Abstract
To visualize the tumor cavity after lumpectomy, the tumor cavity was coated with the liquid tissue marker sucrose acetate isobutyrate (SAIB) with its radiopaque electron dense SAIB analogue (x-SAIB) and assessed for radiotherapy planning. SAIB/x-SAIB enhanced the confidence for target structure definition. Tissue displacement after oncoplasty may be revealed by SAIB/x-SAIB.
1. Introduction
Adjuvant radiotherapy after breast conserving tumor resection can be reduced to partial breast irradiation (PBI) and shortened fractionation schedules for completely resected node-negative disease of limited size, and limited systemic risks [1], [2]. PBI has been shown to be adequate and may yield better cosmetic results [3]. In contrast to preoperative radiotherapy, when tumor can be visualized and target volumes defined easily [4], reproducible and standardized techniques to define the cavity and the CTV suitable for PBI are warranted [5]. In the case of target volume definition after lumpectomy, uncertainties may exceed uncertainties from positioning [6], [7]. Currently, surgical clips are standard to define the former tumor cavity and the target volume [8], [9]. To ascertain good target volume coverage, the margins of safety may expose a substantial amount of healthy breast tissue to ionizing radiation [10]. Precise target volume definition to curtail the CTV relies on the ability to visualize the borders of the resected tissue and the tumor cavity.
We have previously shown, that a radiopaque self-degradable hydrogel marker applied into the tumor cavity can visualize the lumpectomy cavity with high accuracy and reduces inter-observer variation in volume definition [11], and Struik et al. recently confirmed that target volume definition with on a hydrogel is more reliable than clips due to a low interobserver volume variability [12]. In the present series we investigated a novel liquid tissue maker that solidifies after application by means of diffusion of the dissolving carrier agent after application.
2. Materials and methods
2.1. Material
The product used for marking the tissue of interest was approved for use in humans and has been tested previously for non-small cell lung cancer in human patients [13]. The transparent, low-viscous homogenous solution consists of three components and is used as a medical device (BioXmark® by Nanovi, Copenhagen, DK). The solution is composed of sucrose acetate isobutyrate (SAIB), the electron dense SAIB analogue (x-SAIB) and ethanol (EtOH) in the overall ratio of 50:30:20 (w/w%). SAIB/x-SAIB has a low viscosity. Upon injection into soft tissue, EtOH diffuses out of the medical device over a period of 60–120 min causing an increase of the viscosity of the device forming a semi-solid implant (gel-like). SAIB and x-SAIB are both hydrophobic molecules, which self-associate and form a homogenous soft tissue marker once implanted into soft tissue. Once injected into soft tissue the component SAIB/x-SAIB stays in place for several months. SAIB/x-SAIB is visible by MRI and ultrasonography due to basic implant component SAIB, and by X-ray imaging due to the electron dense x-SAIB.
2.2. Preclinical assessment
In order to assess the ability of SAIB/x-SAIB to be visualized on a planning CT, we used a paper-surface phantom and applied various volumes (0.05–0,25 ml) as a film to subsequently verify the volume of the marker on CT.
2.3. Patients
After ethical and institutional approval and signing informed consent, eleven women with clinically node-negative breast cancer underwent breast conserving surgery. Four patients were treated with oncoplastic surgery. Patients were admitted for postoperative radiotherapy one month after surgery (range 49 to 75 days). Radiotherapy was initiated four to eight weeks after surgery. Therapy of patients was partial breast irradiation (PBI) using 38 Gy in 10 fractions delivered within 5 consecutive days in two patients, whole breast irradiation (WBI) with a boost was used for eight patients, and one patient was treated with WBI without any boost.
2.4. Contouring the tumor cavity
The tumor cavity was gated using a region of interest covering the tumor cavity on ARIA 11.0 (Varian Inc., Palo Alto, CA). To visualize the structure of interest, the thresholds were defined in the range of –124 (SDEV +/-4) to 222 (SDEV +/-2) H.U.
2.5. Marker application
In contrast to previous reports [14], [15], when the liquid marker was applied with a syringe, we placed the liquid marker with the fingertip to coat the surface of the tumor cavity after completion of hemostasis. A volume of 0.6 to 1.0 ml was used to paint the tumor cavity. After placement, surgical gloves were changed to proceed with surgery and closure according to good clinical practice.
2.6. Statistical analysis
The Wilcoxon rank–sum test was used for non-parametric comparison of the CTVs of differentially treated groups.
3. Results
3.1. Preclinical assessment
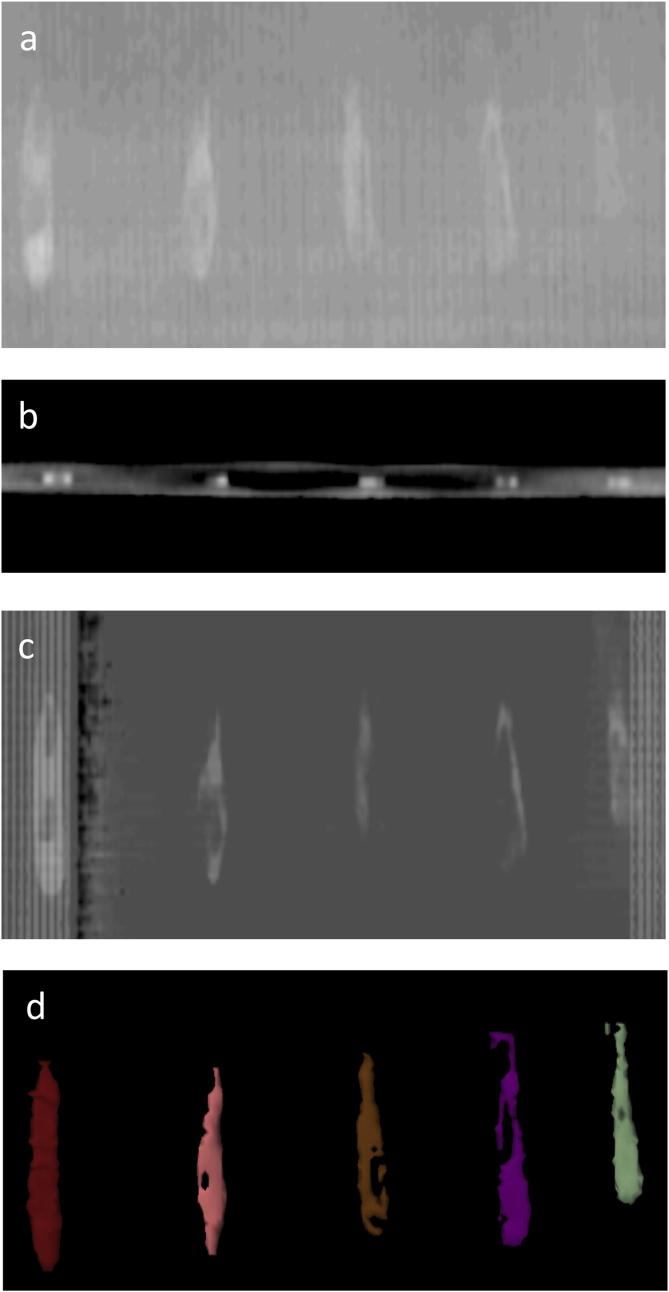
In order to ascertain the visibility of SAIB/x-SAIB when applied as a film on a surface, various amounts of SAIB/x-SAIB were applied as a film over a length of 5 cm on a plain surface with the tip of the finger. Fig. 1 shows that a film of SAIB/x-SAIB is visible on the planning CT an accessible to segmentation.
Fig. 1.
Assessment of visibility of SAIB/X-SAIB for radiotherapy planning on a phantom. Different amounts of SIAB/x-SIAB were applied (a) over a length of 5 cm on a paper carrier with the fingertip loaded with (from left to right) with 50 mikroL, 40 mikrolL, 30 mikroL, 20 mikroL or 10 mikroL. (a) shows a plain X-ray of the marker, (b) the axial imaging on a planning CT at a slice thickness of 3 mm, (c) a digitally reconstructed radiograph, and (d) segmentation of the liquid tissue marker.
3.2. Clinical assessment
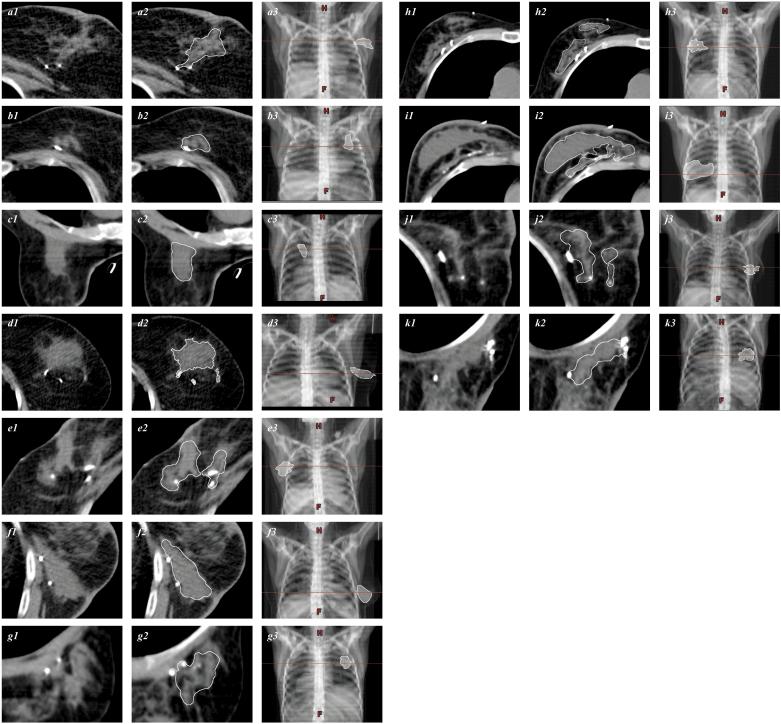
Eleven patients were evaluated. Volumes of 0,6 to 1,0 ml of SAIB/x-SAIB/EtOH was used intraoperatively to define the tumor cavity in proximity to the surgical clips, which served as controls being a part of the CTV (Fig. 3). The mean volume of the tumor cavity was 26,9 ml (SD ± 16,0; median 20,6 ml). Oncoplastic surgery was used in four of eleven patients (Fig. 2h–k). The mean volume of the tumor cavity after oncoplasty was 31,6 ml (SD ± 23,2 ml; median 20,7), compared to 25,4 ml (SD ± 13,0, median 21,1) after simple lumpectomy (p > 0,05). In one of four patients undergoing oncoplastic surgery, the cavity on the planning CT was distorted (Fig. 2i), and whole breast irradiation was chosen as treatment without any boost.
Fig. 3.
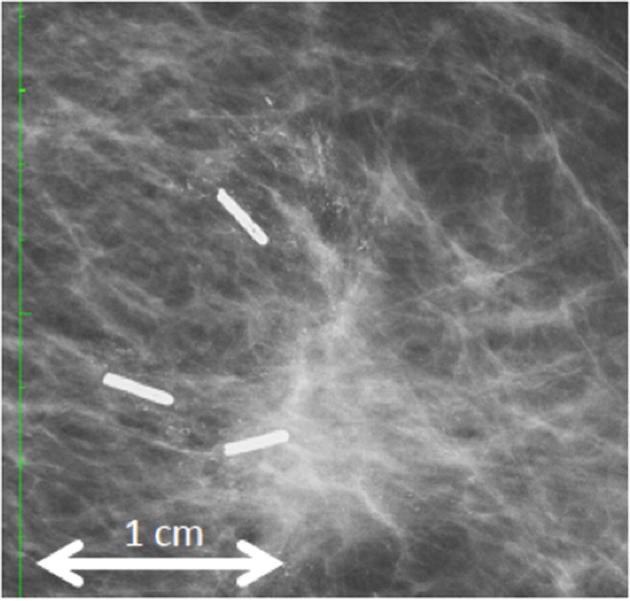
Follow-up X-ray imaging after tumorectomy. The green scale indicates 1 cm between ticks. One year after definitive surgery and adjuvant radiotherapy, a mammogram reveals granular residual radiopaque traces mimicking microcalcifications.
Fig. 2.
Contrast-enhanced visualization of the tumor cavity following lumpectomy with SIAB/x-SIAB. Scans shown are gated from (−132)–(−124) to (201)–(225) Hounsfield units. Patients underwent tumor excision and adjuvant EBRT (a to g), or tumor excision followed by oncoplastic surgery (h to k). Patients in c, g, and k were treated in prone position. Figures a1…k1 indicate the unprocessed imaging data. Manual segmentation target structure definition is shown in a2…k2 (white line). Figures a3…k3 show the digital reconstructed radiograph with the horizontal line passing trough the target structure (white) and the corresponding axial sections.
Manual post-processing of the target structure was necessary in all cases, because the density of the tumor cavity marked with SAIB/x-SAIB were frequently in the range of the structures defined by the ductal glands, and a sphere-like target volume could be only obtained after correcting for the mammary ducts.
3.3. Follow-up
Routine follow-up applied consisting of bi-annual mammography and ultrasound examination and clinical examination q3months. All patients showed unremarkable clinical evaluation. Fibrosis has not been evoked. Mammograms were available for nine of the patients one year after definitive therapy. In all but one patient (89%) micro-calcifications were reported by the radiologist at one year after radiotherapy (Fig. 3). After interdisciplinary assessment with the surgeon, none of the patient had to be reassessed for relapsing or recurrent disease. So far, after a follow-up of 18 to 21 months, all patients remain free of disease at the primary tumor site.
4. Discussion
Image-guided radiotherapy (IGRT) relies on the ability to precisely locate the tissue of interest to be treated. Surgical removal of a breast tumor leaves a lumpectomy cavity, defining the target structure assessed for postoperative RT. The tumor cavity is often difficult to visualize, and clips are widely used to indicate the target structures. However, interobserver target volume variability remains high with metallic clips. To facilitate segmentation, visualization of the tumor cavity harbors the potential to facilitate target volume definition and improve reproducibility [11].
Sucrose acetate isobutyrate (SAIB) has been used as an emulgator in the food and beverage production for many decades, known as E444. X-SAIB is an electron dense analogue of SAIB with high X‐ray visibility due to integrated iodine and suitable for radiological imaging based on a high level of X-ray contrast, exceeding 1000H.U. Ethanol is used to reduce viscosity for handling purposes as a liquid fiducial marker. The product consisting of SAIB/x-SIAB/EtOH is available in vials of 1 ml (BioXmark™; Nanovi Inc. Kopenhagen, DK). The advantage compared to metal based soft tissue markers is a low degree of beam hardening artifacts, thereby creating less image distortion.
SAIB/x-SAIB has been used in clinics injected through a syringe. Marking of a wound surface such as the resection wall after lumpectomy has not been investigated. In the present series, we tested SAIB/x-SAIB for it‘s ability to define the resection surface after lumpectomy. After BioXmarkR is applied into the tumor cavity, efflux of EtOH starts immediately as a result of non-solvent induced phase separation (NIPS). The differences in the hydrophobic/hydrophilic properties cause a passive diffusion of EtOH into the surrounding tissue. As EtOH escapes the marker, the viscosity increases 17000-fold and reaches a viscosity that is ∼360,000-fold the viscosity of blood. The high viscosity of the gel-like state ensures that the marker is locked in its conformation.
Coating of the lumpectomy has been achieved in all patients investigated in the present cohort. In some patients, a spiculated appearance of the cavity surface was observed. Therefore, post-processing to discard normal glandular tissue, as a part of the target structure was required. We hypothesize, that the diffusion of the EtOH intraoperatively might be not fast enough, and that capillary forces allow the liquid marker to diffuse into the tissue at the border of the resection cavity resulting in a coarse surface on the planning CT. Eventually, increasing the volume of the tissue marker or let the liquid at open air to allow some evaporation of the dissolving agent EtOH prior to application might be helpful to enhance the smoothness of cavity surface.
During routine follow-up including mammograms no toxicity of SAIB/x-SAIB was noticed in any of the patients. One year after marker placement and radiotherapy, the aspect of the resorbed marker may mimic microcalcification on the corresponding radiographs in some patients (Fig. 3). As none of the patients underwent re-excision or biopsy, the histological texture remains unclear. A medical history of SAIB/x-SAIB instillation however avoids misinterpretation as local relapse or intraductal carcinoma.
The limitations of the present study are the number of patients’ investigated. A wider use in the context of a large cohort study or registry shall allow to conclude on safety and benefits. Furthermore, we were not able to obtain serial CT images to prove stability of the target structure during the entire RT period. The impact of cavity visualization on local control rates remains to be shown. EBRT-PBI is likely to be more often recommended for postoperative radiotherapy when the confidence level of target volume definition of the responsible radiation oncologist is enhanced by robust segmentation techniques.
Acknowledgments
Acknowledgments
We are indebted to Mr. T. Jepsen (Nanovi, Inc, Copenhagen, DK) for providing BioXmark® to make this work possible.
Disclosures
None.
References
- 1.Kirby A.M. Updated astro guidelines on accelerated partial breast irradiation (apbi): to whom can we offer apbi outside a clinical trial? Br J Radiol. 2018;91:20170565. doi: 10.1259/bjr.20170565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa C., Harris E.E., Leonardi M.C. Accelerated partial breast irradiation: executive summary for the update of an astro evidence-based consensus statement. Pract Radiat Oncol. 2017;7:73–79. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Coles C.E., Griffin C.L., Kirby A.M. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (uk import low trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charaghvandi K.R., van’t Westeinde T., Houweling A.C. Single dose partial breast irradiation using an mri linear accelerator in the supine and prone treatment position. Clin Transl Radiat Oncol. 2019;14:1–7. doi: 10.1016/j.ctro.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez Sanchis A., Brualla Gonzalez L., Fuster Diana C. Tumor bed segmentation: first step for partial breast irradiation. Clin Transl Oncol. 2013;15:39–45. doi: 10.1007/s12094-012-0884-1. [DOI] [PubMed] [Google Scholar]
- 6.van Mourik A.M., Elkhuizen P.H., Minkema D. Multiinstitutional study on target volume delineation variation in breast radiotherapy in the presence of guidelines. Radiother Oncol. 2010;94:286–291. doi: 10.1016/j.radonc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 7.van der Laan H.P., Hurkmans C.W., Kuten A. Current technological clinical practice in breast radiotherapy; results of a survey in eortc-radiation oncology group affiliated institutions. Radiother Oncol. 2010;94:280–285. doi: 10.1016/j.radonc.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Kirova Y.M., Fournier-Bidoz N., Servois V. How to boost the breast tumor bed? A multidisciplinary approach in eight steps. Int J Radiat Oncol Biol Phys. 2008;72:494–500. doi: 10.1016/j.ijrobp.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 9.Furet E., Peurien D., Fournier-Bidoz N. Plastic surgery for breast conservation therapy: how to define the volume of the tumor bed for the boost? Eur J Surg Oncol. 2014;40:830–834. doi: 10.1016/j.ejso.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Cox B.W., Horst K.C., Thornton S. Impact of increasing margin around the lumpectomy cavity to define the planning target volume for 3d conformal external beam accelerated partial breast irradiation. Med Dosim. 2007;32:254–262. doi: 10.1016/j.meddos.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ciernik I.F., Voss H., Wosle M. Standardization of the target volume for boost or partial breast radiation therapy after lumpectomy of breast cancer. Int J Radiat Oncol Biol Phys. 2014;89:690–691. doi: 10.1016/j.ijrobp.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Struik GM, Nienke H, Klem TM, et al. Injection of radiopaque hydrogel at time of lumpectomy improves the target definition for adjuvant radiotherapy. Radiother Oncol, in press. [DOI] [PubMed]
- 13.Rydhog J.S., Mortensen S.R., Larsen K.R. Liquid fiducial marker performance during radiotherapy of locally advanced non small cell lung cancer. Radiother Oncol. 2016;121:64–69. doi: 10.1016/j.radonc.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 14.De Roover R., Crijns W., Poels K. Characterization of a novel liquid fiducial marker for multimodal image guidance in stereotactic body radiotherapy of prostate cancer. Med Phys. 2018 doi: 10.1002/mp.12860. [DOI] [PubMed] [Google Scholar]
- 15.Scherman Rydhog J., Perrin R., Jolck R.I. Liquid fiducial marker applicability in proton therapy of locally advanced lung cancer. Radiother Oncol. 2017;122:393–399. doi: 10.1016/j.radonc.2016.12.027. [DOI] [PubMed] [Google Scholar]