Abstract
Mitochondrial NADP+-dependent isocitrate dehydrogenase 2 (IDH2) is a major NADPH-producing enzyme which is essential for maintaining the mitochondrial redox balance in cells. We sought to determine whether IDH2 deficiency induces mitochondrial dysfunction and modulates auditory function, and investigated the protective potential of an antioxidant agent against reactive oxygen species (ROS)-induced cochlear damage in Idh2 knockout (Idh2−/−) mice. Idh2 deficiency leads to damages to hair cells and spiral ganglion neurons (SGNs) in the cochlea and ultimately to apoptotic cell death and progressive sensorineural hearing loss in Idh2−/− mice. Loss of IDH2 activity led to decreased levels of NADPH and glutathione causing abnormal ROS accumulation and oxidative damage, which might trigger apoptosis signal in hair cells and SGNs in Idh2−/− mice. We performed ex vivo experiments to determine whether administration of mitochondria-targeted antioxidants might protect or induce recovery of cells from ROS-induced apoptosis in Idh2-deficient mouse cochlea. MitoQ almost completely neutralized the H2O2-induced ototoxicity, as the survival rate of Idh2−/− hair cells were restored to normal levels. In addition, the lack of IDH2 led to the accumulation of mitochondrial ROS and the depolarization of ΔΨm, resulting in hair cell loss. In the present study, we identified that IDH2 is indispensable for the functional maintenance and survival of hair cells and SGNs. Moreover, the hair cell degeneration caused by IDH2 deficiency can be prevented by MitoQ, which suggests that Idh2−/− mice could be a valuable animal model for evaluating the therapeutic effects of various antioxidant candidates to overcome ROS-induced hearing loss.
Keywords: Idh2, NADP+, ROS, Hearing loss, Antioxidant, MitoQ
1. Introduction
The balance between reactive oxygen species (ROS) production and antioxidant defenses is essential for maintaining cellular homeostasis. Under physiologic conditions, cells maintain redox balance through the generation and elimination of ROS [1]. Mitochondria are a major endogenous source of cellular ROS, where O2− is generated by electron leakage from complex I to IV of the electron-transport chain [2], [3]. Various enzymatic (superoxide dismutase (SOD) or glutathione peroxidase (GPx)) and nonenzymatic (vitamin C or E) antioxidant systems contribute to eliminating ROS and maintaining redox homeostasis. Excessive ROS constantly attack lipids, proteins, and DNA in cells, leading to severe and irreversible oxidative damage. Several ROS-related studies have demonstrated that increased oxidative damage plays a crucial role in a variety of pathologic conditions, including cancer, neurodegenerative diseases, and aging [4], [5].
Hearing loss, a common sensory disorder that is caused by different genetic or environmental factors, is also triggered by the accumulation of continuous oxidative stress with age [6]. Ototoxic drugs, noise exposure, and aging are common environmental factors that disrupt intracellular redox balance [6], [7], [8], and pathogenic mutations in the genes that are involved in the redox system are the major genetic causes of ROS-induced hearing loss. For example, methionine sulfoxide reductase B3 (MsrB3) that specifically reduces methionine-R-sulfoxide of proteins to methionine, is the causative gene of autosomal recessive non-syndromic hearing loss DFNB74 [9]. Because methionine residues in proteins are a major target of oxidization to methionine sulfoxide by ROS, this enzyme is responsible for preserving the biological activity of proteins after oxidative damage due to ROS [10], [11]. In addition, since cochlear cells are highly sensitive to disturbances in energy metabolism, mitochondrial decay may particularly affect the cochlea. Mutations in mitochondrial DNA (mtDNA) or in several nuclear genes coding mitochondrial proteins have been known to be associated with progressive hearing loss [12]. Major mtDNA mutations occur in the genes encoding mitochondrial oxidative phosphorylation complexes and lead to mitochondrial dysfunction. Hyperaccumulation of mitochondrial ROS decreased mitochondrial membrane potential, and activated apoptotic pathways, eventually causing hair cell death.
Mitochondrial NADP+-dependent isocitrate dehydrogenase 2 (IDH2) catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate, synthesizing NADPH. In mitochondria, the redox balance is mainly determined by the ratios of several redox couples, such as reduced glutathione (GSH)/oxidized glutathione (GSSG) and thioredoxinred/thioredoxinoxid. The GSH/GSSG couple has been considered the primary determinant of the intracellular redox state, and the GSH/GSSG ratio is regulated by antioxidant enzymatic scavengers, including glutathione reductase (GR) and GPX [13], [14]. Given the role of NADPH as the electron donor for GR-mediated GSH generation, IDH2, which increases NADPH levels by converting NADP+ to NADPH, has been highlighted as a major contributor to the antioxidant defense system in various tissues and cells [15], [16], [17], [18], [19], [20], [21]. It has been shown that IDH2 protects organs against various diseases, including cancer, skin pigmentation [22] and renal dysfunction [23]. In addition, endothelium-dependent vasorelaxation is impaired, and the concentration of bioavailable NO is decreased in the aortic ring in Idh2 knockout (Idh2−/−) mice [24].
However, the physiological association between Idh2 and auditory function and its underlying mechanisms are not fully understood. Therefore, we sought to determine whether Idh2 deficiency induces mitochondrial dysfunction and modulates auditory function, and investigated the protective potential of an antioxidant agent against ROS-induced cochlear damage in Idh2−/− mice.
2. Materials and methods
2.1. Animals
Idh2−/− mice were bred [19], and mice of their background strain (C57BL/6N) were used as a wild-type (Idh2+/+) control. For genetic identification of the Idh2−/− mice, tail DNA genotyping was performed. Mice were allowed free access to water and standard mouse chow. Temperature (23 ± 2 °C), humidity (50 ± 5%) and a daily 12 h light–dark cycle were maintained in the Central Laboratory Animal Facility of Kyungpook National University. All animal procedures were conducted in accordance with the Institutional Animal Care guidelines issued by the Committee of Animal Research at Kyungpook National University (2017–0104).
2.2. Reverse-transcription polymerase chain reaction (RT-PCR)
RNA was extracted from the inner ear of mice using an RNeasy® Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. RNA was reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. cDNAs were PCR-amplified. The glyceraldehyde 3-phosphate dehydrogenase (Gapdh) gene was used as an internal control.
2.3. Western blot analysis
The whole-cell lysates or tissue homogenates (20 µg) prepared from cochlear explants was used for western blotting. The proteins were detected using the appropriate primary and secondary antibodies. After a series of washes, membranes were developed using an enhanced chemiluminescent detection system. Values are normalized to β-actin (loading control). The primary antibodies used in this study were as follows: rabbit polyclonal anti-IDH2 (1:1000; Novus Biologicals, Littleton, CO, USA), rabbit polyclonal anti-OXPHOS complex subunits: NDUFA9 (1:500; Thermo Fisher Scientific, Rockford, IL, USA), SDHA (1:500; Thermo Fisher Scientific, Rockford, IL, USA), UQCRC2 (1:5000; Thermo Fisher Scientific, Rockford, IL, USA), COX4 (1:500; Thermo Fisher Scientific, Rockford, IL, USA) and ATP5A1 (1:1000; Thermo Fisher Scientific, Rockford, IL, USA) and rabbit polyclonal anti-β actin (1:2000; Cell Signaling Technology, Danvers, MA, USA). Goat anti-rabbit-lgG-HRP was used as the secondary antibody (1:2000; Cell Signaling Technology, Danvers, MA, USA).
2.4. Auditory brainstem response (ABR) measurement
Mouse auditory function was assessed by measuring ABR with an ABR workstation-System 3 (Tucker Davis Technology, Alachua, FL, USA), as previously described [25]. Auditory function was measured with click stimuli and tone burst sound frequencies of 8, 16, and 32 kHz, and acoustic thresholds of sound pressure level (SPL) were determined using BioSigRP software (Tucker Davis Technology, Alachua, FL, USA).
2.5. Immunofluorescence and histological analysis
Inner ears were harvested and fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). The fixed inner ear tissues were embedded in paraffin. Paraffin-embedded tissues were serially sectioned at 7 µm and then subjected to immunofluorescence analysis or hematoxylin-eosin (H&E) staining. The paraffin sections were incubated for 1 h at 65 °C, deparaffinized with xylene, and rehydrated using a graded ethanol series. The tissue sections were permeabilized with 0.1% Triton X-100 in 1X PBS (PBS-Tx) for 30 min and blocked using a blocking solution containing 5% normal goat serum (NGS) in PBS-Tx for 1 h at room temperature (RT). The sections were then incubated overnight at 4 °C with rabbit anti-NADPH (Biorbyt, Cambridge, UK) and mouse anti-COX4 (Abcam, Cambridge, UK). After washing with PBS, the tissue sections were incubated for 1 h at RT with secondary antibodies. The secondary antibodies used for immunofluorescence were Alexa Fluor 488-conjugated goat anti-mouse IgG and 555-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific, Rockford, IL, USA). 4′-6-Diamidino-2-phenylindole (DAPI, 1 µg/mL) was used to visualize nuclei.
2.6. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
To assess apoptotic cell death in the inner ear, we evaluated DNA fragmentation using the TUNEL assay according to the manufacturer's protocol (Roche Biochemicals, Mannheim, Germany). Paraffin-embedded inner ear sections were deparaffinized and rehydrated. They were then permeabilized with PBS-Tx and 0.1% sodium citrate in distilled water for 20 min at RT and stained with TUNEL working solution for 1 h at 37 °C, without light. The specimens were mounted on glass slides using fluoromount (Sigma-Aldrich, Saint Louis, MO, USA) and visualized using a Zeiss Axio Imager A2 fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
2.7. Enzyme assays
Supernatants from homogenized mitochondrial pellets were each added to 1 mL of 40 mM tris buffer (pH 7.4) containing NADP+ (2 mM), MgCl2 (2 mM), and isocitrate (5 mM). IDH2 activity was measured by monitoring NADPH production at 340 nm at 25 °C. One unit of IDH2 activity was defined as the amount of enzyme needed to catalyze the production of 1 mmol of NADPH per minute. Total GSH levels and the GSH/GSSG ratio were measured using a commercially available GSH/GSSG Ration Detection Assay Kit (Abcam, Cambridge, UK) according to the manufacturer's instructions.
2.8. Culture and histological evaluation of mouse cochlear explants
Primary cochlear explants were prepared from postnatal day (P) 3 mice. The dissected organs of Corti were incubated with culture medium composed of high-glucose Dulbecco's modified Eagle's medium (DMEM; HyClone, Logan, UT, USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and ampicillin (10 μg/mL) in a humidified atmosphere of 5% CO2 at 37 °C. After 16 h incubation, organs of Corti were treated with 500 nM MitoQ in dimethyl sulfoxide (DMSO) or 500 nM decyl triphenylphosphonium (dTPP) in DMSO provided by Prof. Michael P. Murphy. At the same time, some groups were treated with 5 μM rotenone (Sigma-Aldrich, Saint Louis, MO, USA) or 10 μM antimycin A (Sigma-Aldrich, Saint Louis, MO, USA) diluted in culture medium for 1 h. After 1 h incubation, 0.05 mM hydrogen peroxide (H2O2) was added.
2.9. Histological evaluation of mouse cochlear explants
At the end of the 5-day incubation period, all cochlear explants were washed with PBS, fixed with 4% PFA in PBS for 15 min, and permeabilized for 30 min at RT. Permeabilized samples were blocked with 5% NGS diluted in PBS-Tx for 1 h at RT and then stained with Alexa Fluor® 488 or 555-conjugated phalloidin (1:1000; Invitrogen, Eugene, OR, USA) in PBS-Tx for 3 h at RT. The specimens were rinsed three times with PBS and mounted on glass slides using fluoromount (Sigma-Aldrich, Saint Louis, MO, USA). For immunohistochemical quantification, the inner hair cells (IHCs) and outer hair cells (OHCs) were separately counted along the 6 regions from the middle of each cochlear explant. Perfectly shaped hair cells in each region, with a length of 200 µm of basilar membrane, were counted. Each experiment was performed independently and repeated at least three times.
2.10. Determination of mitochondrial ROS levels
MitoSOX-red (Molecular Probes, Eugene, OR, USA) is a fluorogenic indicator of superoxide generated specifically from mitochondria [26]. At the end of the 3-day incubation period, all cochlear explants were washed with PBS and stained with 5 μM MitoSOX-red for 10 min in a humidified atmosphere of 5% CO2 at 37 °C. After washing with PBS, the specimens were visualized using a Zeiss Axio Imager A2 fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
2.11. Determination of mitochondrial membrane potential (ΔYm)
Mitochondrial membrane potential was estimated using the cationic fluorescent dye MitoProbe™ JC-1 (Invitrogen, Eugene, OR, USA) according to the manufacturer's instructions. At the end of the 3 or 5-day incubation period, all cochlear explants were washed with PBS and incubated with 2 μM JC-1 for 50 min in the dark. To confirm the sensitivity of JC-1, the cochlear explants were not treated with any drugs, 50 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was added before treatment with JC-1, and the samples were incubated. If the cells have a normal range of mitochondrial membrane potentials, fluorescence emission shift from green to red.
2.12. Statistical analyses
Statistical analyses were performed using 2-tailed Student's t-tests; P < 0.05 was considered statistically significant. The data were analyzed by comparing treated and untreated contralateral structures or by comparing treated and control mice.
3. Results
3.1. Loss of Idh2 leads to progressive sensorineural hearing loss in mice
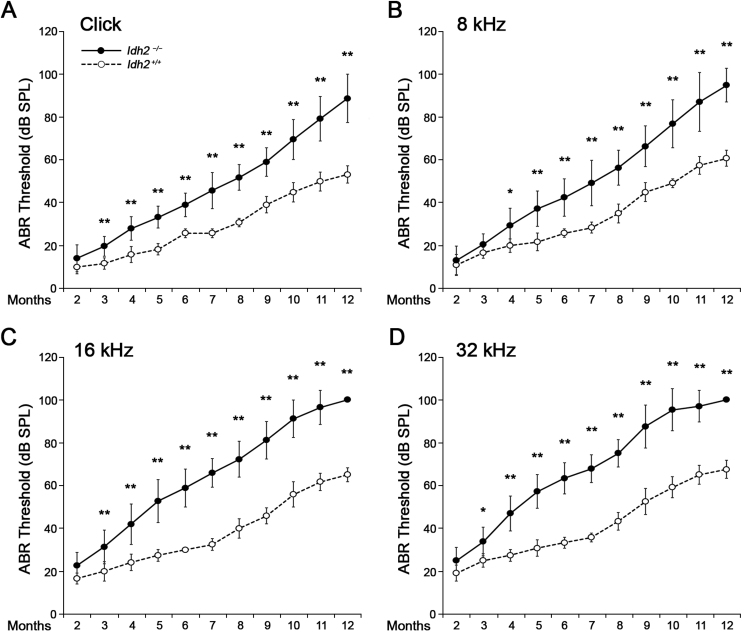
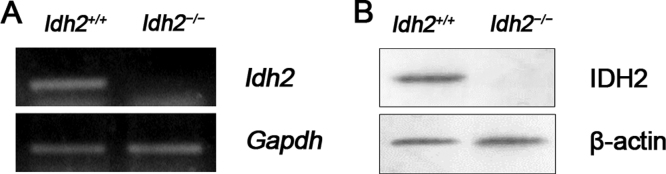
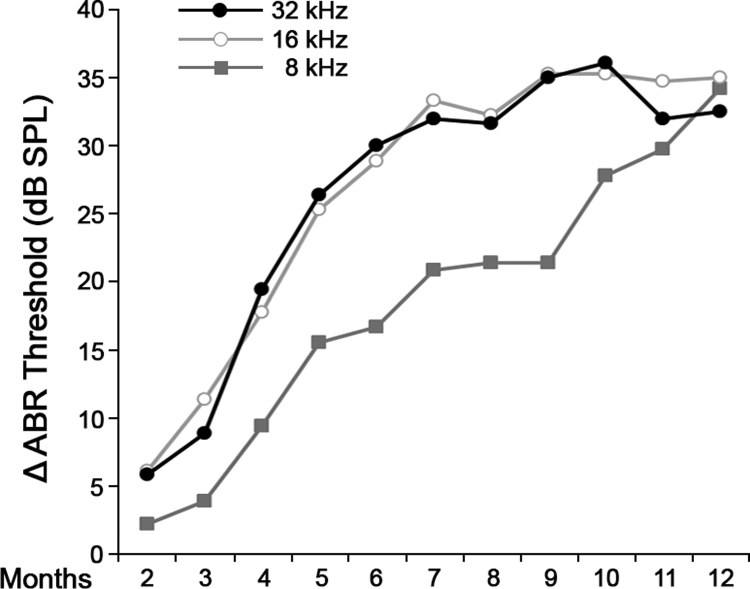
Because inner ear expression of the Idh2 gene in the Idh2−/− mice [27] has not been confirmed in previous studies, we first examined Idh2 expression in the inner ear and confirmed that Idh2 mRNA and protein expression was completely absent in the inner ear of Idh2−/− mice, while it was abundantly expressed in Idh2+/+ mice (Supplementary Fig. 1). The changes in the hearing threshold of Idh2+/+ and Idh2−/− mice were then followed-up for 12 months by ABR tests with a click stimulus and frequency-specific stimuli at 8, 16, and 32 kHz to investigate whether lack of Idh2 affects normal hearing function (Fig. 1). No significant differences were found between Idh2+/+ and Idh2−/− mice until 2 months after birth. However, after 3 months of age, the hearing ability of the Idh2−/− mice began to deteriorate significantly compared with the hearing ability of Idh2+/+ mice, eventually resulting in profound hearing loss after 10 months of age. The ABR threshold gap between the Idh2+/+ and Idh2−/− mice gradually increased at all frequencies (Supplementary Fig. 2), which indicates that Idh2 deficiency leads to the continuous accumulation of hearing damage with age. Moreover, even if this pattern of hearing loss was consistent at all tested frequencies, the progression of hearing loss was more rapid at mid (16 kHz) and high (32 kHz) frequencies than at low (8 kHz) frequencies (Supplementary Fig. 2). This result indicates that Idh2 deficiency leads to progressive sensorineural hearing loss in mice, suggesting an important role of Idh2 in the auditory pathway.
Fig. 1.
ABR hearing thresholds of theIdh2+/+andIdh2−/−mice as a function of age. The changes in the hearing threshold of Idh2+/+ (white circle with dotted line, n = 6) and Idh2−/− (black circle with solid line, n = 18) mice were represented using line graphs. The ABR thresholds were measured for 12 months with a click (A) and tone burst (8, 16, and 32 kHz) (B, C and D) stimuli. Data are shown as the means ± SEM. *p < 0.05, **p < 0.005.
3.2. IDH2 deficiency causes damage to hair cells and spiral ganglion neurons (SGNs), leading to apoptosis
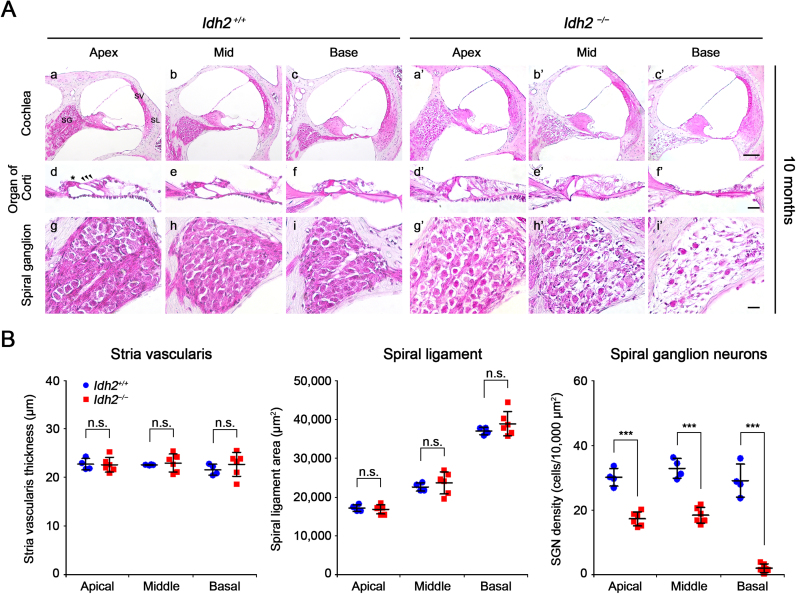
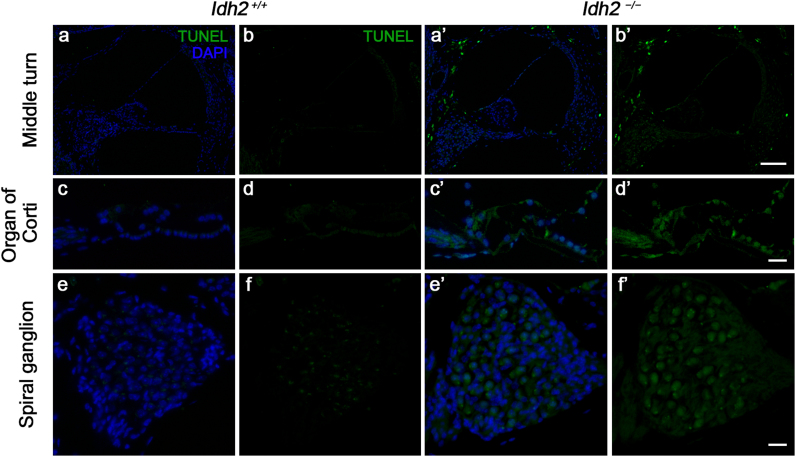
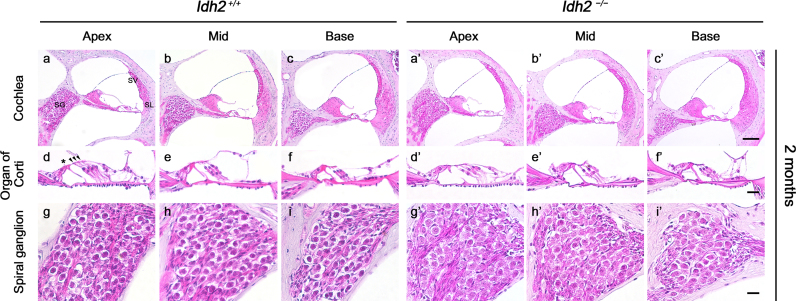
To investigate the immediate cause of hearing loss in Idh2−/− mice, we first examined the histological features of cochlear sections from Idh2+/+ and Idh2−/− mice through H&E staining at 2 months of age when there was no difference in ABR threshold between Idh2+/+ and Idh2−/− mice and at 10 months of age when Idh2−/− mice showed profound hearing loss at 16 and 32 kHz. In the cochlea of 2-month-old mice, no distinguishable differences were detected between Idh2+/+ and Idh2−/− mice (Supplementary Fig. 3). In contrast, at 10 months of age, obvious damage was observed in the organ of Corti and in the SGNs in Idh2−/− cochlea (Fig. 2A). While both the inner and outer hair cells from 10-month-old Idh2+/+ cochlea were intact, Idh2−/− cochlea had morphological degeneration of the hair cells (Fig. 2A d-f, d′-f′). Moreover, the most noticeable difference was found in the spiral ganglion, which showed evident loss of SGNs in the Idh2−/− cochlea (Fig. 2A g-i, g′-i′). Quantitative analysis confirmed that the loss of SGNs significantly differed from apical to basal cochlear turns, whereas there were no significant differences in stria vascularis thickness and spiral ligament area between Idh2+/+ and Idh2−/− cochlea (Fig. 2B). Importantly, all these damages were more severe in the basal turn than in the apical turn, which was consistent with the mid- and high-frequency-dominant progression of hearing loss observed in the ABR test of Idh2−/− mice. A highly increased number of TUNEL-positive cells in the organ of Corti and the SGNs of Idh2−/− cochlea suggested that Idh2 deficiency causes apoptosis of hair cells and SGNs, eventually leading to hearing loss (Fig. 3).
Fig. 2.
Histological evaluation of cochlea fromIdh2+/+andIdh2−/−mice. (A) H&E staining was performed in the inner ear sections of Idh2+/+ (n = 4) and Idh2−/− (n = 6) mice at 10 months of age. SG, spiral ganglion; SV, stria vascularis; SL, spiral ligament. Scale bars; 100 µm in (i′) and 20 µm in (c′ and f′). The arrowheads point to three rows of outer hair cells, and the asterisk (*) indicates an inner hair cell. (B) The thickness of the stria vascularis, the mean area of the spiral ligament, and the density of spiral ganglion neurons at apical, middle, and basal turns of cochlea were measured in the inner ear sections of Idh2+/+ (blue circles) and Idh2−/− (red squares) mice at 10 months of age. ***p < 0.001; n.s., nonsignificant.
Fig. 3.
Detection of apoptotic cell death by TUNEL assay in the cochlea fromIdh2+/+andIdh2−/−mice. Apoptotic DNA degradation was analyzed by TUNEL labeling (green) in Idh2+/+ and Idh2−/− mice at 10 months of age, and the nuclei were counterstained with DAPI (blue). Scale bars: 100 µm in (b′) and 20 µm in (d′ and f′).
3.3. Loss of IDH2 function results in excessive accumulation of ROS due to decreased NADPH levels and disrupted GSH/GSSG balance in mouse cochlea
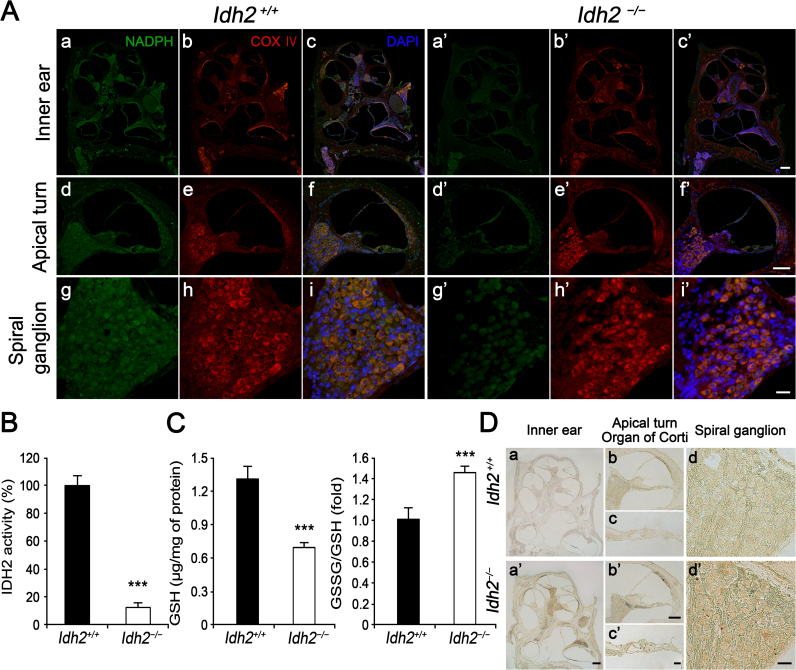
Considering the IDH2 function in mitochondria, we next investigated intracellular levels of NADPH and total ROS using immunofluorescence, IDH2 enzyme activity assay, and 3,3′-diaminobenzidine (DAB) staining in Idh2+/+ and Idh2−/− cochlea at 10 months of age, to determine if the antioxidative function of IDH2 is indispensable for the functional maintenance and survival of hair cells and SGNs (Fig. 4). The immunofluorescence staining results indicated that the fluorescence signal for NADPH was detected throughout the entire inner ear sections in both Idh2+/+ and Idh2−/− mice. However, the signal intensity was much weaker in Idh2−/− cochlea than in Idh2+/+ (Fig. 4A). This result was confirmed by an in vitro enzymatic activity assay, which measures NADPH production by IDH2 in NADP+ containing inner ear lysates from Idh2+/+ and Idh2−/− mice. The relative efficiency of NADPH production in IDH2-deficient inner ear lysate was approximately 12% compared to Idh2+/+ cochlea (Fig. 4B). Consequently, the intracellular level of GSH that is generated by the NADPH-driven reduction in GSSG was significantly decreased, leading to an increase in the GSSG/GSH ratio, which indicated disruption of the redox balance in the IDH2-deficient inner ear (Fig. 4C). Finally, increased intracellular ROS levels in Idh2−/− cochlea, including in the organ of Corti and the spiral ganglia, was detected by DAB staining (Fig. 4D). These results demonstrated that loss of IDH2 activity decreased the levels of NADPH and GSH, leading to redox imbalance, which might cause apoptosis of hair cells and SGNs by abnormal ROS accumulation in Idh2−/− mice.
Fig. 4.
Redox status and oxidative damage observed in the inner ear ofIdh2−/−mice. Intracellular levels of NADPH and ROS accumulation were compared between Idh2+/+ and Idh2−/− mouse cochlea by immunofluorescence and DAB staining at 10 months of age. (A) Intracellular NADPH (green) levels in the inner ears of Idh2+/+ (n = 3) and Idh2−/− (n = 3) mice were compared at 10 months of age. COX IV (red) was used as a mitochondrial marker [32], and the nuclei were counterstained with DAPI (blue). Scale bars: 200 µm in (c′), 100 µm in (f′) and 20 µm in (i′). (B) The enzyme activity of IDH2 that produces NADPH was measured in the inner ear whole protein fractions (n = 3). (C) Total GSH level and the [GSSG]/[GSH] ratio were measured (n = 3). The values were presented as the fold change over the levels observed in Idh2+/+ mice. Data are shown as the means ± SEM. **p < 0.005, *** p < 0.001 (D) Oxidized levels of the inner ear sections from Idh2+/+ (n = 3) and Idh2−/− (n = 3) mice were analyzed using DAB staining. Scale bars: 200 µm in (a′), 100 µm in (b′) and 20 µm in (c′ and d′).
3.4. Mitochondria-targeted antioxidant MitoQ prevents H2O2-induced ototoxicity in Idh2−/− cochlea
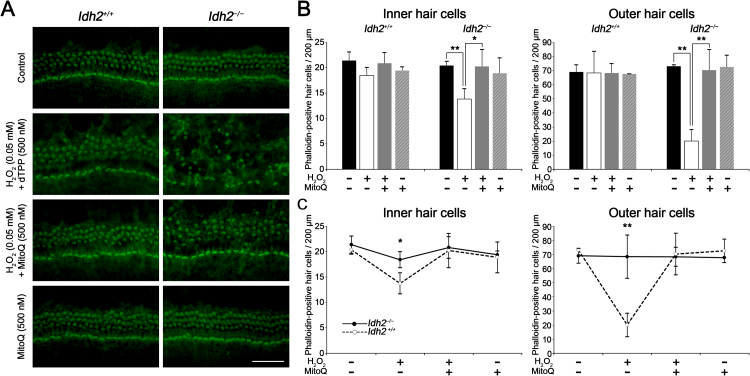
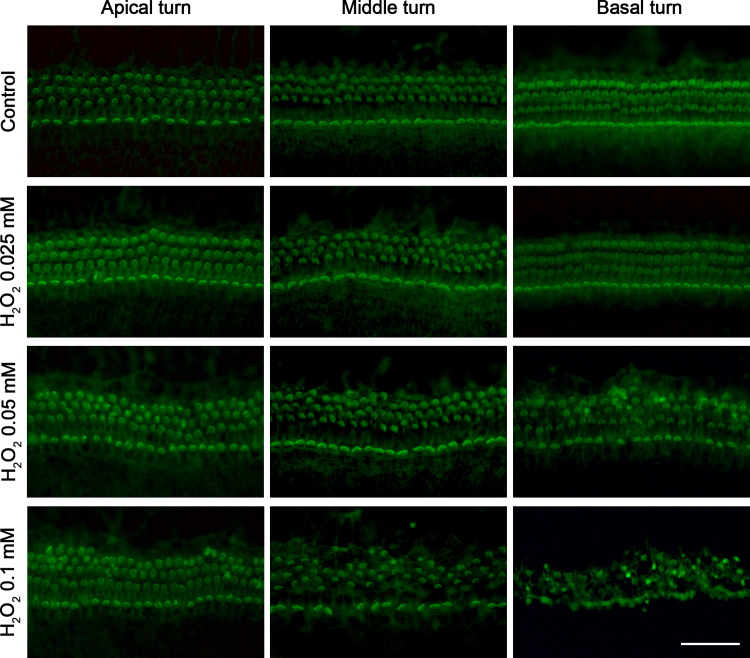
Our in vivo study demonstrated that the most direct cause of hearing loss observed in Idh2−/− mice was the accumulation of excessive ROS, leading to apoptosis of hair cells and SGNs. Because IDH2 is a major NADPH-producing enzyme in the mitochondrial redox system and ROS-induced mitochondrial damage directly affects cell survival [1], we hypothesized that the administration of effective mitochondrial antioxidants might protect or promote the recovery of cells from ROS-induced apoptosis in Idh2-deficient mouse cochlea. To verify our hypothesis, we induced acute oxidative stress in Idh2+/+ and Idh2−/− mouse cochlear explants by treatment with H2O2, leading to ROS-induced cell damage. Before the addition of H2O2, the mitochondria-targeted antioxidant, MitoQ, was pre administered to the cochlear explants to examine whether it could protect Idh2-deficient hair cells from H2O2 (ROS)-induced apoptosis. First, Idh2+/+ cochlear explants were treated with 0.025, 0.05, or 0.1 mM H2O2 for 5 days to determine the optimal toxic dose, and hair cell damage was visualized by immunostaining of cochlear whole-mount with phalloidin. Treatment with H2O2 led to the degeneration of stereocilia and the loss of hair cells in a dose-dependent manner, and the damage gradually became more severe from the apical to basal turn (Supplementary Fig. 4). Finally, a 0.05 mM concentration that contributed to obvious but mild hair cell defects in Idh2+/+ was selected as the H2O2 treatment dose for all subsequent experiments.
The protective effect of MitoQ against H2O2-induced hair cell loss was investigated by pre-treatment with 500 nM MitoQ, followed by post-treatment with 0.05 mM H2O2 in Idh2+/+ and Idh2−/− cochlear explants. When the cochlear explants were exposed to only H2O2 and a negative control of MitoQ, dTPP, Idh2-deficient cochlear explants exhibited highly severe damage in both inner and outer hair cells showing disarrangement of hair cell rows and stereocilia degeneration, while only mild hair cell migration was observed without noticeable loss of hair cells in Idh2+/+ cochlea (Fig. 5A). This means that loss of IDH2 function makes hair cells much susceptible to H2O2-induced oxidative stress. However, MitoQ pre-treatment before adding H2O2 remarkably reduced hair cell loss in Idh2−/− cochlear explants, suggesting that the mitochondrial antioxidative effect of MitoQ protects hair cells from H2O2-induced degeneration even when the cells are deficient in IDH2. Treatment with only MitoQ showed no evident cytotoxicity. These results were quantitatively measured by counting the average number of stereocilia-positive hair cells in the 200-μm cochlear region of the middle turn. In Idh2+/+ cochlea, neither H2O2-induced damage nor MitoQ-dependent recovery of hair cells were significant. In contrast, in Idh2−/− cochlea, hair cell loss by H2O2 treatment was highly significant and was dramatically recovered by pre-treatment with MitoQ (Fig. 5B). Importantly, although H2O2 treatment led to a significant decrease in hair cell survival in Idh2−/− cochlea compared with Idh2+/+ cochlea (Fig. 5C), MitoQ almost completely neutralized the H2O2-induced ototoxicity, contributing to restoration of the survival rate of Idh2−/− hair cells to normal levels in both inner and outer hair cells. This phenomenon was more prominent in OHCs than in IHCs. This result strongly suggests that IDH2 plays an indispensable role in removing mitochondrial ROS, which is essential for the functional maintenance and survival of hair cells. Furthermore, it suggests a strong possibility that the protective or therapeutic effect of various mitochondria-targeted antioxidant reagents could be examined to overcome mitochondrial ROS-induced ototoxicity using this model.
Fig. 5.
Evaluation of protective effect of MitoQ on H2O2-induced ototoxicity inIdh2+/+andIdh2−/−cochlear explants. (A) Immunofluorescent images represent the organ of Corti explants from each experimental group. Hair cells were stained with phalloidin (green). Scale bars: 50 µm. (B and C) Quantitative comparison of phalloidin-positive inner and outer hair cells in Idh2+/+ and Idh2−/− mice within a 200-μm region of the organ of Corti (n = 3). Data are shown as the means ± SEM. *p < 0.05, ** p < 0.005.
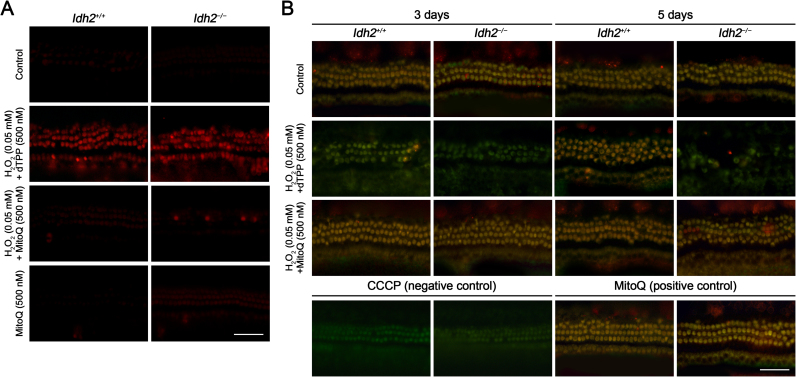
Direct evidence of mitochondrial damage due to oxidative insults in Idh2−/− mouse cochlea was confirmed by analyzing mitochondrial ROS levels and ΔΨm in cochlear explants using the mitochondrial ROS indicator MitoSOX-red and the mitochondria-accumulated fluorescent dye MitoProbe JC-1, respectively. The data showed that H2O2 stimuli led to increased mitochondrial ROS levels (Fig. 6A) and depolarization of ΔΨm (Fig. 6B) in Idh2−/− mouse cochlea 3 days after H2O2 treatment, but this damage was effectively prevented by MitoQ. These results provide strong evidence that the antioxidative activity of MitoQ protects hair cells from H2O2 by reducing ROS levels and by maintaining ΔΨm. Interestingly, increased ROS levels and loss of ΔΨm were also detected in Idh2+/+ cochlea 3 days after H2O2 treatment, indicating that an excessive amount of ROS might lead to temporary mitochondrial damage even in normal cells. However, 5 days after H2O2 treatment, ΔΨm of Idh2+/+ hair cells was substantially restored by the endogenous antioxidative system, while Idh2-deficient hair cells finally failed to survive. This means that a lack of IDH2 leads to the depolarization of ΔΨm by excessive ROS, resulting in hair cell loss caused by mitochondrial damage.
Fig. 6.
MitoQ-mediated hair cell protection against ROS accumulation and loss of mitochondrial membrane potential caused by H2O2insults. The organ of Corti explants of the Idh2+/+ and Idh2−/− mice were treated with H2O2 and/or MitoQ for 3 or 5 days. (A) Level of mitochondrial ROS accumulation in the hair cells (red) was examined using MitoSOX-red staining 3 days after H2O2 treatment. Scale bars: 50 µm. (B) Mitochondrial membrane potential was tested using MitoProbe™ JC-1 assay in the Idh2+/+ and Idh2−/− cochlea, 3 and 5 days after H2O2 treatment. Shift of the fluorescence emission from green to red were detected in the cells that are maintaining normal range of mitochondrial membrane potential. Scale bars: 50 µm.
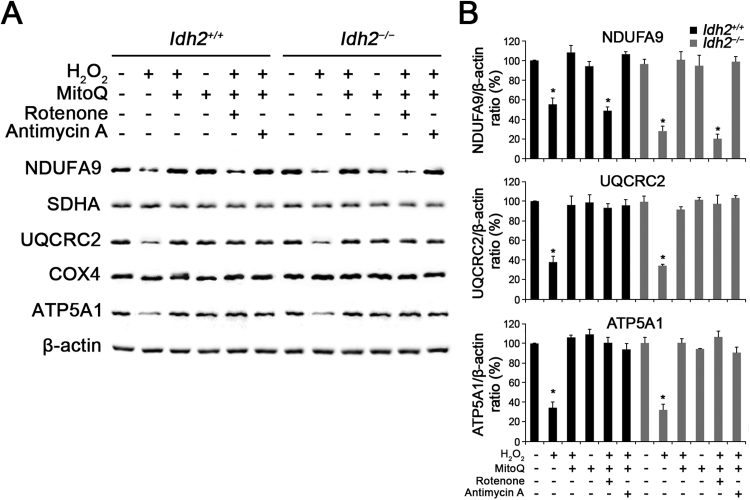
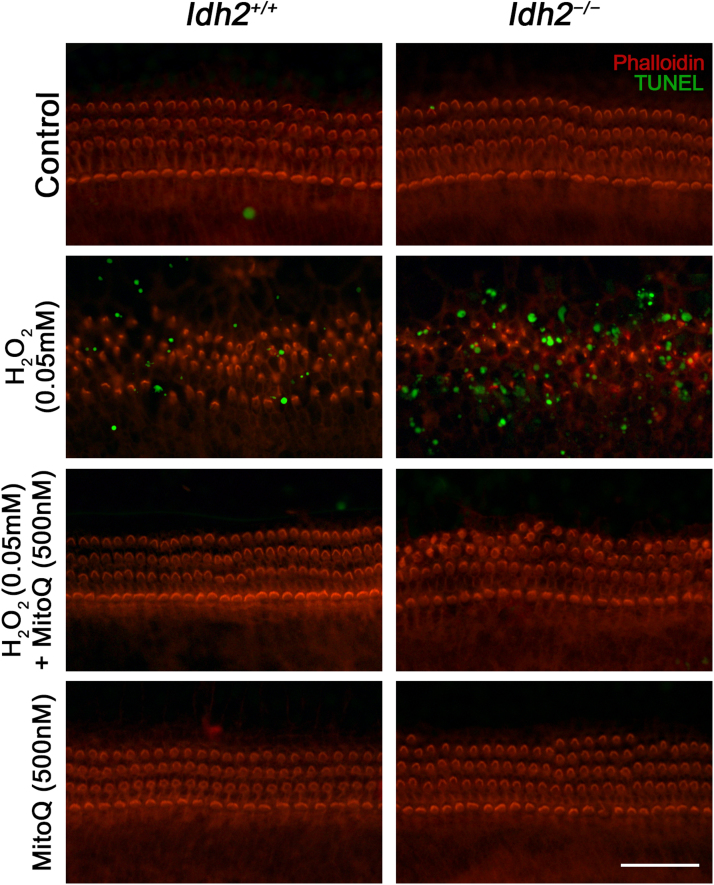
To determine the underlying cause of depolarized ΔΨm, we investigated the expression of the mitochondrial respiratory chain. Because ΔΨm is known to be generated by the mitochondrial respiratory chain composed of four enzyme complexes (complex I – IV), cytochrome c, and ATP synthase (complex V) [28], [29], changes in the expression levels of these protein complexes were examined by detecting subunits of each complex using western blot analysis in Idh2+/+ and Idh2−/− cochlear explants, 3 days after H2O2 treatment. The results showed that subunits of three enzyme complexes, NDUFA9 (complex I), UQCRC2 (complex III), and ATP5A1 (complex V), were decreased by H2O2-induced oxidative stress, while MitoQ pre-administered hair cells maintained intact expression of these subunits at normal levels (Fig. 7). Particularly, although rotenone, a proven complex I inhibitor, decreased the NDUFA9 (complex I) expression even in the presence of MitoQ, subsequent complex subunits (II – V) were not affected by the NDUFA9 damage. In addition, complex III inhibition by treatment of antimycin A that is a complex III inhibitor, was prevented by MitoQ pre-treatment (Fig. 7A). It suggests that MitoQ might have a significant role for maintenance of normal expression and function of electron transport chain, as well as its antioxidative function. Eventually, ROS-induced mitochondrial dysfunctions triggered apoptosis, and depletion of IDH2 more strongly promoted apoptosis (Fig. 8). It suggests that the loss of ΔΨm in hair cells lacking IDH2 was due to ROS-induced degradation of mitochondrial respiratory complexes resulting in mitochondrial dysfunctions and subsequent apoptosis, and that MitoQ has a powerful protective effect against mitochondrial damage through its antioxidative activity.
Fig. 7.
Decreased expression levels of mitochondrial respiratory chain complexes in H2O2-treated cochlear explants. (A) The organ of Corti explants of the Idh2+/+ and Idh2−/− mice were treated with H2O2 and/or MitoQ for 3 days. Altered expression levels of the oxidative phosphorylation (OXPHOS) subunits I, III and V were detected in the total protein lysates from H2O2-treated cochlea by Western blot analysis. β-actin served as a loading control. (B) Protein levels of OXPHOS complexes were calculated by densitometric analysis using ImageJ software (n = 3) Data are shown as the means ± SEM. *p < 0.05.
Fig. 8.
Inhibition of apoptosis by MitoQ in H2O2-treated cochlear hair cells. Apoptotic cell death were determined by a TUNEL assay in cochlear explants of Idh2+/+ and Idh2−/− mice. Microscopic images represent TUNEL (green) and phalloidin (red) labeled cells. Scale bars: 50 µm.
Together, our ex vivo studies using cochlear explants demonstrated that IDH2 plays a crucial role in hair cell survival by regulating mitochondrial ROS levels and that hair cell degeneration caused by IDH2 deficiency can be surprisingly prevented by MitoQ. Therefore, Idh2−/− mice could be used as a valuable animal model to evaluate the therapeutic effects of various antioxidant candidates to overcome ROS-induced hearing loss.
4. Discussion
In the present study, we identified that IDH2 deficiency cause progressive hearing decline in mice. Excessively accumulated mitochondrial ROS induced depolarization of the ΔΨm, which resulted in mitochondrial dysfunction leading to apoptosis of hair cells and SGNs in Idh2−/− mice and their cochlear explants. Thus, IDH2 is indispensable for the functional maintenance of mitochondria and survival of hair cells and for the SGNs that play critical roles in the hearing pathway. Extensive expression of IDH2 in cochlea, including hair cells and SGNs [27], and its mitochondrial-targeted intracellular localization [27] strongly support our results obtained in Idh2−/− mice. In particular, the western blot analysis suggested that the most direct cause of the decreased ΔΨm observed in Idh2−/− cochlea might be the loss of mitochondrial respiratory chain complexes I, III and ATP synthase (complex V). The mitochondrial respiratory chain, which consists of four enzyme complexes (I – IV) and the ATP synthase, generates ΔΨm by transferring protons from the mitochondrial matrix to the interspace between the inner and outer mitochondrial membranes, and it is also well known as a major source of superoxide (·O2-). It has been demonstrated that functional impairment in respiratory chain complexes is highly linked to mitochondria-triggered apoptosis accompanied by cytochrome c release and caspase activation [28], [29]. Inhibited complex I function led to apoptosis through caspase-3-like protease activation in ML-1a cells and cultures of dopaminergic cells [30], [31], and respiratory chain dysfunctions induced by pathogenic gene mutations altered the level of caspase-3 activation in response to mitochondrial stress-mediated apoptotic stimuli [32]. Interestingly, each enzyme complex has differential sensitivity to endogenous oxidative stress, and their levels were restored by the administration of specific antioxidants. In optic nerve head astrocytes, H2O2-induced oxidative stress significantly increased the only complexes I, II and IV, and pre-treatment with coenzyme Q10 preserved their expression levels at a normal state [33]. Another in vivo study using SOD2 null mice revealed that complexes I – IV, but not V, were sensitive to mitochondrial ROS in Sod2−/− mice [34]. Generally, protein oxidation by ROS occurs at particular amino acid residues of a certain protein, rather than at random. Moreover, transition metals bound to proteins are known as the strongest target of initial oxidation, reacting with ROS and form hydroxyl radicals (·OH) [35]. Our data suggest that only mitochondrial respiratory chain complexes I, III and ATP synthase were particularly sensitive to oxidative insult by H2O2 in hair cells lacking IDH2, which finally resulted in apoptosis. Complex I, II and III commonly have transition metal cofactors (iron-sulfur clusters) that are sensitive to mitochondrial ROS, and they are functionally associated with each other [36]. Complex I and III share important physiological characteristics. As the major contributors of mitochondrial ROS generation,·O2- produced from complexes I and III is converted to H2O2, which mediates intracellular signaling under normal physiological conditions, while·O2- from complex II has been proposed to be used to open mitochondrial ATP-sensitive potassium channels [37]. Given the functional significance of respiratory complexes I, III and ATP synthase, which are responsible for the majority of ROS and ATP synthesis, respectively, our result that consistent downregulation of these complexes was confirmed by repeating three independent experiments strongly suggests that IDH2 plays a critical role in maintaining mitochondrial functions and cell survival by protecting the mitochondria from the excessive ROS that damages specific respiratory chain complexes.
The pattern of respiratory complex degeneration and loss of ΔΨm induced by H2O2 stimuli were not IDH2-deficient cell-specific defects. Three days after H2O2 treatment, ROS-induced mitochondrial damage did not significantly differ between Idh2+/+ hair cells and Idh2−/− hair cells. Nevertheless, the intact function of IDH2 induced the hair cells to restore the mitochondrial redox balance to overcome the damage, while a lack of IDH2 eventually caused apoptotic cell death due to failure to remove mitochondrial H2O2. However, this ROS-induced mitochondrial damage leading to apoptosis was dramatically preserved by MitoQ in Idh2−/− cochlea. MitoQ, an analog of ubiquinone, is a respiratory chain component and a highly effective antioxidant that prevents lipid peroxidation in mitochondria [38], [39]. By covalent conjugation of a lipophilic triphenylphosphonium cation to ubiquinone, MitoQ can pass through the phospholipid bilayers and largely accumulate into the mitochondrial inner membrane by the ΔΨm [38]. Thus, MitoQ has been widely used as an antioxidant that targets mitochondria [38], [40]. MitoQ is known to inhibit the final step of lipid peroxidation by blocking ·OH assault and is continuously recycled by returning to the active ubiquinol form by mitochondrial respiratory chain complex II [40]. As a nontoxic and effective mitochondria-targeted antioxidant, the protective effect of MitoQ on lipid peroxidation and mitochondrial damage has been determined in various studies of ROS-related diseases, including renal and liver dysfunction in sepsis, cardiac hypertrophy, and neurodegenerative disease [41], [42], [43]. Moreover, two previous in vivo studies found protective effects of MitoQ in the inner ear. Using gentamicin- or amikacin-treated guinea pigs, it was shown that the administration of MitoQ attenuated aminoglycoside-induced ototoxicity, leading to cochlear damage and hypoacusia [44], [45]. Although the protective effect of MitoQ against ototoxicity was determined in previous studies, an important significance of this study is the suggestion of the possibility to reverse genetic hearing loss. By providing experimental evidence of the protective potential of an antioxidant to inhibit ROS-induced cochlear damage in a transgenic mouse model, we are the first to suggest the possibility that continuous application of nontoxic supplemental agents, such as promising antioxidants, might be effective in controlling the symptoms of genetic hearing loss caused by a lack of genes involved in the cellular redox system, although not a fundamental and direct genetic correction such as virus-mediated gene transfer. In addition, although all the experiment for protective effect of MitoQ was verified using ex vivo organotypic culture system of cochlea, not in vivo, cochlea is highly distinctive organ that has structural and physiological complexity, which brings diverse limits in functional investigations in vivo. In that respect, organotypic culture of cochlear explants have strong advantages to explore the underlying mechanisms for a particular pathogenesis or development, because the cultured cochlear explants have been proven to maintain intact conformation of the hair cells and neuronal innervation with following normal processes of development during it is cultured [46], [47]. Importantly, most of hearing loss studies that performed both ex vivo and in vivo experiments have shown strong consistency in the results between these two systems [48], [49], [50], [51], which suggest that ex vivo studies also could provide significant and reliable massages.
Thus far, aging has been considered as the process of ROS-induced damage accumulation [52], which causes mitochondrial dysfunction resulting in age-related disorders [53]. Hearing loss caused by constant accumulation of ROS in inner ear cells is also known to be a consequence of aging; thus, the genes contributing to intracellular redox balance have been concatenated with ARHL, i.e., presbycusis [54]. IDH2, which converts NADP+ to NADPH to maintain the GSH/GSSH ratio, has been highlighted as the major contributor to the antioxidant defense system in various tissues or cells [15], [16], [21], and several in vivo studies found that IDH2 deficiency causes organ dysfunctions by mitochondrial damage with aging in old mice [23], [55], [56]. A recent study performed by White et al. demonstrated that lack of IDH2 resulted in apoptosis of hair cells and SGNs due to accumulation of mitochondrial oxidative stress, which accelerates ARHL in CBA/CaJ male mice. They suggested that decreased NADPH redox status caused by IDH2 deficiency disables thioredoxin pathway particularly, rather than glutathione pathway in mitochondria, eventually leading damages of hair cells and SGNs [56]. This result showed two major differences with our study, the onset age of hearing loss and its major underlying mechanism. First in this study, a significant shift in the ABR threshold was found after 3 months of age in Idh2−/− mice, which was much earlier than the general onset age of ARHL in mice [57]. A possible explanation can be found in the genetic background of the C57BL/6 mouse strain of the Idh2−/− mice used in this study. The C57BL/6 mouse strain is known as homozygous for a specific mutation in the Cdh23 gene (c.753A>G). Because Cdh23 encodes a component of the tip-link that connects the stereocilia of hair cells, dysfunctional CDH23 generated by the c.753A>G mutation makes the mice susceptible to ARHL [57], [58]. The C57BL/6 strain, which exhibits a critical pattern of ARHL by 12–15months of age, has been used for various studies associated with aging [59], [60]. This genetic background might accelerate the onset age of hearing loss caused by IDH2 deficiency. Although this strain essentially has ARHL even in wild type, the Idh2−/− mice exhibited obvious and significantly greater hearing deterioration and histological defects than the Idh2+/+ mice. Moreover, only Idh2+/+ and Idh2−/− mouse littermates were used in all experiments to minimize individual differences and variations in the maternal environment. Therefore, we conclude that our data are sufficient to provide evidence that the obviously defective phenotype found in Idh2−/− mice and the disrupted redox balance observed in IDH2-deficient cochlear explants were caused by the loss of IDH2 function. Second, our study verified that IDH2 deficiency lead to an increase in the GSSG/GSH ratio leading mitochondrial damage, suggesting that mitochondrial glutathione pathway is significantly regulated by IDH2. This result was highly consistent with other IDH2 studies. In several organs, the role of IDH2 has been determined to be essential for maintenance of mitochondrial glutathione status to prevent diseases including cardiac hypertrophy [18], and renal dysfunction [20], [61]. Considering that IDH2 is an upstream regulator of the glutathione-dependent antioxidant defense pathway in mitochondria, it could be expected that IDH2 function might influence the overall mitochondrial redox balance. Thus, using this Idh2−/− mouse model for functional studies of ROS-related hearing loss will allow us to extend the range of applicable antioxidants. This means that various types of mitochondria-targeted antioxidant that interventions at different stages of the ROS cascade can be evaluated for their therapeutic effect using this valuable Idh2−/− mouse model.
In summary, our in vivo and ex vivo studies using Idh2−/− mice demonstrated that IDH2 plays a crucial role in the survival of hair cells and SGNs by regulating mitochondrial ROS levels, which proves the powerful impact of IDH2 in the functional maintenance of cochlear cells. Degeneration of the cells caused by IDH2 deficiency can be prevented by MitoQ, suggesting a possibility that genetic hearing loss caused by functional loss of ROS-related genes might be prevented by nongenetic agents. Finally, this study suggests that this Idh2−/− mouse model might be of great value in the development of novel therapeutic agents to overcome mitochondrial ROS-induced hearing loss in humans.
Acknowledgements
Our research was supported by the Bio & Medical Technology Development Program of the National Research Foundation of Korea, Republic of Korea: Grant 2014M3A9D5A01073865 (to J.B. and U.K.K), 2018R1A2B2004606 (to U.K.K.), 2017R1C1B2009705 (to J.I.B.). The Korea Health technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea, also supported this study: HI16C1501 (to K.Y.L.) and HI18C0160 (U.K.K.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.11.013
Contributor Information
Kyu-Yup Lee, Email: kylee@knu.ac.kr.
Jeen-Woo Park, Email: parkjw@knu.ac.kr.
Un-Kyung Kim, Email: kimuk@knu.ac.kr.
Appendix A. Supplementary material
Fig. 1.
Analysis of IDH2 expression in the inner ear ofIdh2+/+andIdh2−/−mice. (A) Loss of Idh2 mRNA expression in the inner ear of Idh2−/− mice at P20 was confirmed by RT-PCR analysis. Gapdh was used as a loading control. (B) Lack of IDH2 protein in the inner ear of Idh2−/− mice at P20 was confirmed by western blot analysis using an anti-IDH2 antibody. β-actin was used as a loading control.
Fig. 2.
Relative comparison of hearing loss progression at each frequency. Differences in the mean ABR threshold between Idh2+/+ and Idh2−/− mice are shown in the graph for 8 (squares), 16 (white circles) and 32 kHz (black circles).
Fig. 3.
Histological evaluation of cochlea fromIdh2+/+andIdh2−/−mice. H&E staining was performed in the inner ear sections of Idh2+/+ and Idh2−/− mice at 2 months of age. SG, spiral ganglion; SV, stria vascularis; SL, spiral ligament. Scale bars, 100 µm in (i′) and 20 µm in (c′ and f′). The arrowheads point to three rows of outer hair cells, and the asterisk (*) indicates an inner hair cell.
Fig. 4.
Hydrogen peroxide-induced oxidative damage to hair cells in the cochlear explants fromIdh2+/+mice. The organs of Corti explants of newborn Idh2+/+ mice were treated with the indicated concentrations of hydrogen peroxide for 5 days. The cochlear explants were stained with phalloidin to label the outline of the hair cells. Scale bars: 50 µm.
References
- 1.Kamata H., Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11(1):1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 2.Le Bras M., Clement M.V., Pervaiz S., Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol. Histopathol. 2005;20(1):205–219. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan A., Lehmler H.J., Robertson L.W., Ludewig G. Production of DNA strand breaks in vitro and reactive oxygen species in vitro and in HL-60 cells by PCB metabolites. Toxicol. Sci. 2001;60(1):92–102. doi: 10.1093/toxsci/60.1.92. [DOI] [PubMed] [Google Scholar]
- 4.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Orr W.C., Sohal R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263(5150):1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 6.Kamogashira T., Fujimoto C., Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed. Res. Int. 2015;2015:617207. doi: 10.1155/2015/617207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darrat I., Ahmad N., Seidman K., Seidman M.D. Auditory research involving antioxidants. Curr. Opin. Otolaryngol. Head Neck Surg. 2007;15(5):358–363. doi: 10.1097/MOO.0b013e3282efa641. [DOI] [PubMed] [Google Scholar]
- 8.Ohlemiller K.K., Wright J.S., Dugan L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurootol. 1999;4(5):229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed Z.M., Yousaf R., Lee B.C., Khan S.N., Lee S., Lee K., Husnain T., Rehman A.U., Bonneux S., Ansar M., Ahmad W., Leal S.M., Gladyshev V.N., Belyantseva I.A., Van Camp G., Riazuddin S., Friedman T.B., Riazuddin S. Functional null mutations of MSRB3 encoding methionine sulfoxide reductase are associated with human deafness DFNB74. Am. J. Hum. Genet. 2011;88(1):19–29. doi: 10.1016/j.ajhg.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissbach H., Etienne F., Hoshi T., Heinemann S.H., Lowther W.T., Matthews B., St John G., Nathan C., Brot N. Peptide methionine sulfoxide reductase: structure, mechanism of action, and biological function. Arch. Biochem. Biophys. 2002;397(2):172–178. doi: 10.1006/abbi.2001.2664. [DOI] [PubMed] [Google Scholar]
- 11.Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic. Biol. Med. 1995;18(1):93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- 12.Kujoth G.C., Bradshaw P.C., Haroon S., Prolla T.A. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3(2):e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebrin I., Sohal R.S. Pro-oxidant shift in glutathione redox state during aging. Adv. Drug Deliv. Rev. 2008;60(13–14):1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang L., Su S.M. Isocitrate dehydrogenase mutation and (R)-2-hydroxyglutarate: from basic discovery to therapeutics development. Annu. Rev. Biochem. 2017;86:305–331. doi: 10.1146/annurev-biochem-061516-044732. [DOI] [PubMed] [Google Scholar]
- 16.Reitman Z.J., Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J. Natl. Cancer Inst. 2010;102(13):932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakkar P., Singh B.K. Mitochondria: a hub of redox activities and cellular distress control. Mol. Cell Biochem. 2007;305(1–2):235–253. doi: 10.1007/s11010-007-9520-8. [DOI] [PubMed] [Google Scholar]
- 18.Ku H.J., Park J.W. Downregulation of IDH2 exacerbates H2O2-mediated cell death and hypertrophy. Redox Rep. 2017;22(1):35–41. doi: 10.1080/13510002.2015.1135581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S., Kim S.Y., Ku H.J., Jeon Y.H., Lee H.W., Lee J., Kwon T.K., Park K.M., Park J.W. Suppression of tumorigenesis in mitochondrial NADP(+)-dependent isocitrate dehydrogenase knock-out mice. Biochim. Biophys. Acta. 2014;1842(2):135–143. doi: 10.1016/j.bbadis.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Han S.J., Jang H.S., Noh M.R., Kim J., Kong M.J., Kim J.I., Park J.W., Park K.M. Mitochondrial NADP(+)-dependent isocitrate dehydrogenase deficiency exacerbates mitochondrial and cell damage after kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2017;28(4):1200–1215. doi: 10.1681/ASN.2016030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo S.H., Son M.K., Koh H.J., Lee S.M., Song I.H., Kim Y.O., Lee Y.S., Jeong K.S., Kim W.B., Park J.W., Song B.J., Huh T.L. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2001;276(19):16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 22.Park J.H., Ku H.J., Lee J.H., Park J.W. IDH2 deficiency accelerates skin pigmentation in mice via enhancing melanogenesis. Redox Biol. 2018;17:16–24. doi: 10.1016/j.redox.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S.J., Cha H., Lee S., Kim H., Ku H.J., Kim S.H., Park J.H., Lee J.H., Park K.M., Park J.W. Idh2 deficiency accelerates renal dysfunction in aged mice. Biochem. Biophys. Res. Commun. 2017;493(1):34–39. doi: 10.1016/j.bbrc.2017.09.082. [DOI] [PubMed] [Google Scholar]
- 24.Park J.B., Nagar H., Choi S., Jung S.B., Kim H.W., Kang S.K., Lee J.W., Lee J.H., Park J.W., Irani K., Jeon B.H., Song H.J., Kim C.S. IDH2 deficiency impairs mitochondrial function in endothelial cells and endothelium-dependent vasomotor function. Free Radic. Biol. Med. 2016;94:36–46. doi: 10.1016/j.freeradbiomed.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Kim M.A., Cho H.J., Bae S.H., Lee B., Oh S.K., Kwon T.J., Ryoo Z.Y., Kim H.Y., Cho J.H., Kim U.K., Lee K.Y. Methionine sulfoxide reductase B3-targeted in utero gene therapy rescues hearing function in a mouse model of congenital sensorineural hearing loss. Antioxid. Redox Signal. 2016;24(11):590–602. doi: 10.1089/ars.2015.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson K.M., Janes M.S., Pehar M., Monette J.S., Ross M.F., Hagen T.M., Murphy M.P., Beckman J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA. 2006;103(41):15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.R., Kim K.H., Lee S., Oh S.K., Park J.W., Lee K.Y., Baek J.I., Kim U.K. Expression patterns of members of the isocitrate dehydrogenase gene family in murine inner ear. Biotech. Histochem. 2017;92(7):536–544. doi: 10.1080/10520295.2017.1367034. [DOI] [PubMed] [Google Scholar]
- 28.Wallace D.C. Mitochondrial diseases in man and mouse. Science. 1999;283(5407):1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 29.Wallace D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartley A., Stone J.M., Heron C., Cooper J.M., Schapira A.H. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson's disease. J. Neurochem. 1994;63(5):1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- 31.Higuchi M., Proske R.J., Yeh E.T. Inhibition of mitochondrial respiratory chain complex I by TNF results in cytochrome c release, membrane permeability transition, and apoptosis. Oncogene. 1998;17(19):2515–2524. doi: 10.1038/sj.onc.1202485. [DOI] [PubMed] [Google Scholar]
- 32.Kwong J.Q., Henning M.S., Starkov A.A., Manfredi G. The mitochondrial respiratory chain is a modulator of apoptosis. J. Cell Biol. 2007;179(6):1163–1177. doi: 10.1083/jcb.200704059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noh Y.H., Kim K.Y., Shim M.S., Choi S.H., Choi S., Ellisman M.H., Weinreb R.N., Perkins G.A., Ju W.K. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013;4:e820. doi: 10.1038/cddis.2013.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinerfeld D., Traini M.D., Weinberger R.P., Cochran B., Doctrow S.R., Harry J., Melov S. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J. Neurochem. 2004;88(3):657–667. doi: 10.1046/j.1471-4159.2003.02195.x. [DOI] [PubMed] [Google Scholar]
- 35.Stadtman T.C. Biosynthesis and function of selenocysteine-containing enzymes. J. Biol. Chem. 1991;266(25):16257–16260. [PubMed] [Google Scholar]
- 36.Drose S. Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre- and postconditioning. Biochim. Biophys. Acta. 2013;1827(5):578–587. doi: 10.1016/j.bbabio.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y.R., Zweier J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014;114(3):524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelso G.F., Porteous C.M., Coulter C.V., Hughes G., Porteous W.K., Ledgerwood E.C., Smith R.A., Murphy M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 2001;276(7):4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 39.Ernster L., Forsmark P., Nordenbrand K. The mode of action of lipid-soluble antioxidants in biological membranes: relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. Biofactors. 1992;3(4):241–248. [PubMed] [Google Scholar]
- 40.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 41.Lowes D.A., Thottakam B.M., Webster N.R., Murphy M.P., Galley H.F. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 2008;45(11):1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cocheme H.M., Murphy M.P., Dominiczak A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54(2):322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 43.McManus J.M., Lu H., Cullins M.J., Chiel H.J. Differential activation of an identified motor neuron and neuromodulation provide Aplysia's retractor muscle an additional function. J. Neurophysiol. 2014;112(4):778–791. doi: 10.1152/jn.00148.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojano-Dirain C.P., Antonelli P.J., Prell C.G. Le. Mitochondria-targeted antioxidant MitoQ reduces gentamicin-induced ototoxicity. Otol. Neurotol. 2014;35(3):533–539. doi: 10.1097/MAO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 45.Dirain C.O., Ng M., Milne-Davies B., Joseph J.K., Antonelli P.J. Evaluation of mitoquinone for protecting against amikacin-induced ototoxicity in guinea pigs. Otol. Neurotol. 2018;39(1):111–118. doi: 10.1097/MAO.0000000000001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobkowicz H.M., Bereman B., Rose J.E. Organotypic development of the organ of Corti in culture. J. Neurocytol. 1975;4(5):543–572. doi: 10.1007/BF01351537. [DOI] [PubMed] [Google Scholar]
- 47.Kelley M.W., Xu X.M., Wagner M.A., Warchol M.E., Corwin J.T. The developing organ of Corti contains retinoic acid and forms supernumerary hair cells in response to exogenous retinoic acid in culture. Development. 1993;119(4):1041–1053. doi: 10.1242/dev.119.4.1041. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Water T.R. Van De, Bonny C., de Ribaupierre F., Puel J.L., Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J. Neurosci. 2003;23(24):8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Q., Jia Z., Zhang Y., Ren X. Morin hydrate promotes inner ear neural stem cell survival and differentiation and protects cochlea against neuronal hearing loss. J. Cell Mol. Med. 2017;21(3):600–608. doi: 10.1111/jcmm.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lalwani A.K., Han J.J., Castelein C.M., Carvalho G.J., Mhatre A.N. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope. 2002;112(8 Pt 1):1325–1334. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Gyorgy B., Sage C., Indzhykulian A.A., Scheffer D.I., Brisson A.R., Tan S., Wu X., Volak A., Mu D., Tamvakologos P.I., Li Y., Fitzpatrick Z., Ericsson M., Breakefield X.O., Corey D.P., Maguire C.A. Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol. Ther. 2017;25(2):379–391. doi: 10.1016/j.ymthe.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckman K.B., Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 53.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Fujimoto C., Yamasoba T. Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxid. Med Cell Longev. 2014;2014:582849. doi: 10.1155/2014/582849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chae U., Park N.R., Kim E.S., Choi J.Y., Yim M., Lee H.S., Lee S.R., Lee S., Park J.W., Lee D.S. IDH2-deficient mice develop spinal deformities with aging. Physiol. Res. 2018;67(3):487–494. doi: 10.33549/physiolres.933711. [DOI] [PubMed] [Google Scholar]
- 56.White K., Kim M.J., Han C., Park H.J., Ding D., Boyd K., Walker L., Linser P., Meneses Z., Slade C., Hirst J., Santostefano K., Terada N., Miyakawa T., Tanokura M., Salvi R., Someya S. Loss of IDH2 accelerates age-related hearing loss in male mice. Sci. Rep. 2018;8(1):5039. doi: 10.1038/s41598-018-23436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Q.Y., Johnson K.R., Erway L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130(1–2):94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Someya S., Xu J., Kondo K., Ding D., Salvi R.J., Yamasoba T., Rabinovitch P.S., Weindruch R., Leeuwenburgh C., Tanokura M., Prolla T.A. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA. 2009;106(46):19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keithley E.M., Canto C., Zheng Q.Y., Fischel-Ghodsian N., Johnson K.R. Age-related hearing loss and the ahl locus in mice. Hear. Res. 2004;188(1–2):21–28. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han C., Linser P., Park H.J., Kim M.J., White K., Vann J.M., Ding D., Prolla T.A., Someya S. Sirt1 deficiency protects cochlear cells and delays the early onset of age-related hearing loss in C57BL/6 mice. Neurobiol. Aging. 2016;43:58–71. doi: 10.1016/j.neurobiolaging.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han S.J., Choi H.S., Kim J.I., Park J.W., Park K.M. IDH2 deficiency increases the liver susceptibility to ischemia-reperfusion injury via increased mitochondrial oxidative injury. Redox Biol. 2018;14:142–153. doi: 10.1016/j.redox.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]