The use of chimeric antigen receptor (CAR) T cells has shown promising results in preclinical and early clinical trials for glioblastoma (GBM). Antigen escape, the downregulation or loss of target antigens, occurs after CAR T-cell therapy targeting single antigens and culminates in tumor recurrence. We have previously shown a clear advantage to combinatorial targeting of the 2 GBM antigens human epidermal growth factor receptor 2 (HER2) and interleukin-13 receptor subunit alpha-2 (IL-13Rα2), in offsetting antigen escape and enhancing T-cell performance.1 Our data indicated that the interpatient variability in surface antigen expression hinders the clinical impact of targeting 2 antigen pairs, though. In Bielamowicz et al,2 we therefore studied whether a CAR T cell targeting a third GBM antigen, ephrin-A2 (EphA2), would broaden the T-cell spectrum enough to overcome this obstacle—thereby increasing the probability of eligibility of GBM patients to a single trivalent product.
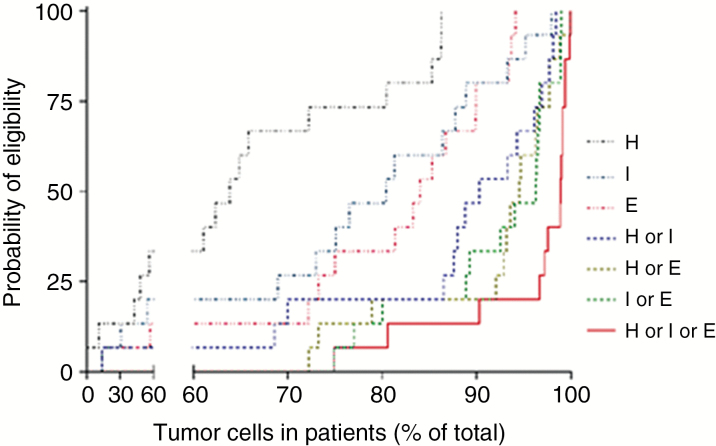
To create a probability model we first studied the pattern of surface protein expression of these 3 target antigens in a cohort of 15 serially diagnosed primary GBM surgical samples. Specifically, we assessed the immunoreactivity to HER2, IL-13Rα2, and EphA2 in 100000–200000 single cells to interrogate the probability of targeting >95% of cells within individual tumors (an overarching indicator of eligibility for the proposed trivalent product). This study was prospectively powered, wherein data from 12 or more tumors were anticipated to reach statistical significance. Next we performed hierarchical comparisons of the most prevalent single versus most prevalent 2 versus 3 antigens of interest (8 permutations) with an adjusted P-value of <0.0001 as a cutoff (these data are detailed in Supplementary Tables S1–S3 in Bielamowicz et al). Accordingly, we built a Boolean “OR” routine of tumor coverage as a function of probability of eligibility to 7 potential cellular products. Figure 1 is a nonparametric probability estimator that shows that bivalent products favorably bundle together above univalent products, yet the trivalent product significantly surpasses their mean probability of eligibility (P < 0.0001).
Fig. 1.
Probability of patient eligibility based on tumor antigen positivity. H = HER2, I = IL-13Rα2, E = EphA2.
An invaluable alternative for studying targeted therapeutics is The Cancer Genome Atlas (TCGA), an atlas of nucleic acid profiles. We interrogated data from the reports from 2008 (208 GBMs)3 and 2013 (152 GBMs)4 cited by Caruso and Heimberger (Supplementary Figure S1). Unfortunately, we found several fold discrepancies between the expression in TCGA of HER2, IL-13Rα2, and EphA2 and their immunoreactivity as assessed by us and as repeatedly reported in the literature.5–8 There are several potential explanations for such genome/proteome discrepancies, such as (i) epigenetic changes that are rampant in GBM especially after chemoradiation and (ii) posttranslational modifications.9 Importantly, TCGA databases are derived from tumor bulks and are “internally controlled” using arbitrary cutoffs, which makes the absolute incidence of target expression and the definition of normalcy, such as for HER2, which is overexpressed but not amplified in GBM, very elusive. Equally important, overexpression is not a prerequisite for CAR T-cell–based targeting.10 For these reasons, we used the data from TCGA to assess “trends” of target expression and coexpression but deemed it unsuitable for assessing the targetability of surface proteins using multivalent CAR T cells. The development of The Human Protein Atlas (THPA) is under way and would represent a more appropriate resource when complete (www.proteinatlas.org).11,12
We concluded that trivalent CAR T cells represent a single product that can be used across this cohort as an index sample representing larger cohorts of GBM patients. Further studies using THPA data or building on our strategy above in larger numbers could significantly substantiate our findings. Nevertheless, the trends in the cohort of patients we used justified the creation of a novel trivalent CAR T-cell product that enhanced the effector functions, which were thereafter extensively tested using patient CAR T-cell products against autologous GBM/patient-derived xenografts. Several patient sample sets were used (as biological replicates, rather than to account for heterogeneity). The superiority of the product being tested was demonstrated above the best univalent and bivalent products based on traditional immunoassays, subcellular imaging, and in vivo studies of primary GBM.
In conclusion, our data indicate that the inclusion of EphA2 as a target antigen, to HER2 and IL-13Rα2, does indeed broaden the T-cell spectrum enough to overcome the interpatient variability in target expression and that trivalent CAR T cells are better poised to more effectively target a varying GBM profile, making them applicable to a wider cohort of patients.
References
- 1. Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bielamowicz K, Fousek K, Byrd TT, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20(4):506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–551. [DOI] [PubMed] [Google Scholar]
- 6. Chow KK, Naik S, Kakarla S, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16(2):474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67(17):7983–7986. [DOI] [PubMed] [Google Scholar]
- 9. Parker NR, Hudson AL, Khong P, et al. Intratumoral heterogeneity identified at the epigenetic, genetic and transcriptional level in glioblastoma. Sci Rep. 2016;6:22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed N, Salsman VS, Yvon E, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17(10):1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pontén F, Jirström K, Uhlen M. The Human Protein Atlas—a tool for pathology. J Pathol. 2008;216(4):387–393. [DOI] [PubMed] [Google Scholar]
- 12. Thul PJ, Lindskog C. The Human Protein Atlas: a spatial map of the human proteome. Protein Sci. 2018;27(1):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]