Abstract
Background
Hydatid disease is a global problem. We report our experience with such cases where the dominant cysts were located outside the liver and lungs. In particular, these cysts were found in the peritoneum which is an uncommon location.
Methods
Between 1967 and 2007 a total of 34 patients were operated for primary or secondary peritoneal cysts. Most of the patients were asymptomatic or had atypical symptoms. The diagnosis was based on the preoperative history, rupture of the cysts, serology, ultrasound (USS) and computer tomography (CT). Open surgery was the procedure of choice with conservative (18 cysts) and radical (25 cysts) methods.
Results
The outcome of surgery was good without postoperative mortality or severe morbidity and the recurrence rate was 23.5%.
Conclusions
Conservative surgery can provide good results in symptomatic peritoneal cysts. Radical therapy is also ideal but only in properly selected cases. The management of this situation is difficult requiring sound operative experience preferably with a one-stage procedure after an appropriate preoperative preparation.
Keywords: Surgery, Internal medicine
1. Introduction
Hydatid disease (HD) is endemic in many parts of the world, including Greece where it is an important public health problem [1]. HD is caused by Echinococcus granulosus, a parasite that has a definite host, usually a dog, and an intermediate host that is commonly a sheep. Humans can accidentally become affected by ingesting contaminated water or vegetables from parasitic ova of dog faeces and thus becoming intermediate hosts [2, 3]. Evidence has shown that the most common location of hydatid cysts (HC) is the liver (50–60%) followed by the lung (10–30%). However, HC can also present in other unusual places of the body such as heart, muscles, peritoneum, spleen, pancreas, brain, kidneys, omentum, bones and ovaries [4, 5, 6].
The signs and symptoms of HD include abdominal pain, nausea and vomiting, while complications with a high mortality rate increase if cysts rupture [7]. For uncomplicated cases of HD, hydatid cysts can be detected via ultrasonography (USS) which has high sensitivity and specificity rates (93–100% respectively), computed tomography (CT) or magnetic resonance (MRI) which are noninvasive tests. Other useful diagnostic tools are antibody assays where the disease is detected via an immune response mechanism produced by hydatid cysts; one of them is the enzyme immunoassay (ELISA) test and another one is the indirect hemaglutination test (IHA) [8, 9].
In many cases, the peritoneum can be contaminated by hydatid disease either through the arterial circulation or via lymphatics and this is known as primary peritoneal hydatidosis which is rare. Contamination may also be due to spontaneous or intra-operative spillage of other cysts or rupture of the cysts in the abdominal cavity; this occurs more frequently and is known as secondary peritoneal hydatidosis [10]. Saidi has stated that peritoneal cysts account for 20% of the cases and are mainly the result of concomitant liver cyst rupture [10]. The current literature reports lower incidence of about 5% to 14% but there is no question that next to the liver and lungs, cysts of the peritoneum comprise a great number of hydatid cysts in the body [11, 12]. Peritoneal cysts can be multiple and can be located anywhere in the abdomen and they can enlarge causing abdominal distention or obstruction [13]. The most common symptom of peritoneal HD is abdominal pain and if the cysts rupture the patient may present with acute abdominal pain or acute allergic reaction secondary to antigenic fluid that is released in the abdomen [14]. Rupture of hydatid cysts can occur due to increased intracystic pressure or trauma and requires emergency surgical intervention [15]. A study by Monaqit et al in 2013 showed that the most common surgical procedure for patients with peritoneal cyst rupture was partial pericystectomy and drainage. These patients had a median hospital stay of 8 days and a recurrence rate post operatively of 7.7% [16]. According to the literature, peritoneal HD is a challenging situation for a surgeon, which requires resection of the protruding dome as well as adjuvant therapy with anti-parasitic treatment such as Albendazole to reduce the recurrence rate [17]. A study in 2009 has shown the effectiveness of combined surgical and anti-parasitic treatment with Albendazole pre and post-operatively for 3 months respectively in patients with primary and secondary hydatidosis. Patients underwent de-roofing and omentoplasties for larger cysts and removal of hydatid cysts and after 8 months follow up had a full recovery [18]. In this study we report our experience with peritoneal cysts and we present the existent technical operative details regarding this location of HD.
2. Methods
We performed a retrospective review of patients operated for peritoneal hydatid cysts in our University hospital. In the period between 1967 and 2007, a total number of 634 patients underwent surgery for HD.
Using a standardized form, data was collected for the eligible patients from their medical records and in the second phase, patients were separated into two groups: those with primary and those with secondary peritoneal HC. The term primary HC is used for hydatid cysts, which are formed by embryos through the haematogenous route of spread. Secondary HC applies to the cysts developed after the rupture of other cysts before or during the operation with the protoscolices spreading into the peritoneal cavity. A total of 34 patients were operated for primary or secondary peritoneal cysts.
In the earlier decades of the study, it was not easy to distinguish between secondary (more frequently seen than primary) and primary peritoneal cysts, based on the clinical picture of an acute rupture after trauma or sometimes indolent with only some anaphylaxis, on the radiological signs of hepatic air-fluid, the vague isotopic liver defects of hepatic cyst or on the intraabdominal adhesions with coexistent ruptured hepatic cysts. In the last decades, a higher preoperative diagnostic distinction rate was achieved based on the characteristic findings of modern diagnostic technology.
In this study, the initial diagnostic work-up consisted, in the earlier two decades, of blood tests such as full blood count, liver function tests, Casoni skin test, hydatid serology with complement fixation test (CF or Weinberg), indirect hemagglutination test (IHA) and the enzyme-linked immunosorbed assay (ELISA). The latter two tests have been used in the last two decades for diagnosis. The pre-operative evaluation also comprised (in the earlier decades of the study) of plain abdominal and chest x-rays while beyond 1980's hepatic scintigraphy, USS, CT, MRI and endoscopic cholangiopangreatography (ERCP) were also involved in the diagnosis of hydatid cysts.
Our methodology in the management of those cases was the same for the whole study. The operative strategy in many cases was conservative and was directed toward cavity drainage and removal of the parasitic elements without spillage. The choice of operative access was based on number, size and location of cysts, nature of complications and previous surgery. The puncture site of hydatid cysts was covered with gauzes soaked in hypertonic saline solution (5% to 15%) to produce local scolicidal effect and avoid peritoneal contamination. The cysts were aspirated at their most superficial point via a special transparent cannula with beveled tip for culture and initial decompression. Then cystic cavities were evacuated with a trocar under continuous suction. Digital destruction of septations and loculations led to one cystic cavity and local inactivation/sterilization was performed with hypertonic saline. The peritoneal cysts after wide de-roofing were left open in most cases. In concomitant hepatic cysts the opening of the pericyst was sutured, or the cavity was left open, obliterated by capitonnage (infolding of the cystic wall by successive suturing layers) or filled with omentum. In infected cysts or hepatic cysts with intrabiliary rupture, external drainage of the cavities was usually undertaken. In liver cysts that ruptured into the biliary tree, the operative technique was also directed towards the suturing of possible cystobiliary communication and drainage of the common bile duct. If the gallbladder was affected, it was removed. In biliobronchial fistula due to thoracic extension of cysts in the dome of the liver, besides management of the parasitic cavity, suturing of the pulmonary side of the fistula and closure of the diaphragmatic defect was the procedure of choice. In calcified cysts of the liver, an operation (pericystectomy) that involved the removal of the calcareous plaques was performed on the largest cyst, while cysts secondary in size or with concomitant infection were drained. In huge calcified cysts an elective secondary operation was sometimes carried out for intervening inflammation, an easy bloodless chiseling of the calcification. In some patients with peripheral or pedunculated cysts, a radical approach was adapted that included either cystoperisystectomy or liver resection.
Antiparasitic treatment with albendazole (Eskazol or Zentel, 400mgr tablets) was given in few patients. The total oral daily dosage of the drug varied between 600 and 800 mg, in two divided doses. It was given preoperatively for 7 days and postoperatively for two to three monthly cycles (for simple cases) or included a more prolonged administration in disseminated HD. The anthelminthic drugs except their use routinely in the last 6 years of the study, were only used in selected cases with multiple or difficultly position cysts.
The following data was analyzed for each admission according to age, gender, clinical laboratory and imaging/instrumental evaluation, number, size and location of cysts, choice of operative access, surgical procedures performed, histological and anatomo-pathological examinations, previous surgery for liver hydatidosis, length of stay, postoperative morbidity, mortality and recurrence rate.
Follow-up was organized by our team or by the primary care physicians and included blood tests and abdominal ultrasonography at regular intervals, as well as CT in some cases, after six months and routinely every six months thereafter for three years and occasionally afterwards if needed according to their history. Patients were sent a questionnaire asking about any recurrence. A 4 -year follow up was completed in 24 cases and partially completed in 10 cases. The Casoni, CFT and ELISA tests became postoperatively negative after 3 years, 3 months and for more than 3 years respectively. The sensitivity and antibody titers were raised again when secondary hydatid cysts developed. According to the literature, recurrence (with or without daughter cysts) is defined either as a growing cystic cavity at the original operative site or as the appearance of a cyst in a new site at the same or another anatomic region. Cystic cavities that preserved the same size for 3 years post-operatively were excluded finally from the statistical analysis due to the limited probability of recurrence. For the same reason, as we noted previously, the cases were followed for recurrence for at least 6 months postoperatively.
3. Results
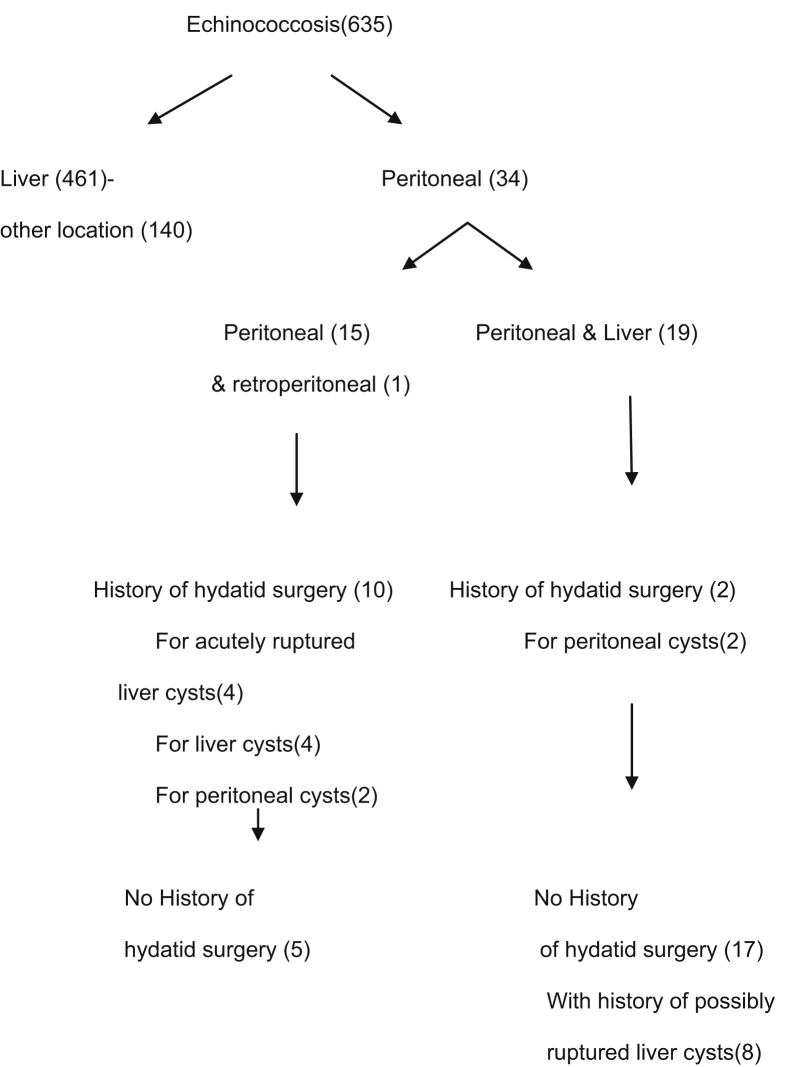
Thirty-four cases (5.35%) had involvement in the peritoneal cavity (Fig. 1). There were 18 men and 16 women with an age range of 20 to 70 years (mean age of 50). Fourteen patients had solitary peritoneal cysts, nineteen had at the same time peritoneal cysts and HD of the liver and one patient had HD in the retroperitoneum.
Fig. 1.
Distribution of patients with hydatid disease.
Eight patients with coexistent peritoneal and liver cysts had signs indicating preoperative rupture (history of trauma pain, mild allergic reaction). Four patients with peritoneal cysts (four to ten years before their admission) had another admission elsewhere for spontaneous acute rupture of hepatic cysts into the peritoneum. These patients had severe allergic reactions in the skin, fever and right abdominal pain with a palpable liver. One of these four patients, in his previous admission had an exploratory operation for acute abdomen without definite management of the disease. According to our records (four to ten years retrospectively) two patients with peritoneal and liver cysts had undergone removal of peritoneal cysts, 3 patients with peritoneal involvement had removal of liver cysts, one patient with peritoneal cysts underwent liver excision and lung cysts removal and 2 patients with peritoneal cysts had removal of peritoneal cysts.
In 5 patients with single peritoneal cysts and in 9 patients with coexistent peritoneal and liver cysts, indications for apparent rupture of the coexistent HD weren't noticed. We assume that certainly the first and possibly the second of the above groups might have had primary cysts in their peritoneal cavity (14 cases). The remainder of the patients was strongly considered to have secondary peritoneal cysts (20 cases).
The most common symptoms of peritoneal cysts were abdominal pain and discomfort (16 cases), some reported weight loss (4 cases), nausea, vomiting (4 cases) and dysuria (4 cases). In 6 patients, the disease was almost asymptomatic and it was discovered by USS. The symptomatology in concomitant peritoneal and liver cysts was frequently indistinguishable from that of solitary cysts of the liver. Some of these patients were complaining for indefinite problems in their liver and had a weak allergic manifestation (8 cases). The clinical examination of 6 out of the 13 patients with coexistent liver and peritoneal cysts revealed smooth round fixed abdominal nodules besides hepatomegaly. Hydatid thrill (sense of vibration to the palpating hand after a gentle tap in univesicular cysts close to the abdominal wall) was rarely elicited (4 cases). Hydatid cysts of the ovary in female patients was difficult to differentiate amongst other possible diagnosis such as ovarian tumors even with careful bimanual examination (in 2 cases).
Casoni's intradermal test was carried out in 17 patients (14 positive results), CFT in 17 patients (9 positive results), IHA in 14 (12 positive results), ELISA in 6 patient (5 positive results) and an eosinophil count in 23 patients (22 positive results). Chest and abdominal x- rays revealed a calcified cyst wall in 6 cases. Hepatic scintigraphy was performed in 20 patients and it was helpful for the diagnosis of liver cysts (18 positive results). Ultrasonography and computed tomography of the abdomen was performed in 13 and 14 cases respectively and showed an accurate picture of the cystic lesion. A few asymptomatic cases were also discovered with the above investigations during a routine follow up after their operation for hepatic echinococcosis. MRI and ERCP were utilized successfully in 3 patients respectively. MRI with gadolinium correctly detected all the hydatid cysts on both T1 and T2 weighted images and ERCP led to the detection of gross cystobiliary communication in hepatic cysts.
Perioperative anthelminthic therapy with albedazole was administered in 8 patients. In the group of peritoneal cysts, the surgical incision was performed vertically in the first decade or double subcostal in the last three decades; the method was adequate to permit a thorough examination of the entire abdominal cavity and management of any coexistent hepatic cysts. The strategy chosen was performed in one stage (31 cases) or two stages (3 cases), and was based on patient's general condition and the adhesions around the cysts, with the possible danger of bleeding or spillage during dissection.
The operations performed in the peritoneal cysts in the group with the single peritoneal cysts (14 patients had 20 dominant cysts), in the group with the liver and peritoneal cysts (19 patients had 20 dominant cysts) and in the retroperitoneum and peritoneal (1 patient had 3 cysts) are the following: un-roofing of the cysts with parasite evacuation in 18 cysts and removal of intact cysts in 25 cysts. During the operation, 1 or more than 15 peritoneal cysts in some cases were noticed (maximum diameter 10 cm). In three patients, after removing a number of cysts it was necessary to terminate the operation, with some small cysts without tension left behind and the treatment considered as palliative. The coexistent hepatic cysts were usually simultaneously treated.
The patients' liver cyst's characteristics concomitant with or without peritoneal cysts are compared statistically in Table 1. Statistical Package for Social Sciences (SPSS) 16 for Windows was used for statistical analyses. Data was tabulated comparing 2 groups: one with liver and peritoneal cysts in comparison with that with solitary liver cysts according to gender, age, and cyst characteristics [multiplicity, multivesicularity, superficial location, rupture (apparent or postulated by intraabdominal adhesions, hydatid surgery, trauma or pain/allergy), intrabiliary rupture, suppuration, calcification, and size of dominant cyst]. Statistical comparisons were performed using Chi-square Test for Independent and for categorical dependent variables and the Independent Samples T-Test for the continuous dependent variable (patient age). The assumptions that apply to methods used were not violated. P -values below 0.05 were considered as significant. The risk of formation of secondary peritoneal echinococcosis is higher in patients with multiple, multivesicular, preoperatively with pain allergy ruptured infected small liver cysts.
Table 1.
Characteristics of liver cysts in patients with and without concomitant peritoneal cysts.
| Patients and cyst | With | Without | Pearson Chi-Square. Yates' Continuity Correction |
Asymp. Sig. (2-sided) P value |
|---|---|---|---|---|
| Mean age (years) | 40 | 42 | 0,344 | |
| Male | 9 | 200 | 0,682 | 0,409 |
| Female | 18 | 261 | ||
| Multiple | 19 | 142 | 16,318 | 0,000 |
| Multivesicular – Viable | 19 | 187 | 8,108 | 0,004 |
| Superficial | 17 | 359 | 2,419 | 0,120 |
|
Rupture Apparent |
8 | 20 | 25,670 | 0,000 |
| Rupture Intrabdominal adhesions |
8 | 100 | 0,529 | 0,467 |
| Rupture Hydatid surgery |
2 | 10 | 1,137 | 0,286 |
|
Rupture Trauma |
8 | 20 | 25,670 | 0,000 |
|
Rupture Pain, allergy |
8 | 40 | 10,374 | 0,001 |
| Intrabiliary ruptured | 2 | 70 | 0,686 | 0,407 |
| Suppurated | 0 | 77 | 4,172 | 0,041 |
| Calcified | 2 | 80 | 1,164 | 0,281 |
|
Size of dominant cyst <10cm |
7 | 250 | 7,101 | 0,008 |
| Size of dominant cyst 10–20cm |
13 | 150 | 2,136 | 0,144 |
| Size of dominant cyst >20 cm | 7 | 61 | 2,450 | 0,118 |
| Number | 27 | 461 |
Major operative problems such as dense adhesions (8 cases), intraoperative hemorrhage (3 cases), multiple cysts below the incision (19 cases) and intestinal obstruction (1 case) were the causative mechanisms of recurrence and therefore needed special management.
All the patients had an uneventful recovery. The postoperative hospitalization ranged from 9 to 30 days (average number of 17 days). This period was extended in 7 patients owing to a number of complications related to concomitant hydatid liver surgery that required drainage. There were 8 post-operative patients with recurrence (three of them with new recurrences). One patient was initially operated for large retroperitoneal and small peritoneal cysts and had a recurrence of the peritoneal cysts 5.7 and 10 years later. One patient with peritoneal and hepatic cysts developed hydatid cysts in the liver and later in the spleen and tail of the pancreas in the first year and 12 years postoperatively. In this case, in addition to the management of the liver cysts, a splenectomy for the splenic cysts and cystopericystectomy for the pancreatic cyst were also performed. One patient who was managed initially for multiple peritoneal and liver cysts had a recurrence 3 years postoperatively, for a cyst in the lung, 22 years later she developed renal, uterine and liver hydatid cysts. Secondary to hydronephrosis and serious infection a nephrectomy was carried out. The uterine cyst was treated by partial pericystectomy and drainage.
The interval between operation of patients with peritoneal cysts and recurrence was ranging from one to 15 years. Two patients had recurrence in the peritoneum, one had recurrence in the liver, one in the liver and peritoneum and one had recurrence in the spleen.
The postoperative course was uneventful in all reoperated cases. During the follow up period, the patients were not reoperated.
4. Discussion
HD is usually found in the liver (60–70%) and lung (10–15%) and it can develop in the abdominal or pelvic cavity and more rarely elsewhere in the body [1, 10, 11, 12]. Peritoneal hydatid cysts (5–16% of all cases) aren't structurally different from those found in other locations with a slow enlargement without any significant reaction to the host [11, 12]. Peritoneal surface is not spared and hydatids in the area of the intestine, spleen, pancreas, omentum, and ovaries have been noticed [10, 11]. Our study has found that among 635 patients with HD, 34 patients had peritoneal involvement, 14 had single peritoneal cysts, 19 had coexisting peritoneal and liver cysts and one patient had peritoneal and retroperitoneal cysts.
Primary peritoneal hydatidosis as suggested in the literature is around 2% of the cases. We have seen that the disease was considered to be secondary to liver involvement in 5 cases [19]. The mechanism of formation of primary peritoneal cysts is not so clear. The hexacanth embryos are carried in the peritoneum by arterial circulation or they might penetrate the bowel wall to enter the peritoneal cavity [10]. In contrast to this it is well established that secondary peritoneal cysts are mainly a result of spontaneous or traumatic rupture of concomitant liver cysts as well as leakage of cystic content during surgery. The material of extrusion in the abdominal cavity varies from mitute scolices and brood capsules of the parasite, to daughter cysts, or both [10]. The slow contamination of the peritoneum from asymptomatic microrupture of cysts or microscopic intraoperative spillage of echinococcal fluid account for 5% to 10% of the cases in the general population and for 50% of the cases of our patients [12]. On the other side it is reported in 2%–7% of the cases (in comparison to 4 of our patients), that a big quantity of contents of a ruptured cyst can cause severe acute peritoneal spillage, anaphylactic reactions and severe secondary infection [15, 20]. Depending on the nature of the primary liver cyst, complications with biliary rupture or infection, choleperitoneum or bacterial peritonitis in a number of cases may arise [2]. Despite the necessary urgent surgery with meticulous irrigation of abdomen (usually with 0.5% silver nitrate, 3% hypertonic saline or rarely with povidone iodine 2.5%), disseminated secondary hydatidosis probably can later develop. In many cases according to the sensitization of the patient and the amount of liberated and absorbed hydatid fluid, transient pain and allergic phenomena are present, as in our series, in up to 25% of the cases [20]. With the patient being well between the acute phases, the self-limited initial episode of rupture may repeat itself initiating additional secondary, tertiary or quaternary cysts for a long time [10]. In case of liberated intact daughter cysts, the parasites remain rarely without pericyst between the surrounding peritoneal surfaces or usually they invade the viscera and become indistinguishable from primary hydatids of such organs [10]. The rupture site in the liver cyst may be small and the inflammatory adhesions seal it, thus leaving a fine scar in the liver [10].
In comparison with other studies, when our patients with concomitant liver secondary peritoneal cysts were compared with patients with single liver cysts the multiplicity multivesicularity, history of spontaneous or traumatic rupture, the pain or allergy and suppuration, less than 10 cm size, of liver cysts were statistically important factors of peritoneal spillage and involvement [1]. What distinguishes primary from secondary parasites is based on the free of previous rupture history, the newer technology, the absence of preexisted ruptured hepatic or peritoneal cysts or the adhesions in the abdominal cavity [10, 12, 19]. We have to consider that in order to distinguish secondary from primary type of peritoneal HC it is sometimes impossible due to silent ruptures, or due to difficulties in exploration of small rupture sites in the liver or in the abdominal cavity. Moreover, the most important factor in the prognosis of this disease is not the definition of the primary or secondary type of parasites but the big number of cysts (diffuse abdominal hydatidosis) that are formed in few cases, as it occurred in 3 of our patients [10].
Peritoneal HD is not usually detected until pressure symptoms develop due to space occupying lesions (peritoneal and concomitant liver cysts) or due to infection or parasite rupture (resulting in sepsis, allergy, local or generalized peritonitis, cholangitis). Therefore high risk patients for peritoneal HD need surveillance for prompt management of the disease [12]. A study in 2013 has shown that a patient with peritoneal hydatidosis presented into hospital with acute abdominal pain and underwent surgery for ruptured hydatid cysts and a non-ruptured cyst in the omentum. In addition, surgeons performed a resection of the protruding dome of the cysts with drainage and peritoneal cleansing with isotonic solution. The patient had further treatment with albendazole for 3 months [21]. In our series, some of the patients were asymptomatic, most of them had allergy reaction, atypical complaints in the liver or abdominal pain and few presented with more typical picture of peritoneal hydatidosis. There is no doubt that there were, especially in the past, diagnostic problems in HD of uncommon sites as in peritoneum [11]. The surgeon must be aware of this possibility so as to follow the necessary therapeutic policy and to avoid dangerous spillage of the parasite intraoperatively.
In general, the diagnosis of HD requires a variety of laboratory and imaging investigations. It must be noted that sonography and CT scans were not available in our early cases and the diagnosis was made very often intraoperatively. However, the newer organ imaging methods (US, CT, MRI) with their accurate pictures have simplified the diagnostic process [22]. CT yields important information regarding the position and extent of intra-abdominal cysts and is the imaging modality of choice for peritoneal disease [23]. Serologic examinations have the problem of low diagnostic sensitivity and specificity and therefore have limited use [22]. Evidence has shown that CFT, IHA and ELISA tests that we have used and are performed by some laboratories, have given satisfactory results [11, 22].
The main goals of surgery in the treatment of peritoneal HD are to eliminate local disease, prevent complications and reduce the disease recurrence. Open surgery, where possible, remains the treatment of choice in large symptomatic peritoneal and coexistent hepatic cysts with good prognosis [24, 25]. The operative management follows the general rules of hydatid surgery. The methods for surgical treatment of peritoneal cysts are conservative with the evacuation of the contents followed by wide de-roofing; thus leaving the cyst open or radical with total removal of the intact parasite. In liver coexistent cysts, either a conservative approach with evacuation, partial pericystectomy as well as unroofing capitonage pericyst suturing, cavity filling with or without drainage or radical policy with cystopericystectomy or resection is preferred [24, 25, 26, 27, 28]. Conservative management in comparison to radical technique is time efficient, simpler to perform, and is favorable in endemic areas [19]. In small peritoneal or hepatic superficially located pedunculated cysts the radical methods can have good rates of surgical success when the patients are stable at the time of surgery without acute complications (cholangitis, suppurated liver cysts, lung problems) [24, 29].
In hydatid disease the administration of antiparasitic treatment is required pre and post operatively to prevent the dissemination of protoscolices via the blood stream and reduce the recurrence rate. The incidence of complications, the benefit and the duration of bezimidazoles pre and post operatively is questionable. Based on the existent studies, these drugs that have also been used in our study, they are recommended for multiple cysts or for cases in which spillage of protoscoleces have occurred preoperatively or may have happened intraoperatively [18, 19, 26]. In addition literature has recommended the use of scolicidal agent (such as silver -nitrate, chlorhexidine, praziquatel, povidone-iodine) irrigation in the peritoneal cavity [30]. The effectiveness of intraoperative intracystic injection of scolicidal agents in the recurrence rate is unknown due to their unpredictable dilution in the cyst contents. In addition to the limited usefulness, they are associated with dose dependent toxicity [10, 22]. We believe that the cautious operative technique itself is the crucial factor to avoid recurrence. However, it is difficult to conclude in the long term the intraoperative use of the already existent or newest safer drug [31].
Another aspect of hydatid disease that should always be taken into consideration is that hydatid cysts are not limited in the abdominal cavity and can be also found in the lungs (9–30%), the spleen (0.9–8%), the kidney (2–3%), the brain (1%) and the musculoskeletal system [32, 33, 34]. These cysts consist of three layers: 1) the outermost pericyst, which is composed of host cells that encase the parasite, 2) the middle laminated membrane and 3) the inner germinal layer [36].
In our study, as 18 peritoneal cysts were incorporated into the organ between adhesions, they were evacuated safely, followed by unroofing and they were left open with abdominal drainage. Intracystic drainage or filling with omentum after evacuation was the usual policy in hepatic cysts coexisting with peritoneal cysts. The total removal of the peritoneal cysts was feasible in 25 pendunculated peritoneal cysts in the omentum or ligaments, and among these, 22 were operated three times. The treatment of peritoneal cysts is dangerous with the possibility of considerable amount of blood loss and spillage and has to be performed in multiple stages with the surgeon mapping the position of the cysts in the initial operation as it has occurred in 3 of our cases. In few seriously ill patients in order to achieve decompression of the abdominal pressure, some cysts can only be aspirated and not removed, and the deflated or calcified cysts, being aborted, can be left alone not posing danger to the patient [10]. Due to pressure of the alimentary tract, resulting from the space-occupying lesions, chronic anorexia and anoxia, a thorough preoperative preparation, is necessary and surgery is considered apparently as palliative, with a close surveillance and a long coverage with anthelmintics.
In general, the outcome of surgery was good in our patients with hydatid cysts. Our results have shown that in general we didn't have postoperative mortality or severe morbidity in the cases with peritoneal cysts in comparison to other studies [12]. The mean hospital stay despite the fact that was prolonged it was not associated with the treatment of the peritoneal cysts but rather it was related to coexistent hepatic cysts [12]. It was especially prolonged in the case where an intracystic drainage was used. The recurrence rate of peritoneal cysts as noted in the literature is 13% in comparison to our rate which was 23.5% [12]. Some intraoperative problems such as dense adhesions, hemorrhage or cysts in the incision line were unpreventable causative mechanisms for recurrence of the disease.
In conclusion, our results for the diagnosis of peritoneal HD seem satisfactory. A well-organized surgery is still the treatment of choice for large and symptomatic peritoneal cysts treated with devious evacuation and unroofing and sometimes with total removal. Lastly, we would like to consider that the anthelmitic perioperative coverage is necessary for a period depending on the radiologic evolution of the disease. With the collaboration of genetic, biologic, diagnostic and prevalence data of all echinococcal forms the future outlook for treating the disease is promising.
Declarations
Author contribution statement
Christophoros Kosmidis: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Christophoros Efthimiadis, Vasileiadou Kelly, Triantafyllia Koetsa: Contributed reagents, materials, analysis tools or data.
Georgios Anthimidis: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Georgios Koimtzis, Epameinondas Fahantidis: Analyzed and interpreted the data.
Ioanna Tzeveleki: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
John Prousalidis, Antonios Michalopoulos: Conceived and designed the experiments.
Georgios Basdanis: Conceived and designed the experiments; Wrote the paper.
Isaac Kesisoglou: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Prousalidis J., Kosmidis Ch, Fahantidis E. Surgical treatment of multiple cystic echinococcosis. HPB. 2004;6(2):110–114. doi: 10.1080/16515320410026068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirican A., Yilmaz M., Unal B., Tatli F., Piskin T., Kayaalp C. Ruptured hydatid cysts in the peritoneum: a case series. Eur. J. Trauma Emerg. Surg. 2010;36(4):375–379. doi: 10.1007/s00068-009-9056-6. [DOI] [PubMed] [Google Scholar]
- 3.Akbulut S., Senol A., Ekin A., Bakir S., Bayam K., Dursum M. Primary retroperitoneal hydatid cyst: report 2 cases and review of 41 published cases. Int. Surg. 2010;95(3):189–196. PMID: 21066995. [PubMed] [Google Scholar]
- 4.Ahmadi N., Badi F. Human Hydatidosis in Tehran, Iran: a retrospective epidemiological study of surgical cases between 1999 and 2009 of two University medical centres. Trop. Biomed. 2011;28(2):450–456. PMID: 22041768. [PubMed] [Google Scholar]
- 5.Rochan R., Rice C., Carrico C. Hydatid disease of the lung. In: Shields T.W., editor. General Thoracic Surgery. fourth ed. Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 1021–1038. [Google Scholar]
- 6.Wani R., Wani J., Malik A., Parray F., Wanni A., Dar A. Hydatid disease at unusual sites. Int. J. Case Rep. Images. 2012;3(6):1–6. [Google Scholar]
- 7.Moro P., Schantz P. Ecchinococcosis: a review. Int. J. Infect. Dis. 2009;13(2):125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., McManus D. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol. Med. Microbiol. 2006;47:24–41. doi: 10.1111/j.1574-695X.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 9.Wuestenberg J., Gruner B., Oeztuerk S., Kratzer W. Diagnostics in cystic echinococcosis serology versus ultrasonography. Turk. J. Gastroenterol. 2014;25(4):398–404. doi: 10.5152/tjg.2014.7112. [DOI] [PubMed] [Google Scholar]
- 10.Saidi F. WB Saunders Comp Ltd; London- Philadelphia – Toronto: 1976. Surgery of Hydatid Disease; pp. 282–301. [Google Scholar]
- 11.Prousalidis J., Tzardinoglou E., Sgouradis L. Uncommon sites of hydatid disease. World J. Surg. 1998;22:17–22. doi: 10.1007/s002689900343. PMID: 9465756. [DOI] [PubMed] [Google Scholar]
- 12.Karavias D., Vagianos C., Androulakis J. Peritoneal echinococcosis. World J. Surg. 1996;20:337–340. doi: 10.1007/s002689900054. PMID: 8661841. [DOI] [PubMed] [Google Scholar]
- 13.Ilica A., Kocaoglu M., Zeybek N., Guven S., Adaletli I., Basgul A., Coban H., Bilici A., Bukte Y. Extrahepatic abdominal hydatid disease caused by echinococcosis granulosus: imaging findings. AJR. 2007;189(2):337–343. doi: 10.2214/AJR.07.2255. [DOI] [PubMed] [Google Scholar]
- 14.Vuitton D. Echinococcosis and allergy. Clin. Rev. Allergy Immunol. 2004;26(2):93–104. doi: 10.1007/s12016-004-0004-2. [DOI] [PubMed] [Google Scholar]
- 15.Beyrouti M., Beyrouti R., Abbes I., Kharrat M., Ben Amar A., Frikha F., Ellench S., Gharbi W., Chaabouni M., Ehorbel A. Acute rupture of hydatid cysts in the peritoneum 17 cases. Presse Med. 2004;33:378–384. doi: 10.1016/s0755-4982(04)98600-9. PMID: 15105779. [DOI] [PubMed] [Google Scholar]
- 16.Mouaqit O., Hibatallah A., Oussaden A., Maazaz K., Ait Taleb K. Acute intraperitoneal rupture of hydatid cysts : a surgical experience in 14 cases. World J. Emerg. Surg. 2013;8:28. doi: 10.1186/1749-7922-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majbar M., Sonadka A., Sabbah F., Raiss M., Hrora A., Ahallat M. Peritoneal echinococcosis: anatomical features and surgical treatment. World J. Surg. 2012;36(5):1030–1035. doi: 10.1007/s00268-012-1475-6. PMID: 1859117. [DOI] [PubMed] [Google Scholar]
- 18.Acharya A., Gupta S. Peritoneal hydatidosis : a review of seven cases. Trop. Gastroenterol. 2009;30(1):32–34. PMID: 19624085. [PubMed] [Google Scholar]
- 19.Akcan Alpez, Akyildiz Hiziz, Actis Tarik. Peritoneal perforation of liver hydatid cysts: clinical presentation, predisposing factors and surgical outcome. WJS. 2007;31:1284–1291. doi: 10.1007/s00268-007-9024-4. [DOI] [PubMed] [Google Scholar]
- 20.Derici H., Tonsug T., Reyhan E. Acute intraperitoneal rupture of hydatid cysts. WJS. 2007;31:1526–1527. doi: 10.1007/s00268-005-0699-0. [DOI] [PubMed] [Google Scholar]
- 21.Benhamiche H., Sottier D., Funes M., Cuisenier B., Mejean N., Krause D. Peritoneal hydatidosis and hepatic hydatid cyst perforation. Diagn. Interventional Imag. 2013;94(11):1157–1160. doi: 10.1016/j.diii.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Milicevic M. third ed. Blumgart LH & Fong Y. Saunders WB; 2003. Hydatid Disease: in Surgery in the Liver and Biliary Tract; pp. 1167–1204. [Google Scholar]
- 23.Yilmaz M., Akbulut S., Kahraman A., Yilmaz S. Liver hydatid cysts rupture into peritoneal cavity after abdominal trauma: cases report and literature review. Int. Surg. 2012;97:239–244. doi: 10.9738/CC116.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kammerer WS, Schantz PM. Echinococcal disease. Infect. Dis. Clin. 1992;7:605-618. PMID: 8254162. [PubMed]
- 25.Eren S., Yildirgan I., Kantarci A. An asymptomatic ruptured hepatic hydatid cyst case presenting with subdiaphragmatic gas in traumatic patient. Emerg. Radiol. 2005;12(1-2):50–52. doi: 10.1007/s10140-005-0433-0. [DOI] [PubMed] [Google Scholar]
- 26.Amman R.W., Eckert J. Cestodes echinococcus. Gastr. Clin. North Am. 1996;25:655–689. doi: 10.1016/s0889-8553(05)70268-5. PMID: 8863045. [DOI] [PubMed] [Google Scholar]
- 27.Prousalidis J., Kosmidis Ch, Kapoutzis K. Intrabiliary rupture of hydatid cysts of the liver. Am. J. Surg. 2009;197:193–198. doi: 10.1016/j.amjsurg.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 28.El Mufti M. In: The Simple Hepatic Hydatid Cyst in Surgical Management of Hydatid Disease. El Mufti M., editor. Butterworth; London: 1989. pp. 31–54. [Google Scholar]
- 29.Prousalidis J., Kosmidis C., Anthimidis G. Forty-four years' experience (1963–2006) in the management of primarily infected hydatid cyst of the liver. HPB. 2008;10:18–24. doi: 10.1080/13651820701854669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filippou D., Kolimpiris C., Anemodouras N., Rizos S. Modified capitonage in partial cystectomy performed for liver hydatid disease:report of 2 cases. BMC Surg. 2004;4:8. doi: 10.1186/1471-2482-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omurbecov T.O. 2004. New Methods of Diagnosis and Intraoperational Sterilization of Echinococcosis of Children in Torgerson P and Shaikenov P Echinococcosis in central Asia: Problem and Solutions; pp. 224–234. [Google Scholar]
- 32.Golzari S.E., Sokouti M., Ghaffari A., Bazzazi A.M., Ghabili K. Ultrasonography in diagnosis of pulmonary hydatid cysts. Lancet Infect. Dis. 2013;13(4):294. doi: 10.1016/S1473-3099(13)70070-7. [DOI] [PubMed] [Google Scholar]
- 33.Sokouti M., Golzari S.E., Tizro P., Khanli H.M., Ghabili K. Genitourinary hydatid disease. Int. Urol. Nephrol. 2013;45(3):757–758. doi: 10.1007/s11255-013-0440-0. [DOI] [PubMed] [Google Scholar]
- 34.Golzari S.E., Sokouti M., Bazzazi A.M., Khanli H.M., Ghabili K. Serodiagnostic tests in musculoskeletal hydatid disease. Spine. 2013;38(20):1797. doi: 10.1097/BRS.0b013e3182a0038c. [DOI] [PubMed] [Google Scholar]