Abstract
Pheochromocytoma is known from a wide range of clinical manifestations and can mimic other disorders which can lead to delay in diagnosis. We report a case of a young female presenting with chest pain, electrocardiographic changes, and episodes of ventricular tachycardia, finally diagnosed with this catecholamine-producing tumor.
<Learning objective: Pheochromocytoma is a rare catecholamine-producing tumor that can pose a diagnostic challenge due to its multiple manifestations mimicking various conditions, including cardiovascular disorders. Despite its infrequent occurrence, pheochromocytoma should be considered as a possible cause of life-threatening cardiac arrhythmias and electrocardiographic changes in patients with diagnostic difficulties and primarily suspected of having cardiovascular disease. Furthermore, the method of treatment is entirely different.>
Keywords: Pheochromocytoma, Arrhythmias, Ventricular tachycardia, Torsades de pointes, Cardiac memory
Introduction
Pheochromocytoma is a rare and most often adrenally located catecholamine-producing tumor with diverse and vicious clinical manifestations and thus difficult to diagnose. Typically it expresses itself as a triad of abrupt episodes of headache, palpitations, and profuse sweating, accompanied by either sustained or paroxysmal hypertension or a labile blood pressure [1]. However, several case reports illustrate unexpected onset of the disease with failure of organs and systems, including multiple organ failure, as well as severe cardiovascular disorders, including life-threatening arrhythmias 2, 3, 4, 5, 6, 7, 8, 9.
Case report
A 37-year-old healthy woman, smoker, with a family history of coronary artery disease (CAD), was admitted to our department with exercise-induced palpitations and chest discomfort. She complained about similar episodes increasing in frequency and severity over the previous year. Over the previous six months the episodes occurred several times a week, and lasted for 5–15 min. Three months prior to the admission, she was examined with echocardiography and an ambulatory Holter monitoring, and both tests were normal.
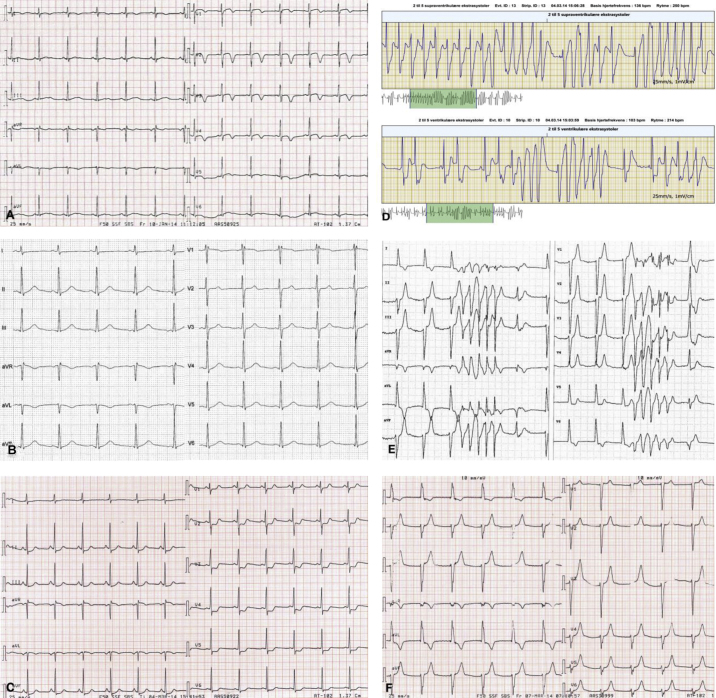
On admission the patient was stable with unremarkable objective examination. Electrocardiography (ECG) showed sinus rhythm 75/min and negative T waves in V1–V5 (Fig. 1A). Additionally, slightly elevated cardiac biomarkers with maximum troponin T (TNT) 26 ng/l (reference: <14 mg/l) and maximum creatinine kinase-MB (CK-MB) 6.6 mg/l (reference: <4.0 mg/l) led to subacute coronary angiography (CAG), which was normal. The remaining blood tests were normal and so were echocardiography, cardiac rhythm monitoring during hospitalization, and exercise test. ECG was normalized over the observation period (Fig. 1B). During the first admission she was normotensive (blood pressure ranged from 100/55 mmHg to 145/90 mmHg) and did not complain of symptoms characteristic for pheochromocytoma. Finally, her episodes were attributed to suspected paroxysmal supraventricular tachycardia, and she was discharged with a planned ambulatory ECG monitoring (R-test) in order to detect arrhythmia and a cardiac magnetic resonance imaging (MRI) to disclose possible ischemic changes.
Fig. 1.
Electrocardiogram (ECG) and R-test tracing. (A) ECG on the first admission mimicking evolved anterior wall myocardial infarction with negative T waves in V1–V5. (B) Prior observed ECG changes normalized during the observation period. (C) ECG on the second admission showing generalized ST-segment depressions of maximum 2 mm in V3–V4. (D) R-test tracing illustrating episodes of non-sustained polymorphic ventricular tachycardia. (E) ECG during an episode, showing nodal rhythm with non-sustained polymorphic ventricular tachycardia. (F) ECG during another episode, showing exclusively nodal rhythm.
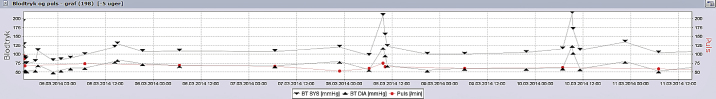
Two months later, the patient was readmitted with similar symptoms, with exercise-induced palpitations and severe chest pain. On admission she was pale and hypotensive at 90/53 mmHg. Her pulse was 67/min, respiratory rate was 18/min, O2Sat 100%, and temperature was 36.7 °C. The physical examination was normal. An arterial blood gas analysis showed respiratory alkalosis with a decrease in PaCO2 due to hyperventilation, an increased plasma lactate 5.8 mmol/l (reference: 0.5–2.5 mmol/l), and plasma glucose 12.8 mmol/l (reference: 4.2–7.8 mmol/l). Renal, liver, and cholesterol blood samples were all normal. The initial ECG showed sinus rhythm 77/min with generalized ST-segment depressions of maximum 2 mm in V3–V4 (Fig. 1C). An echocardiography revealed slightly reduced left ventricular ejection fraction (LVEF) with hypokinesia in the basal parts of the septum and the inferior wall. The chest X-ray was normal. The patient's symptoms, electrocardiographic and echocardiographic changes, as well as elevated cardiac biomarkers, maximum TNT 415 ng/l and maximum CK-MB 12.8 μg/l, led to the suspicion of non-ST-segment elevation myocardial infarction (non-STEMI) and the patient underwent subacute CAG. The examination again ruled out CAD and coronary vasoconstriction as possible causes of the symptoms. To our advantage, the day before the second admission, she had an ambulatory R-test that revealed frequent episodes of an aggressive non-sustained ventricular tachycardia (VT) 250/min, resembling torsades de pointes (Fig. 1D). A diagnostic electrophysiological study was performed without induction of the arrhythmia, despite aggressive stimulation protocols and even despite high doses of isoprenaline intravenously. Presence of an accessory pathway was ruled out. Subsequent cardiac MRI confirmed slightly reduced LVEF at 55% and a suspicion of regional ischemic lesion, corresponding to right coronary artery supply area. During the second hospitalization, we reported further symptomatic episodes of VT as well as a nodal rhythm (Fig. 1E and F) with concomitant labile blood pressure, ranging from 86/65 mmHg to 224/125 mmHg (Fig. 2). Differential diagnostics led to the suspicion of pheochromocytoma, which was supported by observed episodes of pallor and profuse sweating related to the events.
Fig. 2.
Blood pressure fluctuations during the first week of the second hospitalization. Graph illustrating labile blood pressure with episodes of paroxysmal hypertension of up to 224/125 mmHg.
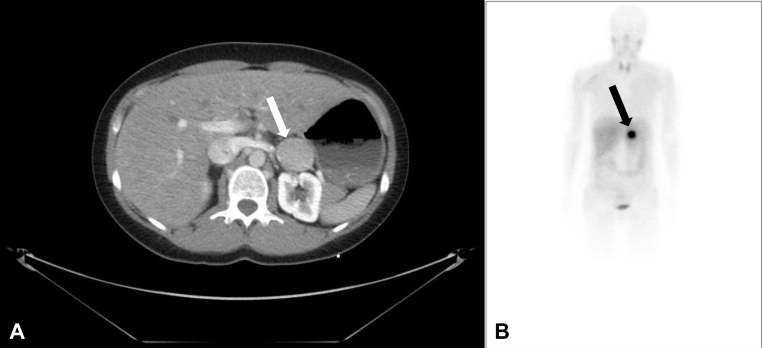
Pheochromocytoma was confirmed by elevated levels of plasma metanephrines 1377 ng/l (reference: <90 ng/l), plasma 3-normetanephrines 296 ng/l (reference: <180 ng/l), and by a 24-h urine sample that showed an elevated urine epinephrine of 2208 nmol/l (reference: <125 nmol/d), and a normal urine norepinephrine of 513 nmol/l (reference: <620 nmol/d). An abdominal computed tomography scan revealed an approximately 33 mm × 32 mm × 35 mm large tumor in the left adrenal area (Fig. 3A), and the metaiodobenzylguanidine scintigraphy demonstrated uptake by the left adrenal mass (Fig. 3B) and simultaneously ruled out metastases. The patient was successfully treated with phenoxybenzamine, followed by beta-blocker. Three weeks later, she underwent endoscopic retroperitoneal adrenalectomy. Histopatological examination confirmed the diagnosis of benign pheochromocytoma. The patient has been asymptomatic to date.
Fig. 3.
Computed tomography (CT) scan of the abdomen and metaiodobenzylguanidine scintigraphy. (A) CT scan of the upper abdomen revealing an approximately 33 mm × 32 mm × 35 mm large tumor of the left adrenal gland. (B) Metaiodobenzylguanidine scintigraphy demonstrating a pathological uptake by the left adrenal mass.
Discussion
Our case report illustrates how difficult the diagnosis of pheochromocytoma may be, if a patient with this rare clinical condition presents with atypical symptoms.
In patients with hypertension, an estimated prevalence of pheochromocytoma is 0.1–0.6% 4, 5. Accurate data reporting the incidence of life-threatening arrhythmias in this group of patients are not available [10]. Only few cases describing pheochromocytoma-related monomorphic or polymorphic VT have been reported 2, 3, 4, 5, 6, 7, 8, 9, 10. Moreover, patients with pheochromocytoma may present with a variety of non-specific symptoms, which can obscure or delay correct diagnosis. In our patient, initial clinical presentation with episodes of abrupt palpitations and chest discomfort pointed at paroxysmal supraventricular tachycardia, while afterwards occurring chest pain with concomitant ECG changes mimicked non-STEMI. Consequently, the patient underwent several investigations, including an invasive electrophysiological study and CAG twice in a short time, and was exposed to potential complications. As we excluded structural heart disease as an etiology of VT, one of the diagnoses of exclusion was pheochromocytoma. Typical symptoms of pheochromocytoma occurred late in the diagnostics process, after the performance of invasive diagnostics during the second hospitalization. Finally, imaging modalities and positive specific biomarkers confirmed the disease.
Pheochromocytoma may primarily manifest itself as a life-threatening and demanding aggressive treatment cardiovascular disorder. The tumor can lead to a dissecting aortic aneurysm, cardiogenic shock, acute heart failure, catecholamine-induced myocarditis, or cardiomyopathies, including Takotsubo cardiomyopathy 2, 3, 4. Others have reported pheochromocytoma causing an acute myocardial infarction, both in patients with and without atherosclerosis 3, 5. Moreover, this neuroendocrine tumor can be associated with a wide range of ECG changes, such as ST-T segment deviations, as in our patient, or with prolongation of the QT interval. Furthermore, several arrhythmias have been reported from asystolic arrest and nodal rhythm to supraventricular and ventricular tachyarrhythmias, including VT, torsades de pointes, or even ventricular fibrillation 2, 3, 4, 5, 6, 7, 8, 9. Thus, palpitations, reported by approximately 50–70% of all patients with the tumor [3], can indicate one of the life-threatening arrhythmias, as seen in our patient. A high amount of catecholamines, abruptly released into the blood, stimulated the β1-receptors and induced an aggressive VT, whereas observed episodes with nodal rhythm illustrated the body's homeostatic mechanisms with increased vagal tonus and reflex bradycardia in response to an abrupt rise in blood pressure.
The effects of catecholamines on different organs are determined by both their blood concentration and the types of the adrenergic receptors in the organs. While in healthy individuals catecholamines lead to vasodilatation of coronary arteries, in order to cover increased oxygen demand under stress, in patients with pheochromocytoma they may lead to vasoconstriction. This is a result of catecholamine overload and thus excessive stimulation of α-receptors. Concomitant β1-receptor stimulation inducing positive inotropic and chronotropic effects may lead to myocardial ischemia or even infarction.
Our patient presented with chest pain and generalized ST-segment depressions. She underwent CAGs that ruled out atherosclerosis and vasospasm. Thus, the subendocardial ischemia observed in the cardiac MRI might be secondary to the increased myocardial oxygen demand and the direct toxic effects of catecholamine oxidation products, inducing injury to cardiomyocytes and their apoptosis 2, 3, 4. An alternative explanation for the abnormal ECGs on both admissions could be cardiac memory phenomenon. The ST-segment depressions and inverted T waves could reflect cardiac memory in a post-tachycardia syndrome after paroxysmal episodes of VT. Similar changes are observed in patients with ventricular pacing. The differentiation of ECG changes due to cardiac memory and those caused by myocardial ischemia can be challenging [11].
In the presented case, several short runs of VT accompanied by the paroxysmal nature of the symptoms and fluctuating blood pressure, as well as exclusion of particular cardiovascular conditions, led to the diagnosis of pheochromocytoma. This rare clinical entity is usually a diagnostic challenge when a patient presents with atypical symptoms, although it should be kept in mind as possible etiology of acute ECG changes and cardiac arrhythmias.
Conflict of interest
None declared.
References
- 1.Bravo E., Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev. 2003;24:539–553. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- 2.Brouwers F., Eisenhofer G., Lenders J., Pacak K. Emergencies caused by pheochromocytoma, neuroblastoma, or ganglioneuroma. Endocrinol Metab Clin North Am. 2006;35:699–724. doi: 10.1016/j.ecl.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Prejbisz A., Lenders J., Eisenhofer G., Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29:2049–2060. doi: 10.1097/HJH.0b013e32834a4ce9. [DOI] [PubMed] [Google Scholar]
- 4.Galetta F., Franzoni F., Bernini G., Poupak F., Carpi A., Cini G., Tocchini L., Antonelli A., Santoro G. Cardiovascular complications in patients with pheochromocytoma: a mini-review. Biomed Pharmacother. 2010;64:505–509. doi: 10.1016/j.biopha.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Methe H., Hinterseer M., Wilbert-Lampen U., Beckmann B., Steinbeck G., Kääb S. Torsades de pointes: a rare complication of an extra-adrenal pheochromocytoma. Hypertens Res. 2007;30:1263–1266. doi: 10.1291/hypres.30.1263. [DOI] [PubMed] [Google Scholar]
- 6.Leite L., Macedo P., Santos S., Quaglia L., Mesas C., De Paola A. Life-threatening cardiac manifestations of pheochromocytoma. Case Rep Med. 2010;2010 doi: 10.1155/2010/976120. 976120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ter Bekke R., Crijns H., Kroon A., Gorgels A. Pheochromocytoma-induced ventricular tachycardia and reversible cardiomyopathy. Int J Cardiol. 2011;147:145–146. doi: 10.1016/j.ijcard.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Li W., Huang C., Su C., Chai C., Wu W., Chou Y. Extra-adrenal pheochromocytoma presenting with life-threatening ventricular tachycardia: a case report. Kaohsiung J Med Sci. 2004;20:612–615. doi: 10.1016/S1607-551X(09)70268-2. [DOI] [PubMed] [Google Scholar]
- 9.Paulin F., Klein G., Gula L., Skanes A., Yee R., Krahn A. QT prolongation and monomorphic VT caused by pheochromocytoma. J Cardiovasc Electrophysiol. 2009;20(8):931–934. doi: 10.1111/j.1540-8167.2008.01405.x. [DOI] [PubMed] [Google Scholar]
- 10.Zipes D.P., Camm A.J., Borggrefe M., Buxton A.E., Chaitman B., Fromer M., Gregoratos G., Klein G., Moss A.J., Myerburg R.J., Priori S.G., Quinones M.A., Roden D.M., Silka M.J., Tracy C. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 11.Patberg K., Shvilkin A., Plotnikov A., Chandra P., Josephson M., Rosen M. Cardiac memory: mechanisms and clinical implications. Heart Rhythm. 2005;2:1376–1382. doi: 10.1016/j.hrthm.2005.08.021. [DOI] [PubMed] [Google Scholar]