Highlights
-
•
Diabetes is present in 15% of pediatric patients with mucormycosis [1–2].
-
•
Treatment includes amphotericin B, as well as surgical debridement for invasive disease.
-
•
High index of suspicion is required as delay of treatment leads to disseminated disease.
-
•
The mortality rate is near 50%, while for disseminated disease is almost 100%.
Keywords: Mucormycosis, Diabetes mellitus, Invasive fungal infections, Complications of diabetes mellitus
Abstract
Mucormycosis is a relatively rare, life-threatening and opportunistic infection that affects immunocompromised patients. We present the unusual case of pulmonary mucormycosis in a 13-year-old Caucasian female that had recently been diagnosed with type 1 diabetes. Our case serves as an example to healthcare providers treating immunosuppressed patients with pneumonia to have a high clinical suspicion for fungal infections, as delay in diagnosis and treatment can result in disseminated disease and higher patient mortality risk.
Introduction
Mucormycosis is a relatively rare, life-threatening and opportunistic infection caused by ubiquitous filamentous fungi of the Mucorales order of Zygomycetes. Rhizopus, Rhizomucor (mucor), and Apophysomyces are the organisms most commonly isolated from clinical specimens. Together, this group of fungi have emerged as the third most common invasive mycosis after aspergillosis and candidiasis [1,2]. These fungi are ubiquitous in the environment, with clinical disease generally being diagnosed in immunocompromised patients [1,2].
Diabetes mellitus is a common predisposing factor for mucormycosis in adults, present in 36–88% of cases [1]. In children, diabetes is present in 15% of patients with mucormycosis, trailing neutropenia, prematurity and malignancy [2]. The five most common sites of infection are rhinocerebral, pulmonary, cutaneous, gastrointestinal, and disseminated [2,3]. Classically, microscopic evaluation reveals filamentous, non-septate hyphae that branch at right angles [4]. Histologically, mucormycosis is characterized by fungal hyphal invasion into blood vessels with subsequent thrombosis and tissue ischemia [4]. Acidosis and hyperglycemia are ideal environments for fungal growth.
In this report we present an unusual case of pulmonary mucormycosis in a 13-year-old Caucasian female that had recently been diagnosed with type 1 diabetes. The initial workup for the patient did not include mucormycosis as part of the differential diagnosis and delayed treatment. Our aim in this report is to highlight the need for a high clinical suspicion for fungal infections in immunocompromised and diabetic patients.
Case report
The patient was a 13-year-old Caucasian female that presented to the hospital with complaints of fever, nasal congestion, and worsening cough over four weeks. This was her second admission within a one-month period. On her previous hospitalization, she had presented to the emergency department with ketoacidosis, was diagnosed with type 1 diabetes mellitus, and started on insulin therapy. An outpatient chest CT had demonstrated pneumonia. Laboratory workup via serology studies immediately prior to admission included Aspergillus, Blastomyces, Coccidiodes, Histoplasma, Mycoplasma, Chlamydia, and tuberculosis, which were all negative. She was consequently placed on four separate courses of antimicrobials including cefdinir, azithromycin, clindamycin, and levofloxacin. However, she developed a brown, purulent sputum and her respiratory function progressively declined.
On admission, the patient was febrile at 38.3 C, heart rate was 148 beats per minute, respiratory rate was 36 breaths per minute, blood pressure was 138/99 mmHg, and O2 saturation was 94% on room air. Physical examination revealed decreased breath sounds in right lower lobe and scattered rhonchi. Initial laboratory investigations demonstrated total WBC count 15.2 x cells per liter (81.3% neutrophil), hemoglobin 10.8 g/dL, ESR 76 mm, and CRP 312 mg/L Chest radiography indicated right lower lobe homogenous opacity suggesting consolidation, and chest CT demonstrated right middle and lower lobe pneumonia. Blood cultures were drawn, and she was empirically started on ceftriaxone and vancomycin.
Overnight, the patient had increased oxygen requirement and was transferred to the pediatric intensive care unit. Infectious disease recommended sputum culture, screenings for pneumococcus and legionellosis, and change antimicrobial regimen to linezolid and meropenem. Further laboratory investigations for potential source of infection including Legionella, Pneumococcus, chlamydia, mycoplasma, and atypical mycobacteria were negative. The patient was initially improving. However, by hospital day 7, she began to experience low-grade fever.
Pediatric pulmonology was consulted due to persistent tachypnea and accessory muscle use. The patient was recommended budesonide and racemic epinephrine. For the next two days, the patient and her family reported improvement. However, after four days, the patient’s respiratory function declined again, bronchoscopy and bronchoalveolar lavage were performed.
During the bronchoscopy, only the larynx was visualized before the patient desaturated, suffered a cardiopulmonary arrest and required immediate resuscitative efforts including CPR, epinephrine, endotracheal intubation, central line placement, and vasopressor support. The patient was subsequently placed on high-flow oscillating ventilator, and the respiratory arrest was believed to be caused by increased secretions and mucus plugging within the airways. Infectious disease changed from meropenem to cefepime once she was stabilized, and subsequently added voriconazole due to prolonged broad-spectrum coverage. While the patient stabilized over the next four days before re-attempting another bronchoscopy, all previous cultures of blood, sputum, and urine continued to remain negative for any suspected pathogens.
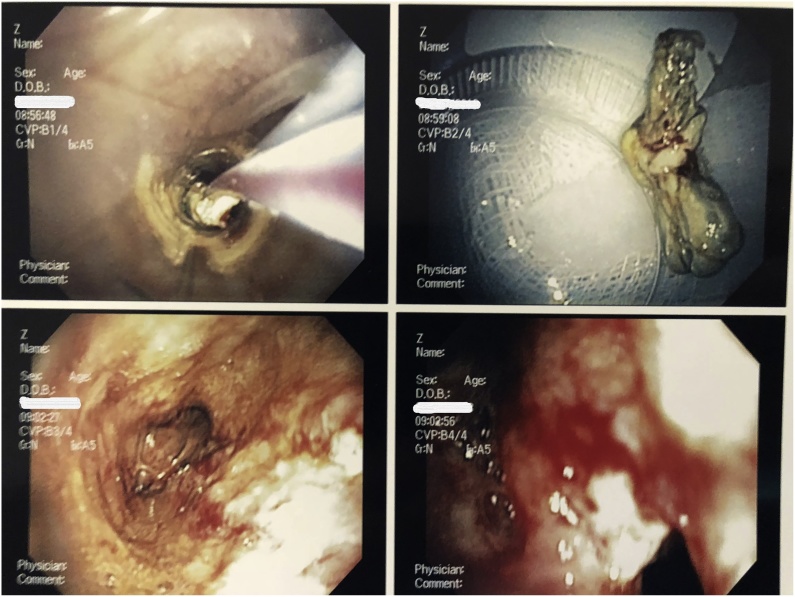
A second bronchoscopy was performed successfully. During the procedure, a large foreign mass was removed from the patient’s trachea (Fig. 1). Direct visualization revealed severe tracheitis, fibrosis of the left mainstem bronchus, and lavage was performed in the right lower lobe. Initial pathology report from the foreign body suggested possible mucormycosis, and she was placed on liposomal amphotericin B. CT of the chest demonstrated bilateral infiltrates involving right upper, middle, and lower lobes, as well as the left lower lobe with a left-side pleural effusion.
Fig. 1.
Direct visualization of Pulmonary mucomycosis in a Adolescent Female. Bronchoscopy was performed that showed severe tracheitis, fibrosis of the left mainstem bronchus.
On hospital day 20, the throat culture confirmed the diagnosis of mucormycosis, and both cefepime and linezolid were discontinued. Repeat chest CT revealed dilatation of the trachea and right mainstem bronchus, and an occluded left mainstem bronchus was noted. The final tracheal biopsy showed the presence of broad, non-septate hyphae in both fibroconnective tissues and cartilage, indicating invasive disease. In spite of clinical improvement of respiratory symptoms, complemented by serial chest radiography, the patient was transferred on hospital day 40 to a tertiary medical center for surgical debridement.
Discussion
The association between mucormycosis and diabetes is not completely understood, but likely results from multifactorial effects of hyperglycemia and ketoacidosis acting to suppress the innate immune system response and provide a facultative environment for fungal proliferation [4]. Hyperglycemia and acidosis have been shown to diminish chemotaxis and oxidative burst of neutrophils, as well as chemotaxis and phagocytosis of macrophages. [5]. Acidosis can lead to increased levels of free iron in the blood due to weakened binding of iron to transferrin, thereby allowing the fungus to utilize this available iron for its own growth [6]. Chronic hyperglycemia impairs release of interleukin-1 and interleukin-6 by mononuclear cells, as well as the proliferative function of CD4 T-lymphocytes.
Current treatment recommendations for pulmonary mucormycosis are timely initiation of intravenous amphotericin B, as well as surgical debridement for invasive disease [[8], [9], [10], [11]]. Previous studies have shown ketoconazole and miconazole to be ineffective against mucormycosis [9]. Surgical intervention early in the patient’s clinical course can provide utility in removing small foci of infection in conjunction with medical management [9]. While wedge resection is occasionally sufficient in treating local infection, lobectomy is frequently required due to extensive, invasive spread of the disease [9]. In addition to antifungal and surgical measures, correcting any underlying predisposing conditions should be immediately addressed, and both immunosuppressants and deferoxamine should be discontinued promptly [1]. However, the mortality rate continues to remain above 50% for mucormycosis overall, and disseminated disease has nearly 100% predicted mortality [[5], [6], [7], [8]]. Patients presenting with endobronchial mucormycosis, commonly seen in diabetics, have been demonstrated to have decreased mortality risk with prompt surgical resection of all affected lung parenchyma [[4], [5], [6]].
Our case serves as an example to healthcare providers treating immunosuppressed patients with pneumonia to have a high clinical suspicion for fungal infections, as delay in diagnosis and treatment can result in disseminated disease and higher patient mortality risk. Prompt recognition of at-risk patient populations, urgent evaluation for sources of infection including fungal species, effective inter-departmental communication with consulting physicians such as infectious disease and pulmonology, as well as immediate initiation of treatment can lead to improved patient outcomes in managing this rare but devastating disease.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Approval was not required.
Conflict of interest
All authors have no conflict of interest to disclose.
Acknowledgements
None.
References
- 1.Spellberg B., Edwards J., Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3) July):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrory M.C., Moore B.A., Nakagawa T.A., Givner L.B., Jason D.R., Palavecino E.L. Disseminated mucormycosis in an adolescent with newly diagnosed diabetes mellitus. Pediatr Infect Dis J. 2014;33(10) October):1094–1096. doi: 10.1097/INF.0000000000000383. doi: [DOI] [PubMed] [Google Scholar]
- 3.Rammaert B., Lanternier F., Poirée S., Kania R., Lortholary O. Diabetes and mucormycosis: a complex interplay. Diabetes Metab. 2012;38(3) June):193–204. doi: 10.1016/j.diabet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1, Febuary):S23–S34. doi: 10.1093/cid/cir866. doi: [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi A., Mehdizadeh A., Ghasemi-Rad M., Habibpour H., Esmaeli A. Pulmonary mucormycosis in patients with diabetic ketoacidosis: a case report and review of literature. Tuberk Toraks. 2012;60(1):66–69. doi: 10.5578/tt.2464. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim A.S., Spellberg B., Walsh T.J., Kontoyiannis D.P. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1, Febuary):S16–S22. doi: 10.1093/cid/cir865. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artis W.M., Fountain J.A., Delcher H.K., Jones H.E. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31(12) December):1109–1114. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- 8.Waldorf A.R., Ruderman N., Diamond R.D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984;74(1) July):150–160. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis J.R., Villanueva P., Bryant P., Blyth C.C. Mucormycosis in children: review and recommendations for management. J Pediatric Infect Dis Soc. 2018;7(2)May):159–164. doi: 10.1093/jpids/pix107. doi: [DOI] [PubMed] [Google Scholar]
- 10.Lee F.Y., Mossad S.B., Adal K.A. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159(12) June):1301–1309. doi: 10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 11.Tedder M., Spratt J.A., Anstadt M.P., Hedge S.S., Tedder S.D., Lowe J.E. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4) April):1044–1050. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]