Abstract
OBJECTIVES: Numerous studies investigated relationships between positron emission tomography and proliferation index Ki-67 in breast cancer (BC) with inconsistent results. The aim of the present analysis was to provide evident data about associations between standardized uptake value (SUV) and expression of Ki-67 in BC. METHODS: MEDLINE library, SCOPUS and EMBASE data bases were screened for relationships between SUV and Ki-67 in BC up to April 2018. Overall, 32 studies with 1802 patients were identified. The following data were extracted from the literature: authors, year of publication, number of patients, and correlation coefficients. Associations between SUV and Ki-67 were analyzed by Spearman's correlation coefficient. RESULTS: Associations between SUVmax derived from 18F-FDG PET and Ki-67 were reported in 25 studies (1624 patients). The pooled correlation coefficient was 0.40, (95% CI = [0.34; 0.46]). Furthermore, 7 studies analyzed associations between SUVmax derived from 18F-fluorthymidin (FLT) PET and Ki-67 (178 patients). The pooled correlation coefficient was 0.54, (95% CI = [0.37; 0.70]). CONCLUSION: SUVmax correlated moderately with expression of Ki-67 and, therefore, cannot be used as a surrogate marker for tumor proliferation. Further studies are needed to evaluate associations between PET parameters and histopathological findings like hormone receptor status in breast cancer.
Introduction
Breast cancer (BC) is one of the most frequent malignancies in humans [1]. Imaging methods, especially mammography, ultrasound and magnetic resonance imaging, play a fundamental role in the diagnosis of BC [2]. Nowadays, also positron emission tomography (PET) is increasingly used in BC [3], [4], [5], [6], [7]. It has been shown that PET besides detection of distant metastases can also provide additional information about tumor histopathology [5], [6], [7], [8]. For example, numerous reports suggested that PET parameters, such as the maximal standardized uptake value (SUVmax), depended on the histologic and biologic characteristics of the breast tumor [5], [6]. Invasive tumors classified exhibit higher uptake than lower-grade tumors [5], [6], [7]. Furthermore, PET parameters can also predict behavior of BC [8], [9], [10], [11], [12]. For instance, Higuchi et al. showed that the prognosis of patients with high SUVmax of primary tumors at baseline is significantly worse than that of patients with low SUVmax in operated BC [9]. In addition, PET can predict patient’s response to chemotherapy in BC [10]. According to Ohara et al., the 3-year disease-free survival rates were 90.9 % for patients with a tumor of SUVmax <8.6 and only 42.9 % for patients with a tumor of SUVmax >8.6 (P < .002) [11].
Presumably, metabolic activity showing by PET may be associated with biological patterns of BC. In fact, some studies also indicated that PET parameters can also provide information about tumor microstructure in BC [4], [5], [7]. For example, Heudel et al. observed significant correlations between SUV and histological grade (P < 0.0001), histological type (P = 0.001), tumor size (P < 0.0435), estrogen receptor status (P < 0.0005), and progesterone receptor status (P = 0.002) [13].
In clinical practice, associations between PET parameters like SUVmax and expression of proliferation marker Ki-67 are of great importance. Ki-67 is a non-histone, nuclear protein synthesized throughout the whole cell cycle except the G0 phase [14]. According to the literature, BC with high expression of Ki-67 (<25%) are associated with a greater risk of death compared with lower expression rates [14]. Moreover, a higher Ki-67 labeling index is associated with a greater risk of recurrence (64 % increased risk) [14].
However, the reported data about relationships between PET and Ki-67 are controversial. While some authors identified significant correlations between the parameters, others did not. Therefore, it is unclear, if SUVmax can be used as imaging biomarker reflecting proliferation activity in BC or not.
The purpose of this meta-analysis was to provide evident data about associations between SUVmax and expression of Ki-67 breast cancer.
Materials and Methods
Data Acquisition
MEDLINE library, EMBASE data base and SCOPUS data base were screened for associations between PET parameters and proliferation marker Ki-67 in breast cancer up to April 2018. The strategy of data acquisition is shown in Figure 1.
Figure 1.
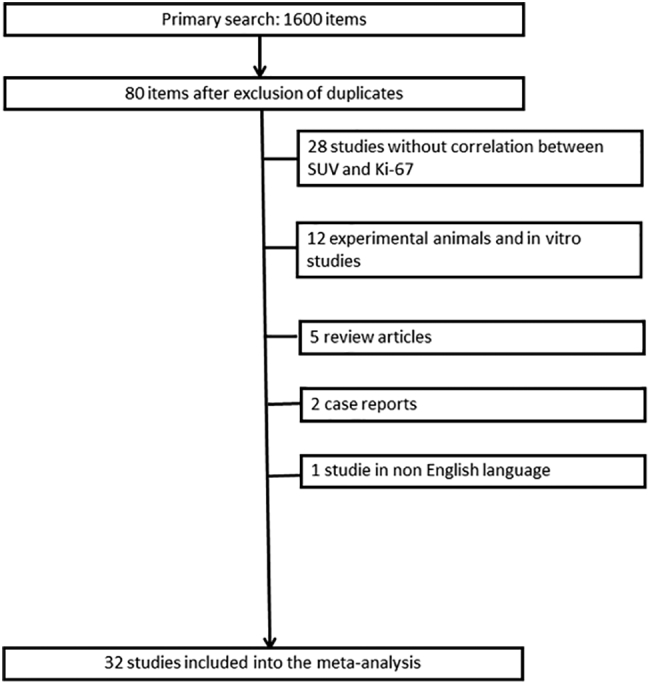
Flowchart of the data acquisition.
The following search words were used: “breast cancer OR breast carcinoma AND PET OR positron emission tomography OR SUV OR standardized uptake value AND Ki-67 OR KI 67 OR Ki 67 OR KI67 OR ki67 OR ki-67 OR mitotic index OR proliferation index OR MIB 1 OR MIB-1 OR mitosis index”. Secondary references were also recruited. Overall, 1600 records were identified. Duplicate articles, review articles, case reports, non-English publications, and articles, which not contain correlation coefficients between PET and Ki-67 were excluded (n= 1568). Therefore, the present analysis comprises 32 studies with 1801 patients (Table 1) [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. The following data were extracted from the literature: authors, year of publication, number of patients, histopathological parameters, and correlation coefficients.
Table 1.
Data About the Involved Studies
| Autors | Year | Country | Design | Histo-pathology | Patients | Receptor status | Tracer |
|---|---|---|---|---|---|---|---|
| Avril et al. [15] | 2001 | Germany | retrospective | different | 46 | different | 18F-FDG |
| Bitencourt et al. [16] | 2014 | Brazil | prospective | different | 50 | different | 18F-FDG |
| Buck et al. [17] | 2002 | Germany | retrospective | different | 75 | different | 18F-FDG |
| Cheng et al. [18] | 2013 | China | retrospective | n.r. | 20 | ER positive | 18F-FDG |
| Choi et al. [19] | 2018 | South Korea | retrospective | different | 117 | Triple negative | 18F-FDG |
| Cochet et al. [20] | 2012 | France | prospective | n.r. | 40 | different | 18F-FDG |
| Contractor et al. [21] | 2011 | United Kingdom | prospective | n.r. | 18 | different | 18F-FLT |
| Crippa et al. [22] | 2015 | Italy | prospective | different | 15 | different, none triple negative | 18F-FLT |
| De Cremoux et al. [23] | 2018 | France | retrospective | different | 75 | Luminal types | 18F-FDG |
| Ege Aktas et al. [24] | 2017 | Turkey | retrospective | IDC | 65 | different | 18F-FDG |
| Garcia Vicente et al. [26] | 2012 | Spain | prospective | different | 68 | different | 18F-FDG |
| García-Esquinas et al. [25] | 2014 | Spain | prospective | n.r. | 43 | different | 18F-FDG |
| Groheux et al. [27] | 2018 | France | prospective | different | 55 | Triple negative | 18F-FDG |
| Humbert et al. [28] | 2014 | France | prospective | different | 61 | Luminal types | 18F-FDG |
| Ikenaga et al. [29] | 2007 | Japan | retrospective | different | 45 | different | 18F-FDG |
| Jacobs et al. [30] | 2011 | USA | prospective | different | 6 | different | 18F-FDG |
| Jena et al. [31] | 2017 | India | retrospective | IDC | 69 | different | 18F-FDG |
| Kenny et al. [32] | 2005 | United Kingdom | prospective | different | 15 | n.r. | 18F-FLT |
| Koo et al. [33] | 2015 | South Korea | retrospective | different | 103 | Triple negative | 18F-FDG |
| Koolen et al. [34] | 2012 | The Netherlands | prospective | different | 214 | different | 18F-FDG |
| Kostakoglu et al. [35] | 2015 | USA | prospective | different | 72 | different | 18F-FLT |
| Kurland et al. [36] | 2012 | USA | prospective | different | 40 | different | 18F-FDG |
| Marti-Climent et al. [37] | 2014 | Spain | prospective | different | 30 | different | 18F-FLT |
| Nishimukai et al. [38] | 2017 | Japan | retrospective | n.r. | 163 | different, none triple negative | 18F-FDG |
| Shimoda et al. [39] | 2007 | Japan | retrospective | different | 37 | different, none triple negative | 18F-FDG |
| Smyczek-Gargya et al. [40] | 2004 | Germany | prospective | n.r. | 12 | different | 18F-FLT, 18F-FDG |
| Soussan et al. [41] | 2014 | France | retrospective | different | 54 | different | 18F-FDG |
| Tchou et al. [42] | 2010 | USA | retrospective | different | 41 | different | 18F-FDG |
| Tokes et al. [43] | 2015 | Hungary | retrospective | different | 42 | different | 18F-FDG |
| Tural et al. [44] | 2015 | Turkey | retrospective | different | 73 | different | 18F-FDG |
| Woolf et al. [45] | 2014 | United Kingdom | prospective | IDC and ILC | 19 | different | 18F-FLT |
| Yang et al. [46] | 2013 | China | retrospective | n.r. | 18 | different | 18F-FDG |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor; HER 2, human epidermal growth factor receptor 2; 18F-FLT, 18F-fluorthymidin; 18F-FDG (fluoro-D-glucose)
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) was used for the research [47].
Meta-Analysis
On the first step, the methodological quality of the acquired 32 studies was independently checked by two observers (A.S. and H.J.M.) using the Quality Assessment of Diagnostic Studies (QUADAS) instrument [48]. The results of QUADAS proving are shown in Table 2. The involved studies originated from several work groups world-wide and included different breast carcinomas. Study design was reported for all researches and it was prospective in 50% and retrospective in other 50%.
Table 2.
Methodological Quality of the Involved 32 Studies According to the QUADAS Criteria
| Quadas criteria | No bias (%) |
Bias (%) |
Unclear (%) |
|---|---|---|---|
| Was the spectrum of patients representative of the patients who will receive the test in practice? | 32 (100) | ||
| Were selection criteria clearly described? | 19 (59.38) | 4 (12.5) | 9 (28.12) |
| Is the reference standard likely to correctly classify the target condition? | 32 (100) | ||
| Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | 32 (100) | ||
| Did the whole sample or a random selection of the sample, receive verification using a reference standard of diagnosis? | 32 (100) | ||
| Did patients receive the same reference standard regardless of the index test result? | 32 (100) | ||
| Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | 32 (100) | ||
| Was the execution of the index test described in sufficient detail to permit replication of the test? | 32 (100) | ||
| Was the execution of the reference standard described in sufficient detail to permit its replication? | 32 (100) | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | 16 (50.0) | 16 (50.0) | |
| Were the reference standard results interpreted without knowledge of the results of the index test? | 12 (46.15) | 20 (30.77) | |
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | 32 (100) | ||
| Were uninterpretable/intermediate test results reported? | 30 (93.76) | 1 (3.12) | 1 (3.12) |
| Were withdrawals from the study explained? | 27 (84.38) | 3 (9.37) | 2 (6.25) |
Secondly, the acquired correlations between SUVmax and Ki-67 were re-analyzed by Spearman's correlation coefficient. Therefore, the reported Pearson`s correlation coefficients in some studies were converted into Spearman`s correlation coefficients according to the previous description [49].
Furthermore, the meta-analysis was undertaken by using RevMan 5.3 (Computer program, version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Heterogeneity was calculated by means of the inconsistency index I2 [50], [51]. Finally, DerSimonian and Laird random-effects models with inverse-variance weights were performed without any further correction [52].
Results
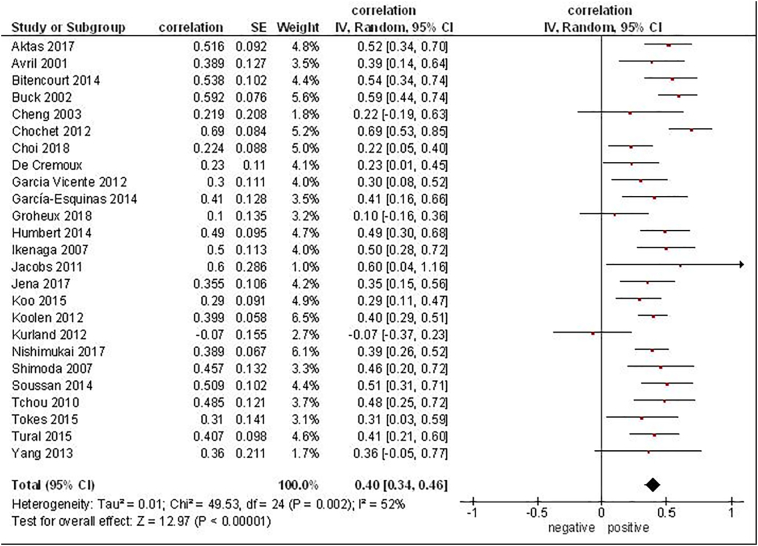
Associations between SUVmax derived from 18F-FDG (fluoro-D-glucose) PET and Ki-67 were reported in 25 studies (Figure 2). Overall, these studies included 1620 patients. The correlation coefficients between SUVmax and Ki-67 ranged from -0.07 to 0.69 (Figure 2). The pooled correlation coefficient was 0.40, (95% CI = [0.34; 0.46]).
Figure 2.
Forest plots of correlation coefficients between SUVmax derived from 18F-FDG PET and Ki-67 in patients with breast cancer.
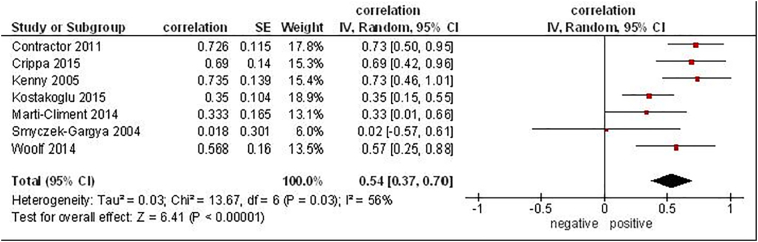
Furthermore, 7 studies analyzed associations between SUVmax derived from 18F-fluorthymidin (FLT) PET and Ki-67 (181 patients) [21], [22], [32], [35], [37], [40], [45]. The reported correlation coefficients between the parameters ranged from 0.02 to 0.73 (Figure 3). The pooled correlation coefficient was 0.54, (95% CI = [0.37; 0.70]).
Figure 3.
Forest plots of correlation coefficients between SUVmax derived from 18F-fluorthymidin PET and Ki-67 in patients with breast cancer.
Discussion
The possibility to predict biological features and, therefore, tumor behavior based on imaging, in particular on PET findings, is very important. In fact, if PET parameters, for instance, SUVmax, can reflect proliferation activity of lesions, so PET can be used as surrogate biomarker. Theoretically, metabolic activity measuring by PET may predict tumor cellularity and proliferation in malignancies. Therefore, PET parameters like SUVmax may well correlate with expression of Ki-67. However, some reports suggested that this does not apply for all tumors. In fact, it has been shown that different tumor types exhibited varied degree of correlation between SUVmax and Ki-67 [53]. So, correlation coefficients ranged from 0.81 in thymic carcinoma to -0.22 in malignant melanoma [53]. In most malignancies, only moderate correlations between the analyzed parameters were identified [53].
Previously, numerous reports analyzed relationships between PET parameters and expression of Ki-67 in BC with very inconsistent results. So, a very wide spectrum of correlation coefficients between SUVmax and Ki-67 was reported. Furthermore, most studies investigated small samples ranging from 6 to 75 patients/tumors and only four studies investigated samples over 100 patients [20], [33], [34], [38]. Therefore, the reported data cannot be considered as evident. These facts question the possibility to use PET parameters as surrogate biomarkers for proliferation activity in BC.
The present analysis identified moderate correlations between SUVmax derived from FDG PET and expression of Ki-67 in BC (0.40). In most acquired studies, slightly-to-moderate correlations were observed. Only in the study of Kurland et al., the reported correlation coefficient was -0.07 [36] and must be seen as an outlier [36]. In this study, the decline of FDG-PET uptake and Ki-67 after aromatase inhibitors and trastuzumab therapy was investigated [36]. The negative correlation at baseline might be caused by a heterogenous patient sample comprising metastasized and not metastasized patients. However, in the aromatase group a strong correlation regarding therapeutic induced decline of Ki-67 and FDG-PET was identified (r=0.77), indicating that in specific subgroups the associations between imaging and histopathology substantial can differ.
Furthermore, we found that SUVmax derived from FLT PET had stronger associations with Ki-67 in BC (0.54). This finding is not unusual. Similar results were reported also for other malignancies. For example, in a meta-analysis investigated relationships between SUVmax and Ki-67 in lung cancer, FLT-PET showed a higher correlation coefficient (r = 0.65) than FDG-PET (r=0.45) [54]. FLT is phosphorylated by thymidine kinase-1 and is a marker of cells in the S-phase of the cell cycle [55], [56]. Therefore, the uptake of FLT is linked to cell proliferation rate. Thus, FLT PET is more sensitive than FDG PET to predict proliferation potential.
However, overall, our findings suggest that it is problematically to use SUVmax as predictor of proliferation activity in BC. This finding is difficult to explain. Presumably, glucose metabolism and cell proliferation are not associated directly. Furthermore, Ki-67 is one of numerous proliferation markers. Possibly, glucose metabolism might be stronger associated with other proliferation factors than with Ki-67. In fact, Nishimukai et al. showed that SUVmax correlated stronger with proliferation marker geminin than with Ki-67 [38]. The authors also suggested that geminin is preferable to Ki-67 evaluating the proliferative activity of breast cancer cells [38].
The present analysis identified another interesting aspect. BC represents a heterogenous group of carcinomas with different histopathological features and behavior. Presumably, different subtypes of BC may have also different associations between PET and Ki-67. Further prospective studies are needed to confirm this hypothesis.
Our analysis identified also methodological problems of the previous reports. Half of the acquired studies were of retrospective design. Furthermore, according the QUADAS criteria, 28.12% of the acquired studies had unclear selection criteria. Additionally, 30.77% of the studies may have diagnostic review bias. Clearly, studies with well-defined inclusion and exclusion criteria are needed to investigate associations between PET and Ki-67 in BC.
In conclusion, the present meta-analysis showed that SUVmax correlated moderately with expression of Ki-67 and, therefore, cannot be used as a surrogate marker for tumor proliferation. Further studies are needed to evaluate associations between PET parameters and histopathological findings like hormone receptor status in breast cancer.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The data that support the findings of this study are available from professor Surov but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of professor Surov.
Authors' Contributions
-
•
AS, HJM, AW made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data;
-
•
HJM, AW been involved in drafting the manuscript or revising it critically for important intellectual content;
-
•
HJM, AW given final approval of the version to be published. Each author have participated sufficiently in the work to take public responsibility for appropriate portions of the content; and
-
•
AS, HJM, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
None.
Footnotes
All authors contributed equally for this work.
Competing Interests: The authors declare that they have no competing interests.
Funding: None.
Contributor Information
Alexey Surov, Email: Alexey.Surov@medizin.uni-leipzig.de.
Hans Jonas Meyer, Email: Hans-Jonas.Meyer@medizin.uni-leipzig.de.
Andreas Wienke, Email: Alexey.Surov@medizin.uni-leipzig.de.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Fiorica JV. Breast Cancer Screening, Mammography, and Other Modalities. Clin Obstet Gynecol. 2016;59(4):688–709. doi: 10.1097/GRF.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 3.Jafari SH, Saadatpour Z, Salmaninejad A, Momeni F, Mokhtari M, Nahand JS, Rahmati M, Mirzaei H, Kianmehr M. Breast cancer diagnosis: Imaging techniques and biochemical markers. J Cell Physiol. 2018;233(7):5200–5213. doi: 10.1002/jcp.26379. [DOI] [PubMed] [Google Scholar]
- 4.Mankoff D. Imaging in breast cancer – breast cancer imaging revisited. Breast Cancer Res. 2005;7:276–278. doi: 10.1186/bcr1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K. Prognostic value of FDG-PET and DWI in breast cancer. Ann Nucl Med. 2018;32(1):44–53. doi: 10.1007/s12149-017-1217-9. [DOI] [PubMed] [Google Scholar]
- 6.Gil-Rendo A, Martínez-Regueira F, Zornoza G, García-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96(2):166–170. doi: 10.1002/bjs.6459. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki M, Tozaki M, Kubota K, Murakami W, Yotsumoto D, Sagara Y, Ohi Y, Oosako S, Sagara Y. Simultaneous whole-body and breast 18F-FDG PET/MRI examinations in patients with breast cancer: a comparison of apparent diffusion coefficients and maximum standardized uptake values. Jpn J Radiol. 2018;36(2):122–133. doi: 10.1007/s11604-017-0707-y. [DOI] [PubMed] [Google Scholar]
- 8.Ahn SG, Park JT, Lee HM, Lee HW, Jeon TJ, Han K, Lee SA, Dong SM, Ryu YH, Son EJ. Standardized uptake value of 18F-fluorodeoxyglucose positron emission tomography for prediction of tumor recurrence in breast cancer beyond tumor burden. Breast Cancer Res. 2014;16:502. doi: 10.1186/s13058-014-0502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higuchi T, Nishimukai A, Ozawa H, Fujimoto Y, Yanai A, Miyagawa Y, Murase K, Imamura M, Takatsuka Y, Kitajima K. Prognostic significance of preoperative 18F-FDG PET/CT for breast cancer subtypes. Breast. 2016;30:5–12. doi: 10.1016/j.breast.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Shih TT, Yen RF. Multiparametric Evaluation of Treatment Response to Neoadjuvant Chemotherapy in Breast Cancer Using Integrated PET/MR. Clin Nucl Med. 2017;42(7):506–513. doi: 10.1097/RLU.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 11.Ohara M, Shigematsu H, Tsutani Y, Emi A, Masumoto N, Ozaki S, Kadoya T. Role of FDG-PET/CT in evaluating surgical outcomes of operable breast cancer: usefulness for malignant grade of triple-negative breast cancer. Breast. 2013;22:958–963. doi: 10.1016/j.breast.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Kadoya T, Aogi K, Kiyoto S, Masumoto N, Sugawara Y, Okada M. Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: a multi-institute study. Breast Cancer Res Treat. 2013;141(269) doi: 10.1007/s10549-013-2687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15(6):588–593. doi: 10.1007/s10147-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 14.Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153(3):477–491. doi: 10.1007/s10549-015-3559-0. [DOI] [PubMed] [Google Scholar]
- 15.Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, Nathrath W, Schwaiger M. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42(1):9–16. [PubMed] [Google Scholar]
- 16.Bitencourt AG, Lima EN, Chojniak R, Marques EF, de Souza JA, Graziano L, Andrade WP, Osório CA. Correlation between PET/CT results and histological and immunohistochemical findings in breast carcinomas. Radiol Bras. 2014;47(2):67–73. doi: 10.1590/S0100-39842014000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, Dankerl A, Glatting G, Reske S, Mattfeldt T. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29(10):1317–1323. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, Lei L, Xu J, Sun Y, Zhang Y, Wang X, Pan L, Shao Z, Zhang Y, Liu G. 18F-fluoromisonidazole PET/CT: a potential tool for predicting primary endocrine therapy resistance in breast cancer. J Nucl Med. 2013;54(3):333–340. doi: 10.2967/jnumed.112.111963. [DOI] [PubMed] [Google Scholar]
- 19.Choi SH, Chang JS, Koo JS, Park JW, Sohn JH, Keum KC, Suh CO, Kim YB. Differential Prognostic Impact of Strong PD-L1 Expression and 18F-FDG Uptake in Triple-negative Breast Cancer. Am J Clin Oncol. 2018 doi: 10.1097/COC.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 20.Cochet A, Pigeonnat S, Khoury B, Vrigneaud JM, Touzery C, Berriolo-Riedinger A, Dygai-Cochet I, Toubeau M, Humbert O, Coudert B. Evaluation of breast tumor blood flow with dynamic first-pass 18F-FDG PET/CT: comparison with angiogenesis markers and prognostic factors. J Nucl Med. 2012;53(4):512–520. doi: 10.2967/jnumed.111.096834. [DOI] [PubMed] [Google Scholar]
- 21.Contractor KB, Kenny LM, Stebbing J, Challapalli A, Al-Nahhas A, Palmieri C, Shousha S, Lewis JS, Hogben K, De Nguyen Q. Biological basis of [11C]choline-positron emission tomography in patients with breast cancer: comparison with [18F]fluorothymidine positron emission tomography. Nucl Med Commun. 2011;32(11):997–1004. doi: 10.1097/MNM.0b013e328349567b. [DOI] [PubMed] [Google Scholar]
- 22.Crippa F, Agresti R, Sandri M, Mariani G, Padovano B, Alessi A, Bianchi G, Bombardieri E, Maugeri I, Rampa M. 18F-FLT PET/CT as an imaging tool for early prediction of pathological response in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy: a pilot study. Eur J Nucl Med Mol Imaging. 2015;42(6):818–830. doi: 10.1007/s00259-015-2995-8. [DOI] [PubMed] [Google Scholar]
- 23.de Cremoux P, Biard L, Poirot B, Bertheau P, Teixeira L, Lehmann-Che J, Bouhidel FA, Merlet P, Espié M, Resche-Rigon M. 18FDG-PET/CT and molecular markers to predict response to neoadjuvant chemotherapy and outcome in HER2-negative advanced luminal breast cancers patients. Oncotarget. 2018;9(23):16343–16353. doi: 10.18632/oncotarget.24674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ege Aktas G, Taştekin E, Sarikaya A. Assessment of biological and clinical aggressiveness of invasive ductal breast cancer using baseline 18F-FDG PET/CT-derived volumetric parameters. Nucl Med Commun. 2018;39(1):83–93. doi: 10.1097/MNM.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 25.García García-Esquinas M, García-Sáenz JA, Arrazola García J, Enrique Fuentes Ferrer M, Furió V, Rodriguez Rey C, Román JM, Carreras Delgado JL. 18F-FDG PET-CT imaging in the neoadjuvant setting for stages II-III breast cancer: association of loco regional SUVmax with classical prognostic factors. Q J Nucl Med Mol Imaging. 2014;58(1):66–73. [PubMed] [Google Scholar]
- 26.García Vicente AM, Castrejón ÁS, Relea Calatayud F, Muñoz AP, León Martín AA, López-Muñiz IC, Del Mar Muñoz Sánchez M, Cordero García JM, Becerra Nakayo EM. 18F-FDG retention index and biologic prognostic parameters in breast cancer. Clin Nucl Med. 2012;37(5):460–466. doi: 10.1097/RLU.0b013e31823926c9. [DOI] [PubMed] [Google Scholar]
- 27.Groheux D, Biard L, Lehmann-Che J, Teixeira L, Bouhidel FA, Poirot B, Bertheau P, Merlet P, Espié M, Resche-Rigon M. Tumor metabolism assessed by FDG-PET/CT and tumor proliferation assessed by genomic grade index to predict response to neoadjuvant chemotherapy in triple negative breast cancer. Eur J Nucl Med Mol Imaging. 2018;45(8):1279–1288. doi: 10.1007/s00259-018-3998-z. [DOI] [PubMed] [Google Scholar]
- 28.Humbert O, Berriolo-Riedinger A, Cochet A, Gauthier M, Charon-Barra C, Guiu S, Desmoulins I, Toubeau M, Dygai-Cochet I, Coutant C. Prognostic relevance at 5 years of the early monitoring of neoadjuvant chemotherapy using (18)F-FDG PET in luminal HER2-negative breast cancer. Eur J Nucl Med Mol Imaging. 2014;41(3):416–427. doi: 10.1007/s00259-013-2616-3. [DOI] [PubMed] [Google Scholar]
- 29.Ikenaga N, Otomo N, Toyofuku A, Ueda Y, Toyoda K, Hayashi T, Nishikawa K, Tanaka M. Standardized uptake values for breast carcinomas assessed by fluorodeoxyglucose-positron emission tomography correlate with prognostic factors. Am Surg. 2007;73(11):1151–1157. [PubMed] [Google Scholar]
- 30.Jacobs MA, Ouwerkerk R, Wolff AC, Gabrielson E, Warzecha H, Jeter S, Bluemke DA, Wahl R, Stearns V. Monitoring of neoadjuvant chemotherapy using multiparametric, 23Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast Cancer Res Treat. 2011;128(1):119–126. doi: 10.1007/s10549-011-1442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jena A, Taneja S, Singh A, Negi P, Sarin R, Das PK, Singhal M. Reliability of 18F-FDG PET Metabolic Parameters Derived Using Simultaneous PET/MRI and Correlation With Prognostic Factors of Invasive Ductal Carcinoma: A Feasibility Study. AJR Am J Roentgenol. 2017;209(3):662–670. doi: 10.2214/AJR.16.17766. [DOI] [PubMed] [Google Scholar]
- 32.Kenny LM, Vigushin DM, Al-Nahhas A, Osman S, Luthra SK, Shousha S, Coombes RC, Aboagye EO. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 2005;65(21):10104–10112. doi: 10.1158/0008-5472.CAN-04-4297. [DOI] [PubMed] [Google Scholar]
- 33.Koo HR, Park JS, Kang KW, Han W, Park IA, Moon WK. Correlation between (18)F-FDG uptake on PET/CT and prognostic factors in triple-negative breast cancer. Eur Radiol. 2015;25(11):3314–3321. doi: 10.1007/s00330-015-3734-z. [DOI] [PubMed] [Google Scholar]
- 34.Koolen BB, Vrancken Peeters MJ, Wesseling J, Lips EH, Vogel WV, Aukema TS, van Werkhoven E, Gilhuijs KG, Rodenhuis S, Rutgers EJ. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39(12):1830–1838. doi: 10.1007/s00259-012-2211-z. [DOI] [PubMed] [Google Scholar]
- 35.Kostakoglu L, Duan F, Idowu MO, Jolles PR, Bear HD, Muzi M, Cormack J, Muzi JP, Pryma DA, Specht JM. A Phase II Study of 3'-Deoxy-3'-18F-Fluorothymidine PET in the Assessment of Early Response of Breast Cancer to Neoadjuvant Chemotherapy: Results from ACRIN 6688. J Nucl Med. 2015;56(11):1681–1689. doi: 10.2967/jnumed.115.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurland BF, Gadi VK, Specht JM, Allison KH, Livingston RB, Rodler ET, Peterson LM, Schubert EK, Chai X, Mankoff DA. Feasibility study of FDG PET as an indicator of early response to aromatase inhibitors and trastuzumab in a heterogeneous group of breast cancer patients. EJNMMI Res. 2012;2(1):34. doi: 10.1186/2191-219X-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marti-Climent JM, Dominguez-Prado I, Garcia-Velloso MJ, Boni V, Peñuelas I, Toledo I, Richter JA. [18F]fluorothymidine-positron emission tomography in patients with locally advanced breast cancer under bevacizumab treatment: usefulness of different quantitative methods of tumor proliferation. Rev Esp Med Nucl Imagen Mol. 2014;33(5):280–285. doi: 10.1016/j.remn.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Nishimukai A, Inoue N, Kira A, Takeda M, Morimoto K, Araki K, Kitajima K, Watanabe T, Hirota S, Katagiri T. Tumor size and proliferative marker geminin rather than Ki67 expression levels significantly associated with maximum uptake of 18F-deoxyglucose levels on positron emission tomography for breast cancers. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa M. The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer. 2007;14(3):260–268. doi: 10.2325/jbcs.14.260. [DOI] [PubMed] [Google Scholar]
- 40.Smyczek-Gargya B, Fersis N, Dittmann H, Vogel U, Reischl G, Machulla HJ, Wallwiener D, Bares R, Dohmen BM. PET with [18F]fluorothymidine for imaging of primary breast cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2004;31(5):720–724. doi: 10.1007/s00259-004-1462-8. [DOI] [PubMed] [Google Scholar]
- 41.Soussan M, Orlhac F, Boubaya M, Zelek L, Ziol M, Eder V, Buvat I. Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0094017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchou J, Sonnad SS, Bergey MR, Basu S, Tomaszewski J, Alavi A, Schnall M. Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol Imaging Biol. 2010;12(6):657–662. doi: 10.1007/s11307-009-0294-0. [DOI] [PubMed] [Google Scholar]
- 43.Tőkés T, Szentmártoni G, Torgyík L, Somlai K, Kulka J, Lengyel Z, Györke T, Dank M. Complexity of Response Evaluation During Primary Systemic Therapy of Breast Cancer: Scoring Systems and Beyond-Preliminary Results. Anticancer Res. 2015;35(9):5063–5072. [PubMed] [Google Scholar]
- 44.Tural D, Kivrak Salim D, Mutlu H, Erkilic M, Gunduz S, Karakurt M, Musri F, Tuna S, Boz A, Aydin F. Is there any relation between PET-CT SUVmax value and prognostic factors in locally advanced breast cancer? J BUON. 2015;20(5):1282–1286. [PubMed] [Google Scholar]
- 45.Woolf DK, Beresford M, Li SP, Dowsett M, Sanghera B, Wong WL, Sonoda L, Detre S, Amin V, Ah-See ML. Evaluation of FLT-PET-CT as an imaging biomarker of proliferation in primary breast cancer. Br J Cancer. 2014;110(12):2847–2854. doi: 10.1038/bjc.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z, Sun Y, Xue J, Yao Z, Xu J, Cheng J, Shi W, Zhu B, Zhang Y, Zhang Y. Can positron emission tomography/computed tomography with the dual tracers fluorine-18 fluoroestradiol and fluorodeoxyglucose predict neoadjuvant chemotherapy response of breast cancer?--A pilot study. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 48.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalkidou A, Landau DB, Odell EW, Cornelius VR, O'Doherty MJ, Marsden PK. Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur J Cancer. 2012;48:3499–3513. doi: 10.1016/j.ejca.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–897. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 53.Deng SM, Zhang W, Zhang B, Chen YY, Li JH, Wu YW. Correlation between the Uptake of 18F-Fluorodeoxyglucose (18F-FDG) and the Expression of Proliferation-Associated Antigen Ki-67 in Cancer Patients: A Meta-Analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen G, Ma H, Pang F, Ren P, Kuang A. Correlations of 18F-FDG and 18F-FLT uptake on PET with Ki-67 expression in patients with lung cancer: a meta-analysis. Acta Radiol. 2018;59:188–195. doi: 10.1177/0284185117706609. [DOI] [PubMed] [Google Scholar]
- 55.Salskov A, Tammisetti VS, Grierson J, Vesselle H. FLT: measuring tumor cell proliferation in vivo with positron emission tomography and 3′-deoxy-3′-18F-fluorothymidine. Semin Nucl Med. 2007;37:429–439. doi: 10.1053/j.semnuclmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–1217. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from professor Surov but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of professor Surov.