Abstract
There has been a drive to develop new cell based therapies to treat corneal blindness, one of the most common causes of blindness worldwide. Mechanical and physical cues are known to regulate the behavior of many cell types, however studies examining these effects on corneal epithelial cells have been limited in number and their findings have not previously been amalgamated and contrasted. Here, we provide an overview of the different types of mechanical stimuli to which the corneal epithelium is exposed and the influence that these have on the cells. Shear stress from the tear film motion and blinking, extracellular matrix stiffness and external physical forces such as eye rubbing and contact lens wear are among some of the forms of mechanical stimuli that the epithelium experiences. In vivo and in vitro studies examining the mechanobiology on corneal epithelial cells under differing mechanical environments are explored. A greater understanding of the mechanobiology of the corneal epithelium has the potential to lead to improved tissue engineering and cell based therapies to repair and regenerate damaged cornea.
Highlights
-
•
The corneal epithelium is subjected to variety of mechanical forces and stresses.
-
•
Force is applied through tears film motion, blinking and other forms of contact.
-
•
Mechanical stimuli is shown to influence cell behavior and signaling processes.
-
•
Epithelial mechanobiology may assist in developing new regenerative therapies.
1. Introduction
The corneal epithelium is the outermost anterior section of the cornea consisting of 5–7 layers of stratified squamous epithelial cells (Fig. 1). It is maintained by limbal epithelial stem cells located in crypts along the cornea-scleral border (Dua et al., 2005; Dziasko et al., 2014). Damage to the corneal epithelium due to trachoma, limbal stem cell deficiencies or physical abrasion can result in pain, inflammation, vascularization and blindness. Depending on the severity of injury or vision loss, keratoplasty may be required. Corneal tissue is the most transplanted tissue worldwide but in many countries the supply does not meet demand. Graft failure occurs in up to 10% of corneal transplants and normally requires a re-graft which can then fail in 50% of cases (Tan et al., 2012). When combined with a higher demand for donor tissue due to an aging population and a reduction in the pool of suitable tissue donors due to increasing popularity of elective surgical procedures such as LASIK, there is a real need for alternative therapies to treat corneal epithelial blindness.
Fig. 1.
Schematic representation of the structure and composition of the cornea and limbus.
Biomaterial, tissue engineering and cell based therapies have produced promising results to regenerate or repair the corneal epithelium. Biomaterial and tissue engineering approaches have focused on developing suitable materials for transplanting sheets of cells (Deshpande et al., 2009; Nakamura et al., 2003; Sitalakshmi et al., 2009) or have attempted to engineer scaffolds suitable for anterior lamellar keratoplasty (Pang et al., 2010; Zhang et al., 2015). Cell based therapies have primarily focused on optimizing the culture conditions for expanding limbal stem cells and forming epithelial sheets (Miyashita et al., 2008; Pellegrini et al., 1997; Zhang et al., 2005). Most studies have focused on the development and application of different biomaterials and fabrication techniques to generate scaffolds or examined ways of influencing the cells behavior by adding different biological or chemical agents. However, the role of the cells physical environment and the effect of mechanical stimuli on modulating the repair and regeneration of a healthy corneal epithelium is less well understood.
When cells are subjected to physical forces this normally results in a series of intracellular biochemical processes that regulate both the cells physiological and pathological responses (Chen, 2008). Cells can detect changes in their mechanical environment and respond by modulating intracellular chemical signaling pathways in a process called mechanotransduction (Huang et al., 2004). Examples of how mechanical forces can influence the behavior of cells in tissue and organs can be seen throughout the body such as the effect of fluid pressure and shear stress from pumping blood on the regulation of endothelial vasculature (Resnick et al., 2003) or the ability of bone to remodel under load (Orr et al., 2006).
Physical forces have been shown to provide a way of altering the conformation of proteins to generate signals for both widely expressed and specialized mechanosensitive systems (Orr et al., 2006). A wide variety of signaling molecules and structures have been shown to contribute to mechanotransductive events including molecules and structures such as integrins, extracellular matrix components, cadherin molecules, nuclei and stretch activated ion channels (Ingber, 2006). For example, integrins link the cells cytoskeleton to its surrounding extracellular matrix and undergo conformational changes in response to force (Hynes, 2002; Ross et al., 2013; Wang et al., 1993). This allows the transduction of mechanically induced signals across the cell membrane. Cell adhesion and migration regulating mechanosensitive mechanisms occur in the nucleus such as nuclear membrane tension and remodeling of nuclear envelope lamina (Aureille et al., 2017).
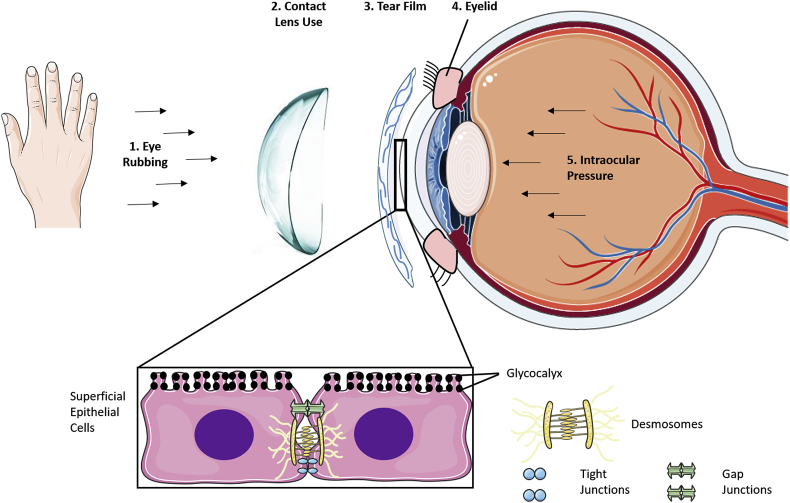
In-vivo, the corneal epithelium is subjected to a variety of extrinsic forces and intrinsic stresses. The most common mechanisms by which mechanical stimuli are applied to the epithelium include intraocular pressure, eyelid motion, tear film motion, contact lens use and eye rubbing (Fig. 2). Here we present an overview of the effect that different mechanical stimuli have on the epithelial layer of the cornea, examine how to replicate these conditions in-vitro and discuss how this information can be used to aid in developing biomaterials to repair or regenerate the corneal epithelium.
Fig. 2.
Image depicting different sources of mechanical stimuli that can be applied to the cornea and the subcellular structures in superficial epithelial cells that respond to these mechanical signals.
2. Impact of fluid flow on the epithelium
2.1. Tear film dynamics in vivo
The corneal epithelium is covered by a thin tear film to protect the eye against bacteria and other pathogens. It plays an important role in the optical properties of the eye by refracting light entering the eye (Albarran et al., 1997; Montés-Micó et al., 2005; Montes-Mico et al., 2010). The dynamic nature of the tear film means its motion results in shearing forces being applied to the underlying cells (Braun et al., 2015). The amount of force applied is regulated by a number of factors including the tear film thickness, composition, viscosity and flow pattern resulting from blinking.
The tear film must be stable with uniform thickness over the entire corneal surface to maintain the vision and health of the ocular surface (Svitova and Lin, 2016). While early studies on tear film thickness and protein composition were inconclusive, more recent studies have established that tear lipids exist as multi layered films that range between 30 and 100 nm in thickness and that the total tear film thickness varies between individuals and is dependent on the measurement techniques applied with values ranging from 3 to 10 μm (Hodson and Earlam, 1994).
During blinking, the tear film is partially displaced by the eyelid. Several mathematical and computational models have been developed to predict tear film motion during blinking (Braun et al., 2015; Heryudono et al., 2007; Winter et al., 2010). In most models the blink cycle can be broken into different distinct phases (e.g. downstroke, turning point, upstroke and interblink) each of which affect the tear film dynamics in differing ways. For example during the downstoke lipids are compressed into a thick layer under the upper eyelid. At the start of the upstroke these lipids form a thick and narrow band. A ‘rippling’ effect in the tear film is also observed during lid motion (Braun et al., 2015).
The flow pattern of the tear film can be altered by several medical conditions such as Sjögrens syndrome or rheumatoid arthritis both of which can result in dry eye syndrome (Fujita et al., 2005). Wavefront aberrations are optical flaws that occur in the eye preventing light focusing effectively onto the retina. These aberrations can be of low order (e.g. astigmatism) and can be corrected using glasses or surgery. However higher order aberrations are untreatable and the tear film flow can affect these aberrations (Resan et al., 2012). Variations in the tear film dynamics associated with dry eye can affect wavefront aberrations after blinking (Montes-Mico et al., 2005; Wang et al., 2009). For some medical conditions, artificial tear drops can be used to relieve pain but they increase the viscosity of tears and significantly increase shear stress on the ocular surface during a blink. This in turn increases the cell shedding rate of corneal epithelial cells (McElwain et al., 2007).
Evaporation can influence the dynamics of the tear film and is affected by environmental factors including temperature and humidity. The lipid layer of the tear film modulates the evaporation rate and has an impact on tear film dynamics, stability and osmolality (Stahl et al., 2012). As the lipid layer becomes thinner the tear film becomes less stable with a shorter break-up time (Maissa and Guillon, 2010). Lipid layer thinning increases as people get older and is more pronounced in women than in men (Maissa and Guillon, 2010).
2.2. Simulation of fluid flow in vitro
To improve our understanding of the precise mechanisms by which fluid shear forces affect corneal epithelial cells it is useful to study the cells in vitro under simulated flow conditions. Ren and Wilson (1996, 1997) used an in vitro whole eye perfusion system to study the effects of fluid shear on the epithelium (Ren and Wilson, 1996, 1997). A magnetic stir plate and tube containing tear solution was used to allow the entire corneal surface to be exposed to the solution for 6 to 8 hours. There was an increase in the amount of apoptosis when cells were subjected to shear conditions (Ren and Wilson, 1996). The rate of cell shedding also increased in shear conditions with 3500 cells shed under static conditions compared to 20,000 cells shed under dynamic conditions (Ren and Wilson, 1997). Both studies suggest a role for fluid motion in the maintenance and disposal of corneal epithelial cells.
Kang et al. (2014) used a purpose built bioreactor to examine the effect of flow induced shear stress on the stemness of limbal epithelial stem cells (Kang et al., 2014). The flow induced shear stress mimicked that during blinking. It was found that steady flow had a positive effect on maintenance of stemness of the cells while intermittent flow caused the cells to differentiate into transient amplifying cells as evident from the appearance of holoclones.
Molladavoodi et al. (2017) used a flow chamber to study the effect of fluid to passing over cells adhered to a substrate (Molladavoodi et al., 2017). Shear stress resulting from a flow rates of 4 dyn/cm2 being applied for 24 hours was found to up-regulate integrin β1 expression which is normally associated with cell adhesion. Under the same conditions there was downregulation of intercellular adhesion molecule-1 (ICAM-1) which is known to interact with α-actinin (Carpen et al., 1992) and has been associated with cell motility (Iwata et al., 2003). In this study, the changes in integrin β1 and ICAM-1 appears to correlate with changes in the cells cytoskeletal organization with actin filaments forming dense, aligned bundles under flow conditions. Changes to the cytoskeleton resulting from shear forces were also found to enhance the rate of wound healing although fluid shear needed to be applied prior to the damage occurring.
Hampel et al. (2018) used an IBIDI pump system to replicate the stress applied during blinking on an immortalized corneal epithelial cell line (hTCEpi) (Hampel et al., 2018). The application of shear stress resulted in fewer extracellular gaps and increased mRNA expression of E-cadherin, occludin, desmoplakin and tight junction protein. Flow direction did not induce alignment of stress fibers.
Together these studies show that shear stress induced from fluid flow has an impact on regulating the behavior of corneal epithelial and limbal cells. A summary of the methods used to simulate fluid flow in vitro is shown in Table 1.
Table 1.
Studies on the effects of fluid flow on the corneal epithelium.
| Author(s) | Methods | Results |
|---|---|---|
| Ren and Wilson 1996 | In vitro whole-eye perfusion | Shear stress increased apoptosis and terminal differentiation |
| Ren and Wilson 1997 | In vitro whole-eye perfusion | Increased cell shedding with shear force |
| Kang et al., 2014 | Bioreactor flow chamber | Increased proliferation and maintenance of stemness – dependent on flow type |
| Molladavoodi, S. et al., 2017 | Parallel plate flow chamber + Syringe pump | Migratory behavior and wound healing ability affected by shear stress levels |
| Hampel et al., 2018 | IBIDI pump system | Shear stress resulted in less extracellular gaps between cells and increased gene expression of cell junction proteins. Cells did not align in the direction of the flow |
3. Role of matrix substrate stiffness on regulation of epithelial cells
Several studies have shown that the mechanical characteristics of the substrate on which cells adhere can influence their phenotype (Discher et al., 2005; Engler et al., 2006). Early studies using corneal epithelial cells cultured on different corneal substrates showed changes in distribution of cytoskeletal and adhesion proteins in response to the materials properties which may contribute to wound healing dynamics in the cornea (Wu et al., 1995). In the cornea, changes to the stroma or Bowman's layer resulting from damage or disease can affect how the epithelial cells behave. Extracellular matrix proteins secreted by the stroma in response to chronic inflammation or in homeostatic conditions can alter the mechanical integrity of the extracellular matrix and lead to the activation of YAP/TAZ and β-catenin signaling pathways that in turn promotes the epidermal differentiation of the epithelium. This can lead to corneal squamous cell metaplasia that can lead to blindness. (Nowell et al., 2016). The regulation of YAP/TAZ has also been shown to be dependent on topographical cues (Raghunathan et al., 2014). This pathway has been shown in multiple studies to be involved in the relaying of mechanical signals resulting from cell shape and extracellular matrix rigidity. The pathway is required to control the differentiation of mesenchymal stem cells induced by varying matrix stiffness (Dupont et al., 2011). Hence it is not surprising that it has a role to play in the corneal epitheliums response to underlying matrix stiffness.
Collagen hydrogels have been used as a substrate to examine the influence of stiffness on Yap expression by limbal cells. Plastic compression of collagen hydrogels can be used to vary their stiffness by removing water and increasing the overall collagen density and stiffness (Abou Neel et al., 2006; Cheema et al., 2007; Levis et al., 2010). Foster et al. (2014) showed that the expression YAP by epithelial cells varied on collagen hydrogels of differing stiffness (Foster et al., 2014). Localization of YAP was selected to assess the effect of substrate stiffness on differentiation and centripetal migration of limbal epithelial cells during normal homeostasis. The expression of YAP was compared between the limbus and central cornea in situ and in vitro using limbal epithelial stem cells on collagen hydrogels. The nuclear expression of YAP was increased with increasing stiffness and this was also used as a molecular probe to look at the mechanical microenvironment in situ in a normal ocular surface. Localization of YAP in situ was cytoplasmic in the basal limbal epithelial cells and nuclear in the basal central corneal epithelial cells. The study concluded that YAP and the distinct cell populations studied indicated that cells experience a different mechanical environment between the central cornea and limbus. This has led to a new hypothesis in which differences in substrate stiffness drives migration and differentiation of limbal epithelial cells controlling homeostasis.
Jones et al. (2012) used collagen substrates to demonstrate that stiffness can affect the phenotype of limbal derived cells (Jones et al., 2012). The authors showed using immunohistochemistry and western blotting that when these cells were cultured on stiffer collagen substrates (2.9 kPa) they had a greater expression of cytokeratin 3 and a higher cell number after 2 weeks when compared to cells on softer collagen substrates (0.003 kPa). The modulus of the stiffer collagen substrate was similar to the modulus of the anterior basement membrane which is believed to be between 2 kPa and 15 kPa (Last et al., 2009, 2012).
Human corneal epithelial cells cultured on Polyacrylamide (Paam) gels with differing elastic moduli (Compliant 1.3 kPa, Medium 3.2 kPa and Stiff 9.2 kPa) displayed no significant variation in cell proliferation, integrin expression and ICAM-1 expression although apoptosis and necrosis were increased on softer substrates (Molladavoodi et al., 2015). The migration of cells was also analyzed and showed that cells on medium and high stiffness substrates migrated significantly more than the softer. When the cytoskeletal structure of the cells was assessed, cells on compliant or softer substrates were lacking stress fibers while the formation of stress fibers increased on a high substrate stiffness. Actin is known to be vital for cells to exert pulling forces onto their environment (Ahearne et al., 2010), hence the lack of stress fiber polymerized actin filaments as well as a higher number of apoptotic cells on soft substrates could explain the cells inability to migrate. One limitation with using Paam gels is that their surface topography and the availability of adhesion sites can vary with varying stiffness introducing additional parameters that could affect cell behavior (Trappmann et al., 2012).
Fluctuations in intraocular pressure (IOP) can also lead to changes in the corneal epithelium. As IOP increases, the shape of the cornea may change leading to mechanical strain (the percentage deformation relative to original shape) being applied to the corneal epithelial cells although the overall stiffness (ability to resist deformation) of the cornea is not believed to change significantly (Johnson et al., 2007; Pierscionek et al., 2007).
Table 2 summarizes the studies carried out in the literature on the effect of stiffness on corneal epithelium.
Table 2.
Studies on the effects of stiffness on the corneal epithelium.
| Author(s) | Methods | Results |
|---|---|---|
| Wu et al., 1995 | Corneal epithelial cells cultures on living modified stromal substrates | Changes in cytoskeletal and adhesion protein distribution in response to different substrates |
| Johnson et al., 2007 | Saline infusion to corneas mounted on an artificial anterior chamber | Elastic properties of corneas ex vivo demonstrate a buffering mechanism protecting the eye from intraocular pressure surges in vivo |
| Pierscionek et al., 2007 | Saline intravitreal injections into fresh porcine eyes | Elastic moduli of cornea and sclera are independent of intraocular pressure but does affect scleral curvature |
| Jones et al., 2012 | Compressed collagen substrates | Increased differentiation and cell number on stiffer gels |
| Foster et al., 2014 | Collagen gels of increasing stiffness | Centripetal increase in nuclear localization of Yap with higher substrate stiffness |
| Raghunathan et al., 2014 | Polymeric topographically patterned substrates | Differential expression of YAP and TAZ observed in limbus influenced by substratum topography |
| Molladavoodi et al., 2015 | Polyacrylamide gels | Compliant substrates increase apoptosis and necrosis, less visible actin filaments and impaired cell migration |
| Nowell et al., 2016 | Corneal wounding | Chronic inflammation can promote abnormal cell fate through mechanotransduction |
4. External forces on the epithelium
While several studies have examined the mechanical and viscoelastic characteristics of the cornea using techniques such as tensile testing, inflation or micro-indentation (Ahearne et al., 2007; Elsheikh et al., 2007; Hatami-Marbini and Rahimi, 2014; Hjortdal, 1994), a different method is required to characterize the mechanical properties of the epithelium or epithelial cells. The epithelium is too thin to be accurately measured by these techniques while the mechanical properties of individual cells or cell layers are more commonly examined using techniques such as nano-indentation, which involves pushing an indenter nanometers into a cell and measuring the resultant force (Zhou et al., 2012), or micropipette aspiration, which involves sucking part of a cell into a capillary tube and calculating the cells mechanical properties based in the suction force and cell deformation (Hochmuth, 2000). One of the only studies that examined the mechanical properties of corneal epithelial cells using nano-indentation obtained a value of 16.5 kPa for its elastic modulus (Straehla et al., 2010). Further study of the mechanical properties of epithelial cells may be warranted since changes to the modulus of cells have been shown to be indicators of cell abnormalities and human diseases (Guz et al., 2014), for example diabetes increases the modulus of cardiomyocytes (Benech et al., 2014) while some cancer cells have a lower modulus than non-cancerous cells (Lekka et al., 1999).
Since the corneal epithelium is subjected to a wide range of mechanical stimuli it needs to have sufficient strength and stiffness to withstand any applied forces. Two common examples of methods by which external forces are applied to the cornea are by rubbing and wearing contact lenses. The following section describes studies which have examined their effects on the epithelium.
4.1. Rubbing of the eyelid
Eyelids are composed of skin, fibrous tissue, muscle and a mucous membrane to protect the eye from injury and excess light. They also spread the tear film evenly over the ocular surface while blinking. Electrohydrodynamic studies have shown that the tear film lubricates and protects the cornea from the eyelid during blinking and eye lid closure (Jones et al., 2008). However, when the eyelid is being rubbed, it is pushed back against the corneal epithelium, reducing the tear film thickness under the compressed area. In addition to compression, the motion during rubbing can result in shear forces being applied to the epithelium. The combination of these forces can have a negative effect on the health of the cornea.
A number of cases have reported an association between eye rubbing and the progression of keratoconus (Ioannidis et al., 2005; Jafri et al., 2004; Lindsay et al., 2000). For example, Lindsay et al. (2000) reported a patient who experienced frequent eye rubbing due to epiphora from punctal agenesis developed unilateral keratoconus in the affected eye (Lindsay et al., 2000). The mechanical stimulation applied to the epithelium during rubbing results in an increase in proteases and inflammatory markers such as MMP-13, IL-6 and TNF released into the tear film after 60 seconds of rubbing (Balasubramanian et al., 2013).
Rubbing may also lead to changes in the thickness of the epithelium. One study found that there was an 18.4% reduction in the thickness of the epithelium immediately after rubbing (McMonnies et al., 2010). It was hypothesized that the applied force resulted in cell displacement and flattening. However, a more recent study found no significant change in the epithelium thickness after rubbing (Prakasam et al., 2012).
In additional to having detrimental effects on the epithelium, it has been suggested that gentle rubbing or massage of the eyelid may have some beneficial effects on the eye. For example, eyelid massage could assist patients suffering blepharitis, a condition resulting in eyelid inflammation and blocking of the meibomian glands (Yun et al., 2015). In addition gentle rubbing of the eyelid has been shown to have no adverse effects on the cornea (Riede-Pult et al., 2017).
One of the difficulties in investigating the effect of rubbing on the epithelium is the wide variations in how people rub their eyes, the frequency of rubbing and the amount of force they apply. There is no set of standard rubbing forces or techniques resulting in wide variations in data collected by researchers. For this reason, while the force applied by rubbing does appear to affect corneal epithelial cells, the precise effect of rubbing on the health of the cornea remains unclear.
4.2. Contact lenses
Contact lenses are commonly used to improve vision for myopic patients without the need for eye-glasses. The advantages of contact lenses over glasses include they are more suitable to worn during sports, particularly contact sports where glasses could easily come off, lenses don't get fogged up or scratched like glasses and lenses don't limit the wearers field of vision. Despite these advantages there are some risks associated with their use (Killpartrick, 2016; Willcox, 2013).
One risk is that while contact lenses are designed to be oxygen permeable they still result in a decrease of oxygen uptake by the epithelium (Takatori et al., 2013). Contact lenses have also been shown to inhibit cell proliferation in the central cornea after prolonged wear (Ladage et al., 2003) and delay the migratory capacity of cells (Robertson, 2013). Poor oxygen permeability may lead to hypoxic conditions that can cause various conditions including epithelial keratitis and loss of corneal transparency. Under closed eyelid conditions this is made worse with the minimum oxygen requirement for the cornea not being met (Lee et al., 2015).
Hypoxic conditions activate Polo-like kinase 3(Plk3) signaling and c-Jun/AP-1 transcription complexes that in turn lead to apoptosis of corneal epithelial cells (Wang et al., 2016). Corneal hypoxia also leads to corneal edema which is well documented in low oxygen transmissible contact lenses. One study reported a biochemical description of the cornea to quantify hypoxic swelling (Leung et al., 2011). Inflammation has also been associated with hypoxia of the cornea and this can lead to vascularization and have detrimental effects on vision (Safvati et al., 2009). For these reasons contact lens manufacturers have been interested in developing lenses with higher oxygen permeability.
Contact lenses may also impart a mechanical effect on the epithelium by restricting tear film flow over the corneal surface (Mann and Tighe, 2013; Muntz et al., 2015). As previously mentioned, the motion of the tear film results in shear forces being applied to the epithelium. By restricting the tear flow under the lens, the mechanical stimulation that these cells are normally subjected to is inhibited. Proteins and lipids also may become entrapped under the contact lens leading to an immune reaction (Mann and Tighe, 2013). Furthermore, the thickness of the tear film has been shown to change pre- and post-contact lens wear (Lin et al., 1999; Nichols and King-Smith, 2003; Wang et al., 2003). This would likely result in changes to the magnitude of the shear force being applied to the cells.
An additional risk with wearing contact lenses is adhesion occurring between the lens and the cornea. Proteins and other molecules from the tear film may bind to the contact lens and allow cells from the epithelium to adhere. Depending on the adhesion strength, this can result in damage to the epithelium when the lens is being removed from the eye. This type of adhesion of the lens to the cornea is rare but may occur when the user has fallen asleep while wearing their lenses or when the lenses have been left in for a longer period of time than recommended by the manufacturer. One study found that this adhesion to the contact lens can tear the epithelium upon removal (Elkins et al., 2014). However, a limitation to the study was the use of cell monolayers rather than a stratified multilayer model hence further study will be required to fully elucidate the mechanical effects of contact lens adhesion on the corneal epithelium.
Understanding the effects that a contact lens has on the mechanobiological behavior of the corneal epithelium provides valuable information on how to improve the design of biomaterials for culturing and transplanting these cells. Table 3 summarizes several studies that have examined the effect of externally applied forces on the corneal epithelium.
Table 3.
Studies on the effects of external forces on the corneal epithelium.
| Author(s) | Methods | Results |
|---|---|---|
| Lin et al., 1999 | Measurement of tear layer by optical pachometry after application of soft contact lenses. | Showed the use of optical pachometry as a method to measure tear film thickness between eye and contact lens. Further investigation required to determine if post lens tear thickness is affected by lens wear. |
| Ladage et al., 2003 | One rabbit eye assigned to wear either silicone hydrogel or disposable contact lenses or eyelid suturing or no intervention. Cell proliferation using BrdU staining performed after 24hrs and 1 week. | Decrease in cell proliferation in all test groups at day 2. After 1 week of suturing or silicon hydrogel lens wear there was a significant increase in proliferation. Limbal staining for BrdU in all groups not significantly different from control. |
| Nichols and King-Smith, 2003 | Use of interferometric method to measure tear film thickness pre and post lens application. | Superior method of measuring tear film thickness after lens use compared to pachymetric method. |
| Wang et al., 2003 | Measurement of tear film thickness pre- and post-lens using optical coherence tomography (OCT). | Tear film thickness increased after lens fitting. Post-lens tear film is thicker than pre-lens tear film with soft contact lenses. Thickness independent of lens types. |
| McMonnies et al., 2010 | Eye rubbing of live subjects followed by measurement of epithelial thickness. | Reduction in epithelial thickness observed after rubbing. |
| Liu et al., 2011 | Measurement of corneal hysteresis, corneal resistance factor, corneal compensated IOP (IOPcc) and Goldman equivalent IOP (IOPg) in 40 subjects before and after 2 episodes of eye rubbing and breath holding. | Corneal hysteresis, corneal resistance factor and IOPg significantly lower after eye rubbing. This was not the case for IOPcc. Breath holding made no difference in any of the measurements. |
| Prakasam et al., 2012 | Eye rubbing over closed eyelids of live subjects followed by spectral domain OCT. | Eye rubbing in a circular motion over closed eyelids did not affect total corneal, epithelial and bowman's membrane thickness. |
| Balasubramanian et al., 2013 | Tear collection before and after 60 s of eye rubbing followed by measurement of tear collagenase activity and inflammatory molecules. | Increased level of MMP-13, IL-6 and TNFα but no significant alteration in collagenase activity. |
| Takatori et al., 2013 | Use of micro-polarographic Clark electrode to measure in situ corneal oxygen uptake during soft contact lens wear. | 12 different lenses varied oxygen uptake from low to high permeability depending on the lens. High oxygen permeable lenses have high oxygen uptake rates similar to a no lens eye. |
| Elkins et al., 2014 | Live cell rheometer to quantify cell adhesion to contact lenses in vitro with shear stress | Lens deposited tear film proteins that affect corneal epithelial cell adhesion |
5. Impact of mechanobiology on biomaterial design
Biomaterials are materials that are suitable for use in biological applications. They may be synthetic, natural or biological in origin and they play a pivotal role in the field of tissue engineering and regenerative medicine (Hubbell, 1995). For corneal epithelial regeneration, amniotic membrane isolated from the placenta is a biomaterial that has been used to treat ocular surface conditions due to their high biocompatibility, anti-inflammatory properties and promotion of epithelial wound healing in the eye due to the presence of growth factors (Meller et al., 2011; Rahman et al., 2009). However, drawbacks include transmission of bacterial infections due to a lack of donor screening, improper storage and processing of membranes and donor variability (Malhotra and Jain, 2014). This has increased the demand for alternative biomaterials to be designed to treat corneal epithelial defects.
One important factor that needs to be considered when choosing a biomaterial is its physical and mechanical characteristics since these will affect how the cells respond (Mitragotri and Lahann, 2009). For the corneal epithelium the biomaterial must have sufficient strength and stiffness to withstand the external and internal forces experienced by the corneal epithelium as discussed earlier. For the corneal epithelium the elastic modulus of the biomaterial must be sufficiently high to withstand these forces and may need to be strong enough to withstand suturing if required. It may also be beneficial to try to replicate the mechanical characteristics of the basement membrane or Bowmans layer to create a more in-vivo like environment. This can create a problem since there may be some discrepancies between what the ideal Young's modulus would be for resisting deformation and the modulus of the basement membrane. Taking the amniotic membrane as an example, the Young's modulus of full term membranes ranges from approximately 1.5-3 MPa (Benson-Martin et al., 2006) which is considerably higher than the Young's modulus of the anterior basement membrane on which the corneal epithelium rests with a value between 2 and 15 kPa (Last et al., 2009). It should also be noted that there is considerable variation in reported values for the Young's modulus of the cornea, primarily due to different methods of measurement, differing methods of sample preparation, a non-linear stress-strain relationship and the viscoelastic nature of the tissue (Boyce et al., 2007; Elsheikh et al., 2007; Hatami-Marbini and Rahimi, 2014). Despite these issues, studies that have examined the effect that the mechanical environment has on the corneal epithelium should assist researchers trying to optimize parameters to drive cells to form a healthy epithelium.
In addition to mimicking the mechanical characteristics of tissues, many biomaterials aim to mimic in vivo biochemical and biophysical conditions in order to create microenvironments that allow for effective in vitro studies and assist in tissue regeneration. Many biomaterials incorporate specific biological components such as growth factors and cytokines to replicate conditions cells experience in vivo (Chen and Liu, 2016) or to promote matrix deposition (Ahearne et al., 2014). These biological components can influence how cells behave and the matrix proteins they deposit which in turn affect the mechanical properties of the environment (Glatt et al., 2016). As cells change the mechanical properties of their environment, the new environment can affect how the cells behave thus creating a dynamic reciprocity between cells and environment (Ahearne, 2014). Recently, one study found that ascorbic acid promotes stemness of the corneal epithelial stem cells though regulation of extracellular matrix components and accelerates wound healing (Chen et al., 2017). Other studies have shown that the extracellular matrix, which provides structural and mechanical support, also plays a role in limbal epithelial stem cell homeostasis (Kabosova et al., 2007; Ljubimov et al., 1995; Mei et al., 2012; Schlötzer-Schrehardt et al., 2007). These studies highlight the importance of studying the effect of mechanical environment on the corneal epithelium to aid in biomaterial or 3D microenvironment design and optimize the chemical environment to which the cells are subjected.
6. Conclusion
Research into the effects of mechanical stimuli on the corneal epithelium could provide novel ways to control the behavior of the corneal epithelial cells. Here we outlined the many ways in which mechanical stimuli affects how the corneal epithelial cells behave. Further research into corneal epithelium mechanobiology could aid in developing new regenerative therapies and in turn help ease the burden of donor shortages of corneas worldwide.
Acknowledgements
This research has received funding from the European Research Council under the European Union's Horizon 2020 research and innovation program (grant no. 637460) and from Science Foundation Ireland (15/ERC/3269).
References
- Abou Neel E.A., Cheema U., Knowles J.C., Brown R.A., Nazhat S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006;2:986–992. doi: 10.1039/b609784g. [DOI] [PubMed] [Google Scholar]
- Ahearne M. Introduction to cell-hydrogel mechanosensing. Interface Focus. 2014;4:20130038. doi: 10.1098/rsfs.2013.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearne M., Liu K.K., El Haj A.J., Then K.Y., Rauz S., Yang Y. Online monitoring of the mechanical behavior of collagen hydrogels: influence of corneal fibroblasts on elastic modulus. Tissue Eng. Part C. 2010;16:319–327. doi: 10.1089/ten.TEC.2008.0650. [DOI] [PubMed] [Google Scholar]
- Ahearne M., Liu Y., Kelly D.J. Combining freshly isolated chondroprogenitor cells from the infrapatellar fat pad with a growth factor delivery hydrogel as a putative single stage therapy for articular cartilage repair. Tissue Eng. 2014;20:930–939. doi: 10.1089/ten.tea.2013.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearne M., Yang Y., Then K.Y., Liu K.K. An indentation technique to characterize the mechanical and viscoelastic properties of human and porcine corneas. Ann. Biomed. Eng. 2007;35:1608–1616. doi: 10.1007/s10439-007-9323-9. [DOI] [PubMed] [Google Scholar]
- Albarran C., Pons A.M., Lorente A., Montes R., Artigas J.M. Influence of the tear film on optical quality of the eye. Contact Lens Anterior Eye. 1997;20:129–135. doi: 10.1016/s1367-0484(97)80011-2. [DOI] [PubMed] [Google Scholar]
- Aureille J., Belaadi N., Guilluy C. Mechanotransduction via the nuclear envelope: a distant reflection of the cell surface. Curr. Opin. Cell Biol. 2017;44:59–67. doi: 10.1016/j.ceb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S.A., Pye D.C., Willcox M.D. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin. Exp. Optom. 2013;96:214–218. doi: 10.1111/cxo.12038. [DOI] [PubMed] [Google Scholar]
- Benech J.C., Benech N., Zambrana A.I., Rauschert I., Bervejillo V., Oddone N., Damian J.P. Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation. Am. J. Physiol. Cell Physiol. 2014;307:C910–C919. doi: 10.1152/ajpcell.00192.2013. [DOI] [PubMed] [Google Scholar]
- Benson-Martin J., Zammaretti P., Bilic G., Schweizer T., Portmann-Lanz B., Burkhardt T., Zimmermann R., Ochsenbein-Kolble N. The Young's modulus of fetal preterm and term amniotic membranes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;128:103–107. doi: 10.1016/j.ejogrb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Boyce B.L., Jones R.E., Nguyen T.D., Grazier J.M. Stress-controlled viscoelastic tensile response of bovine cornea. J. Biomech. 2007;40:2367–2376. doi: 10.1016/j.jbiomech.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Braun R.J., King-Smith P.E., Begley C.G., Li L., Gewecke N.R. Dynamics and function of the tear film in relation to the blink cycle. Prog. Retin. Eye Res. 2015;45:132–164. doi: 10.1016/j.preteyeres.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpen O., Pallai P., Staunton D.E., Springer T.A. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J. Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema U., Chuo C.B., Sarathchandra P., Nazhat S.N., Brown R.A. Engineering functional collagen scaffolds: cyclical loading increases material strength and fibril aggregation. Adv. Funct. Mater. 2007;17:2426–2431. [Google Scholar]
- Chen C.S. Mechanotransduction - a field pulling together? J. Cell Sci. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- Chen F.M., Liu X.H. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lan J., Liu D., Backman L.J., Zhang W., Zhou Q., Danielson P. Ascorbic acid promotes the stemness of corneal epithelial stem/progenitor cells and accelerates epithelial wound healing in the cornea. Stem Cells Transl. Med. 2017;6:1356–1365. doi: 10.1002/sctm.16-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande P., Notara M., Bullett N., Daniels J.T., Haddow D.B., MacNeil S. Development of a surface-modified contact lens for the transfer of cultured limbal epithelial cells to the cornea for ocular surface diseases. Tissue Eng. 2009;15:2889–2902. doi: 10.1089/ten.tea.2008.0528. [DOI] [PubMed] [Google Scholar]
- Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Dua H.S., Shanmuganathan V.A., Powell-Richards A.O., Tighe P.J., Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Dziasko M.A., Armer H.E., Levis H.J., Shortt A.J., Tuft S., Daniels J.T. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS One. 2014;9:e94283. doi: 10.1371/journal.pone.0094283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins C.M., Qi Q.M., Fuller G.G. Corneal cell adhesion to contact lens hydrogel materials enhanced via tear film protein deposition. PLoS One. 2014;9:e105512. doi: 10.1371/journal.pone.0105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikh A., Wang D., Pye D. Determination of the modulus of elasticity of the human cornea. J. Refract. Surg. 2007;23:808–818. doi: 10.3928/1081-597X-20071001-11. [DOI] [PubMed] [Google Scholar]
- Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Foster J.W., Jones R.R., Bippes C.A., Gouveia R.M., Connon C.J. Differential nuclear expression of Yap in basal epithelial cells across the cornea and substrates of differing stiffness. Exp. Eye Res. 2014;127:37–41. doi: 10.1016/j.exer.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Fujita M., Igarashi T., Kurai T., Sakane M., Yoshino S., Takahashi H. Correlation between dry eye and rheumatoid arthritis activity. Am. J. Ophthalmol. 2005;140:808–813. doi: 10.1016/j.ajo.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Glatt V., Evans C.H., Tetsworth K. A concert between biology and biomechanics: the influence of the mechanical environment on bone healing. Front. Physiol. 2016;7:678. doi: 10.3389/fphys.2016.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz N., Dokukin M., Kalaparthi V., Sokolov I. If cell mechanics can Be described by elastic modulus: study of different models and probes used in indentation experiments. Biophys. J. 2014;107:564–575. doi: 10.1016/j.bpj.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel U., Garreis F., Burgemeister F., Essel N., Paulsen F. Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model. Ocul. Surf. 2018;16:341–351. doi: 10.1016/j.jtos.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Hatami-Marbini H., Rahimi A. Effects of bathing solution on tensile properties of the cornea. Exp. Eye Res. 2014;120:103–108. doi: 10.1016/j.exer.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Heryudono A., Braun R.J., Driscoll T.A., Maki K.L., Cook L.P., King-Smith P.E. Single-equation models for the tear film in a blink cycle: realistic lid motion. Math. Med. Biol. 2007;24:347–377. doi: 10.1093/imammb/dqm004. [DOI] [PubMed] [Google Scholar]
- Hjortdal J.O. Young's modulus of elasticity for the human cornea. J. Cataract Refract. Surg. 1994;20:672. doi: 10.1016/s0886-3350(13)80665-7. [DOI] [PubMed] [Google Scholar]
- Hochmuth R.M. Micropipette aspiration of living cells. J. Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Hodson S., Earlam R. Of an extracellular matrix in human pre-corneal tear film. J. Theor. Biol. 1994;168:395–398. doi: 10.1006/jtbi.1994.1119. [DOI] [PubMed] [Google Scholar]
- Huang H., Kamm R.D., Lee R.T. Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am. J. Physiol. Cell Physiol. 2004;287:C1–C11. doi: 10.1152/ajpcell.00559.2003. [DOI] [PubMed] [Google Scholar]
- Hubbell J.A. Biomaterials in tissue engineering. Nat. Biotechnol. 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ingber D.E. Cellular mechanotransduction: putting all the pieces together again. Faseb. J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Ioannidis A.S., Speedwell L., Nischal K.K. Unilateral keratoconus in a child with chronic and persistent eye rubbing. Am. J. Ophthalmol. 2005;139:356–357. doi: 10.1016/j.ajo.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Iwata M., Fushimi N., Suzuki Y., Suzuki M., Sakimoto T., Sawa M. Intercellular adhesion molecule-1 expression on human corneal epithelial outgrowth from limbal explant in culture. Br. J. Ophthalmol. 2003;87:203–207. doi: 10.1136/bjo.87.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri B., Lichter H., Stulting R.D. Asymmetric keratoconus attributed to eye rubbing. Cornea. 2004;23:560–564. doi: 10.1097/01.ico.0000121711.58571.8d. [DOI] [PubMed] [Google Scholar]
- Johnson C.S., Mian S.I., Moroi S., Epstein D., Izatt J., Afshari N.A. Role of corneal elasticity in damping of intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2007;48:2540–2544. doi: 10.1167/iovs.06-0719. [DOI] [PubMed] [Google Scholar]
- Jones M.B., Fulford G.R., Please C.P., McElwain D.L., Collins M.J. Elastohydrodynamics of the eyelid wiper. Bull. Math. Biol. 2008;70:323–343. doi: 10.1007/s11538-007-9252-7. [DOI] [PubMed] [Google Scholar]
- Jones R.R., Hamley I.W., Connon C.J. Ex vivo expansion of limbal stem cells is affected by substrate properties. Stem Cell Res. 2012;8:403–409. doi: 10.1016/j.scr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Kabosova A., Azar D.T., Bannikov G.A., Campbell K.P., Durbeej M., Ghohestani R.F., Jones J.C., Kenney M.C., Koch M., Ninomiya Y., Patton B.L., Paulsson M., Sado Y., Sage E.H., Sasaki T., Sorokin L.M., Steiner-Champliaud M.F., Sun T.T., Sundarraj N., Timpl R., Virtanen I., Ljubimov A.V. Compositional differences between infant and adult human corneal basement membranes. Invest. Ophthalmol. Vis. Sci. 2007;48:4989–4999. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.G., Shin J.W., Park S.H., Oh M.J., Park H.S., Shin J.W., Kim S.H. Effects of flow-induced shear stress on limbal epithelial stem cell growth and enrichment. PLoS One. 2014;9:e93023. doi: 10.1371/journal.pone.0093023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killpartrick M.R. Disposable lens risk factors and posterior lens surface contamination. Contact Lens Anterior Eye. 2016;39:400. doi: 10.1016/j.clae.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Ladage P.M., Ren D.H., Petroll W.M., Jester J.V., Bergmanson J.P., Cavanagh H.D. Effects of eyelid closure and disposable and silicone hydrogel extended contact lens wear on rabbit corneal epithelial proliferation. Invest. Ophthalmol. Vis. Sci. 2003;44:1843–1849. doi: 10.1167/iovs.02-0897. [DOI] [PubMed] [Google Scholar]
- Last J.A., Liliensiek S.J., Nealey P.F., Murphy C.J. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J. Struct. Biol. 2009;167:19–24. doi: 10.1016/j.jsb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last J.A., Thomasy S.M., Croasdale C.R., Russell P., Murphy C.J. Compliance profile of the human cornea as measured by atomic force microscopy. Micron. 2012;43:1293–1298. doi: 10.1016/j.micron.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Kim S.R., Park M. Oxygen permeability of soft contact lenses in different pH, osmolality and buffering solution. Int. J. Ophthalmol. 2015;8:1037–1042. doi: 10.3980/j.issn.2222-3959.2015.05.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekka M., Laidler P., Gil D., Lekki J., Stachura Z., Hrynkiewicz A.Z. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J. 1999;28:312–316. doi: 10.1007/s002490050213. [DOI] [PubMed] [Google Scholar]
- Leung B.K., Bonanno J.A., Radke C.J. Oxygen-deficient metabolism and corneal edema. Prog. Retin. Eye Res. 2011;30:471–492. doi: 10.1016/j.preteyeres.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis H.J., Brown R.A., Daniels J.T. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials. 2010;31:7726–7737. doi: 10.1016/j.biomaterials.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Lin M.C., Graham A.D., Polse K.A., Mandell R.B., McNamara N.A. Measurement of post-lens tear thickness. Invest. Ophthalmol. Vis. Sci. 1999;40:2833–2839. [PubMed] [Google Scholar]
- Lindsay R.G., Bruce A.S., Gutteridge I.F. Keratoconus associated with continual eye rubbing due to punctal agenesis. Cornea. 2000;19:567–569. doi: 10.1097/00003226-200007000-00034. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Lee S.M., Graham A.D., Lin M.C. Effects of eye rubbing and breath holding on corneal biomechanical properties and intraocular pressure. Cornea. 2011;30:855–860. doi: 10.1097/ICO.0b013e3182032b21. [DOI] [PubMed] [Google Scholar]
- Ljubimov A.V., Burgeson R.E., Butkowski R.J., Michael A.F., Sun T.T., Kenney M.C. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab. Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- Maissa C., Guillon M. Tear film dynamics and lipid layer characteristics--effect of age and gender. Contact Lens Anterior Eye. 2010;33:176–182. doi: 10.1016/j.clae.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Malhotra C., Jain A.K. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J. Transplant. 2014;4:111–121. doi: 10.5500/wjt.v4.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann A., Tighe B. Contact lens interactions with the tear film. Exp. Eye Res. 2013;117:88–98. doi: 10.1016/j.exer.2013.07.013. [DOI] [PubMed] [Google Scholar]
- McElwain S., Jones M.B., Fulford G., Please C.P., Collins M.J. 16th Australasian Fluid Mechanics Conference. 2007. Effect of tear additives on the shear stress and normal stress acting on the ocular surface; pp. 616–620. [Google Scholar]
- McMonnies C.W., Alharbi A., Boneham G.C. Epithelial responses to rubbing-related mechanical forces. Cornea. 2010;29:1223–1231. doi: 10.1097/ICO.0b013e3181d3d660. [DOI] [PubMed] [Google Scholar]
- Mei H., Gonzalez S., Deng S.X. Extracellular matrix is an important component of limbal stem cell niche. J. Funct. Biomater. 2012;3:879–894. doi: 10.3390/jfb3040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller D., Pauklin M., Thomasen H., Westekemper H., Steuhl K.-P. Amniotic membrane transplantation in the human eye. Deutsches Ärzteblatt International. 2011;108:243–248. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S., Lahann J. Physical approaches to biomaterial design. Nat. Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita H., Shimmura S., Higa K., Yoshida S., Kawakita T., Shimazaki J., Tsubota K. A novel NIH/3T3 duplex feeder system to engineer corneal epithelial sheets with enhanced cytokeratin 15-positive progenitor populations. Tissue Eng. 2008;14:1275–1282. doi: 10.1089/ten.tea.2007.0212. [DOI] [PubMed] [Google Scholar]
- Molladavoodi S., Kwon H.J., Medley J., Gorbet M. Human corneal epithelial cell response to substrate stiffness. Acta Biomater. 2015;11:324–332. doi: 10.1016/j.actbio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Molladavoodi S., Robichaud M., Wulff D., Gorbet M. Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviour. PLoS One. 2017;12:e0178981. doi: 10.1371/journal.pone.0178981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes-Mico R., Alio J.L., Charman W.N. Dynamic changes in the tear film in dry eyes. Invest. Ophthalmol. Vis. Sci. 2005;46:1615–1619. doi: 10.1167/iovs.05-0017. [DOI] [PubMed] [Google Scholar]
- Montés-Micó R., Alió J.L., Charman W.N. Dynamic changes in the tear film in dry eyes. Invest. Ophthalmol. Vis. Sci. 2005;46:1615–1619. doi: 10.1167/iovs.05-0017. [DOI] [PubMed] [Google Scholar]
- Montes-Mico R., Cervino A., Ferrer-Blasco T., Garcia-Lazaro S., Madrid-Costa D. The tear film and the optical quality of the eye. Ocul. Surf. 2010;8:185–192. doi: 10.1016/s1542-0124(12)70233-1. [DOI] [PubMed] [Google Scholar]
- Muntz A., Subbaraman L.N., Sorbara L., Jones L. Tear exchange and contact lenses: a review. J. Optom. 2015;8:2–11. doi: 10.1016/j.optom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Koizumi N., Tsuzuki M., Inoki K., Sano Y., Sotozono C., Kinoshita S. Successful regrafting of cultivated corneal epithelium using amniotic membrane as a Carrier in severe ocular surface disease. Cornea. 2003;22:70–71. doi: 10.1097/00003226-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Nichols J.J., King-Smith P.E. Thickness of the pre- and post-contact lens tear film measured in vivo by interferometry. Invest. Ophthalmol. Vis. Sci. 2003;44:68–77. doi: 10.1167/iovs.02-0377. [DOI] [PubMed] [Google Scholar]
- Nowell C.S., Odermatt P.D., Azzolin L., Hohnel S., Wagner E.F., Fantner G.E., Lutolf M.P., Barrandon Y., Piccolo S., Radtke F. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat. Cell Biol. 2016;18:168–180. doi: 10.1038/ncb3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A.W., Helmke B.P., Blackman B.R., Schwartz M.A. Mechanisms of mechanotransduction. Dev. Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pang K., Du L., Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials. 2010;31:7257–7265. doi: 10.1016/j.biomaterials.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Pellegrini G., Traverso C.E., Franzi A.T., Zingirian M., Cancedda R., DeLuca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- Pierscionek B.K., Asejczyk-Widlicka M., Schachar R.A. The effect of changing intraocular pressure on the corneal and scleral curvatures in the fresh porcine eye. Br. J. Ophthalmol. 2007;91:801–803. doi: 10.1136/bjo.2006.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakasam R.K., Schwiede M., Hutz W.W., Guthoff R.F., Stachs O. Corneal responses to eye rubbing with spectral domain optical coherence tomography. Curr. Eye Res. 2012;37:25–32. doi: 10.3109/02713683.2011.622850. [DOI] [PubMed] [Google Scholar]
- Raghunathan V.K., Dreier B., Morgan J.T., Tuyen B.C., Rose B.W., Reilly C.M., Russell P., Murphy C.J. Involvement of YAP, TAZ and HSP90 in contact guidance and intercellular junction formation in corneal epithelial cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I., Said D.G., Maharajan V.S., Dua H.S. Amniotic membrane in ophthalmology: indications and limitations. Eye. 2009;23:1954–1961. doi: 10.1038/eye.2008.410. [DOI] [PubMed] [Google Scholar]
- Ren H., Wilson G. Apoptosis in the corneal epithelium. Invest. Ophthalmol. Vis. Sci. 1996;37:1017–1025. [PubMed] [Google Scholar]
- Ren H., Wilson G. The effect of a shear force on the cell shedding rate of the corneal epithelium. Acta Ophthalmol. Scand. 1997;75:383–387. doi: 10.1111/j.1600-0420.1997.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Resan M., Vukosavljevic M., Millvojevic M. Wavefront abberations. In: Rumelt S., editor. Advances in Ophthalmology. InTech; 2012. [Google Scholar]
- Resnick N., Yahav H., Shay-Salit A., Shushy M., Schubert S., Zilberman L.C., Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. Prog. Biophys. Mol. Biol. 2003;81:177–199. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- Riede-Pult B.H., Evans K., Pult H. Investigating the short-term effect of eyelid massage on corneal topography. Optom. Vis. Sci. 2017;94:700–706. doi: 10.1097/OPX.0000000000001076. [DOI] [PubMed] [Google Scholar]
- Robertson D.M. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye Contact Lens. 2013;39:67–72. doi: 10.1097/ICL.0b013e31827c5b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T.D., Coon B.G., Yun S., Baeyens N., Tanaka K., Ouyang M., Schwartz M.A. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 2013;25:613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safvati A., Cole N., Hume E., Willcox M. Mediators of neovascularization and the hypoxic cornea. Curr. Eye Res. 2009;34:501–514. doi: 10.1080/02713680902919557. [DOI] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt U., Dietrich T., Saito K., Sorokin L., Sasaki T., Paulsson M., Kruse F.E. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007;85:845–860. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Sitalakshmi G., Sudha B., Madhavan H.N., Vinay S., Krishnakumar S., Mori Y., Yoshioka H., Abraham S. Ex vivo cultivation of corneal limbal epithelial cells in a thermoreversible polymer (Mebiol Gel) and their transplantation in rabbits: an animal model. Tissue Eng. 2009;15:407–415. doi: 10.1089/ten.tea.2008.0041. [DOI] [PubMed] [Google Scholar]
- Stahl U., Willcox M., Stapleton F. Osmolality and tear film dynamics. Clin. Exp. Optom. 2012;95:3–11. doi: 10.1111/j.1444-0938.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- Straehla J.P., Limpoco F.T., Dolgova N.V., Keselowsky B.G., Sawyer W.G., Perry S.S. Nanomechanical probes of single corneal epithelial cells: shear stress and elastic modulus. Tribol. Lett. 2010;38:107–113. [Google Scholar]
- Svitova T.F., Lin M.C. Dynamic interfacial properties of human tear-lipid films and their interactions with model-tear proteins in vitro. Adv. Biochem. Eng. Biotechnol. 2016;233:4–24. doi: 10.1016/j.cis.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Takatori S.C., Lazon de la Jara P., Holden B., Ehrmann K., Ho A., Radke C.J. In vivo corneal oxygen uptake during soft-contact-lens wear. Invest. Ophthalmol. Vis. Sci. 2013;54:3472–3479. doi: 10.1167/iovs.12-11169. [DOI] [PubMed] [Google Scholar]
- Tan D.T., Dart J.K., Holland E.J., Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- Trappmann B., Gautrot J.E., Connelly J.T., Strange D.G.T., Li Y., Oyen M.L., Stuart M.A.C., Boehm H., Li B.J., Vogel V., Spatz J.P., Watt F.M., Huck W.T.S. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- Wang J., Fonn D., Simpson T.L., Jones L. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2003;44:2524–2528. doi: 10.1167/iovs.02-0731. [DOI] [PubMed] [Google Scholar]
- Wang L., Gonzalez S., Dai W., Deng S., Lu L. Effect of hypoxia-regulated polo-like kinase 3 (Plk3) on human limbal stem cell differentiation. J. Biol. Chem. 2016;291:16519–16529. doi: 10.1074/jbc.M116.725747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Butler J.P., Ingber D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xu J., Sun X., Chu R., Zhuang H., He J.C. Dynamic wavefront aberrations and visual acuity in normal and dry eyes. Clin. Exp. Optom. 2009;92:267–273. doi: 10.1111/j.1444-0938.2009.00354.x. [DOI] [PubMed] [Google Scholar]
- Willcox M.D. Microbial adhesion to silicone hydrogel lenses: a review. Eye Contact Lens. 2013;39:61–66. doi: 10.1097/ICL.0b013e318275e284. [DOI] [PubMed] [Google Scholar]
- Winter K.N., Anderson D.M., Braun R.J. A model for wetting and evaporation of a post-blink precorneal tear film. Math. Med. Biol. 2010;27:211–225. doi: 10.1093/imammb/dqp019. [DOI] [PubMed] [Google Scholar]
- Wu X.Y., Svoboda K.K., Trinkaus-Randall V. Distribution of F-actin, vinculin and integrin subunits (alpha 6 and beta 4) in response to corneal substrata. Exp. Eye Res. 1995;60:445–458. doi: 10.1016/s0014-4835(05)80101-0. [DOI] [PubMed] [Google Scholar]
- Yun S.T., Woo D.M., Chong C.W., Liu Y., Francis K.E., Shah S.A., Agar A., Francis I.C. Utilisation of a novel test to measure severity and treatment efficacy of posterior blepharitis. J. Ophthalmol. 2015;2015:617019. doi: 10.1155/2015/617019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Sisley A.M., Anderson A.J., Taberner A.J., McGhee C.N., Patel D.V. Characterization of a novel sollagen scaffold for corneal tissue engineering. Tissue Eng. 2015 doi: 10.1089/ten.TEC.2015.0304. [DOI] [PubMed] [Google Scholar]
- Zhang X., Sun H., Tang X., Ji J., Li X., Sun J., Ma Z., Yuan J., Han Z.C. Comparison of cell-suspension and explant culture of rabbit limbal epithelial cells. Exp. Eye Res. 2005;80:227–233. doi: 10.1016/j.exer.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zhou Z.L., Ngan A.H., Tang B., Wang A.X. Reliable measurement of elastic modulus of cells by nanoindentation in an atomic force microscope. J Mech Behav Biomed Mater. 2012;8:134–142. doi: 10.1016/j.jmbbm.2011.11.010. [DOI] [PubMed] [Google Scholar]