation test was not performed because the patient's lower back pain made her unsuitable for the insertion of a temporary pacemaker. To assess the underlying etiology, the coronary microcirculation was evaluated using a pressure/temperature-sensing coronary wire. The CFR and IMR were measured in the LAD, where the values were 1.6 and 23, respectively (Fig. 2A). Although the association between IMR and microvascular angina has not been well established, the decreased CFR suggested microvascular dysfunction. Nicorandil treatment (15 mg/day) was initiated but was discontinued four months later because the patient suffered from severe headaches.
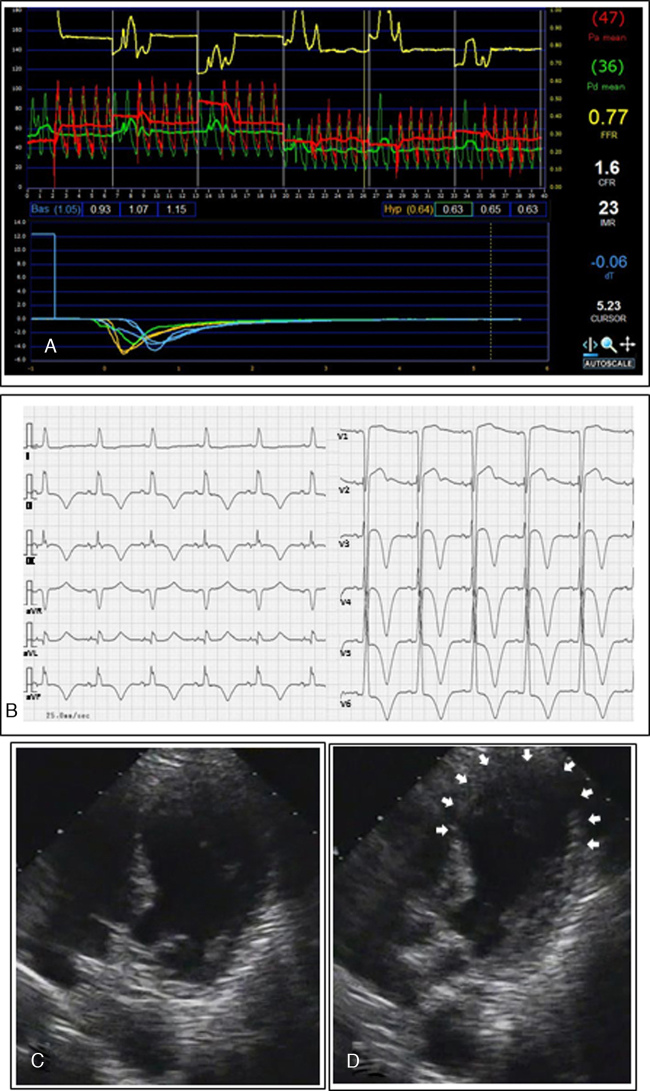
Fig. 2.
(A) The coronary flow reserve (CFR) and index of microcirculatory resistance (IMR) values measured in the left anterior descending coronary artery were 1.6 and 23, respectively. (B) An electrocardiogram performed at the time of the second admission showed T wave inversions with prolonged QT intervals in leads II, III, aVF, and V3 to V6. (C and D) An echocardiography revealed apical ballooning (C, end-diastole; D, end-systole).
Several days after treatment discontinuation, she was admitted to our hospital because of the recurrence of chest pressure occurring while she was doing housework. Her ECG showed T wave inversions with prolonged QT intervals in leads II, III, aVF, and V3 to V6 (Fig. 2B). An echocardiography revealed apical ballooning, supporting a diagnosis of TC (Fig. 2C and D). Given these findings, diltiazem (200 mg/day) was started instead of nicorandil, and metoprolol was discontinued. The wall motion abnormality recovered fully within a week.
Discussion
To the best of our knowledge, this is the first report of TC spontaneously occurring in patients with microvascular angina. Many researchers have reported the impairment of coronary microcirculation during the acute phase of TC 3, 4, 5, 6. Even though microcirculatory dysfunction has been observed in some clinical settings, such as hyperlipidemia, diabetes mellitus, cigarette smoking [7], and idiopathic dilated cardiomyopathy [8], the findings from these cases suggest that microvascular dysfunction, which is neither influences of concomitant coronary risk factors nor a secondary phenomenon of contractile dysfunction, may play a primary role in the pathophysiology of TC.
Kume et al. measured the coronary flow velocity reserve (CFVR) using a Doppler guidewire as an index of microvascular dysfunction within 24 h of symptom onset and three weeks later [3]. They reported that a decreased CFVR, which had been observed during the acute phase, improved normally during the follow-up period. Galiuto et al. performed myocardial contrast echocardiography within five days of symptom onset and found that the reduction in myocardial perfusion observed in the region with myocardial dysfunction had improved significantly after one month [4]. Daniels et al. first described high microvascular resistance in TC using a novel IMR [5], and Layland et al. also reported that high IMR level observed soon after disease onset decreased to normal in six weeks [6]. Although there is some dissent regarding microvascular dysfunction in TC, the above findings suggest that microvascular dysfunction and myocardial damage are only observable during the first few days of TC and then disappear within a relatively short time.
Epicardial vasomotor abnormality is less frequent in TC. In a previous systematic review, only 24 of 84 (28.6%) patients experienced multivessel epicardial spasm after the injection of ergonovine or ACH [9]. Recently, Patel et al. reported that the magnitude of vasomotor dysfunction was greater in the microcirculation than in the epicardial arteries for this entity [10]. Importantly, they stated that 90% of patients had abnormal microvascular vasomotion in response to either ACH or adenosine. This finding was quite consistent with our cases: one had endothelium-dependent microvascular dysfunction (microvascular spasm), and the other had endothelium-independent microvascular dysfunction (decreased coronary flow reserve). Microcirculatory vasoconstriction is predominantly mediated by α2-adrenoceptors; thus, α-adrenergic microvascular spasm, which occurs after catecholamine excess, appears to be the most plausible mechanism of TC. It remains to be established whether endothelium-dependent and -independent microvascular dysfunctions can co-exist in a patient with microvascular angina.
Additionally, in our cases, it seems to be important to understand why diltiazem and the discontinuation of nicorandil failed to prevent the occurrence of TC. In case 1, the clinical course including the ECG and echocardiography changes was consistent with TC, but findings of these examinations were not as marked as those of case 2. Diltiazem is widely used for the treatment of vasospastic angina as a calcium channel blocker; so, even though the patient had developed TC while taking diltiazem, the coronary microvascular contractility might have improved, to some extent. Nicorandil activates adenosine triphosphate-sensitive potassium channels and increases coronary blood flow. In a previous experimental study, the intracoronary infusion of nicorandil significantly dilated smaller vessels less than 100 μm in diameter [11]. Consequently, the abrupt withdrawal of nicorandil in case 2 might have destabilized the coronary microcirculation. These findings also suggest the pathophysiological relevance of TC and coronary microcirculatory instability.
Both TC and microvascular angina commonly occur in elderly women. Even though some variant forms of left ventricular dysfunction have been reported, the unique abnormal apical and midventricular wall motion of TC could be explained by a difference in microvascular distribution. Further research is needed to validate the potential role of microvascular angina in TC, and further consideration should be given to assessments of coronary microcirculation, especially in postmenopausal women.
Conclusion
These cases suggest that microvascular dysfunction is one of the plausible mechanisms underlying the pathophysiological findings of TC.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgment
The authors thank Dr Takae Asai for assistance with the manuscript preparation.
References
- 1.Bybee K.A., Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 2.Mohri M., Koyanagi M., Egashira K., Tagawa H., Ichiki T., Shimokawa H., Takeshita A. Angina pectoris caused by coronary microvascular spasm. Lancet. 1998;351:1165–1169. doi: 10.1016/S0140-6736(97)07329-7. [DOI] [PubMed] [Google Scholar]
- 3.Kume T., Akasaka T., Kawamoto T., Yoshitani H., Watanabe N., Neishi Y., Wada N., Yoshida K. Assessment of coronary microcirculation in patients with Takotsubo-like left ventricular dysfunction. Circ J. 2005;69:934–939. doi: 10.1253/circj.69.934. [DOI] [PubMed] [Google Scholar]
- 4.Galiuto L., De Caterina A.R., Porfidia A., Paraggio L., Barchetta S., Locorotondo G., Rebuzzi A.G., Crea F. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31:1319–1327. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 5.Daniels D.V., Fearon W.F. The index of microcirculatory resistance (IMR) in Takotsubo cardiomyopathy. Catheter Cardiovasc Interv. 2011;77:128–131. doi: 10.1002/ccd.22599. [DOI] [PubMed] [Google Scholar]
- 6.Layland J., Whitbourn R., Macisaac A., Somaratne J., Wilson A. Takotsubo cardiomyopathy: reversible elevation in microcirculatory resistance. Cardiovasc Revasc Med. 2012;13:66–68. doi: 10.1016/j.carrev.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Camici P.G., Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 8.Tsagalou E.P., Anastasiou-Nana M., Agapitos E., Gika A., Drakos S.G., Terrovitis J.V., Ntalianis A., Nanas J.N. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52:1391–1398. doi: 10.1016/j.jacc.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 9.Gianni M., Dentali F., Grandi A.M., Sumner G., Hiralal R., Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 10.Patel S.M., Lerman A., Lennon R.J., Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy) Eur Heart J Acute Cardiovasc Care. 2013;2:147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akai K., Wang Y., Sato K., Sekiguchi N., Sugimura A., Kumagai T., Komaru T., Kanatsuka H., Shirato K. Vasodilatory effect of nicorandil on coronary arterial microvessels: its dependency on vessel size and the involvement of the ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 1995;26:541–547. doi: 10.1097/00005344-199510000-00006. [DOI] [PubMed] [Google Scholar]