Abstract
A 66-year-old man was admitted for congestive heart failure with tachycardiac atrial fibrillation (AF). Heart failure was improved by diuretics, but control of heart rate by verapamil was insufficient, and bisoprolol was prescribed. After taking 2.5 mg of bisoprolol, he developed a general malaise followed by sinus bradycardia and shock. In addition to catecholamines, the patient was treated with intra-aortic balloon pumping and a pacemaker. With intensive therapy, the general condition was improved, and acute elevation of liver enzymes after bisoprolol was normalized by the 17th hospital day. The blood sample taken 30 h after the intake of bisoprolol showed abnormally high levels. Although the patient was CYP2D6*10 heterozygote, the precise mechanism for excess accumulation of bisoprolol and refractory shock remains unknown.
<Learning objective: Bisoprolol has been used for heart failure, but it may need caution to avoid hemodynamic deterioration. In our case, refractory shock and acute liver injury were induced by bisoprolol. The blood concentration was excessively high. The patient was CYP2D6*10 heterozygote, but the precise mechanism and shock are to be studied.>
Keywords: Bisoprolol, Cardiogenic shock, CYP2D6 polymorphism
Introduction
The beneficial long-term outcomes through bisoprolol have been confirmed in chronic heart failure (HF) [1], and the beneficial outcomes are related mainly to heart rate reduction 2, 3.
Rapid atrial fibrillation (AF) can be a cause of acute HF in patients with preserved heart function [4], and control of heart rate would be essential in them 5, 6.
We experienced a case with severe refractory shock and bradycardia which were induced by excessive accumulation of bisoprolol given for the rate control of AF.
Case report
A 66-year-old male visited our hospital for suddenly developed dyspnea. He had been treated for hypertension, diabetes, dyslipidemia, and AF. On physical examination, his blood pressure was 160/80 mmHg. Coarse crackles were heard diffusely in both lungs, but there was no evidence of neck vein distension or peripheral edema.
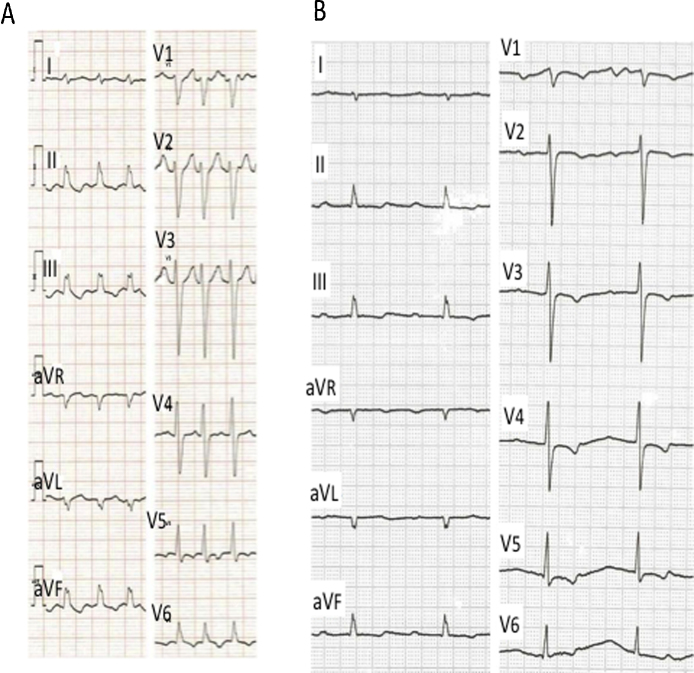
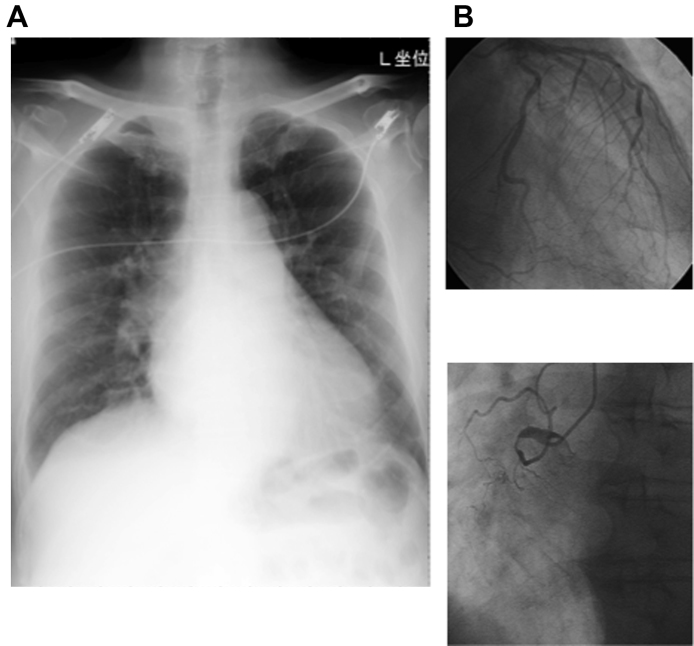
His electrocardiogram (ECG) showed AF with a heart rate of about 150 bpm and wide QRS complexes suggesting intraventricular conduction delay (Fig. 1A). The chest X-ray revealed an increased cardio-thoracic ratio to 57%, pulmonary congestion, and pleural effusion (Fig. 2A). Echocardiography revealed a left ventricle with end-systolic diameter 51 mm, and diffusely reduced left ventricular wall motion with an ejection fraction of 25%. Moderate regurgitation was observed in mitral, tricuspid, and aortic valves. He was admitted for treatment of acute HF.
Fig. 1.
Electrocardiograms. (A) Electrocardiogram on admission showed atrial fibrillation with ventricular rate of 150 bpm. The wide QRS complexes and ST–T changes were evident. (B) After bisoprolol, atrial fibrillation was converted to normal sinus rhythm at 52 beats/min with prolonged PR interval (400 ms). ST depression is evident in inferior and precordial leads.
Fig. 2.
The chest X ray and the coronary angiograms. (A) The heart was enlarged with pulmonary congestion. (B) Coronary angiography revealed total occlusion in the proximal site of the right coronary artery, and about 75% stenosis in the left anterior descending artery. The collaterals to the right were well developed.
Complete blood cell counts showed a decrease in platelets (12.3 × 104/μL). C-reactive protein was normal (0.28 mg/dl). Lactic dihydrogenase (LDH = 355 U/L), alkaline phosphatase (ALP = 257 U/L), and γ-glutamyl transpeptidase (γ-GTP = 76 U/L) were mildly elevated. Creatine kinase and MB fraction were 242 U/L and 20 ng/ml, respectively. Serum creatinine was 1.29 mg/dl with estimated glomerular filtration rate of 41.3 ml/m/1.73 m2.
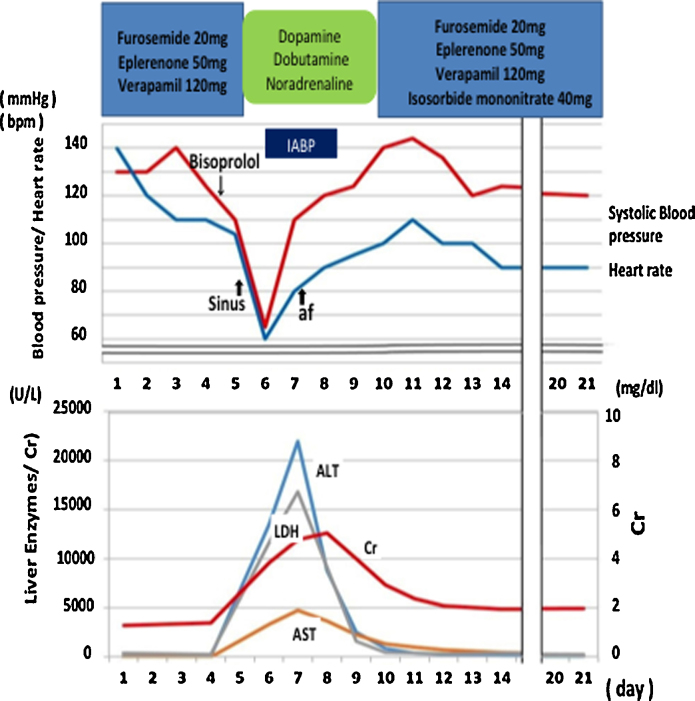
After admission, diuretics (furosemide and eplerenone) and verapamil were given, and dyspnea and pleural effusion were improved in 4 days. The O2 saturation was improved to >96% with room air, and the liver enzymes were normalized. In spite of verapamil (120 mg/day), heart rate remained above 120 bpm, and 2.5 mg of bisoprolol was given on the 5th hospital day.
Heart rate decreased to 100 bpm, and then to 52 bpm with sinus rhythm after bisoprolol (Fig. 1B). ECG showed a first-degree atrio-ventricular block and ST depression in inferior and precordial leads. Within hours, he developed a general malaise. Blood pressure declined to 104/72 mmHg, and by the next morning, systolic blood pressure gradually fell to 74 mmHg, and the O2 saturation was 90% with room air.
Liver enzymes, alanine transaminase and aspartate transaminase elevated sharply again to 7700 U/L and 2600 U/L by the next morning, and then to 21,933 U/L and 4727 U/L, respectively on the 7th day after taking bisoprolol (Fig. 2). Other enzymes were also elevated: LDH = 16,821 U/L, ALP = 690 U/L, γ-GTP = 124 U/L. Abdominal computed tomography was negative for cholangiectasis or biliary tract stone.
Systolic blood pressure remained low at 74 mmHg, and catecholamines (dobutamine, dopamine, and noradrenaline) were administered (Fig. 3). In addition, hemodyamics were supported by intra-aortic balloon pumping (IABP) and bradycardia was treated with a temporary pacemaker. At the time of insertion of IABP, coronary angiography was performed and there was total occlusion in the right and approximately 75% stenosis in the distal left coronary artery (Fig. 2B). However, no intervention was attempted because of good collateral circulation via septal branches of left descending artery. Left ventriculography showed a diffuse decrease in wall motion and depressed ejection fraction to 27%. Pulmonary arterial pressure was 32 mmHg.
Fig. 3.
The clinical course of the patient. After admission, diuretics (furosemide and eplerenone) were given together with verapamil which relieved symptoms of heart failure. Bisoprolol was given to control ventricular rate further. Following a general malaise, the patient developed shock that needed catecholamines (dopamine, dobutamine, and noradrenaline) and IABP. The liver enzymes were elevated to peaks on 7th day and returned to the normal levels with hemodynamic improvement. bpm, beats per minute; IABP, intra-aortic balloon pumping; LDH, lactic dehydrogenase; Cr, creatine kinase; ALT, alanine transaminase; AST, aspartate transaminase.
With intensive therapy, the circulatory state was improved, and the liver enzymes normalized by the 17th hospital day (Fig. 3). The ejection fraction of the left ventricle improved to 43% in echocardiography. AF appeared within days and after full recovery, he was discharged on the 30th day on verapamil (80 mg/day) and digoxin (0.125 mg/day) with control of ventricular rate around 80 bpm (Fig. 3).
The blood concentration of bisoprolol was measured at 30 h after taking the drug (2.5 mg), and was found to be excessively high: 45.1 ng/ml. By the screening of CYP2D6 variants, he was found to be CYP2D6*10 heterozygote.
Discussion
We experienced a case of acute HF. Although the patient had coronary artery disease, HF was considered to be precipitated by AF tachycardia. After addition of bisoprolol 2.5 mg for rate control, he developed a general malaise followed by shock and liver injury. The critical state was improved by intensive care that included IABP and cardiac pacing. Excessively high blood level of bisoprolol was confirmed 30 h after intake. Although he was a heterozygote of CYP2D6*10, the precise mechanisms of abnormal metabolism of bisoprolol and refractory shock were not confirmed.
Rapid AF can be one of the triggers of worsening of HF, and may result in tachycardia-induced cardiomyopathy [4]. In such cases, β-blocker is most effective for rate control followed by verapamil and digoxin [5]. In the present patient, the coronary artery disease was underlying, but verapamil was given first to control heart rate with insufficient efficacy. Then, bisoprolol was given cautiously at a low dose (2.5 mg), and the patient fell into shock and needed intensive care. The blood level of the drug was found to be excessively high: 45.1 ng/ml. Bisoprolol is absorbed with bioavailability >80% and peak plasma concentrations occur within 2–4 h. Since the blood concentration of a single dose of 5 mg of bisoprolol is reported to be 23.7 ng/ml at l3.1 ± 0.41 h after oral administration [7], the level of our patient was high.
Bisoprolol is known to be metabolized in the liver mainly via CYP3A4 and CYP2D6 6, 7, 8, 9, 10. Our patient was a CYP2D6*10/1 heterozygote, but the functional impact of CYP2D6 allele is substrate-dependent and the precise genotype–phenotype correlation can be variable among β-blockers (Table 1) 6, 7, 8, 9, 10. It is unlikely that the CYP2D6 variant was the main cause of the high concentration of bisoprolol 8, 9, and preexisting HF, shock, mildly impaired renal function, and/or excess responsiveness to the drug agent might be involved for the abnormal metabolism of the drug. Furthermore, prior administration of verapamil (120 mg/day) which is metabolized by CYP3A4 might affect bisoprolol metabolism [6]. Further attempts of genetic screening for CYP3A4 or CYP2D6 are desired for optimal pharmacotherapy.
Table 1.
Main cardiac drugs metabolized by CYP2D6.
| β-Blockers | Timolol |
| Propranolol | |
| Metoprolol | |
| Alprenolol | |
| Carvedilol | |
| Bisoprolol | |
| Antiarrhythmic | Encainide |
| Flecainide | |
| Mexiletine | |
| Propafenone | |
In summary, we had a patient with acute HF due to rapid AF. Bisoprolol was given to control heart rate, but its blood concentration elevated excessively resulting in refractory shock. Although the patient was a CYP2D6*10 heterozygote, its role was not clear, and the precise cause of abnormal bisoprolol metabolism and shock remains unknown.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 2.Flannery G., Gehrig-Mills R., Billah B., Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol. 2008;101:865–869. doi: 10.1016/j.amjcard.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Boehm M., Swedberg K., Komajda M., Borer J.S., Ford I., Dubost-Brama A., Lerebours G., Tavazzi L., SHIFT Investigators Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 4.Fujino T., Yamashita T., Suzuki S., Sugiyma H., Sagara K., Sawada H., Aizawa T., Igarashi M., Yamazaki J. Characteristics of congestive heart failure accompanied by atrial fibrillation with special reference to tachycardia-induced cardiomyopathy. Circ J. 2007;71:936–940. doi: 10.1253/circj.71.936. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad K., Dorian P. Rate control in atrial fibrillation: looking beyond the average heart rate. Curr Opin Cardiol. 2006;21:88–93. doi: 10.1097/01.hco.0000210303.33866.c7. [DOI] [PubMed] [Google Scholar]
- 6.Leopold G. Balanced pharmacokinetics and metabolism of bisoprolol. J Cardiovasc Pharmacol. 1986;8(Suppl. 11):S16–S20. doi: 10.1097/00005344-198511001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Horikiri Y., Suzuki T., Mizobe M. Pharmacokinetics and metabolism of bisoprolol enantiomers in humans. J Pharm Sci. 1998;87:289–294. doi: 10.1021/js970316d. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi M., Nozawa T., Igawa A., Inoue H., Takesono C., Tahara K., Hasimoto Y. Pharmacokinetic variability f routinely administered bisoprolol in middle-aged and elderly Japanese patients. Biol Pharm Bull. 2005;28:876–881. doi: 10.1248/bpb.28.876. [DOI] [PubMed] [Google Scholar]
- 9.Saluyama K., Sasaki T., Ujiie S., Obata K., Mizugaki M., Ishikawa M., Hiratsuka M. Functional characterization of 17 CYP2D6 allelic variants (CYP2D6.2, 10, 14A–B, 18, 27, 36, 39, 47–51, 53–55, and 57) Drug Metab Dispos. 2008;36:2460–2467. doi: 10.1124/dmd.108.023242. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner S.J., Begg E.J. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol Rev. 2006;58:521–590. doi: 10.1124/pr.58.3.6. [DOI] [PubMed] [Google Scholar]