Abstract
Alzheimer's disease (AD) was discovered and the pathological hallmarks were revealed more than a century ago. Subsequently, many remarkable discoveries and breakthroughs provided us with mechanistic insights into the pathogenesis of AD. The identification of the molecular underpinning of the disease not only provided the framework of AD pathogenesis but also targets for therapeutic inventions. Despite all the initial successes, no effective treatment for AD has emerged yet as all the late stages of clinical trials have failed. Many factors ranging from genetic to environmental factors have been critically appraised as the potential causes of AD. In particular, the role of stress on AD has been intensively studied while the relationship between sleep and circadian rhythm disruption (SCRD) and AD have recently emerged. SCRD has always been thought to be a corollary of AD pathologies until recently, multiple lines of evidence converge on the notion that SCRD might be a contributing factor in AD pathogenesis. More importantly, how stress and SCRD intersect and make their concerted contributions to AD phenotypes has not been reviewed. The goal of this literature review is to examine at multiple levels – molecular, cellular (e.g. microglia, gut microbiota) and holistic – how the interaction between stress and SCRD bi-directionally and synergistically exacerbate AD pathologies and cognitive impairment. AD, in turn, worsens stress and SCRD and forms the vicious cycle that perpetuates and amplifies AD.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative dementia that currently affects more than 40 million people worldwide and is expected to affect more than 131 million people by 2050 (Prince, 2015). A deeper understanding of its pathophysiology and potential risk factors is critical to develop effective strategies at preventing or delaying development of AD. Intriguingly, stress, sleep disturbance, and circadian rhythm disruption share many common molecular signaling and anatomic pathways that promote the neurodegeneration of AD. For example, the hypothalamus-pituitary-adrenal (HPA) axis – the main mediator of stress – is regulated by the circadian rhythm (Girotti et al., 2009). In particular, cortisol levels exhibits circadian fluctuations (Cohen et al., 2015) and is elevated and deregulated in people with AD (Brureau et al., 2013; Huang et al., 2009; Swaab et al., 1994; Pomara et al., 2003). Stress, sleep disturbance, and circadian disruption interact at many levels to affect neurogenesis, neuroinflammation, and metabolic disruption. In this review, we will discuss circadian rhythm, sleep, and stress physiologies, their bidirectional relationships, and how these interactions promote the development of AD.
2. Physiology of circadian rhythms, sleep and stress
2.1. Circadian rhythms
Living organisms evolved to adopt a circadian rhythm (circa-: about, -dia: a day) that matches with the light-dark cycle for their protection, feeding, mating and survival (Takahashi et al., 2008). In humans, the intrinsic period of the circadian rhythm is slightly longer than 24 h while in mice, its period is about 23.5 h (Scheer et al., 2007). This period compresses daily to 24 h by using cues from the environment, such as light, and from the organism's behavior, such as feeding and activity (Czeisler et al., 1999). The circadian system governs several physiologic functions, such as sleep and wake, activity, temperature, and cognitive function (Wyatt et al., 1999).
At the molecular level, the circadian rhythm is controlled by oscillations of transcriptional-translational negative feedback loops (Shearman et al., 2000). Brain and Muscle ARNT-Like-1 (BMAL1) heterodimerizes with Circadian Locomotor Output Cycles gone Kaput (CLOCK), forming a complex that binds to E-box enhancers of circadian-controlled genes. The CLOCK/BMAL1 heterodimer also activates transcription of Period (Per) and Cryptochrome (Cry), forming the positive-limb of the loop. Per and Cry heterodimerize and inhibit the Clock-BMAL1 heterodimers, thereby inhibiting their own transcription and forming the negative loop (Ko and Takahashi, 2006). In addition, an accessory loop is formed by CLOCK/BMAL1 heterodimers enhancing transcription of ROR and REV-Erb genes. These genes bind to the RORE region of the BMAL1 gene and regulate the core circadian gene loop (Kondratova and Kondratov, 2012). Colcontrol sleep and wakecycleslectively, these interwoven loops result in rhythmic oscillations of circadian-controlled gene expression. This feedback loop is the molecular basis of the circadian rhythm and sets the timing and period of central and peripheral clocks in animals.
Nearly all peripheral tissues in the body have a circadian rhythm – with the ability to oscillate independently (Schibler and Sassone-Corsi, 2002; Nagoshi et al., 2004), at least in the short term – and are regulated and synchronized by a central circadian pacemaker, the suprachiasmatic nucleus (SCN) (Saper et al., 2005; Akhtar et al., 2002). This master clock is in the ventral most part of the hypothalamus, just above the optic chiasm. The SCN sends output signals via direct or indirect neural projections or humoral controls to areas to regulate timing and period of peripheral rhythms (Akhtar et al., 2002; Dai et al., 1998). For example, the SCN sends signals to the various nuclei in the hypothalamus and areas beyond that control sleep and wake cycles (Sherin et al., 1996; Chou et al., 2002), temperature (Buhr et al., 2010), and hunger/satiety (Kalsbeek et al., 2001).
The SCN receives input from various time cues to regulate the circadian timing and synchronize the peripheral circadian rhythms. Specifically, the SCN receives light exposure input from the retina by a direct pathway. In the retina are specialized melanopsin-containing retinal ganglion cells (mRGCs) (Gooley et al., 2001) which carry light input via the retinohypothalamic tract to the SCN (Berson et al., 2002; Hattar et al., 2002). Light exposure can compress or expand the circadian period and therefore change the timing, or phase, of the circadian rhythm; the direction and magnitude of change depend on the timing, intensity, and duration of light exposure (Khalsa et al., 2003). In blind people who lack light signaling to the SCN, the circadian period can oscillate closer to its intrinsic period, resulting sleep-wake cycle that is slightly longer than 24 h (Sack et al., 1992). Another example of a timing input to the SCN is melatonin. Melatonin is naturally produced by the pineal gland in a circadian manner and is under control by the SCN (Teclemariam-Mesbah et al., 1999). During the day, melatonin levels are low; at night, melatonin levels rise just prior to circadian timing for sleep, peak during sleep, and fall around the circadian timing for wake (Lewy and Sack, 1989). Melatonin feeds back to the SCN to adjust timing of the circadian rhythm. Melatonin administration can also adjust the phase of circadian rhythm depending on the timing and dose (Lewy et al., 1998).
Circadian rhythm outputs can be measured in various means. For example, the rest/activity rhythm, which can be used as a proxy for sleep/wake rhythms, can be measured using actigraphy over several days (Ancoli-Israel et al., 2003). Melatonin levels can be measured in plasma (Benloucif et al., 2008) or saliva (Voultsios et al., 1997), and melatonin metabolites can be measured in urine (Benloucif et al., 2008). These outputs can be used to examine the period, phase, and amplitude of the circadian rhythm. In people with circadian rhythm sleep-wake phase disorders, the circadian timing of the sleep-wake cycle is misaligned, either early or late, with respect to the environment (American Academy of Sleep Medicine, 2014). Loss of amplitude and therefore rhythmicity of the sleep-wake rhythm results in irregular sleep-wake disorder, in which patients – typically those with dementia – have no major bout of sleep and instead have frequent bouts of sleep and bouts of wake throughout the 24-h cycle (American Academy of Sleep Medicine, 2014).
The circadian system also influences cognitive function. Cognitive performance varies throughout the 24-h cycle day and is high during the day about 24 hrs after waking except for a dip in the afternoon and low at night (Wertz et al., 2006; Burke et al., 2015). Several cognitive processes are affected by circadian timing; for reviews, see (Wright et al., 2012; Krishnan and Lyons, 2015). The SCN controls cognitive function indirectly through its effects on sleep and wakefulness and perhaps through more direct effects (Sherin et al., 1996; Chou et al., 2002; Wright et al., 2012). Circadian misalignment, where the timing of daily activities – such as sleep and wake – are not aligned to the endogenous circadian timing, results in impaired cognitive function. Chronic jet lag leads to cognitive performance and is associated with increased cortisol levels (Cho, 2001; Cho et al., 2000) Experimental jet lag in rodents result in long term cognitive deficits (Gibson et al., 2010). Thus when circadian rhythm is misaligned and circadian amplitude is lowered in old age and dementia patients, it also coupled with cognitive impairment (Smarr et al., 2014).
2.2. Sleep
A vast array of literature has described various functions of sleep, including cognitive function, metabolism, and inflammation. Although a detailed review of the structure, function and regulation of sleep are beyond the scope of this paper, we will describe in general the physiology, anatomy, and functions of sleep as it relates to cognitive function and AD.
Sleep and wake states are regulated by a 2-process model: the circadian system, as described above, and a homeostatic process (Borbely, 1982). The homeostatic drive for sleep indicates that the greater period of wakefulness, the greater need for sleep an organism has. As the sleep homeostat increases, the circadian alerting system also increases to maintain wakefulness during the day. At night, the circadian alerting signal drops; this, in conjunction with a high sleep need, results in transition to sleep (Borbely, 1982).
From a brain physiology level, sleep is characterized by two general sleep states measured by polysomnography: non-rapid eye movement sleep (NREM) – which consists of three stages, N1, N2, and N3 – and rapid eye movement (REM) sleep. As NREM sleep becomes deeper, there is slowing of electroencephalographic frequencies and increasing synchronization of cortical neuronal activity. At its deepest point – stage N3 or slow wave sleep (SWS) – the neuronal synchrony appears as large slow wave activity on the electroencephalogram (Berry et al., 2012). SWS is considered a marker of the sleep homeostat, as it rebounds during recovery sleep after prolonged wakefulness (Dijk et al., 1990). REM sleep is characterized by cortical desynchrony and rapid eye movements and is associated with dream states (Berry et al., 2012).
From an anatomical standpoint, sleep and wake states are controlled by a balance between sleep-promoting nuclei and wake-promoting nuclei. The sleep-promoting nuclei are the GABAergic ventrolateral preoptic (VLPO) and the median preoptic area located in the hypothalamus (Szymusiak and McGinty, 2008). These areas inhibit the wake-promoting centers in the lateral hypothalamus, the tuberomammillary nucleus, and several brainstem nuclei (Szymusiak and McGinty, 2008). Conversely, the monoaminergic wake-promoting nuclei inhibit the VLPO (Szymusiak and McGinty, 2008). These two systems form a flip-flop switch, in which the orexinergic neurons in the lateral hypothalamus promote the activity of wake-promoting nuclei and inhibit the VLPO, thereby stabilizing the wake state (Saper et al., 2010). In addition, the VLPO also receives input from the SCN to regulate circadian rhythm of sleep/wake states (Saper et al., 2005a). REM sleep is generated by neurons in the peri-locus ceruleus area and sublaterodorsal area in the upper pons. The sublaterodorsal area projects to the basal forebrain and cortex, leading to dream states, and to medullary and spinal cord areas to inhibit movements during REM sleep (Peever et al., 2014).
Sleep plays an important role in various cognitive functions. Acute sleep deprivation impairs attention and vigilance, but even chronic sleep deprivation of 6 h nightly can cumulatively impact cognitive functions (Van Dongen et al., 2003). Beyond just sleep duration, SWS and REM sleep each have roles in impacting cognitive performance. SWS has been linked primarily to learning and memory, particularly declarative memory, which includes remembering facts and events (Wilckens et al., 2018; Leger et al., 2018). In contrast, REM sleep appears to play a role in non-declarative memory, or procedural memory, and emotional memory (Boyce et al., 2017). However, some data suggest that SWS and REM sleep have complementary roles in memory consolidation, as SWS does also benefit procedural memory (Mednick et al., 2011). In addition, sleep spindles, which are predominantly in stage N2 and SWS, are also linked to cognitive function and appear to be involved in memory consolidation during sleep (Rasch and Born, 2013).
Much research has focused on the role of SWS in memory. On a physiologic level, two hypotheses – which are not mutually exclusive – have emerged to explain sleep's role in memory consolidation. The synaptic homeostasis hypothesis postulates that during wakefulness, synaptic strength increases, and during sleep – particularly SWS – synaptic strength decreases (Tononi and Cirelli, 2006). This downscaling maintains synaptic homeostasis. The active system consolidation model postulates that slow wave activity strengthens synapses that are reactivated during sleep and facilitates transfer of memory from the hippocampus to neocortex for long-term storage (Diekelmann and Born, 2010).
2.3. Stress
Stress is an aversive stimulus that elicits physiological responses both at the central (arousal, vigilance, attention) and peripheral systems (metabolism and oxidation) to minimize the effects of that stressor. Stressors acutely engage the autonomic nervous system to prepare the body for the classic “fight or flight” response to avoid immediate danger (Ulrich-Lai and Herman, 2009). Chronic effects of stressors engage a slower kinetic neuroendocrine response of the hypothalamus-pituitary-adrenal (HPA) axis, leading to deleterious effects on the organs.
The HPA axis is the major pathway for the stress response and is activated in allostatic response to return the body to homeostatic state. The hypothalamus releases two neuro-hormones: corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) into the blood that directly activates the pituitary via the hypophysial portal system. These two hormones stimulate the release of adrenocorticotropic hormone (ACTH) into the main circulation system. ACTH then stimulates the adrenal cortex to release glucocorticoid in rodents and cortisol in humans (Stephens and Wand, 2012) Glucocorticoid, in turn, provides negative feedback by binding to the mineralocorticoid receptors (MRs) and the glucocorticoid receptors (GRs) both in the hypothalamus and pituitary gland and shuts off its own production. Glucocorticoid (G) is a steroid hormone whose primary function is to facilitate the metabolism of glucose, protein and fat to supply energy for the body during stressful events. Besides these specific effects, G binds to GR and activates various signaling pathways with wide-range effects influencing learning and memory, immune response, arousal, and others. For the healthy individual, cortisol rapidly increases during a stressful event and quickly declines following the removal of an acute stressor.
The effects of both acute and chronic stress on the brain are very broad and beyond the scope of this review but are covered in previous reviews (Ulrich-Lai and Herman, 2009; Chrousos, 2009). Psychological and physical stressors incur a wide array of deleterious effects such as sleep and circadian rhythm disruption, neuroinflammation, oxidative stress and more. Specifically, the role of stress on AD has been intensively and extensively discussed in these reviews (Pomara et al., 2003; Machado et al., 2014; Johansson et al., 2010; Futch et al., 2017). Chronic stress has detrimental consequences on the brain and is thought to be a contributing to neurodegenerative disease. Both chronic psychological and physical stress also lead to cellular stress. Multiple insults from the environment could damage the macromolecules such as DNA, lipids, and proteins. These two pathways then integrate signaling and elicit appropriate responses from the cellular stress signaling response network. For example, if DNA is damaged from stress, chromatin modeling machinery will be recruited for DNA repair. Eventually, if all attempts to nullify the environment stressor fail, cell apoptosis engages as the final solution (Kultz, 2005).
2.4. Interactions between circadian rhythms, sleep, and stress
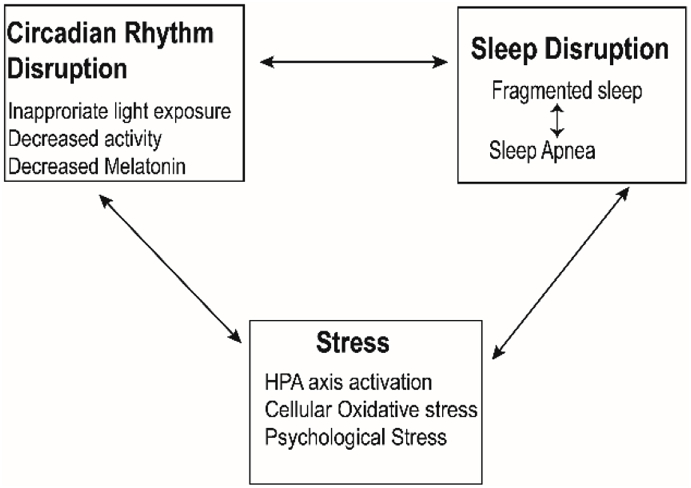
Stress, circadian rhythms, and sleep all have interactive relationships (Fig. 1). For example, psychological stressors result in sleep fragmentation and reduced sleep duration, SWS, and REM sleep (Kim and Dimsdale, 2007). One major area where stress responses and sleep and circadian rhythm disruption intersect is the HPA axis and cortisol levels. Sleep restriction and circadian misalignment, such as with shift work, increase the stress response with elevated cortisol levels (Spiegel et al., 1999) and inflammation (Vgontzas et al., 1999). Circadian misalignment can also result in sleep disruption (American Academy of Sleep Medicine, 2014) and alter cortisol production (Wright et al., 2015). In turn, cortisol can reset other peripheral rhythms, such as that in adipose tissue (Kolbe et al., 2015).
Fig. 1.
A model of interactions among sleep disruption, circadian disruption and stress.
Obstructive sleep apnea (OSA) is a sleep disorder that is particularly relevant to stress. OSA is characterized by recurrent obstructions in the upper airway when asleep, leading to intermittent hypoxemia and arousals. The prevalence of OSA increases with age and affects 9% of women and 17% of men age 50–70 years (Peppard et al., 2013). With frequent arousals, OSA can cause sleep to be fragmented. OSA impairs cognitive function, especially executive function. Furthermore, OSA may increase cortisol activity (Vgontzas et al., 2007), and greater nighttime cortisol correlates with worse cognitive function beyond that expected for OSA alone (Edwards et al., 2014).
The HPA axis and its main effector, cortisol in humans and corticosterone (where glucocorticoid is the main example) in rodents, are also under circadian regulation. The release of G is controlled by a dual-rhythm regulation: circadian rhythm for 24-h control and pulsatile ultradian rhythm for hourly control (Windle et al., 1998). Since the levels of CRF, ACTH, and G are circadian regulated (Girotti et al., 2009; Chrousos, 1998), it is not surprising that the level of stress responses is time dependent (Dunn et al., 1972). Rodents, which are nocturnal animals, have heightened stress responses at the beginning of the light cycle (when corticosterone is already high) than at the beginning of the dark cycle even with the same stressor (Kant et al., 1986). Furthermore, Bmal1 (Leliavski et al., 2014) and Clock (Turek et al., 2005) positively regulate the level of glucocorticoid. Ablating the Bmal1 gene results in lower basal G level and blunted effects on the stress-induced responses (Leliavski et al., 2014). In contrast, Cry represses glucocorticoid production; in the absence of Cry gene, glucocorticol is elevated (Barclay et al., 2013; Lamia et al., 2011).
The molecular clock is critical for adrenal function and therefore HPA axis. Through different experiments, it was unambiguously demonstrated that the molecular clock of the adrenal gland is cell-autonomous. Specifically, the mRNA levels of Bmal1, Per1, and Per2 in the adrenal gland are still rhythmic following hypophysectomy (Fahrenkrug et al., 2008), while in vitro experiments demonstrate that ACTH controls phase setting of the adrenal core molecular clock (Yoder et al., 2014). Genetic ablation of Bmal1 (Son et al., 2008) or Per1/Per2 (Oster et al., 2006) demonstrate that rhythmic GC release is due to localized peripheral clock in the adrenal gland. Through the HPA axis and potentially other pathways, the SCN still regulates this peripheral rhythm to control the adrenal activity (Chung et al., 2011). Thus, circadian activity of the adrenal gland is controlled both by the upstream signaling and localized molecular clock machinery.
In short, sleep and circadian rhythm functions modulate the HPA axis in basal conditions; however, under chronic stress, the HPA axis becomes constantly activated and therefore overrides the circadian rhythm control and can disrupt the circadian rhythm. It is reasonable to conclude that these interconnected relationships, when dysfunctional, will form a perpetual feedback loop that synergistically exacerbates pathophysiologic changes in each other.
3. Alzheimer's disease (AD)
AD is a progressive dementia associated with complex etiologies and affects an increasing aging population (Huang and Mucke, 2012; Masters et al., 2015). The pathological hallmarks of AD are the extracellular plaques, intracellular tangles, and neuronal loss (Masters et al., 1985; Glenner and Wong, 1984; Hardy and Higgins, 1992). Following the identification of amyloid peptide at the core of the plaques, the amyloid precursor protein (APP) and the enzymes that cleave APP were identified (Hardy, 2017). The mechanistic insights integrated from multidisciplinary discoveries constitute the elements of the of amyloid cascade hypothesis (ACH), which postulates that aberrant accumulation of amyloid beta (Aβ) leads to intracellular fibrillary tangle build up and ultimately cellular death (Hardy and Higgins, 1992; Hardy, 2017). The newly emerged pathological signature of AD is neuroinflammation, which has recently become the center of research interest in AD (Heneka et al., 2015; VanItallie, 2017; Regen et al., 2017). Neuroinflammation contributes to formation of plaques and reduction of synapses through over-pruning by the microglia (Hong et al., 2016a). Thus, inflammation is a tangible therapeutic avenue (Tan et al., 2012).
The ACH accentuates the role of amyloidosis and places the amyloidocentric perspective at its center, but it is inadequate to fully explain the disease pathologies. For instance, it remains enigmatic as to why there is a long quiescent period, where patients remain asymptomatic for decades despite accumulation of amyloid plaques. Nevertheless, the ACH provides an important frame work for all the recent therapeutic approaches. As such, many clinical trials aimed at reducing amyloid plaques have yielded no tangible successes. Among all the probable confounders, limitations, and technical challenges, one important reason may be the missed therapeutic window; patients even at mild and moderate stage at the time of commencing the clinical trials were already at potentially irreversible stage due to significant synaptic and neuronal losses (Selkoe and Hardy, 2016; Karran and De Strooper, 2016).
Alternative theories were thus engendered to explain the seemingly contradictory evidence, including the temporal course of disease progression and the spatial discordance of the disease. These theories diverge on the origins and the primary factors of AD pathogenesis, yet all evidence converges on the perspective that AD must be detected early to maximize the effectiveness of treatment. Thus, it is imperative to have a reliable, simple, and non-invasive biomarker to detect early stage of AD. One potential early biomarker for detecting AD is sleep and circadian rhythm dysfunction (SCRD). It has been reported many decades ago that some AD patients exhibited confusion in the early evening hours, hence the term “sundowning” (Volicer et al., 2001). Recent and mounting evidence further strengthened the connection between SCRD and AD (Holth et al., 2017) as we discuss below.
3.1. The challenges of early detection and treatment of AD
At the mechanistic level, according to the ACH, Aβ peptides are generated by a sequential protease-mediated cleavage of the APP by β-secretase enzyme 1 (BACE1) followed by γ-secretase. Aβ42 is synaptotoxic and is the main constituent of neuritic plaques. The elevation of Aβ42 initiates a cascade of failure resulting in accumulating intracellular Tau and ultimately neuronal demise (Hardy and Higgins, 1992; Selkoe and Hardy, 2016; Hardy and Selkoe, 2002). In support of the ACH, people who inherit mutations in the APP, Presenilin 1 (PSEN1) or PSEN2 genes produce the longer forms of Aβ peptides (>42aa) and have an accelerated symptomatic onset. In contrast, most AD patients have late-onset symptoms of dementia with undefined causes. Recent results from genome wide association studies (GWAS) provide several genes that associated with AD (Lambert et al., 2013; Moustafa et al., 2018; Van Cauwenberghe et al., 2016); however, the mechanisms between these genes and AD pathophysiology remain to be elucidated.
Much efforts in identifying the genetic underpinnings of AD had yielded only a handful of genes. Mutations in APP, PS1, and PS2 were identified as causes of early onset AD but are seen in less than 1% of people with AD overall (Alzheimer's, 2013). Apolipoprotein E ε4 (APOE ε4) mutation is cause of late onset AD and consistently comes up in many genome wide association studies (GWAS) (Castellano et al., 2011; Ma et al., 2016). Genetically, APOE ε4 poses the highest risk factor for late onset AD. We will mention a few important points about APOE. First, APOE encodes for a lipid/cholesterol carrier, a lipoprotein that binds to the APOE receptor (Mahley, 1988). Individuals with one APOE ε4 allele have a 4-fold higher risk of AD, and those with two alleles have a 10-fold higher risk of AD compared to those without an APOE ε4 allele. Second, the APOE ε2 allele appears to have a protective effect on AD. Recent experiments in mice have shown the APOE ε4 is associated with greater amyloid plaques compared to APOE ε2 isoform (Castellano et al., 2011). Third, APOE transcription appears to fluctuate in a circadian manner (Ma et al., 2016); the APOE protein, however, does not fluctuate across the day (Ulrich et al., 2013), so the significance of these fluctuations and the role of circadian rhythm control on APOE is unclear (Zhao et al., 2018; Liu et al., 2013; Yu et al., 2014; Bu, 2009; Kanekiyo et al., 2014). Fourth, another relevant aspect of APOE is its protective role in oxidative stress. Specifically, a significant increase in lipid peroxidation in plasma and lipoprotein was observed in mice with APOE ablated (Hayek et al., 1994). Furthermore, APOE ε4 confers higher risk of cell death compared to APOE ε2 in when cells are exposed to hydrogen peroxide. (Miyata and Smith, 1996). Consistently, freshly dissected prefrontal cortex postmortem tissues of people with AD showed that APOE ε 4 is strongly associated with higher levels of lipid oxidation (Ramassamy et al., 1999). Taken together, these results showed that APOE has an essential role in protecting the cells against oxidative damage in an allele specific manner, where APOE ε2 is the most protective and APOE ε4 is the most harmful allele.
The ACH is not universally accepted for several reasons (for reviews, see (Karran and De Strooper, 2016; Herrup, 2015; De Felice, 2013)). The ACH parsimoniously expounds the temporal and spatial discordance between the region of brain where Aβ and tangles originate. Aβ plaques first appear in the precuneus and the frontal cortex, but hyper-phosphorylated Tau first appears in the entorhinal cortex (Musiek and Holtzman, 2015). Recently, therapeutic strategies aimed at reducing Aβ peptides, such as the BACE1 inhibitor verubecestat (Mullard, 2017) and the anti-Aβ antibody treatments (Solanezumab, Eli Lily) (Mullard, 2016) both failed in phase III clinical trials (Huang et al., 2009). The failures could be due to confounders such as the drug potency, brain penetrance, and stage of AD at the time of treatment (Sperling et al., 2011). Results from a new verubecestat phase III trial that treats early stage (or prodromal) patients will be revealed in early 2019, and positive results from this study would corroborate the validity of the ACH. Nevertheless, the ACH accommodates many findings and discoveries thus far. It is the most validated hypothesis providing framework for the mechanistic understanding and potential therapeutic treatments to date (Selkoe and Hardy, 2016).
Currently available medications for AD only manage symptoms and include cholinesterase inhibitors (donepezil, rivastigmine, galantamine) and an NMDA receptor antagonist (memantine) (Lleo et al., 2006). Although these drugs show some short-term benefits, the potential side effects (e.g nausea, vomiting, dizziness, loss of appetite, confusion) often outweigh the benefits (Lleo et al., 2006; Inglis, 2002).
Due to the recent litany of failure of pharmaceutical approaches to ameliorate cognitive impairments in mild-moderate AD patients, the current goal is to detect and treat AD before it starts, especially in the quiet prodromal period (Sala Frigerio and De Strooper, 2016). Until recently, the only way to confirm the diagnosis of AD was by post-mortem immunostaining of the brain. It is now possible to detect Aβ plaques in living patients with synthesized PET-scan dyes, e.g. the 11C-PIB (Pittsburgh compound B). Although 11C-PIB can detect the fibrillary form of Aβ peptides (mainly Aβ40 and Aβ42) (Klunk et al., 2004) and was heralded as a new diagnostic tool, current large scale usage of this tool is limited because of availability and cost and is not appealing to the general population due to the use of radioactive tracers (Khan and Alkon, 2015). Innovative and less invasive approaches, such as blood-based biomarkers (O'Bryant et al., 2016; Nakamura et al., 2018) and retina scans for Aβ deposition (Hart et al., 2016; Koronyo et al., 2017; Koronyo-Hamaoui et al., 2011), show promise for detection of early signs of AD in general population but are still in their infancy. Alternatively, a wealth of recently accumulating evidence demonstrate that SCRD could serve as early biomarkers (Sterniczuk et al., 2013; Cedernaes et al., 2017; Lim et al., 2014a; Lucey and Bateman, 2014), providing an intriguing possibility to understand the physiology and identify modifiable risk factors to prevent the development or delay the progression of AD.
3.2. Circadian rhythms and AD
Alterations in circadian rhythm function in AD has long been recognized. Most notably, people with AD often develop a loss of the sleep/wake rhythm, resulting in short bouts of wakefulness interspersed with short bouts of sleepiness throughout the 24-h day but no major sleep or wake bout (Witting et al., 1990). This finding has led to further study degeneration of circadian function in AD. In general, such dysfunction may occur due to loss of time cues to the SCN, dysfunction in the SCN itself, or changes in the output rhythms directly, which also lead to impaired feedback to the SCN.
Failure of appropriate inputs to the SCN occur in AD and may also play a role in the associated circadian rhythm disruption. People with AD experience decreased light exposure, even if not institutionalized (Figueiro et al., 2012). Beyond having less light exposure, signaling of light to the SCN may be impaired, as mRGCs are significantly reduced in AD patients (Feng et al., 2016). Evidence from optical coherence tomography and post-mortem histological analysis of AD patients show a significant reduction of nerve fiber layer thickness and a reduced number of mRGCs (La Morgia et al., 2016; La Morgia C et al., 2013). These findings were further corroborated by multiple lines of evidence, both in humans and mice, that Aβ plaque deposits and phosphorylated Tau are present in the retina (Koronyo-Hamaoui et al., 2011; Yoneda et al., 2005; Ning et al., 2008). Reduction of mRGCs may impair proper SCN entrainment, and protecting the mRGCs from degeneration could potentially improve circadian function in AD and perhaps delay the progression of AD.
The SCN anatomically and functionally deteriorates in aging and neurodegenerative diseases (Mattis and Sehgal, 2016), and the degree of SCN degeneration correlates with the degree of circadian disruption (Harper et al., 2008; Stopa et al., 1999). The number of neurons positive for vasoactive intestinal peptide (VIP) and arginine-vasopressin (AVP) are significantly reduced in the SCN of AD patients compared to control cohorts in post-mortem analysis (Wang et al., 2015; Swaab et al., 1985). Moreover, there is a high concordance between the degree of degeneration of these SCN-specific neurons and the severity of sleep-wake disruption. Interestingly, AVP is an anxiogenic and purported to mediate long term stress and depression which often seen in AD (Beurel and Nemeroff, 2014). The loss of these critical neurons results in asynchronous firing patterns among SCN neurons and diminished amplitude outputs, thus rendering the SCN unable to synchronize the peripheral clocks. Degeneration of the SCN invariably disrupts the circadian rhythm with many consequences, including sleep fragmentation (Liu et al., 2012), memory impairment (Phan et al., 2011), and metabolic changes (Kalsbeek et al., 2011).
Besides the sleep-wake cycle, other disrupted circadian rhythms, including a delayed phase of the core body temperature rhythm (Peter-Derex et al., 2015), low amplitude of the melatonin rhythm (Weissova et al., 2016), and changes in circadian gene expression (Cermakian et al., 2011) and circadian period (Sethi et al., 2015; Schneider et al., 2014) are also observed in AD patients. Conversely, recent evidence has implicated that circadian rhythm disruption is not only consequence of AD but also may increase the risk of AD (Musiek, 2015; Musiek and Holtzman, 2016). For example, alterations in the rest/activity rhythm is a predictor of dementia in the elderly (Tranah et al., 2011). In addition, variations in the coding sequence of BMAL1 (Chen et al., 2015) or CLOCK (Chen et al., 2013a, 2013b; Yang et al., 2013) genes increase the propensity of developing AD. By definition, these studies only demonstrate a correlation between the risk of getting the disease and having a variation in the gene but do not imply causation.
In short, the SCN orchestrates a complex symphony of neuronal activity. When the SCN function is compromised as in AD patients, the symphony becomes cacophony. The SCN is “the strange case of Dr. Jekyll or Mr. Hyde”, when functional, it is beneficial, but when it breaks down, it becomes a stressor, leading to sleep disruption and sleep fragmentation.
3.3. Sleep and AD
Sleep disruption manifests in several ways in AD. It is not surprising that AD patients frequently exhibit nighttime sleep fragmentation and daytime sleepiness. With aging, sleep tends to become more fragmented and some sleep stages, particularly SWS, decline. These changes are accentuated in AD, and furthermore, stage REM sleep declines (Mander et al., 2017; Brown et al., 2012; Ju et al., 2014). These sleep problems are also manifestations of underlying circadian rhythm disturbances, such as low amplitude of the rest and activity rhythm, the degree of which correlates with the degeneration of the SCN (Harper et al., 2008; Stopa et al., 1999). Sleep fragmentation may also be related to Aβ accumulation and neuronal loss in structures that regulate sleep-wake states, such as the intermediate nucleus of the hypothalamus, the human homologue of the VLPO (Lim et al., 2014b). Moreover, animal models that were engineered to express human Aβ peptides reveal that a high level of Aβ induces fragmented sleep in Drosophila (Gerstner et al., 2017). Other sleep architecture changes, such as a decline in SWS is seen even in people with mild cognitive impairment, who are at risk for developing AD (Westerberg et al., 2012). This change may be of particular importance as SWS plays a role in cognitive performance as discussed above (Westerberg et al., 2012).
Recent evidence has shown that sleep disruption is a risk factor for AD. Sleep disruption can begin years or even decades before the onset of AD. Sleep fragmentation impairs memory consolidation in animals (Rolls et al., 2011) and has been demonstrated to be a risk for AD (Djonlagic et al., 2012; Lim et al., 2013). Cognitively normal individuals with self-reported sleep problems have a higher likelihood of having AD biomarkers such as lower levels of Aβ42 and higher levels of total Tau and phosphorylated-Tau in the cerebrospinal fluid (Sprecher et al., 2017). Several studies have shown that rest/activity rhythm alterations predict cognitive decline (Walsh et al., 2014) and mild cognitive impairment and dementia (Tranah et al., 2011). Recently, a large meta-analysis reported that both insomnia and sleep disordered breathing increase the risk of AD by 49% (Shi et al., 2017). Although these data do not demonstrate casual effects, recently there are mechanistic studies pointing to this possibility (Bellesi et al., 2017; Roh et al., 2012; Lucey et al., 2017). For example, sleep deprivation decreases leptin levels and increases ghrelin levels (Taheri et al., 2004); these two hormones appear to play protective roles from Aβ peptides.
Two seminal discoveries provide mechanistic insights between circadian rhythm, sleep and AD through production and clearance of Aβ: 1) the relationship between the sleep-wake cycle and Aβ metabolism and 2) clearance of Aβ from the brain. First, the sleep-wake cycle drives the oscillations of interstitial fluid Aβ peptides (mainly Aβ40 and Aβ42). Aβ level peaks during the active phase and reaches a trough during the sleep phase both in mice and in humans (Roh et al., 2012; Huang et al., 2012). During sleep deprivation, Aβ levels increase in humans and mice (Roh et al., 2012; Bateman et al., 2007; Kang et al., 2009). In contrast, inhibition of neuronal activity by tetrodotoxin significantly reduces the level of interstitial fluid Aβ peptide (Cirrito et al., 2005). Second, through experiments using in vivo two-photon imaging, it was shown that clearance of Aβ peptides occurs through the elusive glymphatic system, which is analogous to the lymphatic system but in the central nervous system and mediated by the glia. Toxic metabolites egress through bulk flow that carries metabolic wastes, including Aβ and Tau, more efficiently during natural or substance-induced sleep than during wakefulness (Xie et al., 2013).
Blood brain barrier (BBB) disruption may also be a mechanism by which sleep disruption promotes AD. Breakdown of the BBB – a finding consistently observed in people with AD (Zlokovic, 2011) – can be detected before any symptomatic onset of cognitive impairment and any AD pathology in the brain (Montagne et al., 2017) and even before changes in cerebrospinal fluid Aβ and tau levels (Montagne et al., 2015). Chronic sleep restriction induces the breakdown of the BBB in rodents (Gomez-Gonzalez et al., 2013; He et al., 2014). Selective deprivation of REM sleep also impairs BBB integrity (Gomez-Gonzalez et al., 2013). Further studies are needed to clarify the role of BBB integrity and AD and the effect of chronic sleep disruption on the BBB.
The mechanism of sleep disruption on Aβ following one night of sleep deprivation could be due to either overproduction of Aβ, reduced clearance or a combination of both. Bateman and colleagues present evidence that sleep deprivation increases production of Aβ peptides (Lucey et al., 2018). Experimental sleep disruption, either pharmacologically (Kang et al., 2009) or with sounds (Ju et al., 2017), also increases Aβ levels. Although these studies cannot distinguish between increased Aβ production and impaired glymphatic clearance of Aβ peptides, wakefulness does appear to promote Aβ production. Aβ peptide levels increase in the barrel cortex when mouse whiskers are physically stimulated, and removal of whiskers (thereby inhibiting neuronal activity) decreases Aβ peptide level (Bero et al., 2011). Collectively, these results suggest that neuronal activity drives the production of Aβ peptides. Although the biological function of Aβ42 is not fully understood, evidence indicates that Aβ peptides inhibit synaptic transmission through NMDA receptor activation, perhaps to dampen neuronal activity (Kamenetz et al., 2003). Whether the daily oscillation of Aβ peptide levels has any functional role remains to be explored; however, disruption of Aβ peptide oscillations increases the likelihood of plaque accumulation (Roh et al., 2012; Bateman et al., 2007; Kang et al., 2009). Furthermore, impaired clearance of Aβ could play a role. Indeed, it was demonstrated that the glymphatic system was suppressed before an abundant accumulation of Aβ plaques (Peng et al., 2016), supporting impaired glymphatic clearance as a mechanism in AD pathophysiology. More studies are needed to determine how well glymphatic system clears Aβ and Tau in sleep-deprived and sleep-fragmented states to conclude with certainty the roles of Aβ production and clearance.
Levels of Aβ and sleep fragmentation appear to have a bidirectional relationship. While sleep disruption increases Aβ levels, Aβ may promote sleep disruption. Aβ peptides promote degradation of BMAL1 and Creb-Binding Protein (CBP), which causes further sleep and circadian disruption in an AD mouse model (Song et al., 2015). Therefore, sleep fragmentation increases Aβ release – increasing the risk of amyloid plaque formation and neuronal injury – which in turn exacerbates sleep fragmentation and disturbance. If such damage occurs in structures controlling sleep and wake, further sleep fragmentation would ensue, worsening the neurodegenerative process (Ju et al., 2013).
3.4. Circadian rhythms, sleep, stress and AD
Stress has been extensively demonstrated to be a contributing factor to AD (Machado et al., 2014; Futch et al., 2017; Csernansky et al., 2006; Mravec et al., 2018; Greenberg et al., 2014). Briefly, stress alters the allostatic load and effects the brain directly at three levels. At the cellular level, it alters proteostasis – as observed in hyperphosphorylation and aggregation of Tau – and causes epigenetic changes in neuronal DNA (Mravec et al., 2018). At the tissue level, stress causes reduction of synaptic density and number of neurons and accumulation of extracellular Aβ peptides (Mravec et al., 2018). Stress induces changes in the peripheral organs where it alters metabolic, cardiovascular, and gastrointestinal physiology and deregulates the immune system (Mravec et al., 2018). These effects indirectly worsen brain homeostasis and increase susceptibility to AD (Mravec et al., 2018). In particular, chronic stress activates the HPA axis resulting in many deleterious effects such as increased Aβ plaque load and Tau pathology, memory impairment, and neurodegeneration (Carroll et al., 2011). Stress also increases several cytokines and impairs neurogenesis in rodent AD-models (Ricci et al., 2012). In humans with AD, cortisol is associated with higher rate of cognitive decline, though this relationship was not seen in people without dementia (Csernansky et al., 2006).
Given the intertwined nature of sleep and circadian rhythm disruption and stress and each of their direct relationships with AD pathology, the intersection of these factors likely promotes AD pathology. Circadian rhythm disruption affects sleep and misalignment of other physiologic rhythms, which may worsen stress. Sleep disruption increases stress, and stress increases sleep disruption, both leading to downstream effects on AD pathogenesis. Different forms of stresses alter sleep architecture and activate the HPA axis (Pawlyk et al., 2008). Stress-inducing paradigms such as social defeat, restraint or immobilization, foot shock, or water submersion demonstrate the detrimental effects of stress on sleep (Sanford et al., 2015).
Remarkably, in humans, one night of sleep deprivation increases cortisol levels and potentiates the HPA axis (Minkel et al., 2014). In mice, acute stress potentiates the HPA axis response but chronic stress blunts the HPA axis response (Novati et al., 2008; Hagewoud et al., 2011). On the other hand, chronic stress due to isolation housing and acute stress due to restraints significantly elevate Aβ via a CRF-dependent mechanism (Kang et al., 2007). Multiple studies have shown that sleep deprivation increases cellular oxidative stress (D'Almeida et al., 1998; Mathangi et al., 2012; Ramanathan et al., 2002). Thereafter, cellular oxidative stress by reactive oxygen and reactive nitrogen species instigates AD pathogenesis (Sultana and Butterfield, 2010). Accordingly, oxidative damage was the first event to be observed in human post-mortem AD brains (Nunomura et al., 2001). Furthermore, oxidative stress as indicated by lipid peroxidation presages plaque deposition in an amyloidosis mouse model (Pratico et al., 2001).
OSA is another potential link between stress and AD. Sleep disordered breathing, including OSA, raises the risk of incident AD by 20% (Shi et al., 2017). The sleep fragmentation in OSA may increase neuronal activity and Aβ release and impair Aβ clearance. In addition, the intermittent hypoxemia has several effects that can raise risk of neurodegeneration. For example, intermittent hypoxemia upregulates β-secretase, resulting in increased cleavage of APP to Aβ42 (Ng et al., 2010; Shiota et al., 2013). Cerebrospinal fluid in people with OSA have decreased Aβ42 and increased tau protein, findings that like those with AD (Ju et al., 2016). Furthermore, intermittent hypoxemia increases oxidative stress, reactive oxygen and nitrogen species, and inflammation, promoting AD pathophysiology (Heppner et al., 2015), cortical thinning (Joo et al., 2013), and neuronal apoptosis (Gozal et al., 2003; Nair et al., 2011).
Interestingly, the anatomical areas controlling the sleep-wake cycle, including the locus coeruleus (LC), the VLPO, and others, are damaged by the stress-response in the setting of AD in a manner that can worsen AD pathophysiology (Coogan et al., 2013; Satoh and Iijima, 2017). For instance, LC is a wake-promoting center and is activated when exposed to stress-induced paradigms (McDevitt et al., 2009). Moreover, the LC and VLPO are degenerated in AD, further strengthening the connection between stress and the sleep-wake cycle (Harper et al., 2008; Lim et al., 2014b; Satoh and Iijima, 2017; Mather and Harley, 2016; Saper et al., 2005b). Intriguingly, the LC was demonstrated to be the first region of the brain to be damaged in AD patients (Mather and Harley, 2016). In support of this notion, stress increases phosphorylated Tau, which is a precursor to neurofibillary tangle and is mediated by CRF (Kvetnansky et al., 2016). Furthermore, stress increases phosphorylated Tau in the LC, which might explain why this region is subjected to deterioration in stress-induced circadian disruption conditions (Kvetnansky et al., 2016). It is currently a conundrum to determine which comes first, stress or sleep disruption, to initiate the pathogenesis of AD. However, the evidence supports the notion that stress, sleep disturbance, and circadian disruption collaboratively and synergistically exacerbate AD pathogenesis and cognitive dysfunction in people with AD.
4. Multiple interactions and potential mechanisms
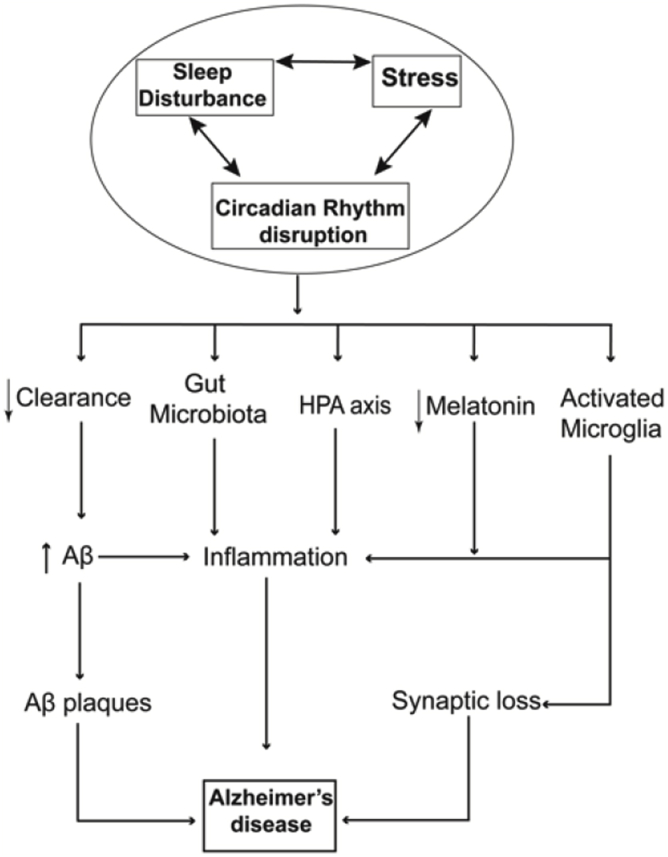
Collectively, each of these factors, sleep, circadian rhythm, and stress could independently or co-operatively influence the pathogenesis or maintenance of AD. To recapitulate the complex interwoven nature of these two factors: the sleep-wake cycle and the circadian rhythm are tightly intertwined, and stress has bidirectional relationships with both circadian rhythm dysfunction and sleep disturbance. Consequently, there are multiple converging pathways where sleep and circadian disruption can synergistically influence on AD pathophysiology (Fig. 2); we will discuss these pathways below.
Fig. 2.
A model of the interaction of stress, sleep disturbance, and circadian rhythm disruption promotes Alzheimer's disease pathogenesis.
4.1. HPA axis
The HPA axis is preeminent converging pathway and was discussed above. However, a few important additional points should be mentioned. First, the HPA axis activation promotes AD pathogenesis (Pomara et al., 2003). Indeed, in a 35-year longitudinal population study the investigators found midlife stress significantly increases the risk of developing AD (Machado et al., 2014; Johansson et al., 2010). In particular, stress increases CRF release which then increases γ-secretase activity, resulting in higher Aβ production (Park et al., 2015). In addition, cortisol levels in AD patients are high compared to controls. Although treatment aimed at reducing cortisol levels, such as by taking dex-amethasone failed to yield any positive effect in AD patients (Swanwick et al., 1998), approaches to modulate and decrease the HPA axis activity could still be a viable and promising venue for treating AD (Futch et al., 2017), particularly if started prior to symptom onset. Second, Aβ promotes HPA axis activation. Intracerebroventricular injection of Aβ peptides increase corticosterone and elevated HPA axis activity. These results demonstrate that the HPA axis contributes significantly to the development and perhaps the progression of AD (Brureau et al., 2013). Taken together, the activation of the HPA axis appears to be a common pathway by which SCRD and stress increase Aβ production and promotes the development of AD. What remains unclear is whether stress and SCRD independently activate the HPA axis, stress causes SCRD leading to HPA axis activation, or SCRD causes stress and therefore HPA axis activation.
4.2. Microglia
A recent intense focus in AD research has involved elucidating the role of neuroinflammation, which is one of the defining hallmarks of AD (Morales et al., 2014). Indeed, there have been multiple studies targeting inflammation as a therapeutic treatment for AD. Specifically, microglia play an essential role in the initiation and progression of neuroinflammation. In fact, it is believed that the overactive and constant activation of microglia-mediated pruning could be the cause of the irreversible synaptic loss. Microglia is as an ideal target for studying the disease mechanism and is a “druggable” target. Microglia are the resident macrophages in the CNS. Using combination of genetic manipulation and in-vivo life microscopy imaging revealed that microglia constantly survey the neuronal milieu and when required phagocytose dendrites such as during development or when marked for pruning (Kettenmann et al., 2011). Together with the complement system, microglia play an essential role in synaptic pruning and sculpting of synapses in the brain (Hong et al., 2016b).
Sleep disruption and stress also affect microglia function. Sleep loss induces microglia activation and astrogliosis in the mouse cerebral cortex (Bellesi et al., 2017; Wisor et al., 2011). Furthermore, chronic, but not acute, sleep loss promotes microglia activation without neuroinflammation and loss of synapses (Bellesi et al., 2017). This loss of synapses, and eventually the loss of neurons, have been consistently reported and widely accepted as pathological hallmark of AD (Selkoe, 2002). Remarkably, a novel and non-invasive approach to activate microglia using light pulses (40 Hz) to the eyes significantly reduces Aβ plaque loads and improves cognitive function in mice. The mechanism is not fully understood but thought to activate microglia and phagocytose Aβ plaques in the visual cortex of an AD mouse model (Iaccarino et al., 2016). In addition, ablation of the prostaglandin E2 receptor, an activator of microglia, results in reduced AD pathology and improved cognitive function (Johansson et al., 2015). Physiological stress induces activation of microglia, and mice subjected to chronic restraint have more Iba-positive microglia, indicating more activated microglia proliferation (Wohleb et al., 2011; Tynan et al., 2010). Taken together, stressors such as sleep deprivation and circadian disruption activate microglia, which may result in excessive synaptic pruning and neuronal loss and ultimately AD.
4.3. Melatonin
Another important converging point between stress and circadian rhythm is melatonin. Melatonin regulates many circadian aspects including sleep-wake cycle and protects cells against oxidative stress. Contrary to conventional thoughts, other localized structures/organs also produce melatonin, such as the gut, skin, platelets, bone marrow and others (Bubenik, 2002; Slominski et al., 2002; Champier et al., 1997; Cardinali et al., 2003; Stefulj et al., 2001). Melatonin is synthesized from tryptophan by three key enzymes: tryptophan hydroxylase, arylalkylamine-N-acetyltransferase and hydroxyindole-O-methyl-transferase. The mRNA level of these three enzymes, and their corresponding protein levels, oscillate with the day/night rhythm (Bernard et al., 1999). Due to the lack of melatonin storage, the plasma melatonin concentration reflects the activity of the pineal gland. Human melatonin levels peak at around 03:00 AM and decline to a barely detectable level during the day (Claustrat et al., 1986). Melatonin increases SWS and possibly stimulates the release of growth hormone and neurotrophin (Monti et al., 1999).
Melatonin has many characteristics of a free radical scavenger, and is excellent at reducing oxidative stress (Galano et al., 2011). In particular, many studies have shown that melatonin protects neurons from secondary damage following ischemia (Manev et al., 1996; Koh, 2008; Kilic et al., 1999; Wakatsuki et al., 1999; Pappolla et al., 1997). Melatonin also protects primary neurons in vitro from damage when incubated with synthetic Aβ peptides through an undefined mechanism (Pappolla et al., 1997). Collectively, melatonin protects neurons against oxidative stress, ischemia, depression, and cognitive impairment. However, the effect of stress on melatonin production is largely unknown, although stress has been reported to decrease the level of melatonin in trout (Lopez-Patino et al., 2014).
The link between melatonin and AD has been well characterized; low melatonin levels at night were noticed in the aging population and correlated with cognitive impairment (Skene and Swaab, 2003) (Lin et al., 2013). The melatonin levels in the prefrontal cortex is inversely correlated with an individual's Braak pathology stage, such that lower melatonin associates with a higher Braak stage (indicating more severe AD) (Lin et al., 2013). In keeping with this, Aβ peptides impede melatonin synthesis in the pineal gland and interfere with melatonin receptor signaling via the ERK/MAPK pathway (Cecon et al., 2015). Given the relationship between melatonin and AD, melatonin has been examined as a treatment in AD. Such studies have reported positive, though modest, effects of melatonin in cognitive deficits and attenuating behavioral disturbances (Riemersma-van der Lek et al., 2008; Haffmans et al., 2001).
4.4. Hypocretin/orexin
Hypocretin (de Lecea et al., 1998) and orexin (Sakurai et al., 1998) are the same neuropeptide that were cloned in parallel by two independent research groups in 1998. One of the primary functions of orexin is to promote and stabilize wakefulness (Kilduff and Peyron, 2000; Sutcliffe and de Lecea, 2002). The orexinergic neurons in the hypothalamus project their fibers to many different nuclei that govern sleep-wake cycle such as the LC, septal nuclei, medullary reticular formation, and others (Peyron et al., 1998). Stressors such as foot shock and restraint increase c-Fos expression in hypocretinergic neurons; however, only restraint stress elicits an increase in orexin mRNA levels (Reyes et al., 2003). The effect of stress on increased c-Fos expression is nullified in CRH-Receptor 1 (CRHR1) knockout mice, suggesting that an increase in orexin occurs through activation of the CRH pathway (Winsky-Sommerer et al., 2004). Subsequent studies have shown that there is an intricate connection between orexin and the HPA axis. Furthermore, higher levels of interstitial Aβ were observed in forced wakefulness using orexin; conversely, lower levels of interstitial Aβ were observed in forced sleep using almorexant, an orexin antagonist (Kang et al., 2009). Moreover, deletion of orexin receptors in the APP/PS1/OR knockout mouse shows a significant reduction of Aβ plaque deposition in 3.5- and 8-month old mice. Mice lacking orexin receptors sleep significantly more while plaque deposition was significantly abated (Roh et al., 2014). When these orexin receptor knockout mice were sleep deprived, amyloid plaque burden worsened, further supporting the role of sleep on AD pathogenesis sleep. In addition, orexin has been shown to modulate circadian oscillation of AD risk genes such as APOE, ABCA1, BACE1, GSKβ, and others (Ma et al., 2016). In summary, these results demonstrate the role of orexin in sleep regulation, its interaction with stress through the HPA axis, and most recently that orexin regulates the oscillatory expression of the AD risk genes. These results warrant the necessity for further investigation of orexin in AD pathogenesis (Liguori et al., 2014).
4.5. ERK/MAPK signaling pathway
Memory impairment is the most prominent type of cognitive dysfunction and the first symptom in AD. Memory formation is a multistep process that requires encoding, consolidation, retrieval, reconsolidation, and extinction (Sindreu et al., 2007; Athos et al., 2002; Chen et al., 2005). The influence of stress on memory has been well studied (for reviews, see (Schwabe et al., 2012; Finsterwald and Alberini, 2014)). Briefly, short-term stress may enhance memory while chronic long-term stress impairs memory. Chronic stress elevates cortisol levels, which subsequently decrease the number of dendritic synapses. Stress and memory impairment are part of the vicious cycle, where memory impairment causes stress and stress causes memory impairment in AD patients.
The molecular pathways that intersect between memory and circadian rhythm has been intensively reviewed (Smarr et al., 2014; Xia and Storm, 2017; Eckel-Mahan, 2012). The ERK/MAPK pathway is at the center of memory consolidation. Notably, levels of phospho-ERK, cAMP and phospho-CREB, and the activity of PKA and MEK were observed to oscillate in a circadian manner (Eckel-Mahan et al., 2008). Remarkably, multiple approaches, including pharmacological, genetic and behavioral tests were utilized to demonstrate that the oscillation of cAMP/PKA/MAPK/CREB is crucial for memory maintenance. Moreover, the cAMP/PKA/ERK/CREB signaling pathway in the hippocampus is regulated by the SCN (Phan et al., 2011). Consistently, ablating the Bmal1 gene resulted in reduced Per1 and pERK levels, dysrhythmia in sleep-wake, and impaired spatial and associative memories compared to controls (Wardlaw et al., 2014). Interestingly, the cAMP/PKA/ERK/CREB signaling pathway is specifically increased during REM sleep (Luo et al., 2013). In addition, Per1 mediates nuclear shuttling of a CREB kinase, P90RSK, and plays a major role in memory formation (Rawashdeh et al., 2016). The role of APP and Aβ on memory are inconclusive, as many studies have shown that short term application of Aβ peptides has a positive effect on long-term potentiation (the molecular correlate of memory) and memory formation (Garcia-Osta and Alberini, 2009). Yet, long term effects of Aβ are detrimental to memory and cognition. In short, stress has been shown to effect memory through the ERK signaling pathway (Shen et al., 2004), and the circadian rhythm influences this same signaling pathway (Masters et al., 2015; Lleo et al., 2006; Inglis, 2002). Furthermore, in AD mouse model, ERK appears overactivated, and pharmacological inhibition of ERK improves memory (Feld et al., 2014). Downstream of the ERK pathway, AD mouse model shows reduction of pCREB level, which leads to a reduction of CRE-mediated transcription, resulting in recognition memory impairment (Bartolotti et al., 2016). Aß (1–42) reportedly interferes with the ERK signaling pathway and impairs working memory (Faucher et al., 2015). Taken together, the ERK signaling pathway is disrupted in AD perhaps due to Aß (1–42) inference binding (Hu et al., 2013). Therefore, the ERK/MAPK signaling pathway plays an integral role not only for memory consolidation but also is a common pathway downstream of stress, circadian rhythm and sleep.
4.6. Gut microbiome: the unexpected union of stress and circadian rhythm
The gut microbiome has recently become an intense field of research interest. Gut bacteria (1014) outnumber total cells in humans (3.7 × 1013), and their collective genome outnumbers the human genome 100 to 1 (Collins et al., 2012). The gut microbiota's density is 1012 cells/ml, and their cumulative mass is around 1.5 kg. Besides facilitating digestion and providing vitamin B and K, the gut microbiota plays many essential roles in inflammation and brain protection by secreting brain derived neurotrophic factor (Maqsood and Stone, 2016). In particular, the gut-brain axis has been implicated in neurodegenerative diseases such as Parkinson's disease and AD (Hill et al., 2014). Comprehensive reviews of the gut-microbiota are covered in these articles (Mancuso and Santangelo, 2018; de la Fuente-Nunez et al., 2018; Zhang et al., 2017). Remarkably, the gut microbiota display a circadian rhythm in both the amount and the composition of the microbiota examined at the transcriptomic level. Furthermore, when the host has been subjected to a jetlag circadian paradigm, the circadian rhythm of gut microbiota also changes. This indicates a crucial role of the SCN master clock on the circadian rhythm of gut microbiota (Thaiss et al., 2016). Although the role of the circadian rhythm on microbiota has only recently been examined, the role of stress on gut microbiota was explored many decades ago. Specifically, rats subjected to multiple stressors (i.e. wet cage bedding) for two days had decreased Lactobacilli and fusiform-shaped bacteria in the small and large bowels while the total number of anaerobic was unchanged (Tannock and Savage, 1974).
Recently, a potential association between gut microbiota and AD has begun to emerge, largely from studies of mouse models of AD. Several experiments have suggested that gut microbial dysbiosis may contribute to AD pathogenesis. First, the 16s rRNA sequence of the gut microbiome in an AD mouse model was significantly different from the microbiome of a control mice (Zhang et al., 2017). Second, treatment of a mouse model of AD with antibiotics ameliorates AD pathologies (Minter et al., 2016). Thus, immune-activated factors released from the gut microbiota are thought to cause inflammation in the brain and exacerbate AD pathology. Reducing the source of inflammation with a broad-spectrum antibiotic reduces Aβ plaques (Minter et al., 2016). Consistently, AD mice housed in a germ-free environment, essentially eliminating gut microbiota from birth, show a similar reduction in Aβ plaques (Harach et al., 2017). Remarkably, germ-free AD mice that received microbiota from an AD mouse housed in normal conditions had worse AD pathologies. In contrast, microbiota from control mice housed in normal conditions transplanted into germ-free AD mice decreases Aβ plaques. A bacteriotherapy approach of feeding probiotics to a mouse model of AD reduced Aβ plaques, inflammatory signals and attenuated cognitive decline (Bonfili et al., 2017). Finally, in a first human study, feeding probiotics to AD patients improved their cognitive functions (Akbari et al., 2016). Collectively, these results support the novel hypothesis that bacteriotherapy could potentially serve as a therapeutic intervention to delay the progress of AD. Both stress and circadian disruption have numerous effects on the gut microbiome (Maqsood and Stone, 2016), and a few initial studies indicate that modifying the gut microbiome has the potential to improve cognition in mouse models of AD and patients. The gut microbiome is therefore an important target in the search of modifying the disease course of AD.
5. Conclusion and future directions
In summary, SCRD can either independently or cooperatively with stress exacerbate AD pathology. SCRD and stress worsen AD pathology by concomitantly increasing production, decreasing clearance of Aβ, or both. SCRD and stress converge at multiple signaling pathways. Intriguingly, all of these converging points are under the control of sleep and the circadian rhythm. Thus, therapeutic strategies should incorporate new insights to approach through different lens, through which we can ameliorate SCRD in hope of abating the disease progress.
We envision that sleep and circadian rhythm disruption assessment might also be used as part of tool for detecting and mitigating risk for AD. Therapies aimed at improving sleep and circadian rhythm function, which by corollary decrease stress, may be necessary to prevent or limit progression of AD. Stress-reduction strategies can also improve sleep and perhaps could be helpful for preventing AD. Approaches to modifying circadian rhythm such as lengthening the photoperiod showed a significant increase of lifespan in a neurodegenerative disease mouse model (Morton, 2017), suggesting that circadian rhythm modification could provide a tangible therapeutic avenue. Alternatively, small molecules could be used to modulate the biological clock and mitigate SCRD (Chen et al., 2013c). Furthermore, interventional behavior modifying approaches such as, physical activity, and bright light therapy have shown limited successes (McCurry et al., 2005, 2011). Lastly, phototherapy in combination with melatonin has shown some successes in improving cognitive functions. Logically, combinatorial approach such as early detection of the disease, bright light therapy and melatonin to restore circadian rhythm, stress-reduction techniques, and modification of the gut microbiome, may be necessary to prevent and treat AD.
Acknowledgements
The authors have no disclosures relevant to this manuscript. Trongha Phan has received grant support from the National Institutes of Health as a Co-investigator. Roneil Malkani has received grant support from the National Institutes of Health, Illinois Department of Public Health, the Alzheimer's Association, and Northwestern University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.10.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Akbari E. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar R.A. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 2002;12(7):540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Alzheimer's A. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine . third ed. American Academy of Sleep Medicine; Darien, IL: 2014. International Classification of Sleep Disorders. [Google Scholar]
- Ancoli-Israel S. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Athos J. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat. Neurosci. 2002;5(11):1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- Barclay J.L. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2013;304(10):E1053–E1063. doi: 10.1152/ajpendo.00512.2012. [DOI] [PubMed] [Google Scholar]
- Bartolotti N., Segura L., Lazarov O. Diminished CRE-induced plasticity is linked to memory deficits in familial Alzheimer's disease mice. J. Alzheimers Dis. 2016;50(2):477–489. doi: 10.3233/JAD-150650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman R.J. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68(9):666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- Bellesi M. Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J. Neurosci. 2017;37(21):5263–5273. doi: 10.1523/JNEUROSCI.3981-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S. Measuring melatonin in humans. J Clin Sleep Med. 2008;4(1):66–69. [PMC free article] [PubMed] [Google Scholar]
- Bernard M. Melatonin synthesis pathway: circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reprod. Nutr. Dev. 1999;39(3):325–334. doi: 10.1051/rnd:19990305. [DOI] [PubMed] [Google Scholar]
- Bero A.W. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat. Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R.B. American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications, Darien, Illinois. [Google Scholar]
- Berson D.M., Dunn F.A., Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Beurel E., Nemeroff C.B. Interaction of stress, corticotropin-releasing factor, arginine vasopressin and behaviour. Curr Top Behav Neurosci. 2014;18:67–80. doi: 10.1007/7854_2014_306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Boyce R., Williams S., Adamantidis A. REM sleep and memory. Curr. Opin. Neurobiol. 2017;44:167–177. doi: 10.1016/j.conb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Brown R.E. Control of sleep and wakefulness. Physiol. Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brureau A. Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer's disease rat model. Neurobiol. Aging. 2013;34(5):1426–1439. doi: 10.1016/j.neurobiolaging.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik G.A. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig. Dis. Sci. 2002;47(10):2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- Buhr E.D., Yoo S.H., Takahashi J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T.M. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J. Sleep Res. 2015;24(4):364–371. doi: 10.1111/jsr.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali D.P. Melatonin effects on bone: experimental facts and clinical perspectives. J. Pineal Res. 2003;34(2):81–87. doi: 10.1034/j.1600-079x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Carroll J.C. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci. 2011;31(40):14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano J.M. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 2016;18(5):421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecon E. Amyloid beta peptide directly impairs pineal gland melatonin synthesis and melatonin receptor signaling through the ERK pathway. Faseb. J. 2015;29(6):2566–2582. doi: 10.1096/fj.14-265678. [DOI] [PubMed] [Google Scholar]
- Cedernaes J. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer's disease. Sleep Med. Rev. 2017;31:102–111. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N. Circadian clock gene expression in brain regions of Alzheimer 's disease patients and control subjects. J. Biol. Rhythm. 2011;26(2):160–170. doi: 10.1177/0748730410395732. [DOI] [PubMed] [Google Scholar]
- Champier J. Evidence for tryptophan hydroxylase and hydroxy-indol-O-methyl-transferase mRNAs in human blood platelets. Life Sci. 1997;60(24):2191–2197. doi: 10.1016/s0024-3205(97)00234-8. [DOI] [PubMed] [Google Scholar]
- Chen X. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat. Neurosci. 2005;8(7):925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Chen H.F. Polymorphism of CLOCK gene rs 4580704 C > G is associated with susceptibility of Alzheimer's disease in a Chinese population. Arch. Med. Res. 2013;44(3):203–207. doi: 10.1016/j.arcmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Chen Q. Functional CLOCK gene rs1554483 G/C polymorphism is associated with susceptibility to Alzheimer's disease in the Chinese population. J. Int. Med. Res. 2013;41(2):340–346. doi: 10.1177/0300060513476430. [DOI] [PubMed] [Google Scholar]
- Chen Z., Yoo S.H., Takahashi J.S. Small molecule modifiers of circadian clocks. Cell. Mol. Life Sci. 2013;70(16):2985–2998. doi: 10.1007/s00018-012-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. Association between ARNTL (BMAL1) rs2278749 polymorphism T >C and susceptibility to Alzheimer disease in a Chinese population. Genet. Mol. Res. 2015;14(4):18515–18522. doi: 10.4238/2015.December.23.39. [DOI] [PubMed] [Google Scholar]
- Cho K. Chronic 'jet lag' produces temporal lobe atrophy and spatial cognitive deficits. Nat. Neurosci. 2001;4(6):567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Cho K. Chronic jet lag produces cognitive deficits. J. Neurosci. 2000;20(6):RC66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.C. Afferents to the ventrolateral preoptic nucleus. J. Neurosci. 2002;22(3):977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G.P. Ultradian, circadian, and stress-related hypothalamic-pituitary-adrenal axis activity–a dynamic digital-to-analog modulation. Endocrinology. 1998;139(2):437–440. doi: 10.1210/endo.139.2.5857. [DOI] [PubMed] [Google Scholar]
- Chrousos G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Chung S., Son G.H., Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim. Biophys. Acta. 2011;1812(5):581–591. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Cirrito J.R. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Claustrat B. A once-repeated study of nocturnal plasma melatonin patterns and sleep recordings in six normal young men. J. Pineal Res. 1986;3(4):301–310. doi: 10.1111/j.1600-079x.1986.tb00753.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. Diurnal fluctuations in HPA and neuropeptide Y-ergic systems underlie differences in vulnerability to traumatic stress responses at different zeitgeber times. Neuropsychopharmacology. 2015;40(3):774–790. doi: 10.1038/npp.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Coogan A.N. The circadian system in Alzheimer's disease: disturbances, mechanisms, and opportunities. Biol. Psychiatry. 2013;74(5):333–339. doi: 10.1016/j.biopsych.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Csernansky J.G. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am. J. Psychiatry. 2006;163(12):2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler C.A. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- D'Almeida V. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9(12):2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- Dai J. Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. J. Comp. Neurol. 1998;400(1):87–102. doi: 10.1002/(sici)1096-9861(19981012)400:1<87::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Brunner D.P., Borbely A.A. Time course of EEG power density during long sleep in humans. Am. J. Physiol. 1990;258(3 Pt 2):R650–R661. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- Djonlagic I. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen H.P. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Dunn J., Scheving L., Millet P. Circadian variation in stress-evoked increases in plasma corticosterone. Am. J. Physiol. 1972;223(2):402–406. doi: 10.1152/ajplegacy.1972.223.2.402. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K.L. Circadian oscillations within the Hippocampus support memory formation and persistence. Front. Mol. Neurosci. 2012;5:46. doi: 10.3389/fnmol.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan K.L. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat. Neurosci. 2008;11(9):1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K.M. Obstructive sleep apnea and neurocognitive performance: the role of cortisol. Sleep Med. 2014;15(1):27–32. doi: 10.1016/j.sleep.2013.08.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Hannibal J., Georg B. Diurnal rhythmicity of the canonical clock genes Per1, Per2 and Bmal1 in the rat adrenal gland is unaltered after hypophysectomy. J. Neuroendocrinol. 2008;20(3):323–329. doi: 10.1111/j.1365-2826.2008.01651.x. [DOI] [PubMed] [Google Scholar]
- Faucher P. Hippocampal injections of oligomeric amyloid beta-peptide (1-42) induce selective working memory deficits and long-lasting alterations of ERK signaling pathway. Front. Aging Neurosci. 2015;7:245. doi: 10.3389/fnagi.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld M. Decrease of ERK/MAPK overactivation in prefrontal cortex reverses early memory deficit in a mouse model of Alzheimer's disease. J. Alzheimers Dis. 2014;40(1):69–82. doi: 10.3233/JAD-131076. [DOI] [PubMed] [Google Scholar]
- De Felice F.G. Alzheimer's disease and insulin resistance: translating basic science into clinical applications. J. Clin. Invest. 2013;123(2):531–539. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R. Melanopsin retinal ganglion cell loss and circadian dysfunction in Alzheimer's disease (Review) Mol. Med. Rep. 2016;13(4):3397–3400. doi: 10.3892/mmr.2016.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiro M.G. Field measurements of light exposures and circadian disruption in two populations of older adults. J. Alzheimers Dis. 2012;31(4):711–715. doi: 10.3233/JAD-2012-120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwald C., Alberini C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Nunez C. Neuromicrobiology: how microbes influence the brain. ACS Chem. Neurosci. 2018;9(2):141–150. doi: 10.1021/acschemneuro.7b00373. [DOI] [PubMed] [Google Scholar]
- Futch H.S. Targeting psychologic stress signaling pathways in Alzheimer's disease. Mol. Neurodegener. 2017;12(1):49. doi: 10.1186/s13024-017-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A., Tan D.X., Reiter R.J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 2011;51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A., Alberini C.M. Amyloid beta mediates memory formation. Learn. Mem. 2009;16(4):267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner J.R. Amyloid-beta induces sleep fragmentation that is rescued by fatty acid binding proteins in Drosophila. J. Neurosci. Res. 2017;95(8):1548–1564.1. doi: 10.1002/jnr.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E.M. Experimental 'jet lag' inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5(12):e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M., Weinberg M.S., Spencer R.L. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am. J. Physiol. Endocrinol. Metab. 2009;296(4):E888–E897. doi: 10.1152/ajpendo.90946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G.G., Wong C.W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalez B. REM sleep loss and recovery regulates blood-brain barrier function. Curr. Neurovascular Res. 2013;10(3):197–207. doi: 10.2174/15672026113109990002. [DOI] [PubMed] [Google Scholar]
- Gooley J.J. Melanopsin in cells of origin of the retinohypothalamic tract. Nat. Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Gozal D. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J. Neurochem. 2003;86(6):1545–1552. doi: 10.1046/j.1471-4159.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- Greenberg M.S. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10(3 Suppl. l):S155–S165. doi: 10.1016/j.jalz.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Haffmans P.M. Bright light therapy and melatonin in motor restless behaviour in dementia: a placebo-controlled study. Int. J. Geriatr. Psychiatr. 2001;16(1):106–110. doi: 10.1002/1099-1166(200101)16:1<106::aid-gps288>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hagewoud R. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J. Sleep Res. 2011;20(2):259–266. doi: 10.1111/j.1365-2869.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- Harach T. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. The discovery of Alzheimer-causing mutations in the APP gene and the formulation of the "amyloid cascade hypothesis". FEBS J. 2017;284(7):1040–1044. doi: 10.1111/febs.14004. [DOI] [PubMed] [Google Scholar]
- Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harper D.G. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131(Pt 6):1609–1617. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart N.J. Ocular indicators of Alzheimer's: exploring disease in the retina. Acta Neuropathol. 2016;132(6):767–787. doi: 10.1007/s00401-016-1613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek T. Increased plasma and lipoprotein lipid peroxidation in apo E-deficient mice. Biochem. Biophys. Res. Commun. 1994;201(3):1567–1574. doi: 10.1006/bbrc.1994.1883. [DOI] [PubMed] [Google Scholar]
- He J. Sleep restriction impairs blood-brain barrier function. J. Neurosci. 2014;34(44):14697–14706. doi: 10.1523/JNEUROSCI.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 2015;18(6):794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- Hill J.M. The gastrointestinal tract microbiome and potential link to Alzheimer's disease. Front. Neurol. 2014;5:43. doi: 10.3389/fneur.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holth J., Patel T., Holtzman D.M. Sleep in Alzheimer's disease - beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2017;2:4–14. doi: 10.1016/j.nbscr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Dissing-Olesen L., Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.S. Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3beta, neurotrophin-3 and CREB signaling. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.W. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. J. Clin. Neurosci. 2009;16(10):1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Huang Y. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch. Neurol. 2012;69(1):51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino H.F. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540(7632):230–235. doi: 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002;(127):45–63. [PubMed] [Google Scholar]
- Johansson L. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(Pt 8):2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Johansson J.U. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer's disease models. J. Clin. Invest. 2015;125(1):350–364. doi: 10.1172/JCI77487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E.Y. Localized cortical thinning in patients with obstructive sleep apnea syndrome. Sleep. 2013;36(8):1153–1162. doi: 10.5665/sleep.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.E. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.E., Lucey B.P., Holtzman D.M. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat. Rev. Neurol. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.E. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann. Neurol. 2016;80(1):154–159. doi: 10.1002/ana.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.S. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain. 2017;140(8):2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142(6):2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011;585(10):1412–1426. doi: 10.1016/j.febslet.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F. APP processing and synaptic function. Neuron. 2003;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T., Xu H., Bu G. ApoE and Abeta in Alzheimer's disease: accidental encounters or partners? Neuron. 2014;81(4):740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.E. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc. Natl. Acad. Sci. U. S. A. 2007;104(25):10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.E. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant G.J., Mougey E.H., Meyerhoff J.L. Diurnal variation in neuroendocrine response to stress in rats: plasma ACTH, beta-endorphin, beta-LPH, corticosterone, prolactin and pituitary cyclic AMP responses. Neuroendocrinology. 1986;43(3):383–390. doi: 10.1159/000124553. [DOI] [PubMed] [Google Scholar]
- Karran E., De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J. Neurochem. 2016;139(Suppl. 2):237–252. doi: 10.1111/jnc.13632. [DOI] [PubMed] [Google Scholar]
- Kettenmann H. Physiology of microglia. Physiol. Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Khalsa S.B. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T.K., Alkon D.L. Alzheimer's disease cerebrospinal fluid and neuroimaging biomarkers: diagnostic accuracy and relationship to drug efficacy. J. Alzheimers Dis. 2015;46(4):817–836. doi: 10.3233/JAD-150238. [DOI] [PubMed] [Google Scholar]
- Kilduff T.S., Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23(8):359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Kilic E. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cerebr. Blood Flow Metabol. 1999;19(5):511–516. doi: 10.1097/00004647-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Dimsdale J.E. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav. Sleep Med. 2007;5(4):256–278. doi: 10.1080/15402000701557383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk W.E. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Ko C.H., Takahashi J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006;15 Spec(2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Koh P.O. Melatonin attenuates the focal cerebral ischemic injury by inhibiting the dissociation of pBad from 14-3-3. J. Pineal Res. 2008;44(1):101–106. doi: 10.1111/j.1600-079X.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- Kolbe I., Dumbell R., Oster H. Circadian clocks and the interaction between stress Axis and adipose function. Int. J. Endocrinol. 2015;2015:693204. doi: 10.1155/2015/693204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratova A.A., Kondratov R.V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 2012;13(5):325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronyo Y. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer's disease. JCI Insight. 2017;2(16) doi: 10.1172/jci.insight.93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronyo-Hamaoui M. Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(Suppl. 1):S204–S217. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H.C., Lyons L.C. Synchrony and desynchrony in circadian clocks: impacts on learning and memory. Learn. Mem. 2015;22(9):426–437. doi: 10.1101/lm.038877.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R. Exaggerated phosphorylation of brain tau protein in CRH KO mice exposed to repeated immobilization stress. Stress. 2016;19(4):395–405. doi: 10.1080/10253890.2016.1183119. [DOI] [PubMed] [Google Scholar]
- Lambert J.C. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat. Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia K.A. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480(7378):552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger D. Slow-wave sleep: from the cell to the clinic. Sleep Med. Rev. 2018;41:113–132. doi: 10.1016/j.smrv.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Leliavski A. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology. 2014;155(1):133–142. doi: 10.1210/en.2013-1531. [DOI] [PubMed] [Google Scholar]
- Lewy A.J., Sack R.L. The dim light melatonin onset as a marker for circadian phase position. Chronobiol. Int. 1989;6(1):93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- Lewy A.J. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol. Int. 1998;15(1):71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Liguori C. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- Lim A.S. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.M., Gerstner J.R., Holtzman D.M. The sleep-wake cycle and Alzheimer's disease: what do we know? Neurodegener. Dis. Manag. 2014;4(5):351–362. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A.S. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain. 2014;137(Pt 10):2847–2861. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. Melatonin in Alzheimer's disease. Int. J. Mol. Sci. 2013;14(7):14575–14593. doi: 10.3390/ijms140714575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.-G. Lesions of suprachiasmatic nucleus modify sleep structure but do not alter the total amount of daily sleep in rats. Sleep Biol. Rhythm. 2012;10(4):293–301. [Google Scholar]
- Liu C.C. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A., Greenberg S.M., Growdon J.H. Current pharmacotherapy for Alzheimer's disease. Annu. Rev. Med. 2006;57:513–533. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- Lopez-Patino M.A. Stress inhibition of melatonin synthesis in the pineal organ of rainbow trout (Oncorhynchus mykiss) is mediated by cortisol. J. Exp. Biol. 2014;217(Pt 8):1407–1416. doi: 10.1242/jeb.087916. [DOI] [PubMed] [Google Scholar]
- Lucey B.P., Bateman R.J. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer's disease pathogenesis. Neurobiol. Aging. 2014;35(Suppl. 2):S29–S34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Lucey B.P. Effect of sleep on overnight cerebrospinal fluid amyloid beta kinetics. Ann. Neurol. 2018;83(1):197–204. doi: 10.1002/ana.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation. J. Neurosci. 2013;33(15):6460–6468. doi: 10.1523/JNEUROSCI.5018-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Jiang W., Zhang E.E. Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer's disease-risk genes. Sci. Rep. 2016;6:36035. doi: 10.1038/srep36035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A. Chronic stress as a risk factor for Alzheimer's disease. Rev. Neurosci. 2014;25(6):785–804. doi: 10.1515/revneuro-2014-0035. [DOI] [PubMed] [Google Scholar]
- Mahley R.W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mancuso C., Santangelo R. Alzheimer's disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018;129:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Mander B.A., Winer J.R., Walker M.P. Sleep and human aging. Neuron. 2017;94(1):19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H. Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. Faseb. J. 1996;10(13):1546–1551. doi: 10.1096/fasebj.10.13.8940301. [DOI] [PubMed] [Google Scholar]
- Maqsood R., Stone T.W. The gut-brain Axis, BDNF, NMDA and CNS disorders. Neurochem. Res. 2016;41(11):2819–2835. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- Masters C.L. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U. S. A. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C.L. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- Mathangi D.C., Shyamala R., Subhashini A.S. Effect of REM sleep deprivation on the antioxidant status in the brain of Wistar rats. Ann. Neurosci. 2012;19(4):161–164. doi: 10.5214/ans.0972.7531.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M., Harley C.W. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cognit. Sci. 2016;20(3):214–226. doi: 10.1016/j.tics.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J., Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol. Metabol. 2016;27(4):192–203. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry S.M. Nighttime insomnia treatment and education for Alzheimer's disease: a randomized, controlled trial. J. Am. Geriatr. Soc. 2005;53(5):793–802. doi: 10.1111/j.1532-5415.2005.53252.x. [DOI] [PubMed] [Google Scholar]
- McCurry S.M. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: results of a randomized, controlled trial. J. Am. Geriatr. Soc. 2011;59(8):1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt R.A. Stress-induced activity in the locus coeruleus is not sensitive to stressor controllability. Brain Res. 2009;1285:109–118. doi: 10.1016/j.brainres.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S.C. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34(10):504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol. 2014;33(11):1430–1434. doi: 10.1037/a0034219. [DOI] [PubMed] [Google Scholar]
- Minter M.R. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci. Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M., Smith J.D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat. Genet. 1996;14(1):55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- Montagne A. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A., Zhao Z., Zlokovic B.V. Alzheimer's disease: a matter of blood-brain barrier dysfunction? J. Exp. Med. 2017;214(11):3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J.M. Polysomnographic study of the effect of melatonin on sleep in elderly patients with chronic primary insomnia. Arch. Gerontol. Geriatr. 1999;28(2):85–98. doi: 10.1016/s0167-4943(98)00129-0. [DOI] [PubMed] [Google Scholar]
- Morales I. Neuroinflammation in the pathogenesis of Alzheimer's disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Morgia C G.R., Sambati L., Provini F., Ross-Cisneros F.N., Pan B., Barboni P., Avanzini P., Cantalupo G., Hannibal J., Sadun A., Carelli V. Melanopsin retinal ganglion cells and circadian dysfunction in Alzheimer's disease. Acta Ophthalmol. 2013;91(Suppl. s252):0. [Google Scholar]
- La Morgia C. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 2016;79(1):90–109. doi: 10.1002/ana.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouk K., Aungier J., Morton J. Prolonged day length exposure improves circadian deficits and survival in a transgenic mouse model of Huntington's disease. Neurobiol. Sleep Circadian Rhythms. 2017;2(January 2017):27–38. doi: 10.1016/j.nbscr.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa A.A. Genetic underpinnings in Alzheimer's disease - a review. Rev. Neurosci. 2018;29(1):21–38. doi: 10.1515/revneuro-2017-0036. [DOI] [PubMed] [Google Scholar]
- Mravec B., Horvathova L., Padova A. Brain under stress and Alzheimer's disease. Cell. Mol. Neurobiol. 2018;38(1):73–84. doi: 10.1007/s10571-017-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A. Alzheimer amyloid hypothesis lives on. Nat. Rev. Drug Discov. 2016;16(1):3–5. doi: 10.1038/nrd.2016.281. [DOI] [PubMed] [Google Scholar]
- Mullard A. BACE inhibitor bust in Alzheimer trial. Nat. Rev. Drug Discov. 2017;16(3):155. doi: 10.1038/nrd.2017.43. [DOI] [PubMed] [Google Scholar]
- Musiek E.S. Circadian clock disruption in neurodegenerative diseases: cause and effect? Front. Pharmacol. 2015;6:29. doi: 10.3389/fphar.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: time, space and 'wingmen. Nat. Neurosci. 2015;18(6):800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek E.S., Holtzman D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119(5):693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Nair D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature. 2018;554(7691):249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- Ng K.M., Lau C.F., Fung M.L. Melatonin reduces hippocampal beta-amyloid generation in rats exposed to chronic intermittent hypoxia. Brain Res. 2010;1354:163–171. doi: 10.1016/j.brainres.2010.07.044. [DOI] [PubMed] [Google Scholar]
- Ning A. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest. Ophthalmol. Vis. Sci. 2008;49(11):5136–5143. doi: 10.1167/iovs.08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novati A. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31(11):1579–1585. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- O'Bryant S.E. A blood screening test for Alzheimer's disease. Alzheimers Dement (Amst) 2016;3:83–90. doi: 10.1016/j.dadm.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metabol. 2006;4(2):163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pappolla M.A. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 1997;17(5):1683–1690. doi: 10.1523/JNEUROSCI.17-05-01683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.J. The stress response neuropeptide CRF increases amyloid-beta production by regulating gamma-secretase activity. EMBO J. 2015;34(12):1674–1686. doi: 10.15252/embj.201488795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk A.C. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci. Biobehav. Rev. 2008;32(1):99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever J., Luppi P.H., Montplaisir J. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014;37(5):279–288. doi: 10.1016/j.tins.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Peng W. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol. Dis. 2016;93:215–225. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard P.E. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Derex L. Sleep and Alzheimer's disease. Sleep Med. Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Peyron C. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.X. The diurnal oscillation of MAP (mitogen-activated protein) kinase and adenylyl cyclase activities in the hippocampus depends on the suprachiasmatic nucleus. J. Neurosci. 2011;31(29):10640–10647. doi: 10.1523/JNEUROSCI.6535-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N. Therapeutic implications of HPA axis abnormalities in Alzheimer's disease: review and update. Psychopharmacol. Bull. 2003;37(2):120–134. [PubMed] [Google Scholar]
- Pratico D. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21(12):4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M.J. Alzheimer's Disease International; 2015. World Alzheimer Report 2015: the Global Impact of Dementia: an Analysis of Prevalence, Incidence, Cost and Trends. [Google Scholar]
- Ramanathan L. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13(11):1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramassamy C. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer's disease is related to the apolipoprotein E genotype. Free Radic. Biol. Med. 1999;27(5–6):544–553. doi: 10.1016/s0891-5849(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Rasch B., Born J. About sleep's role in memory. Physiol. Rev. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawashdeh O. Period1 gates the circadian modulation of memory-relevant signaling in mouse hippocampus by regulating the nuclear shuttling of the CREB kinase pP90RSK. J. Neurochem. 2016;138(5):731–745. doi: 10.1111/jnc.13689. [DOI] [PubMed] [Google Scholar]
- Regen F. Neuroinflammation and Alzheimer's disease: implications for microglial activation. Curr. Alzheimer Res. 2017;14(11):1140–1148. doi: 10.2174/1567205014666170203141717. [DOI] [PubMed] [Google Scholar]
- Reyes T.M. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J. Neurosci. 2003;23(13):5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S. Stress-induced cytokines and neuronal dysfunction in Alzheimer's disease. J. Alzheimers Dis. 2012;28(1):11–24. doi: 10.3233/JAD-2011-110821. [DOI] [PubMed] [Google Scholar]
- Riemersma-van der Lek R.F. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. J. Am. Med. Assoc. 2008;299(22):2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- Roh J.H. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer's disease pathology. Sci. Transl. Med. 2012;4(150) doi: 10.1126/scitranslmed.3004291. 150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.H. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J. Exp. Med. 2014;211(13):2487–2496. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 2011;108(32):13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R.L. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J. Clin. Endocrinol. Metab. 1992;75(1):127–134. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5):1. doi: 10.1016/s0092-8674(02)09256-5. page following 696. [DOI] [PubMed] [Google Scholar]
- Sala Frigerio C., De Strooper B. Alzheimer's disease mechanisms and emerging roads to novel therapeutics. Annu. Rev. Neurosci. 2016;39:57–79. doi: 10.1146/annurev-neuro-070815-014015. [DOI] [PubMed] [Google Scholar]
- Sanford L.D., Suchecki D., Meerlo P. Stress, arousal, and sleep. Curr Top Behav Neurosci. 2015;25:379–410. doi: 10.1007/7854_2014_314. [DOI] [PubMed] [Google Scholar]
- Saper C.B. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28(3):152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Saper C.B., Cano G., Scammell T.E. Homeostatic, circadian, and emotional regulation of sleep. J. Comp. Neurol. 2005;493(1):92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Saper C.B. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A., Iijima K.M. Roles of tau pathology in the locus coeruleus (LC) in age-associatedpathophysiology and Alzhei- mer’s disease pathogenesis: potential strategies to protect the LCagainst aging. Brain Res. 2017;1702:17–28. doi: 10.1016/j.brainres.2017.12.027. [DOI] [PubMed] [Google Scholar]
- Scheer F.A. Plasticity of the intrinsic period of the human circadian timing system. PLoS One. 2007;2(8):e721. doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111(7):919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- Schneider F. Behavioral and EEG changes in male 5xFAD mice. Physiol. Behav. 2014;135:25–33. doi: 10.1016/j.physbeh.2014.05.041. [DOI] [PubMed] [Google Scholar]
- Schwabe L. Stress effects on memory: an update and integration. Neurosci. Biobehav. Rev. 2012;36(7):1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi M. Increased fragmentation of sleep-wake cycles in the 5XFAD mouse model of Alzheimer's disease. Neuroscience. 2015;290:80–89. doi: 10.1016/j.neuroscience.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman L.P. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288(5468):1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- Shen C.P. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin J.E. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271(5246):216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Shi L. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2017 doi: 10.1016/j.smrv.2017.06.010. (in press) [DOI] [PubMed] [Google Scholar]
- Shiota S. Chronic intermittent hypoxia/reoxygenation facilitate amyloid-beta generation in mice. J. Alzheimers Dis. 2013;37(2):325–333. doi: 10.3233/JAD-130419. [DOI] [PubMed] [Google Scholar]
- Sindreu C.B., Scheiner Z.S., Storm D.R. Ca2+ -stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53(1):79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene D.J., Swaab D.F. Melatonin rhythmicity: effect of age and Alzheimer's disease. Exp. Gerontol. 2003;38(1–2):199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- Slominski A. Serotoninergic and melatoninergic systems are fully expressed in human skin. Faseb. J. 2002;16(8):896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- Smarr B.L. A time to remember: the role of circadian clocks in learning and memory. Behav. Neurosci. 2014;128(3):283–303. doi: 10.1037/a0035963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son G.H. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20970–20975. doi: 10.1073/pnas.0806962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. Abeta-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer's disease. Mol. Neurodegener. 2015;10:13. doi: 10.1186/s13024-015-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.A. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7(4):367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Sprecher K.E. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89(5):445–453. doi: 10.1212/WNL.0000000000004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefulj J. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001;30(4):243–247. doi: 10.1034/j.1600-079x.2001.300408.x. [DOI] [PubMed] [Google Scholar]
- Stephens M.A., Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468–483. [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R. Sleep disturbance is associated with incident dementia and mortality. Curr. Alzheimer Res. 2013;10(7):767–775. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- Stopa E.G. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J. Neuropathol. Exp. Neurol. 1999;58(1):29–39. doi: 10.1097/00005072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Sultana R., Butterfield D.A. Role of oxidative stress in the progression of Alzheimer's disease. J. Alzheimers Dis. 2010;19(1):341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J.G., de Lecea L. The hypocretins: setting the arousal threshold. Nat. Rev. Neurosci. 2002;3(5):339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Swaab D.F., Fliers E., Partiman T.S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342(1):37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Swaab D.F. Increased cortisol levels in aging and Alzheimer's disease in postmortem cerebrospinal fluid. J. Neuroendocrinol. 1994;6(6):681–687. doi: 10.1111/j.1365-2826.1994.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Swanwick G.R. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer's disease: lack of association between longitudinal and cross-sectional findings. Am. J. Psychiatry. 1998;155(2):286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- Szymusiak R., McGinty D. Hypothalamic regulation of sleep and arousal. Ann. N. Y. Acad. Sci. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- Taheri S. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS One. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J.S. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B. Manipulation of microglial activity as a therapy for Alzheimer's disease. Front Biosci (Schol Ed) 2012;4:1402–1412. doi: 10.2741/s342. [DOI] [PubMed] [Google Scholar]
- Tannock G.W., Savage D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 1974;9(3):591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J. Comp. Neurol. 1999;406(2):171–182. [PubMed] [Google Scholar]
- Thaiss C.A. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167(6):1495–1510 e12. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tranah G.J. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek F.W. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan R.J. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010;24(7):1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Ulrich J.D. In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol. Neurodegener. 2013;8:13. doi: 10.1186/1750-1326-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanItallie T.B. Alzheimer's disease: innate immunity gone awry? Metabolism. 2017;69S:S41–S49. doi: 10.1016/j.metabol.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Vgontzas A.N. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999;84(8):2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Vgontzas A.N. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J. Clin. Endocrinol. Metab. 2007;92(11):4199–4207. doi: 10.1210/jc.2007-0774. [DOI] [PubMed] [Google Scholar]
- Volicer L. Sundowning and circadian rhythms in Alzheimer's disease. Am. J. Psychiatry. 2001;158(5):704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- Voultsios A., Kennaway D.J., Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J. Biol. Rhythm. 1997;12(5):457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Wakatsuki A. Melatonin protects against ischemia and reperfusion-induced oxidative lipid and DNA damage in fetal rat brain. J. Pineal Res. 1999;26(3):147–152. doi: 10.1111/j.1600-079x.1999.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Walsh C.M. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep. 2014;37(12):2009–2016. doi: 10.5665/sleep.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.L. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann. Neurol. 2015;78(2):317–322. doi: 10.1002/ana.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw S.M. Genetic disruption of the core circadian clock impairs hippocampus-dependent memory. Learn. Mem. 2014;21(8):417–423. doi: 10.1101/lm.035451.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissova K. Moderate changes in the circadian system of Alzheimer's disease patients detected in their home environment. PLoS One. 2016;11(1):e0146200. doi: 10.1371/journal.pone.0146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz A.T. Effects of sleep inertia on cognition. J. Am. Med. Assoc. 2006;295(2):163–164. doi: 10.1001/jama.295.2.163. [DOI] [PubMed] [Google Scholar]
- Westerberg C.E. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2012;18(3):490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens K.A. Slow-wave activity enhancement to improve cognition. Trends Neurosci. 2018;41(7):470–482. doi: 10.1016/j.tins.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle R.J. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology. 1998;139(2):443–450. doi: 10.1210/endo.139.2.5721. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 2004;24(50):11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor J.P., Schmidt M.A., Clegern W.C. Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. Sleep. 2011;34(3):261–272. doi: 10.1093/sleep/34.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol. Psychiatry. 1990;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- Wohleb E.S. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.P., Lowry C.A., Lebourgeois M.K. Circadian and wakefulness-sleep modulation of cognition in humans. Front. Mol. Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.P., Jr. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015;47:24–34. doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J.K. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. 1999;277(4 Pt 2):R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- Xia Z., Storm D. Role of circadian rhythm and REM sleep for memory consolidation. Neurosci. Res. 2017;118:13–20. doi: 10.1016/j.neures.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.K. The polymorphism of CLOCK gene 3111T/C C>T is associated with susceptibility of Alzheimer disease in Chinese population. J. Invest. Med. 2013;61(7):1084–1087. doi: 10.2310/JIM.0b013e31829f91c0. [DOI] [PubMed] [Google Scholar]
- Yoder J.M., Brandeland M., Engeland W.C. Phase-dependent resetting of the adrenal clock by ACTH in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306(6):R387–R393. doi: 10.1152/ajpregu.00519.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn. J. Ophthalmol. 2005;49(2):106–108. doi: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- Yu J.T., Tan L., Hardy J. Apolipoprotein E in Alzheimer's disease: an update. Annu. Rev. Neurosci. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- Zhang L. Altered gut microbiota in a mouse model of Alzheimer's disease. J. Alzheimers Dis. 2017;60(4):1241–1257. doi: 10.3233/JAD-170020. [DOI] [PubMed] [Google Scholar]
- Zhao N. Apolipoprotein E, receptors, and modulation of Alzheimer's disease. Biol. Psychiatry. 2018;83(4):347–357. doi: 10.1016/j.biopsych.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.