Abstract
Introduction:
One class of magnetic nanoparticles is magnetic iron oxide nanoparticles (MIONs) which has been widely offered due to of their many advantages. Owing to the extensive application of MIONs in biomedicine, before they can be used in vivo, their cytotoxicity have to be investigated. Therefore, there is an urgent need for understanding the potential risks associated with MIONs.
Materials and Methods:
Firstly, gold-coated Fe3O4 nanoparticles (GMNP) were synthesized. The size, structure and spectroscopic properties of the nanoparticles were characterized by transmission electron microscopy (TEM), X-ray diffractometry (XRD) and UV-Visible spectrophotometer, respectively. Cytotoxicity of nanoparticles was studied with different concentrations ranging from 10 µg/mL up to 400 µg/mL and for different incubation times (12 hours and 24 hours) on MCF-7 and HFFF-PI6. Cytotoxicity study was performed by MTT assay.
Results:
XRD pattern confirmed the structure of GMNPs and TEM image shows that GMNPs are under 50 nm. For MCF-7 and HFFF-PI6 cells, at concentration of 300 and 400 µg/mL, Fe3O4 nanoparticles are toxic, respectively. Moreover, for both cells, cell viability for GMNPs is higher than %80, therefore, up to 400 µg/mL they are not toxic. Results show that for both cells, Fe3O4 nanoparticles have higher cytotoxicity than GMNPs.
Conclusion:
This finding suggests that gold coating reduces the toxic effects of uncoated Fe3O4 nanoparticles. Less toxicity of GMNP may be attributed to controlled release from Fe2+ ions in intracellular space. Moreover, cell toxicity increased with raise in dose (concentration) and incubation time.
Keywords: Iron Oxide Nanoparticles , Gold-coated , Cytotoxicity , MCF-7
Introduction
Nowadays, nanotechnology and molecular biology are widely developing nanoparticles with useful properties for overcoming the shortcomings of traditional disease diagnostic and therapeutic agents [1]. Among many nanoparticles used, magnetic nanoparticles have attracted great attention due to their specific magnetic properties [2]. One class of magnetic nanoparticles is magnetic iron oxide nanoparticles (MIONs) which has been widely offered because of its reactive surface that can be readily modified by biocompatible coatings as well as targeting, imaging and therapeutic molecules. MIONs are being extensively used in many biomedical applications such as magnetic resonance imaging [3-8]. drug delivery [2,9,10] and magnetic hyperthermia [11,12].
There are several methods for fabricating magnetic iron oxide nanoparticles. The most common method, known as co-precipitation, includes co-precipitation of ferrous and ferric salts in an alkaline medium. In this method, the size and shape of iron oxide nanoparticles depend on several factors such as a type of salt used, temperature, pH value and so on. Another method is thermal decomposition, which usually requires relatively higher temperatures. In the microemulsion method, magnetic nanoparticles are fabricated in oil-in-water micoemulsions by suspending a ferrous salt-surfactant precipitate from an aqueous solution; the next, a base is added. Hydrothermal method includes various wet chemical technologies of crystallizing substance in a sealed container from the high temperature aqueous solution at the high vapor pressure. Another method that has been extensively used is a sonochemical method in which the acoustic cavitation is formed by the ultrasound waves, generating a localized hotspot through adiabatic compression [13].
After synthesis, iron oxide nanoparticles are usually coated in order to improve their stability, facilitate the bonding of various biological ligands to nanoparticle surfaces and reduce their toxicity. Usual coating materials are classified to inorganic and organic materials such as gold, silica, polyethylene glycol (PEG), polyvinyl alcohol (PVA), dextran, chitosan and other polymers [14].
Gold is one of the noteworthy coating materials because of its chemical stability, biocompatibility and various applicability [15]. Gold-coated MIONs (GMNPs) can be heated by an external magnetic field for hyperthermia application and they are useful in photothermal therapy because of the excellent near-infrared (NIR) light sensitivity and strong adsorptive ability of the Au layer [16].
Owing to extensive applications of MIONs in biomedicine, before they can be used in vivo, their cytotoxicity must be investigated. Therefore, there is a distinct need of understanding the potential risks associated with MIONs. The aim of this work is to investigate the cytotoxicity of GMNPs and bare uncoated MIONs (Fe3O4).
In this study, cytotoxicity effects of GMNPs and Fe3O4 on two cell lines were investigated. These two cell lines were human breast adenocarcinoma (MCF-7) and human foreskin fibroblast (HFFF-PI6). MCF-7 was used as a cancerous cell line and HFFF-PI6 was chosen as a normal cell line.
Material and Methods
Synthesis of Gold -coated Fe3O4 Nanoparticles (GMNPs)
The Chemical co-precipitation method was used for the nanoparticle synthesis in four steps.
Step 1
1.28 M of FeCl3.6H2O and 0.64 M of FeCl2.4H2O were dissolved in deoxygenated HCl (0.4 M). Then, 200 mL NH3 was quickly added to the mixture. The black product is Fe3O4 nanoparticles and after collecting, it was washed several times with deionized water.
Step 2
Then, Fe3O4 nanoparticles were coated with silica (Fe3O4@SiO2). Briefly, Fe3O4 nanoparticles (30 mL) were first diluted with water (30 mL), 2-propanol (300 mL), and then ammonia (15 ml) was added. The solution was well-dispersed by ultrasonic vibration for 15 minutes. Finally, 1.9 mL TEOS was added into resultant solution under vigorous stirring for 16 hours. Fe3O4@SiO2 was collected by a magnet and washed with water and ethanol.
Step 3
In the next step, the previous product was amino-functionalized (Fe3O4@SiO2@NH2). The surface of silica-coated nanoparticles can be easily modified by Aminopropyltriethoxysilane (APTES). Briefly, with vigorously stirring, 12 gr Fe3O4@SiO2 and 32 mL APTES were added into 120 mL anhydrous toluene. After that, the product was magnetically collected and washed with acetone.
Step 4
Finally, the gold nanoparticles were deposited in the previous product surface (15 mg Fe3O4@SiO2@NH2 was dispersed in 30 mL water at pH = 4) by the reduction of uric acid (8 mL, 1.71 mM) using NaBH4 solution (8 mL, 0.1 M). Consequently, the product was separated and dispersed in PBS (pH = 7).
Characterization
Transmission electron microscopy (TEM) imaging (Philips CM 30) was used for the evaluation of morphology and size of nanoparticles. The UV/visible spectra were obtained using a JASCO UV-vis spectrophotometer. X-ray diffraction (XRD) pattern was recorded using a Bruker D8/Advance X-ray diffractometer with Cu-Kα radiation.
Cell Culture
Roswell Park Memorial Institute (RPMI-1640) medium, fetal bovine serum (FBS), phosphate-buffered saline (PBS), trypsin-EDTA solution (0.25% trypsin, 1 mM EDTA) and penicillin-streptomycin were obtained from Invitrogen (Carlsbad, CA, USA). 3-(4,5-Dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich.
MCF-7, human breast adenocarcinoma, and HFFF-PI6, human foreskin fibroblast, cell lines were purchased from the National Cell Bank of Iran (Pasteur Institute, Iran). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (100 µg/mL). The cells were grown and maintained at 37 °C in a humidified incubator with 5% CO2.
In-vitro Cytotoxicity
A simple, a non-radioactive and colorimetric assay named MTT was used for a quantitative cytotoxicity assessment of nanoparticles [17]. In this assay, metabolically active cells convert a yellow and water soluble tetrazolium salt to a water insoluble and dark blue Formazan [18]. For the MTT assay viability studies, MCF-7 and HFFF-PI6 cells were seeded into 96-well cell culture plates at the density of 20,000 cells per well and incubated for 24 hours in a humidified incubator with a CO2 concentration of 5% to allow adherence of the cells. Then, the cells were incubated with different concentrations of both Fe3O4 and GMNPs ranging from 10 to 400 µg/mL for 12 and 24 hours. The control wells were a culture medium with no particles.
After 24 hours, the culture medium was removed, 100 µL fresh medium and 20 µL MTT (5 mg/mL) were added into each well and the plates were incubated for 4 hours. Then, the culture medium was carefully removed, and 200 µL dimethyl sulfoxide (DMSO) was added to each well to dissolve the Formazan crystals for 10 minutes. The absorbance was measured at 570 nm using an ELISA reader (Synergy H1, Bio Tek). Experiments were performed in triplicate and cell survival was determined as a percentage of viable cells in comparison with control wells.
Results and Discussion
Figure 1 shows XRD pattern related to GMNPs and demonstrates the crystalline nature of GMNPs. 6 peaks at about 30.3° (220), 35.6° (311), 43.2° (400), 53.4° (422), 57.2° (511), 62.7° (440) could be assigned to Fe3O4 [19]. Also, four peaks positioned at 2θ values of 38.2° (111), 44.4° (200), 64.7° (220) and 77.7° (311) are corresponding to the planes of the cubic-phase Au [20]. XRD analysis shows that the recorded pattern matches the reference pattern for magnetite (Fe3O4) and gold.
Figure1.
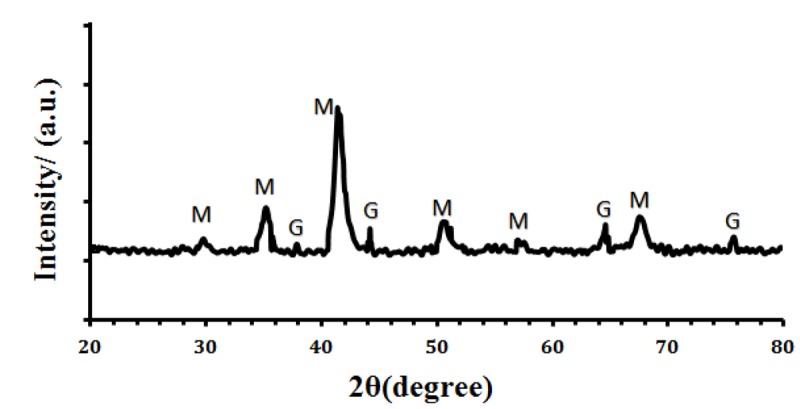
XRD pattern of GMNP (‘M’ and ‘G’ are diffraction peaks for Fe3O4 and GMNP, respectively).
Figure 2 shows TEM image of synthesized GNMPs. The core size and the shell thickness were less than 25 nm and 5 nm, respectively. In addition, nanoparticles are spherical from a morphological point of view.
Figure2.
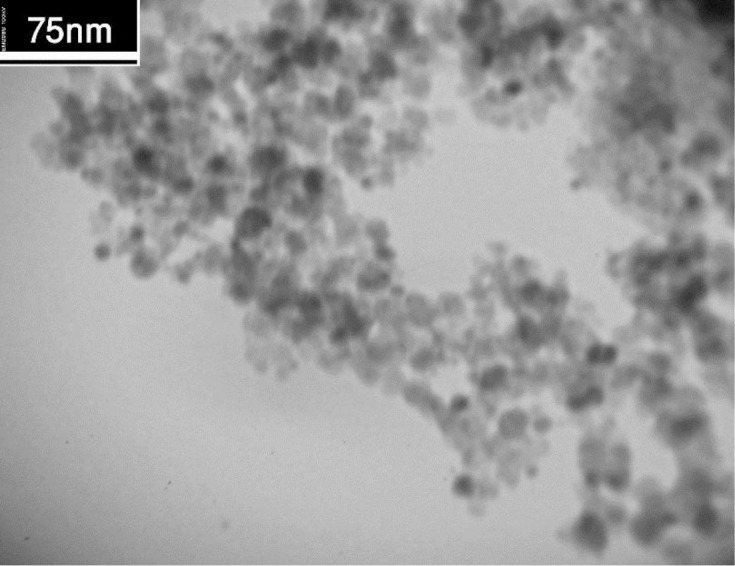
Transmission electron microscopy (TEM) image of GNMP.
Figure 3 demonstrates the UV-Vis absorption spectra for Fe3O4, and GNMP. Fe3O4 shows no measurable features in the visible region, while the aqueous dispersion of GNMP shows a characteristic absorption peak at 530 nm which is attributed to the surface Plasmon resonance bands of gold shell.
Figure3.
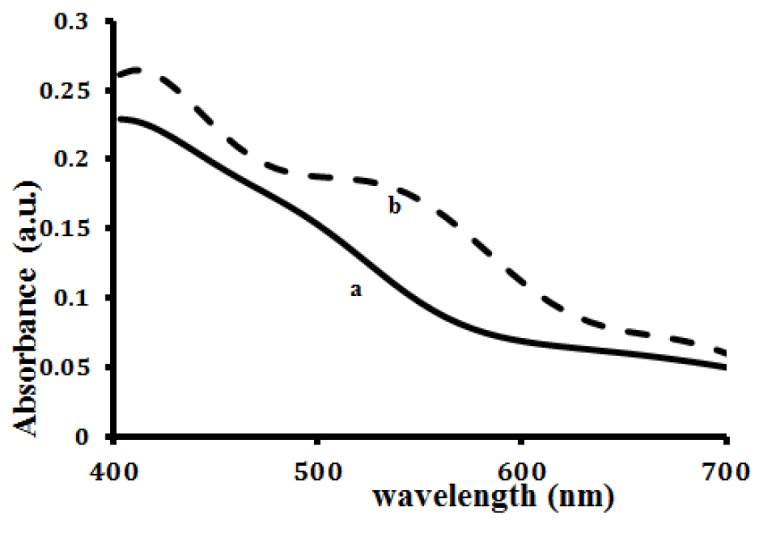
UV-visible spectra of nanoparticles (a) Fe3O4 nanoparticles, (b) GMNP.
To study the viability of two cell lines, MTT assay was performed. In this assay, yellow tetrazolium is reduced into purple formazan crystals by mitochondrial succinate dehydrogenase of viable cell. Thereby, it can be concluded that the rate of formazan crystal formation is directly proportional to the number of viable cells [21].
Cytotoxicity of nanoparticles was studied with different concentrations ranging from 10 µg/mL up to 400 µg/mL and for different incubation times (12 and 24 hours). All experiments were carried out in triplicate. After subtraction of the blank values, the mean values and standard deviation (SD) were calculated. The results are presented in terms of percentage cell viability. It should be noted that materials with cell viability more than 80% can be considered as being biocompatible [17].
Figures 4 and 5 illustrate the cytotoxicity data for MCF-7 and HFFF-PI6 cells, respectively. Results reveal that for both cells, Fe3O4 nanoparticles have higher cytotoxicity than GMNPs (Figures 4a and 5a). For MCF-7 and HFFF-PI6 cells, at concentrations of 300 and 400 µg/mL, Fe3O4 nanoparticles are toxic, respectively. Moreover, by increasing nanoparticle concentrations, cell viability decreases.
Figure4.
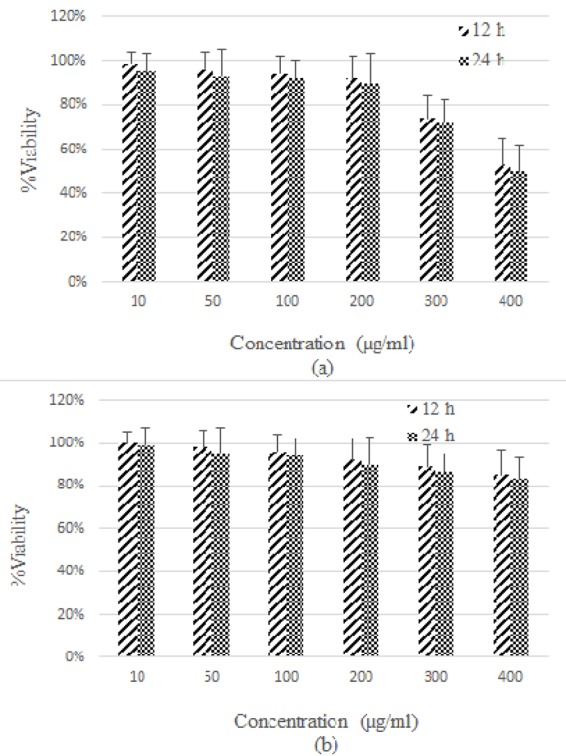
Representation of cytotoxicity of Fe3O4 nanoparticles (a) and GMNPs (b) in MCF-7 cells, with different concentrations at various time intervals.
Figure5.
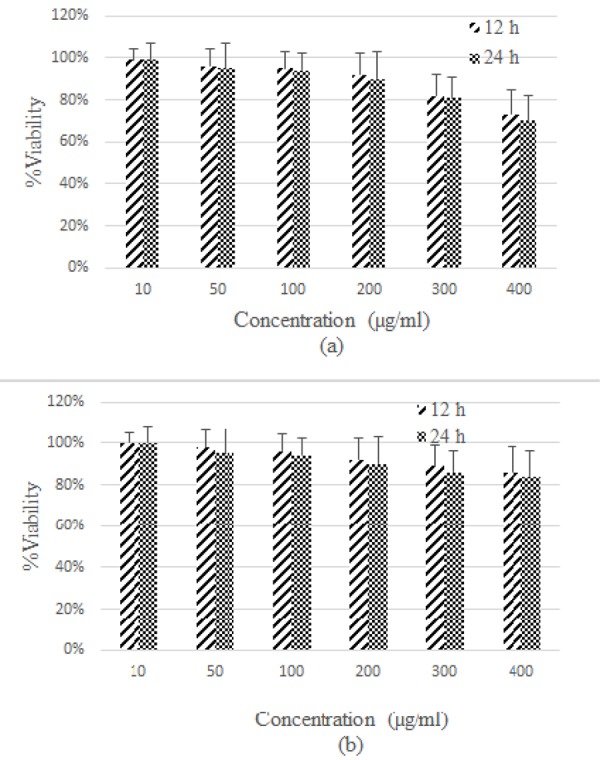
Representation of cytotoxicity of Fe3O4 nanoparticles (a) and GMNPs (b) in HFFF-PI6 cells, with different concentrations at various time intervals.
In 2009, Mahmoudi et al. synthesized superparamagnetic iron oxide nanoparticles with different polymer/iron mass rations (r-ratio) by coating them with polyvinyl alcohol (PVA). MTT assay was used to investigate the cell toxicity of the samples. Their results showed that the biocompatibility of the nanoparticles, based on cell viabilities, could be enhanced by increasing the r-ratio due to increasing particle size causing lower cell toxicity effects [17].
Our results are in good agreement with Gaihre et al. too, where they modified the surfaces of magnetic iron oxide nanoparticles with gelatin in order to investigate their cytotoxicity and cellular uptake. MTT assay was used to assess the cytotoxicity of gelatin-coated nanoparticles and uncoated nanoparticles on human fibroblasts. Their results showed that gelatin coating would increase cell viability [22]
Figures 4b and 5b show the cytotoxicity results are related to GMNP for MCF-7 and HFFF-PI6 cells, respectively. The results clearly indicate that the toxic effect of GMNP on MCF-7 and HFFF-PI6 cells were less as compared to Fe3O4 nanoparticles. This finding suggests that the gold coating reduces the toxic effects of uncoated Fe3O4 nanoparticles. Less toxicity of GMNP may be attributed to controlled release from Fe2+ ions in intracellular space. Higher toxicity of uncoated-MIONs (Fe3O4) could be attributed to high release from iron ions in intracellular space [23]. Moreover, the results show toxicity is time-dependent for all cells and nanoparticles. Notably, cell toxicity increased with raise in dose (concentration) and incubation time.
Conclusion
In this study, the cytotoxicity effects of Fe3O4 nanoparticles and GMNPs were evaluated on MCF-7 and HFFF-PI6 cells by a simple and colorimetric method named MTT assay. Findings represent that for both cells, Fe3O4 nanoparticles have higher cytotoxicity than GMNPs, which could be attributed to high release from iron ions into intracellular space in case of Fe3O4 nanoparticles. Moreover, nanoparticle toxicity is dose- and time-dependent. It should be noted that at any concentrations, all kinds of nanoparticles are toxic depending on cell type.
Acknowledgement
This work is a part of PhD thesis which financially supported by Isfahan University of Medical Sciences (Grant No. 394322).
Conflict of Interest:None
References
- 1.Sanvicens N, Marco MP. Multifunctional nanoparticles--properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26:425–33. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Montazerabadi AR, Oghabian MA, Irajirad R, Muhammadnejad S, Ahmadvand D, Delavari HH, et al. Development of Gold-Coated Magnetic Nanoparticles as a Potential MRI Contrast Agent. NANO. 2015;10(04):1550048. doi: 10.1142/S1793292015500484. [DOI] [Google Scholar]
- 3.Abdolahi M, Shahbazi-Gahrouei D, Laurent S, Sermeus C, Firozian F, Allen BJ, et al. Synthesis and in vitro evaluation of MR molecular imaging probes using J591 mAb-conjugated SPIONs for specific detection of prostate cancer. Contrast Media Mol Imaging. 2013;8:175–84. doi: 10.1002/cmmi.1514. [DOI] [PubMed] [Google Scholar]
- 4.Shahbazi-Gahrouei D, Abdolahi M, Zarkesh-Esfahani SH, Laurent S, Sermeus C, Gruettner C. Functionalized magnetic nanoparticles for the detection and quantitative analysis of cell surface antigen. Biomed Res Int. 2013;2013:349408. doi: 10.1155/2013/349408. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshtkar M, Shahbazi-Gahrouei D, Khoshfetrat SM, Mehrgardi MA, Aghaei M. Aptamer-conjugated magnetic nanoparticles as targeted magnetic resonance imaging contrast agent for breast cancer. J Med Signals Sens. 2016;6(4):243–247. [PMC free article] [PubMed] [Google Scholar]
- 6.Shahbazi-Gahrouei D, Abdolahi M. Superparamagnetic iron oxide-C595: Potential MR imaging contrast agents for ovarian cancer detection. J Med Phys. 2013;38:198–204. doi: 10.4103/0971-6203.121198. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahbazi-Gahrouei D, Abdolahi M. Detection of MUC1-expressing ovarian cancer by C595 monoclonal antibody-conjugated SPIONs using MR imaging. ScientificWorldJournal. 2013;2013:609151. doi: 10.1155/2013/609151. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghasemian Z, Shahbazi-Gahrouei D, Manouchehri S. Cobalt Zinc Ferrite Nanoparticles as a Potential Magnetic Resonance Imaging Agent: An In vitro Study. Avicenna J Med Biotechnol. 2015;7:64–8. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 9.Hola K, Markova Z, Zoppellaro G, Tucek J, Zboril R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol Adv. 2015;33:1162–76. doi: 10.1016/j.biotechadv.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Shahbazi-Gahrouei D, Keshtkar M. Magnetic nanoparticles and cancer treatment. Immunopathologia Persa. 2016;2(1) [Google Scholar]
- 11.Laurent S, Dutz S, Hafeli UO, Mahmoudi M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8–23. doi: 10.1016/j.cis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Hilger I, Kaiser WA. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine (Lond) 2012;7:1443–59. doi: 10.2217/nnm.12.112. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, He Q, Jiang C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett. 2008;3:397–415. doi: 10.1007/s11671-008-9174-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent S, Forge D, Port M, Roch A, Robic C, Vander*Elst L, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 15.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21:10644–54. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Kang KA. Application of novel metal nanoparticles as optical/thermal agents in optical mammography and hyperthermic treatment for breast cancer. Oxygen Transport to Tissue Xxviii: Springer; 2008. pp. 45–52. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoudi M, Simchi A, Milani AS, Stroeve P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci. 2009;336:510–8. doi: 10.1016/j.jcis.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Pieters R, Huismans DR, Leyva A, Veerman AJ. Comparison of the rapid automated MTT-assay with a dye exclusion assay for chemosensitivity testing in childhood leukaemia. Br J Cancer. 1989;59:217–20. doi: 10.1038/bjc.1989.44. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawande MB, Velhinho A, Nogueira ID, Ghumman C, Teodoro O, Branco PS. A facile synthesis of cysteine–ferrite magnetic nanoparticles for application in multicomponent reactions—a sustainable protocol. Rsc Advances. 2012;2:6144–9. doi: 10.1039/c2ra20955a. [DOI] [Google Scholar]
- 20.Wang A-J, Li Y-F, Li Z-H, Feng J-J, Sun Y-L, Chen J-R. Amperometric glucose sensor based on enhanced catalytic reduction of oxygen using glucose oxidase adsorbed onto core-shell Fe 3 O 4@ silica@ Au magnetic nanoparticles. Materials Science and Engineering: C. 2012;32:1640–7. doi: 10.1016/j.msec.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 21.Hussain RF, Nouri AM, Oliver RT. A new approach for measurement of cytotoxicity using colorimetric assay. J Immunol Methods. 1993;160:89–96. doi: 10.1016/0022-1759(93)90012-V. [DOI] [PubMed] [Google Scholar]
- 22.Gaihre B, Hee*Lee Y, Khil MS, Yi HK, Kim HY. In-vitro cytotoxicity and cell uptake study of gelatin-coated magnetic iron oxide nanoparticles. J Microencapsul. 2011;28:240–7. doi: 10.3109/02652048.2011.557747. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION) Nano Rev. 2010;1:0. doi: 10.3402/nano.v1i0.5358. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


