Abstract
Background
Polycystic ovarian syndrome (PCOS) is one of the most common endocrinopathies among reproductive-age women. Its metabolic features often overlap with those associated with metabolic syndrome (MS) and insulin resistance syndrome (IRS). The objective of this study was to determine the prevalence and predictors of MS and IRS in infertile Vietnamese women with PCOS.
Methods
A cross-sectional study was conducted at a tertiary fertility centre at Hue University Hospital from June 2016 to November 2017. A total of 441 infertile women diagnosed with PCOS based on the revised 2003 Rotterdam consensus criteria were enrolled. MS and IRS were defined based on the National Heart, Lung, and Blood Institute/American Heart Association Adult Treatment Panel III 2005 and American College of Endocrinology IRS 2003 criteria, respectively. Complete clinical and biochemical measurements of 318 women were available for analysis. Independent predictors of MS and IRS were identified using multivariate logistic regression.
Results
The overall prevalence of MS and IRS in women with PCOS was 10.4% and 27.0%, respectively. We identified older age (>30 years) and obesity as independent predictors of MS and IRS. Elevated anti-Müllerian hormone levels increased the risk of IRS, but not that of MS.
Conclusion
MS and IRS are prevalent disorders among infertile Vietnamese women with PCOS. PCOS is not solely a reproductive problem. Screening and early intervention for MS and/or IRS based on anthropometric, metabolic, and reproductive hormone risk factors should be an integral part of fertility care.
Keywords: Prevalence, Polycystic ovary syndrome, Metabolic syndrome, Insulin resistance, Infertility
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is one of the most common endocrine disorders among women of reproductive age. Its prevalence ranges from 6% to 10% in unselected populations worldwide [1]. Infertility is a frequent presenting feature of PCOS, and roughly 90% to 95% of cases of anovulatory infertility are caused by PCOS [2]. Apart from reproductive abnormalities, PCOS is also commonly associated with disturbances in glucose and lipid metabolism. Cardiovascular disease (CVD) risk factors, including diabetes and dyslipidaemia, are more prevalent in women with PCOS than in age- and weight-matched women without PCOS [3,4]. Obesity, when present as a comorbidity of PCOS, further compounds insulin resistance and CVD risks [5,6].
Although the aetiology of PCOS is not completely understood, evidence indicates that insulin resistance is intrinsically involved in the pathophysiology of PCOS. To overcome insulin resistance and to maintain adequate glucose uptake in the muscles and adipose tissues, compensatory hyperinsulinaemia occurs. This regulatory feedback mechanism preserves normoglycaemia. However, individuals with insulin resistance/hyperinsulinaemia remain at an increased risk for developing a cluster of physiological abnormalities, known as insulin resistance syndrome (IRS). These abnormalities include some degree of glucose intolerance, abnormal uric acid metabolism, dyslipidaemia, hypertension, endothelial dysfunction, chronic inflammation, and a prothrombotic state [7].
The American College of Endocrinology (ACE) identified four major components that comprise IRS: (1) hypertriglyceridaemia, (2) reduced high density lipoprotein cholesterol (HDL-C), (3) hypertension, and (4) impaired glucose tolerance (IGT). It excludes patients with frank hyperglycaemia fulfilling the criteria for type 2 diabetes mellitus (T2DM). As such, the concept of IRS focuses on identifying high-risk individuals before T2DM and/or CVD occurs. Its clinical implications extend beyond cardiovascular consequences, including non-alcoholic fatty liver disease and certain forms of cancer [7].
Metabolic syndrome (MS) is another cluster of metabolic abnormalities that identifies individuals at increased risk for CVD. Apart from sharing the first three components for IRS mentioned above, the National Heart, Lung, and Blood Institute/American Heart Association (NHLBI/AHA) Adult Treatment Panel III (ATP III) 2005 guidelines include abdominal obesity as a diagnostic criterion for MS [8]. Unlike IRS, evidence of insulin resistance is not a requirement for diagnosing MS [7].
Studies of the association between PCOS and insulin resistance have largely used the euglycaemic-hyperinsulinaemic clamp technique or surrogate markers for insulin sensitivity. Limited data have been reported on the prevalence of IRS based on the ACE 2003 criteria or other comparable working definitions of IRS [9]. In some reports, the term IRS is used interchangeably with MS, further confounding the controversy of their definition and clinical use. The present study examines MS and IRS as two distinct multicomponent syndromes. Our aim was to compare the prevalence and predictors of MS and IRS in a population of Vietnamese women presenting with infertility due to PCOS.
METHODS
Study design
This cross-sectional study was conducted at the Hue Center for Reproductive Endocrinology and Infertility, Hue University Hospital, Vietnam from June 2016 to November 2017. Consecutive women diagnosed with PCOS during this period were enrolled. The inclusion criteria were (1) women from 18 to 45 years of age and (2) at least 1 year of infertility. Anthropometric, metabolic, and reproductive hormone measurements were obtained. The study was approved by the Hue University of Medicine and Pharmacy Ethics Committee (approval number H2016/236). Informed written consent was obtained from all participants.
Participants
Patients with PCOS were identified based on the revised 2003 Rotterdam consensus criteria, in which two out of three of the following conditions must be met: (1) the presence of oligo- and/or anovulation, (2) clinical and/or biochemical signs of hyperandrogenism, and (3) polycystic ovaries on ultrasonography. Other causes of hyperandrogenism, such as congenital adrenal hyperplasia, androgen-secreting neoplasms, and Cushing syndrome, were excluded. Women who had hypothyroidism or hyperprolactinaemia, were on oral contraceptive medication within 3 months prior to the time of enrolment, were on insulin sensitisers, or who had established diabetes (type 1 or type 2) were also excluded from the study.
We defined oligomenorrhea as having fewer than eight menstrual cycles per year, the absence of three to six consecutive menstrual cycles per year, or intermenstrual intervals ≥35 days. Clinical hyperandrogenism was defined as the presence of acne, androgenic alopecia, or hirsutism (modified Ferriman and Gallwey score ≥6). Biochemical hyperandrogenism was defined as serum total testosterone >2.8 nmol/L. Patients were considered to have polycystic ovaries if at least one ovary with 12 or more follicles measuring 2 to 9 mm in diameter was detected on ultrasonography (ALOKA ProSound SSD-3500, Hitachi, Tokyo, Japan) using a 7.5-MHz transvaginal probe.
Measurements
Age at menarche
Age at menarche was defined as the age at the first menstrual bleeding. This information was obtained by patient recall based on an open-ended survey question: “At what age did you have your first menstrual period?” Responses were recorded in full years. Early age at menarche was defined as <12 years old; late age of menarche was defined as ≥16 years old.
Anthropometry and blood pressure
The height and weight of each subject were measured. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in metres. Based on the Asian-specific classification for BMI status, BMI values were categorised as underweight (<18.5 kg/m2), normal (18.5 to 22.9 kg/m2), overweight (23.0 to 24.9 kg/m2), and obese (≥25 kg/m2). Waist and hip circumference were measured at the level of the umbilicus and symphysis pubis, respectively. Waist circumference (WC) was taken at the end of a normal expiration. Abdominal obesity was defined as a WC ≥80 cm and a waist-to-hip ratio (WHR) of 0.80 for Asian women. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in the sitting position after a 5-minute rest.
Biochemical assays
Venous blood samples were collected in the morning after an overnight fast on the second or third day of the patient's spontaneous or progesterone-induced menstrual cycle. Each patient underwent a 2-hour oral glucose tolerance test (OGTT) following 75 g of glucose intake. Fasting glucose, OGTT, and lipid panel analyses including total cholesterol, triglycerides (TGs), HDL-C, and low density lipoprotein cholesterol (LDL-C) were performed using Roche/Hitachi Cobas C systems (Module COBAS 4000/6000, Roche Diagnostics, Indianapolis, IN, USA). Anti-Müllerian hormone (AMH), luteinising hormone (LH), follicle-stimulating hormone (FSH), oestradiol, total testosterone, and prolactin levels were assessed by electrochemiluminescence using Elecsys and Cobas E immunoassay analysers (Cobas 4000/6000). All measurements were performed at the Hue University Hospital laboratory following the manufacturer's instructions.
Definition of variables and outcomes
We defined MS using the NHLBI/AHA ATP III 2005 guidelines [8]. A diagnosis of MS was made when ≥3 of the following were present: (1) WC ≥80 cm, (2) TG ≥1.7 mmol/L, (3) HDL-C <1.3 mmol/L, (4) blood pressure (BP) ≥130/85 mm Hg, and (5) fasting glucose ≥5.6 mmol/L.
Women with IRS were identified based on the modified ACE IRS 2003 criteria [7]. Individuals with ≥2 of the following were considered to have IRS: (1) TG ≥1.7 mmol/L, (2) HDL-C <1.3 mmol/L, (3) BP ≥130/85 mm Hg, and (4) evidence of IGT, indicated by impaired fasting glucose 5.6 to 6.9 mmol/L and a glucose level of 7.8 to 11.0 mmol/L after a 2-hour OGTT.
The primary outcome of this study was the prevalence of MS and IRS, and the clustering of their components, in Vietnamese women with PCOS attending our infertility clinic. The secondary outcome was to identify risk factors that predicted the occurrence of MS and IRS in these women.
Statistical analysis
To estimate the sample size (n) required for this study, we used the equation n=Zα/22×P×(1−P)/Δ2, where α=0.05, Δ=0.05, and Zα/2=1.96. The expected prevalence, P, for MS and IRS was 18.2% and 14.2%, respectively [10]. The minimum sample size required to estimate the prevalence of MS in women with PCOS attending our infertility clinic was 229. At least 187 women with PCOS were required to estimate IRS prevalence. Descriptive statistics were used to determine the characteristics of the study population. Continuous variables between groups were compared using the independent-samples t test or the Mann-Whitney U test. To compare categorical variables, we used the chi-square test or the Fisher exact test, as appropriate. Multivariate logistic regression analysis was performed to test the associations of anthropometric and biochemical variables with MS and IRS. Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs) or 2-sided P values. A P<0.05 was considered to indicate statistical significance. Receiver operating characteristic (ROC) curves were generated for BMI, WC, and WHR. All analyses were performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA).
RESULTS
From June 2016 to November 2017, a total of 441 women who fulfilled the Rotterdam diagnostic criteria for PCOS were enrolled in this study. Complete clinical and biochemical measurements were available for 318 women. The age of these women ranged from 19 to 42 years. Those with missing data (123 women) were excluded from our analyses.
Characteristics of PCOS women with and without MS and IRS
The characteristics of 318 infertile Vietnamese women with PCOS are shown in Table 1. The mean age was 28.3±3.87 years and mean BMI was 21.0±2.88 kg/m2. Their mean age at menarche was 14.24±1.35 years. Subjects with MS and IRS were older and more obese than those without MS and IRS. Compared with the women who did not meet the criteria for MS, those with MS had a significantly higher mean SBP, but not a significantly higher DBP. In contrast, both SBP and DBP were higher in women with IRS than in those without IRS. As expected, we found greater abnormalities in glucose and lipid metabolism among women with MS and IRS than in those without. These included higher mean TG and LDL-C levels, and lower mean HDL-C concentrations. Total cholesterol levels did not differ between each group. In general, women with MS and IRS had higher fasting and 2-hour postprandial glucose concentrations.
Table 1. Characteristics of 318 PCOS Women with and without Metabolic Syndrome and Insulin Resistance Syndrome.
| Characteristic | Total (n=318) | MS | P valuea | IRS | P valuea | ||
|---|---|---|---|---|---|---|---|
| PCOS with MS (n=33) | PCOS without MS (n=285) | PCOS with IRS (n=86) | PCOS without IRS (n=232) | ||||
| Age, yr | 28.27±3.87 | 29.97±3.37 | 28.08±3.88 | 0.01 | 29.30±4.18 | 27.89±3.68 | <0.01 |
| Age at menarche, yr | 14.24±1.35 | 14.09 ±1.47 | 14.25±1.34 | 0.51 | 14.08±1.36 | 14.29±1.35 | 0.22 |
| Anthropometry | |||||||
| BMI, kg/m2 | 21.04±2.88 | 24.41±3.42 | 20.65±2.55 | <0.01 | 22.76±3.58 | 20.40±2.28 | <0.01 |
| WC, cm | 73.64±7.85 | 84.33±8.84 | 72.41±6.72 | <0.01 | 78.41±8.98 | 71.88±6.57 | <0.01 |
| WHR | 0.83±0.06 | 0.88±0.06 | 0.83±0.06 | <0.01 | 0.85±0.06 | 0.82±0.06 | <0.01 |
| Blood pressure | |||||||
| SBP, mm Hg | 108.81±11.97 | 114.39±15.19 | 108.16±11.39 | <0.01 | 112.38±14.67 | 107.48±10.53 | <0.01 |
| DBP, mm Hg | 68.13±7.61 | 69.55±9.05 | 67.96±7.43 | 0.26 | 69.83±8.52 | 67.50±7.16 | 0.02 |
| Hormonal profile | |||||||
| AMH, pmol/L | 59.65±36.34 | 56.70±36.52 | 59.99±36.36 | 0.62 | 51.24±34.66 | 62.77±36.52 | 0.01 |
| LH, IU/L | 10.97±6.43 | 9.04±5.46 | 11.19±6.51 | 0.07 | 9.55±5.13 | 11.50±6.79 | 0.02 |
| FSH, IU/L | 5.44±1.67 | 4.91±1.67 | 5.50±1.67 | 0.05 | 5.08±1.79 | 5.57±1.61 | 0.02 |
| LH/FSH | 2.19±1.54 | 1.92±1.07 | 2.22±1.58 | 0.29 | 2.05±1.19 | 2.23±1.65 | 0.36 |
| Oestradiol, pmol/L | 169.30±104.19 | 129.70±49.10 | 173.89±107.90 | 0.02 | 152.52±99.92 | 175.52±105.27 | 0.08 |
| Testosterone, nmol/L | 1.26±0.70 | 1.26±0.65 | 1.26±0.71 | 0.95 | 1.32±0.73 | 1.24±0.69 | 0.35 |
| Prolactin, ng/mL | 17.97±33.82 | 12.86±8.34 | 18.56±35.57 | 0.36 | 22.08±54.95 | 16.44±21.21 | 0.19 |
| Lipid profile | |||||||
| Total cholesterol, mmol/L | 4.77±2.35 | 5.32±0.92 | 4.70±2.46 | 0.15 | 4.90±0.87 | 4.72±2.70 | 0.55 |
| Triglycerides, mmol/L | 1.36±0.87 | 2.59±1.35 | 1.22±0.67 | <0.01 | 2.11±1.22 | 1.08±0.44 | <0.01 |
| LDL-C, mmol/L | 2.93±0.77 | 3.38±0.83 | 2.87±0.75 | <0.01 | 3.17±0.76 | 2.84±0.76 | <0.01 |
| HDL-C, mmol/L | 1.43±0.56 | 1.26±1.05 | 1.45±0.47 | 0.07 | 1.20±0.74 | 1.51±0.45 | <0.01 |
| Glucose metabolism | |||||||
| Fasting glucose, mmol/L | 5.11±0.52 | 5.69±0.63 | 5.04±0.46 | <0.01 | 5.33±0.60 | 5.03±0.46 | <0.01 |
| 2-Hour OGTT, mmol/L | 6.82±1.78 | 7.94±2.43 | 6.69±1.65 | <0.01 | 8.10±2.09 | 6.35±1.39 | <0.01 |
Values are expressed as mean±SD.
WHR, waist-to-hip ratio; SBP, systolic blood pressure; DPB, diastolic blood pressure; AMH, anti-Müllerian hormone; LH, luteinising hormone; FSH, follicle-stimulating hormone; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; OGTT, oral glucose tolerance test.
aComparison was performed between PCOS women with and without MS or IRS using the independent-samples t test and the chi-square test.
Prevalence of MS, IRS, and individual metabolic abnormalities in PCOS women
Tables 2, 3 show the frequency of individual metabolic abnormalities. The overall prevalence of MS and IRS was 10.4% and 27.0%, respectively. In women with MS (Table 2), the most common metabolic abnormality was low HDL-C (93.9%), followed by abdominal obesity (84.8%), hypertriglyceridaemia (78.8%), elevated fasting glucose (54.5%), and hypertension (18.2%). In women with IRS (Table 3), the most frequent metabolic abnormality was low HDL-C (87.2%), followed by hypertriglyceridaemia and IGT (59.3% for each). The least common metabolic abnormality was hypertension (12.8%). More than half (57.5%) of the women who did not fulfil the criteria for MS already had one or two metabolic abnormalities. Similarly, up to 46.6% of women who did not fulfil the criteria for IRS already had one metabolic abnormality. IGT was present in 59.3% and 10.3% of the women with and without IRS, respectively.
Table 2. Prevalence of Metabolic Abnormalitiesa in PCOS Women with and without MS.
| Metabolic abnormalities | Total (n=318) | PCOS with MS (n=33) | PCOS without MS (n=285) | P valueb |
|---|---|---|---|---|
| WC ≥80 cm | 66 (20.8) | 28 (84.8) | 38 (13.3) | <0.01 |
| TG ≥1.7 mmol/L | 65 (20.4) | 26 (78.8) | 39 (13.7) | <0.01 |
| HDL-C <1.29 mmol/L | 137 (43.1) | 31 (93.9) | 106 (37.2) | <0.01 |
| BP ≥130/85 mm Hg | 18 (5.7) | 6 (18.2) | 12 (4.2) | <0.01 |
| Fasting glucose ≥5.6 mmol/L | 43 (13.5) | 18 (54.5) | 25 (8.8) | <0.01 |
| Presence of metabolic abnormalities | ||||
| 0 | 121 (38.1) | NA | 121 (42.5) | |
| 1 | 108 (34.0) | NA | 108 (37.9) | |
| 2 | 56 (17.6) | NA | 56 (19.6) | |
| 3 | 23 (7.2) | 23 (69.7) | NA | |
| 4 | 10 (3.1) | 10 (30.3) | NA | |
| 5 | 0 | 0 | NA |
Values are expressed as number (%).
PCOS, polycystic ovary syndrome; MS, metabolic syndrome; WC, waist circumference; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; BP, blood pressure; NA, not applicable.
aBased on the Adult Treatment Panel III 2005 criteria [17]; bComparison was performed between PCOS women with and without MS using the chi-square test with asymptotic significance (2-sided) for continuity correction.
Table 3. Prevalence of Metabolic Abnormalitiesa in PCOS Women with and without IRS.
| Component | Total (n=318) | PCOS with IRS (n=86) | PCOS without IRS (n=232) | P valueb |
|---|---|---|---|---|
| TG ≥1.7 mmol/L | 65 (20.4) | 51 (59.3) | 14 (6.0) | <0.01 |
| HDL-C <1.29 mmol/L | 137 (43.1) | 75 (87.2) | 62 (26.7) | <0.01 |
| BP ≥130/85 mm Hg | 18 (5.7) | 11 (12.8) | 7 (3.0) | <0.01 |
| IFGc/IGTd | 75 (23.6) | 51 (59.3) | 24 (10.3) | <0.01 |
| Presence of metabolic abnormalities | ||||
| 0 | 124 (39.0) | NA | 124 (53.4) | |
| 1 | 108 (34.0) | NA | 108 (46.6) | |
| 2 | 66 (20.8) | 66 (76.7) | NA | |
| 3 | 20 (6.3) | 20 (23.3) | NA | |
| 4 | 0 | 0 | NA |
Values are expressed as number (%).
PCOS, polycystic ovary syndrome; IRS, insulin resistance syndrome; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; BP, blood pressure; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NA, not applicable.
aBased on the American College of Endocrinology (ACE) 2003 criteria [14]. Levels modified from the updated Adult Treatment Panel III 2005 guidelines [17]; bComparison was performed between PCOS women with and without IRS using the chi-square test with asymptotic significance (2-sided) for continuity correction; cFasting glucose 5.6 to 6.9 mmol/L. The original ACE 2003 definition identified impaired fasting glucose as 6.1 to 6.9 mmol/L (110 to 125 mg/dL) [14]. This was modified in 2004 by the American Diabetes Association (ADA) from 6.1 to 5.6 mmol/L. This new the cut-off for defining IFG is consistent with the latest ADA 2017 guidelines [66, 67]; d2-Hour oral glucose tolerance test 7.8 to 11.0 mmol/L.
Predictors of MS and IRS in PCOS women using logistic regression analysis
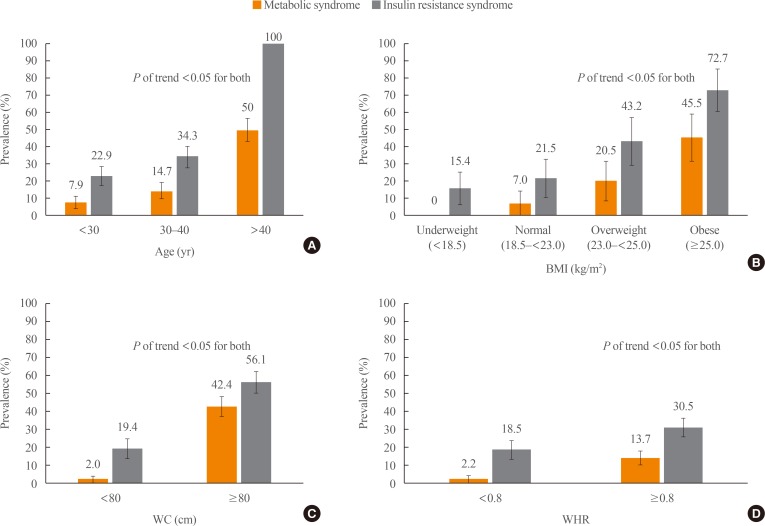
Using multivariate logistic regression (Table 4), we identified age and obesity as independent predictors of MS and IRS. We found that women aged 30 to 40 years had a 2-fold higher risk for MS than younger women <30 years old (OR, 2.00; 95% CI, 0.95 to 4.18), but this difference was not significant (P=0.07). The risk for IRS was comparable to the risk for MS in women aged 30 to 40 (OR, 1.86; 95% CI, 1.11 to 3.11). Only two women in our cohort were older than 40 years of age. Of those two women, only one fulfilled the criteria for MS, while both were found to have IRS.
Table 4. Logistic Regression Analysis for Predictors of MS and IRS in Women with PCOS.
| Variable | MS (n=33) | P value | IRS (n=86) | P value | ||
|---|---|---|---|---|---|---|
| MS/total (%) | OR (95% CI) | IRS/total (%) | OR (95% CI) | |||
| Age, yr | ||||||
| <30 | 17/214 (7.9) | 1 | 49/214 (22.9) | 1 | ||
| 30–40 | 15/102 (14.7) | 2.00 (0.95–4.18) | 0.07 | 35/102 (34.3) | 1.76 (1.05–2.95) | 0.033 |
| >40 | 1/2 (50) | 11.59 (0.69–193.59) | 0.09 | 2/2 (100) | NA | NA |
| Age at menarche, yr | ||||||
| ≥16 | 5/49 (10.2) | 0.98 (0.36–2.67) | 1.00 | 13/49 (26.5) | 0.97 (0.49–1.93) | 1.00 |
| 12–16 | 28/269 (10.4) | 1 | 73/269 (27.1) | 1 | ||
| <12 | 0 (0.0) | NA | NA | 0 (0.0) | NA | NA |
| BMI, kg/m2 | ||||||
| <18.5 (underweight) | 0/52 (0.0) | NA | NA | 8/52 (15.4) | 0.66 (029–1.52) | 0.33 |
| 18.5–22.9 (normal) | 14/200 (7.0) | 1 | - | 43/200 (21.5) | 1 | - |
| 23.0–24.9 (overweight) | 9/44 (20.5) | 3.42 (1.37–8.50) | <0.01 | 19/44 (43.2) | 2.78 (1.40–5.51) | <0.01 |
| ≥25 (obese) | 10/22 (45.5) | 11.07 (4.07–30.08) | <0.01 | 16/22 (72.7) | 9.74 (3.59–26.39) | <0.01 |
| WC, cm | ||||||
| ≥80 | 28/66 (42.4) | 36.4 (13.24–100.04) | <0.01 | 37/66 (56.1) | 5.29 (2.97–9.42) | <0.01 |
| <80 | 5/252 (2.0) | 1 | 49/252 (19.4) | 1 | ||
| WHR | ||||||
| ≥0.80 | 31/226 (13.7) | 7.15 (1.68–30.55) | <0.01 | 69/226 (30.5) | 1.94 (1.07–3.53) | 0.03 |
| <0.80 | 2/92 (2.2) | 1 | 17/92 (18.5) | 1 | ||
| Blood pressure, mm Hg | ||||||
| ≥130/85 | 6/18 (33.3) | 5.06 (1.76–14.55) | <0.01 | 11/18 (41.5) | 4.71 (1.76–12.60) | <0.01 |
| <130/85 | 27/300 (9.0) | 1 | 75/300 (21.0) | 1 | ||
| AMH, pmol/L | ||||||
| ≥36.8 | 12/94 (12.8) | 1.42 (0.67–3.01) | 0.37 | 39/94 (41.5) | 2.67 (1.59–4.50) | <0.01 |
| <36.8 | 21/224 (9.4) | 1 | 47/224 (21.0) | 1 | ||
| LH, IU/L | ||||||
| >10 | 10/145 (6.9) | 0.48 (0.22–1.05) | 0.06 | 34/145 (23.4) | 0.71 (0.43–1.18) | 0.19 |
| ≤10 | 23/173 (13.3) | 1 | 52/173 (30.1) | 1 | ||
| LH/FSH | ||||||
| >2 | 10/134 (7.5) | 0.69 (0.41–1.19) | 0.19 | 36/134 (26.9) | 0.98 (0.59–1.61) | 0.93 |
| ≤2 | 23/184 (12.0) | 1 | 50/183 (27.3) | 1 | ||
| Testosterone, nmol/L | ||||||
| >2.8 | 0/11 (0) | NA | 0.25 | 4/11 (36.4) | 1.57 (0.45–5.50) | 0.48 |
| ≤2.8 | 33/307 (10.7) | NA | 82/307 (26.7) | 1 | ||
| Triglycerides, mmol/L | ||||||
| ≥1.7 | 26/65 (40.0) | 23.43 (9.52–57.65) | <0.01 | 51/65 (78.5) | 22.69 (11.37–45.27) | <0.01 |
| <1.7 | 7/253 (2.8) | 1 | 35/253 (13.8) | 1 | ||
| Cholesterol, mmol/L | ||||||
| ≥5.2 | 16/66 (24.2) | 4.42 (2.09–9.34) | <0.01 | 26/66 (39.4) | 2.08 (1.17–3.69) | 0.01 |
| <5.2 | 17/252 (6.7) | 1 | 60/252 (23.8) | 1 | ||
| ≥3.3 | 18/89 (20.2) | 3.62 (1.73–7.55) | 38/89 (42.7) | 2.81 (1.66–4.76) | <0.01 | |
| <3.3 | 15/229 (6.6) | 1 | <0.01 | 48/229 (21.0) | 1 | |
| HDL-C, mmol/L | ||||||
| <1.3 | 31/137 (22.6) | 26.18 (6.14–111.58) | 75/137 (54.7) | 18.70 (9.32–37.51) | <0.01 | |
| ≥1.3 | 2/181 (1.1) | 1 | <0.01 | 11/181 (6.1) | 1 | |
| Fasting glucose, mmol/L | ||||||
| ≥5.6 | 18/43 (41.9) | 12.48 (5.62–27.74) | 21/43 (48.8) | 3.08 (1.59–5.96) | <0.01 | |
| <5.6 | 15/275 (5.5) | 1 | <0.01 | 65/275 (23.6) | 1 | |
| 2-Hour OGTT, mmol/L | ||||||
| 7.8–11.0 | 13/75 (17.3) | 2.24 (1.10–4.96) | 51/75 (68.0) | 12.63 (6.91–23.08) | <0.01 | |
| <7.8 | 20/243 (8.2) | 1 | 0.02 | 35/243 (14.4) | 1 | |
MS, metabolic syndrome; IRS, insulin resistance syndrome; PCOS, polycystic ovary syndrome; OR, odds ratio; CI, confidence interval; NA, not applicable; BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; AMH, anti-Müllerian hormone; LH, luteinising hormone; FSH, follicle-stimulating hormone; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; OGTT, oral glucose tolerance test.
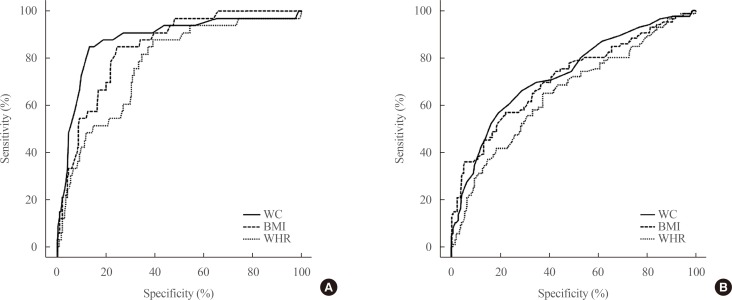
Obesity was an important risk factor for MS in women with PCOS, whether measured by BMI (OR, 11.07; 95% CI, 4.07 to 30.08), WC (OR, 36.4; 95% CI, 13.24 to 100.4), or WHR (OR, 7.15; 95% CI, 1.68 to 30.55). This was also true for IRS when obese women were identified based on BMI (OR, 2.78; 95% CI, 1.40 to 5.51), WC (OR, 5.29; 95% CI, 2.97 to 9.42), or WHR (OR, 1.94; 95% CI, 1.07 to 5.35). Being underweight (BMI <18.5 kg/m2) appeared to have a protective effect against IRS, but this trend was not statistically significant (OR, 0.66; 95% CI, 0.29 to 1.52; P=0.33). The area under the ROC curves for BMI and WC were greater than that for WHR (Fig. 1). Pairwise comparison showed no significant difference between the areas under the ROC curve for BMI and WC in predicting MS and IRS. These characteristics were demonstrated more in Fig. 2.
Fig. 1. Receiver operating characteristic curves of body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHR) for predicting (A) metabolic syndrome and (B) insulin resistance syndrome.
Fig. 2. 2. Prevalence of metabolic syndrome and insulin resistance syndrome stratified by (A) age, (B) body mass index (BMI), (C) waist circumference (WC), and (D) waist-to-hip ratio (WHR).
In terms of the lipid profile, dyslipidaemia (hypertriglyceridaemia and low HDL-C) was the strongest predictor for MS and IRS, in contrast to high total cholesterol or LDL-C levels. Abnormal fasting glucose (≥5.6 mmol/L) was a stronger predictor for MS (OR, 12.48; 95% CI, 5.62 to 27.74) than for IRS (OR, 3.08; 95% CI, 1.59 to 5.96). On the contrary, IGT was associated with a higher risk for IRS (OR, 12.63; 95% CI, 6.91 to 23.08) than for MS (OR, 2.24; 95% CI, 1.10 to 4.96). High AMH levels (≥36.8 pmol/L) significantly increased the risk for IRS (OR, 2.67; 95% CI, 1.59 to 4.50) but not MS (OR, 1.42; 95% CI, 0.67 to 3.01; P=0.37). LH, FSH/LH, and total testosterone were not associated with the occurrence of MS or IRS. No women in our cohort had early menarche (age <12 years). Late menarche (age ≥16 years) was not associated with increased risks for MS and IRS.
DISCUSSION
MS and IRS prevalence
Based on the NHLBI/AHA ATP III 2005 and ACE IRS 2003 criteria, the prevalence of MS and IRS in Vietnamese women with PCOS was 10.4% and 27.0%, respectively. The prevalence of MS in this study was similar to that previously reported in Turkish women (10.3%) [11]. In contrast, the prevalence rates of MS in other Western and Asian countries were much higher: 26.0% in the USA [12], 37.5% in India [13], 24.9% in Hong Kong [14], and 21.2% in Thailand [15]. These differences may be due to genetic and environmental factors such as diet, lifestyle, and the effects of ethnicity on disease phenotype.
In the absence of epidemiological studies on IRS in PCOS women, we compared IGT data from studies that performed OGTT as an index of insulin resistance. The overall prevalence of IGT in our study was 23.6%. This figure is comparable to that reported in Hispanic (22.1%) [16] and Chinese (20.5%) [17] women with PCOS. The prevalence of IGT in women with PCOS was substantially higher in the USA (35.0%) [3] and India (34.8%) [18], but lower in Australia (15.6%) [19] and the Mediterranean region (15.7%) [20]. Interestingly, IGT was detected in 13.6% of Thai women with PCOS living in Bangkok [21], compared with 31.4% in Chiang Mai [22], demonstrating that insulin sensitivity can vary widely in populations within the same country.
Predictors of MS and IRS using multivariate logistic regression analysis
Our study showed that age and obesity were significantly associated with an increased risk of MS and IRS, although age only appeared to be related to IRS, not MS. The ACE Position Statement on IRS advises that all individuals aged >40 years should be evaluated for IRS. A 2-hour OGTT is recommended when high-risk individuals (including women with PCOS and of non-Caucasian ethnicity) do not meet the other criteria for IRS [7]. Due to the small sample size of women over the age of 40 in our study, we are unable to comment about whether routine OGTT would be useful in this age group.
We found BMI, WC, and WHR to be independent predictors for MS and IRS. Our results showed that BMI and WC were more strongly associated with MS and IRS than WHR, and BMI was as sensitive as WC for predicting MS and IRS. Population-based studies have reported inconsistent findings regarding the relative usefulness of different obesity indices in identifying individuals at increased risk for developing insulin resistance, IGT, T2DM, and other CVD risk factors. WC is a more accurate indicator of abdominal visceral fat than BMI [23]. However, it is not uniformly used in clinical practice and may be susceptible to measurement errors [24]. Statistically, BMI has a strong positive correlation with WC, with a correlation coefficient of approximately 0.8 [23]. As such, BMI may be considered a simple and appropriate surrogate for assessing CVD risk.
Women with PCOS have significantly higher AMH levels than those without PCOS [25]. We found a 42% increase in the odds for MS when AMH levels exceeded 36.8 pmol/L, although this was not statistically significant. However, we found elevated AMH levels to be a significant determinant of IRS. Studies examining the role of AMH in cardiovascular risk have produced mixed results. One study reported an inverse relationship between AMH and MS, with an 11% increased risk for MS per unit reduction in AMH [26]. Another study showed a significant positive correlation between insulin resistance and AMH levels [27] while two others reported no relationship between AMH levels and insulin resistance [28,29].
Strengths and limitations
The strength of our study lies in its prospective, cross-sectional design and consecutive sampling method. In addition, this is the first epidemiological study to describe the metabolic profile of women with PCOS in Vietnam. To date, local epidemiological studies for MS have only included female adolescents [30] and middle-aged women (aged 40 to 64 years) from the general population [31], or women with rheumatoid arthritis [32]. Therefore, our study provides valuable information on the prevalence and distribution of the metabolic components of MS and IRS in reproductive-aged women with PCOS in this country.
The main limitation of our study was the absence of an age- and BMI-matched non-PCOS control group. We also did not adjust for confounding factors such as smoking, alcohol intake, or family history of T2DM. In addition, the possibility of selection bias cannot be ruled out. This was a single-centre study with a small sample size. Only women with PCOS who presented to our fertility clinic were assessed. Therefore, the findings from this study should not be generalized to women with PCOS without fertility problems or post-menopausal women.
Clinical implications of screening for MS versus IRS
MS and IRS should not be considered synonymous, as several important distinctions exist between these conditions. Firstly, the ATP III criteria for MS lack sufficient sensitivity for identifying insulin resistant individuals [33]. Secondly, obesity is seen as a risk factor rather than as a criterion for IRS [7]. Our results indicate that approximately 27.3% of obese women did not have IRS, and 81.4% of women with IRS were non-obese. These findings support the view that insulin resistance is independent of obesity in women with PCOS [34]. Last but not least, lean women with PCOS can have normal fasting glucose levels despite being insulin resistant. Compensatory hyperinsulinemia becomes evident only in response to an oral glucose challenge [35]. The 2-hour OGTT can be a valuable tool for detecting insulin resistance in these women. Within the entire cohort of our study, the MS criterion for fasting glucose identified 43 women with dysglycaemia compared with 75 women after an oral glucose challenge. Cost-benefit analyses are needed to determine if the additional effort needed for OGTT justifies its routine use in Asian women with PCOS, particularly in countries where healthcare resources are limited, such as Vietnam.
In conclusion, the prevalence of MS among women with PCOS in Vietnam was found to be low compared to other countries. The proportion of women who had IRS was 2- to 3-fold greater than that of women with MS. Physicians need to be aware that the complications of PCOS extend beyond reproductive implications. Given the high prevalence of IRS in non-obese Vietnamese women with PCOS, this specific group of women should receive further metabolic evaluations. Early recognition of IRS may provide the advantage of enabling intensified interventions to slow or halt the progression to T2DM and CVD. We identified WC and BMI as the strongest anthropometric predictors for MS and IRS, respectively. Another independent predictor for MS and IRS was age ≥30 years; AMH was the only biochemical predictor for IRS. The presence of one or more of these risk factors may be used as an indication to assess a patient for the co-existing metabolic abnormalities that comprise IRS. Preventive measures including patient education, lifestyle modification, and timely initiation of pharmacological treatment in these women should be considered an integral part of fertility care. Long-term prospective studies are needed to determine whether intensified interventions targeting metabolic risk factors will translate into clinically meaningful reductions of T2DM and CVD in women with PCOS.
ACKNOWLEDGMENTS
We thank Dr. Charity Yii Tien Jen for her editorial support in the development of this manuscript.
Footnotes
CONFLICTS OF INTEREST: This work was supported by a research grant from Merck KGaA, Darmstadt, Germany (grant number MERCK-CORP-COMPL-POL16-RT02-v01). The grantor had no influence in the content of the publication nor involvement in the study design, data collection, analysis, or reporting.
- Conception or design: M.T.L., V.Q.H.N., Q,V.T., N.T.C.
- Acquisition, analysis, or interpretation of data: M.T.L., D.D.L., V.N.S.L.
- Drafting the work or revising: M.T.L., V.Q.H.N., D.D.L., V.N.S.L., N.T.C.
- Final approval of the manuscript: M.T.L., V.Q.H.N., Q,V.T., D.D.L., V.N.S.L., N.T.C.
References
- 1.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 2.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 4.Rocha MP, Marcondes JA, Barcellos CR, Hayashida SA, Curi DD, da Fonseca AM, et al. Dyslipidemia in women with polycystic ovary syndrome: incidence, pattern and predictors. Gynecol Endocrinol. 2011;27:814–819. doi: 10.3109/09513590.2010.508852. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30:496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28:777–784. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 7.Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 9.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, et al. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–376. [PubMed] [Google Scholar]
- 10.Li R, Yu G, Yang D, Li S, Lu S, Wu X, et al. Prevalence and predictors of metabolic abnormalities in Chinese women with PCOS: a cross- sectional study. BMC Endocr Disord. 2014;14:76. doi: 10.1186/1472-6823-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27:3067–3073. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 12.Rossi B, Sukalich S, Droz J, Griffin A, Cook S, Blumkin A, et al. Prevalence of metabolic syndrome and related characteristics in obese adolescents with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4780–4786. doi: 10.1210/jc.2008-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandrelle K, Kamath MS, Bondu DJ, Chandy A, Aleyamma T, George K. Prevalence of metabolic syndrome in women with polycystic ovary syndrome attending an infertility clinic in a tertiary care hospital in south India. J Hum Reprod Sci. 2012;5:26–31. doi: 10.4103/0974-1208.97791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung LP, Ma RC, Lam PM, Lok IH, Haines CJ, So WY, et al. Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1431–1438. doi: 10.1093/humrep/den090. [DOI] [PubMed] [Google Scholar]
- 15.Indhavivadhana S, Wongwananuruk T, Rattanachaiyanont M, Techatraisak K, Leerasiri P, Tanmahasamut P, et al. Prevalence of metabolic syndrome in reproductive-aged polycystic ovary syndrome Thai women. J Med Assoc Thai. 2010;93:653–660. [PubMed] [Google Scholar]
- 16.Reyes-Munoz E, Ortega-Gonzalez C, Martinez-Cruz N, Arce-Sanchez L, Estrada-Gutierrez G, Moran C, et al. Association of obesity and overweight with the prevalence of insulin resistance, pre-diabetes and clinical-biochemical characteristics among infertile Mexican women with polycystic ovary syndrome: a cross-sectional study. BMJ Open. 2016;6:e012107. doi: 10.1136/bmjopen-2016-012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Yang D, Li L, Feng S, Wang L. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum Reprod. 2006;21:2027–2032. doi: 10.1093/humrep/del142. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Majumdar A. Prevalence of metabolic syndrome in relation to body mass index and polycystic ovarian syndrome in Indian women. J Hum Reprod Sci. 2015;8:202–208. doi: 10.4103/0974-1208.170394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabadghao P, Roberts BJ, Wang J, Davies MJ, Norman RJ. Glucose tolerance abnormalities in Australian women with polycystic ovary syndrome. Med J Aust. 2007;187:328–331. doi: 10.5694/j.1326-5377.2007.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 20.Gambineri A, Pelusi C, Manicardi E, Vicennati V, Cacciari M, Morselli-Labate AM, et al. Glucose intolerance in a large cohort of Mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes. 2004;53:2353–2358. doi: 10.2337/diabetes.53.9.2353. [DOI] [PubMed] [Google Scholar]
- 21.Wongwananuruk T, Rattanachaiyanont M, Indhavivadhana S, Leerasiri P, Techatraisak K, Tanmahasamut P, et al. Prevalence and clinical predictors of insulin resistance in reproductive-aged thai women with polycystic ovary syndrome. Int J Endocrinol. 2012;2012:529184. doi: 10.1155/2012/529184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantasri T, Vutyavanich T, Sreshthaputra O, Srisupundit K, Piromlertamorn W. Metabolic syndrome and insulin resistance in Thai women with polycystic ovary syndrome. J Med Assoc Thai. 2010;93:406–412. [PubMed] [Google Scholar]
- 23.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 24.Meigs JB, Wilson PW, Nathan DM, D'Agostino RB, Sr, Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 25.Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 26.Feldman RA, O'Neill K, Butts SF, Dokras A. Antimullerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Fertil Steril. 2017;107:276–281. doi: 10.1016/j.fertnstert.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Skalba P, Cygal A, Madej P, Dabkowska-Huc A, Sikora J, Martirosian G, et al. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2011;158:254–259. doi: 10.1016/j.ejogrb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, Shi Y, Cui L, Han T, Gao X, Chen ZJ. Age-specific serum antimüllerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril. 2014;102:230–236. doi: 10.1016/j.fertnstert.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Cassar S, Teede HJ, Moran LJ, Joham AE, Harrison CL, Strauss BJ, et al. Polycystic ovary syndrome and anti-Mullerian hormone: role of insulin resistance, androgens, obesity and gonadotrophins. Clin Endocrinol (Oxf) 2014;81:899–906. doi: 10.1111/cen.12557. [DOI] [PubMed] [Google Scholar]
- 30.Hong TK, Trang NH, Dibley MJ. Prevalence of metabolic syndrome and factor analysis of cardiovascular risk clustering among adolescents in Ho Chi Minh City, Vietnam. Prev Med. 2012;55:409–411. doi: 10.1016/j.ypmed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Binh TQ, Phuong PT, Nhung BT, Tung do D. Metabolic syndrome among a middle-aged population in the Red River Delta region of Vietnam. BMC Endocr Disord. 2014;14:77. doi: 10.1186/1472-6823-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dao HH, Do QT, Sakamoto J. Increased frequency of metabolic syndrome among Vietnamese women with early rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2010;12:R218. doi: 10.1186/ar3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheal KL, Abbasi F, Lamendola C, McLaughlin T, Reaven GM, Ford ES. Relationship to insulin resistance of the Adult Treatment Panel III diagnostic criteria for identification of the metabolic syndrome. Diabetes. 2004;53:1195–1200. doi: 10.2337/diabetes.53.5.1195. [DOI] [PubMed] [Google Scholar]
- 34.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2004;27(Suppl 1):S15–S35. doi: 10.2337/diacare.27.2007.s15. [DOI] [PubMed] [Google Scholar]