Abstract
Background
Following recent studies underlining the differences between de novo and secondary anaplastic meningiomas and the prognostic value of telomerase reverse transcriptase (TERT) promoter mutation, we decided to conduct a multicenter retrospective study to address these questions and determine specific prognostic factors in each of these 2 anaplastic meningioma subgroups.
Methods
Among the 68 meningioma cases initially selected, only 57 were confirmed as anaplastic meningiomas after centralized pathological review. TERT promoter mutation analysis was performed in all cases.
Results
Median overall survival was 2.6 years and 5-year survival rate was 10%. This study confirmed the better prognosis of de novo anaplastic meningiomas (28 tumors) compared with secondary anaplastic meningiomas (29 tumors) (P = 0.02). In the “de novo” group, meningiomas diagnosed on histological anaplasia alone had a better prognosis than those in patients with a high number of mitoses with or without anaplasia (P = 0.01). In the “secondary” group, tumors demonstrate very heterogeneous clinical courses leading to malignant transformation, and time to first relapse as a low-grade tumor was a strong predictor of overall survival (P = 0.0007). TERT promoter mutation in anaplastic meningiomas was rare (14%) and did not influence overall survival but was associated with a shorter recurrence-free survival in the secondary anaplastic meningioma subgroup (P = 0.02). The absence of TERT promoter methylation, although rare (3/33 cases), may be associated with prolonged overall survival (P = 0.02).
Conclusion
This study highlights the different prognoses of de novo and secondary anaplastic meningiomas with specific prognostic factors in each subgroup. The analysis of TERT mutation and methylation could provide additional prognostic insights.
Keywords: anaplastic, grade 3, meningioma, prognosis, TERT
Importance of the study
Anaplastic meningiomas are rare but aggressive tumors with considerably poorer prognosis than lower-grade meningiomas. Due to their rarity, histomolecular prognostic factors are missing to help the clinician identify the most aggressive cases and adapt treatment regimen and surveillance protocols. Based on a large multicentric retrospective cohort, we were able to demonstrate the differential prognosis of de novo and secondary anaplastic meningiomas while determining specific prognostic factors in each of those 2 anaplastic meningioma subgroups. We also clarify the prognostic value of TERT promoter mutations and methylation among those different meningioma subgroups in order to refine its clinical use.
Meningiomas are the most frequent tumors of the central nervous system and are generally benign.1 According to the current World Health Organization (WHO) classification of tumors of the central nervous system, meningiomas are categorized in 3 histological grades: grade I, grade II for atypical meningiomas, and grade III for anaplastic meningiomas. This histological grading is strongly associated with recurrence rate and clinical outcome. WHO grade I meningiomas have a 20%–39% recurrence rate at 10 years2 and WHO grade II meningiomas have a 50% recurrence rate at 5 years.3 While grade III meningiomas are rare and represent 1%–2% of all meningiomas,4 they are characterized by significant morbidity and mortality, with a reported median overall survival (OS) ranging from 2.6 to 5.8 years.5–10 WHO grade III meningiomas are defined by a mitotic index equal to or greater than 20 mitoses per 10 high power fields (HPF; 1.6 mm2), and/or overt anaplasia (loss of meningothelial differentiation associated with pseudo-carcinomatous, -sarcomatous, or -melanomatous features), and/or papillary variants.11 Rhabdoid meningiomas sometimes lack overt features of malignancy and may follow a benign clinical course, even if rhabdoid features are well developed and extensive throughout the tumor,12 and were therefore excluded from this study. Following several recent reports,8,13,14 anaplastic meningiomas are nowadays considered either to arise de novo or to progress from a lower-grade tumor. Several studies have recently underlined the longer survival in de novo anaplastic meningiomas compared with secondary anaplastic tumors.8,14,15
The WHO classification criteria for grade III meningiomas have not changed since 2000,16 and histomolecular factors to refine the prognosis of those tumors are missing. As anaplastic meningiomas are defined by either overt histological anaplasia and/or high mitotic count in the WHO classification,11 there is a significant proportion of de novo anaplastic meningiomas that present with histological anaplasia without high mitotic count. The specific prognosis of these de novo tumors remains poorly understood. In secondary anaplastic meningiomas, previous studies have focused mainly on survival after malignant transformation, thus potentially concealing the heterogeneity of clinical courses leading to malignant transformation and therefore the global prognosis of those patients. Finally, TERT promoter mutation has recently been associated with shorter time to progression in grade III meningiomas,17 but the prognostic value of TERT promoter mutation on overall survival remains unknown. We decided to address these questions while presenting a comprehensive overview of anaplastic meningiomas, based on a multicenter retrospective cohort.
Materials and Methods
Data Collection
The French Brain Tumor18 and the Department of Neuropathology databases at Pitié-Salpêtrière Hospital were queried for all patients treated for anaplastic meningioma. Radiation-induced and neurofibromatosis type 2–related meningiomas were excluded from the study. Patients’ records were retrospectively reviewed and all clinical data were compiled in a single database by 3 authors (M.P., H.L., T.G.). Follow-up information was collected through primary care physician contact. This study was approved by the local institutional review board.
Pathological Analysis
We gathered 68 cases of WHO grade III meningiomas from 7 tertiary surgical neuro-oncology centers (Hôpital Pitié-Salpêtrière, Paris; Hôpital Sainte-Anne, Paris; Hôpital Lariboisière, Paris; Hôpital Beaujon, Clichy; CHRU, Nancy; Hôpital La Timone, Marseille; Hôpital Pellegrin, Bordeaux). All cases were centrally reviewed by one neuropathologist (G.G.) while blinded to clinical outcome, and grading was performed according to the 2016 WHO Classification of Brain Tumors. After central review, only 57 meningiomas were confirmed as WHO grade III tumors, with a mitotic index ≥20 mitoses per 10 HPF and/or overt anaplasia. Eleven meningiomas were excluded from the study, being reclassified as grade II. Papillary and rhabdoid meningiomas were also excluded from the study. Among de novo grade III meningiomas, we discriminated between meningiomas classified as grade III on the number of mitoses and on frank histological anaplasia alone (Fig. 1).
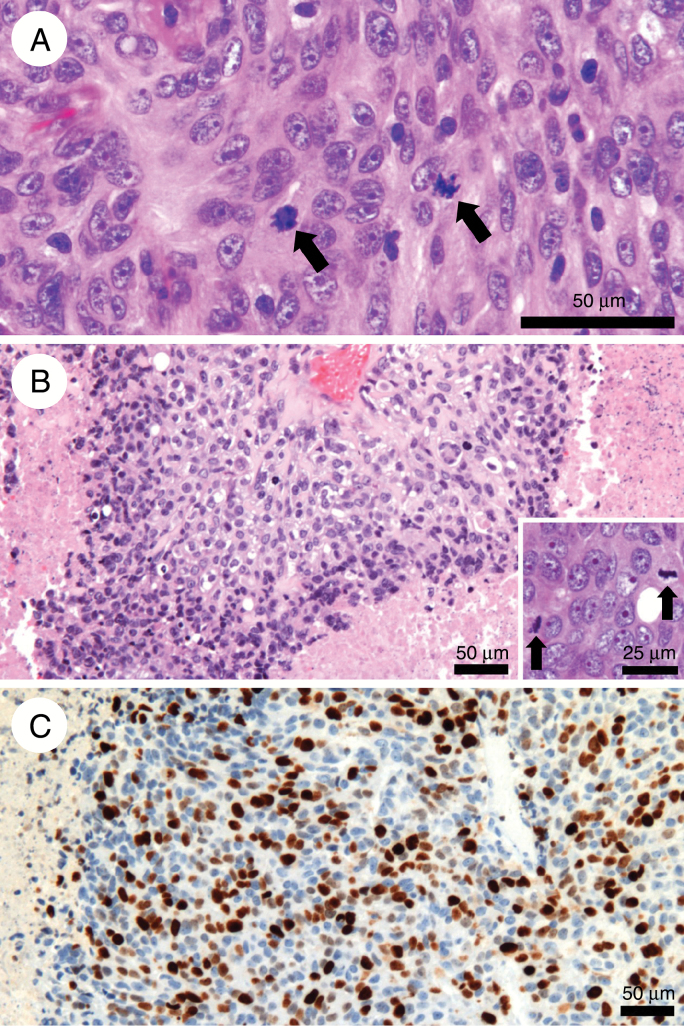
Fig. 1.
(A) Anaplastic meningioma harboring numerous mitoses (>20 / 1.6 mm2) (arrows) and moderate atypia with prominent nuclei (hematoxylin, eosin, and saffron; original magnification: x400). (B) Anaplastic meningioma showing overt anaplasia, with pseudocarcinomatous features, necrosis, and numerous mitoses (>20 / 1.6 mm2; insert, arrows) (hematoxylin, eosin, and saffron; x100; insert: x400). (C) Same case: high Ki-67 proliferative index (immunohistochemistry, x200).
Molecular Analysis
Tumor DNA was extracted using the QIAamp DNA mini kit (Qiagen). Mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene at hotspots chr5:1,295,228 (C228T) and chr5:1,295,250 (C250T) were assessed as previously described.17 For methylation analysis, genomic DNA was subjected to bisulfite conversion (EZ DNA Methylation-Gold-Kit, Zymo Research) and then amplified by methylation-specific PCR as previously described.19 Due to the low quality of DNA extracted from paraffin-embedded tumors, TERT methylation analysis could not be performed on the whole cohort and 24 patients were excluded from the analysis.
Statistical Methods
Statistical analyses were performed using IBM SPSS Statistics software for Windows, version 23.0. Analyses of progression-free survival (PFS) and OS times were performed using a Kaplan–Meier estimation (log-rank test), as well as the Cox proportional hazards regression model, with both univariate and multivariate analyses. For Cox multivariate analyses, only variables that were significant according to univariate analyses (P < 0.05) were integrated into the model. Unless specified, survival data in secondary anaplastic meningiomas are expressed from the time of anaplastic transformation.
Results
Clinical Data
Fifty-seven patients were included in the study. The mean age at diagnosis was 60 years and the median follow-up since diagnosis was 4.8 years (range: 0.6–34.2 years). Of note, the median follow-up duration was also 4.8 years in the 16 patients alive at the time of completion of this study. There were 28 de novo and 29 secondary anaplastic meningiomas. In secondary anaplastic meningiomas, the mean time to anaplastic transformation was 7.7 years (median 4.6 years, range: 0.9–31 years). Figure 1 illustrates the high variability of evolution of secondary anaplastic meningiomas, stressing the existence of slow- and fast-progressing anaplastic tumors. Although there was a slight male predominance in the whole cohort (1.1:1), the proportions were inverted when comparing de novo and secondary anaplastic meningiomas, as female patients prevailed in de novo tumors and males in secondary anaplastic meningiomas. Tumors were located at the convexity (convexity, parasagittal, and falx locations) in 91% of cases. The mean number of craniotomies was 3 per patient and a gross total resection (GTR) was performed in the majority of cases (75%). Unsurprisingly, the mean number of craniotomies was higher in secondary anaplastic tumors (4 vs 2 in de novo tumors). The extent of resection did not differ between de novo and secondary anaplastic meningiomas (P = 0.9, chi-squared test) (Table 1).
Table 1.
Clinical characteristics of anaplastic meningiomas
| De Novo Anaplastic Meningiomas | Progressing Anaplastic Meningiomas | Total | |
|---|---|---|---|
| Sex (male/female) | 11/17 | 19/10 | 30/27 |
| Age at diagnosis, y, mean (range) | 67 (35–83) | 53 (33–79) | 60 |
| Location | |||
| Convexity/ parasagittal/falx | 25 | 27 | 52 |
| Skull base/posterior fossa | 2 | 2 | 4 |
| Intraventricular | 1 | 0 | 1 |
| Number of surgeries | |||
| 1 | 13 | — | 13 |
| 2 | 8 | 8 | 16 |
| 3 | 6 | 8 | 14 |
| 4 | 1 | 4 | 5 |
| 5 | — | 3 | 3 |
| 6 | — | 5 | 5 |
| 7 | — | 1 | 1 |
| Extent of resection | |||
| GTR | 21 | 22 | 43 |
| STR | 6 | 5 | 11 |
| Partial | 1 | 2 | 3 |
| Radiotherapy | |||
| 1 | 20 | 23 | 43 |
| 2 | 2 | 2 | 4 |
| Radiosurgery | |||
| 1 | 2 | 8 | 10 |
| 2 | 5 | 1 | 6 |
| 3 | 1 | 1 | |
| Chemotherapy | |||
| Bevacizumab | 2 | 2 | 4 |
| Sandostatin | 1 | 0 | 1 |
| Belustine | 0 | 2 | 2 |
| Temozolomide | 1 | 0 | 1 |
| Prognosis | |||
| Time to anaplastic transformation, mo (range) | — | 93 (11–373) | — |
| PFS, mo | 15 | 33 | 28 |
| OS after anaplastic transformation, mo | 37 | 25 | 31 |
Adjuvant Treatments: Radiotherapy, Radiosurgery, and Chemotherapy
Forty-seven patients (82%) underwent postoperative conformational radiotherapy (mean dose: 59 Gy). Patients who were not treated with fractionated radiotherapy had radiosurgery instead in 3 cases or had a declined performance status that precluded them from having radiotherapy. Radiosurgery was determined on a case-by-case basis and 7 patients had more than one radiosurgery procedure (mean dose: 16 Gy). Eight patients (14%) had chemotherapy during their treatment, and bevacizumab was the most frequently delivered drug (Table 1). No effect of chemotherapy on tumor progression was encountered in the cohort.
Histological and Molecular Data
Among 29 secondary anaplastic meningiomas, 10 progressed to anaplastic from a grade I tumor and 19 from a grade II tumor. The switch to grade III histology occurred between 1 and 5 relapses after initial diagnosis (Table 1 and Fig. 2). De novo anaplastic meningiomas were diagnosed as grade III based on the number of mitoses in 21 cases and on histological anaplasia without associated elevated mitosis number in 7 cases (less than 5 mitoses in 4 cases, 5 to 10 mitoses in 2 cases, and between 15 and 20 mitoses in 1 case; Fig. 1). Of 57 anaplastic meningiomas, 8 carried a TERT promoter mutation (14%: 7 with C228T variants and 1 with C250T variant). Mutational variants were not associated with either secondary or de novo anaplastic meningiomas. In 13 secondary anaplastic meningiomas, the status of TERT promoter mutation was assessed in at least one previous resection as a lower-grade meningioma. In the only mutated case, the mutation was also found in the previous tumor. In the 12 TERT wild-type secondary anaplastic meningiomas, no mutation was found in the previous tumors. TERT promoter methylation was analyzed in 33 anaplastic meningiomas and was found to be positive in 30 tumors (91%).
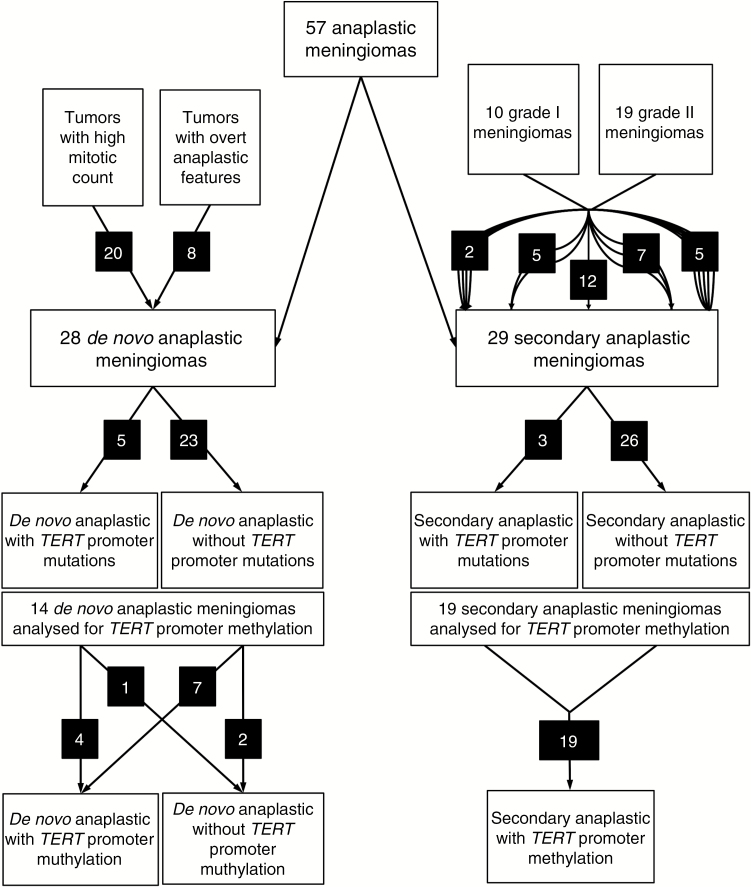
Fig. 2.
Flow chart of histomolecular analysis of anaplastic meningiomas. For secondary anaplastic meningiomas, the number of relapses before anaplastic transformation is represented by the number of arrow lines between low-grade and anaplastic tumors. For example, 2 patients experienced 4 relapses before undergoing malignant transformation.
Outcome and Survival Analysis: Clinical and Histological Parameters
In the whole cohort, mean OS was 2.6 years. Kaplan–Meier estimates of OS were 84% at 2 years with 47 patients alive, and 10% at 5 years with 6 patients alive. Patients harboring de novo anaplastic meningiomas were found to have significantly longer OS (mean: 3.1 y) than patients with secondary tumors (mean: 2.1 y; P = 0.02, log-rank test; Fig. 3A), considering the time of anaplastic transformation. Gross total resection was statistically associated with better OS compared with near-total or partial resection only in de novo anaplastic meningiomas (4.7 vs 2.3 y, P = 0.02, log-rank test). Localization did not influence survival (P = 0.7, log-rank test).
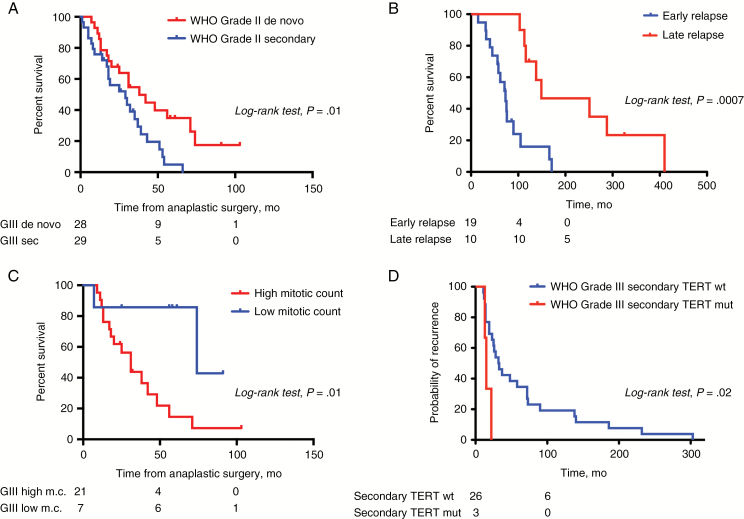
Fig. 3.
Survival analysis of anaplastic meningiomas. (A) Overall survival of de novo vs secondary anaplastic meningiomas. (B) Overall survival of secondary anaplastic meningiomas depending on time to first relapse from a low-grade meningioma. (C) Overall survival of de novo anaplastic meningiomas depending on mitotic count. High mitotic count: meningiomas presenting with >20 mitoses per 10 HPF with or without associated anaplasia. Low mitotic count: meningiomas presenting with <20 mitoses per 10 HPF but with frank anaplasia. (D) Progression-free survival of secondary anaplastic meningiomas depending on TERT promoter mutation status. sec = secondary; m.c. = mitotic count. mut = mutant; wt = wild-type. Numbers of subjects at risk are indicated below plots at time points 0, 50, and 100 months.
Among secondary anaplastic meningiomas, we observed great heterogeneity concerning patterns of relapse and time to anaplastic transformation (Figure 1) and we discovered that patients clustered in 2 groups depending on the time to first relapse after initial surgery as a lower-grade meningioma (<36 mo: 19 patients, and >46 mo: 10 patients). This time to first relapse after initial surgery as a lower-grade meningioma statistically significantly influenced OS (73 mo vs 149 mo) (P = 0.0007, log-rank test; Fig. 3B), while OS (ie, after anaplastic transformation) did not differ statistically significantly between the 2 groups (23 mo vs 26 mo).
Pathological analysis confirmed that all secondary anaplastic meningiomas were diagnosed as WHO grade III on the number of mitoses that progressively increased with subsequent relapses until it reached 20 mitoses per HPF (Table 2). In contrast, all de novo anaplastic meningiomas were not diagnosed as WHO grade III on the number of mitoses, as 7 tumors were diagnosed as WHO grade III on frank histological anaplasia alone. We demonstrated a better survival for meningiomas diagnosed as grade III on anaplasia only (7 tumors—median OS: 6.2 y) compared with meningiomas diagnosed as grade III based on high mitotic count (with or without anaplasia) (21 tumors—median OS: 2.6 y) (P = 0.01, log-rank test; Fig. 3C). Among those 7 patients with histological anaplasia alone, 6 did not have postoperative radiotherapy due to a unilateral decision of the referring surgeon.
Table 2.
Histomolecular characteristics of anaplastic meningiomas
| De Novo Anaplastic Meningiomas n = 28 | Progressing Anaplastic Meningiomas n = 29 | |
|---|---|---|
| Number of relapses before switch to grade III | ||
| 1 | — | 12 |
| 2 | — | 5 |
| 3 | — | 7 |
| 4 | — | 2 |
| 5 | — | 5 |
| Number of mitoses at diagnosis of grade III histology | ||
| >20 | 21 | 29 |
| 15–20 | 1 | 0 |
| 5–10 | 2 | 0 |
| <5 | 4 | 0 |
| Histological anaplasia | ||
| with elevated number of mitoses | 11 | 20 |
| without elevated number of mitoses | 7 | 0 |
| TERT promoter mutation | 5/28 | 3/29 |
| TERT promoter methylation | 11/14 | 19/19 |
Outcome and Survival Analysis: Molecular Parameters
TERT mutated anaplastic meningiomas did not recur significantly earlier than those without mutation (P = 0.3, log-rank test), when considering the whole cohort. There was no difference in OS between TERT mutated and TERT wild-type anaplastic meningiomas (P = 0.6, log-rank test). However, we demonstrated that TERT mutations had a statistically significant impact on prognosis, but only on the PFS of secondary anaplastic meningiomas (P = 0.02, log-rank test; Fig. 3D). This association was not significant in de novo anaplastic meningiomas (P = 0.89). On the other hand, patients without TERT promoter methylation had a better prognosis than patients with TERT promoter methylation (P = 0.01, log-rank test). The 3 patients without TERT promoter methylation had de novo tumors and one also had a TERT promoter mutation. Two were diagnosed on isolated anaplasia and one on the number of mitoses (Fig. 2).
Finally, we conducted univariate and multivariate analyses with the Cox proportional hazards regression model. We confirmed the statistically significant difference in prognosis between de novo and secondary anaplastic meningiomas (OS, hazard ratio [HR] for de novo tumors: 0.455; 95% CI: 0.238–0.868; P = 0.017) and between low and high mitotic count tumors (OS, HR for mitotic index: 1.033; 95% CI: 1.002–1.066) (Table 3). In a multivariate Cox model including these 2 variables, de novo versus secondary status (HR: 0.450; 95% CI: 0.235–0.860; P = 0.035) and mitotic index (HR: 1.034; 95% CI: 1.002–1.066) remained significantly correlated with OS.
Table 3.
Adjusted univariate Cox regression analysis for anaplastic meningioma OS
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.007 | 0.981–1.034 | 0.59 |
| Sex | 1.002 | 0.543–1.850 | 0.99 |
| De novo vs secondary | 0.455 | 0.238–0.868 | 0.02 |
| Mitotic index | 1.033 | 1.002–1.066 | 0.04 |
| Extent of resection | 0.651 | 0.336–1.260 | 0.20 |
| Localization | 1.382 | 0.331–5.763 | 0.66 |
| TERT mutation | 0.757 | 0.296–1.935 | 0.56 |
| TERT methylation | 37.805 | 0.348–4112.806 | 0.13 |
Bold P-values are considered statistically significant (P < 0.05).
Discussion
Anaplastic meningiomas are rare tumors but with a devastating prognosis. Our results are in line with the median OS reported in the literature, ranging between 2.6 and 5.8 years. Surgery remains the cornerstone of anaplastic meningioma treatment. Following a study by Sughrue et al, the utility of GTR compared with subtotal resection (STR) in the treatment of anaplastic tumors was challenged.7,20 Others have indeed demonstrated that GTR is associated with a better outcome than STR.5,8,9,21,22 In our study, we were able to demonstrate a statistically significant difference between the 2 groups only in de novo anaplastic meningiomas. We nonetheless believe that this issue is of moderate interest as all tumors are not amenable to GTR at relapse. In fact, we have demonstrated in a previous case series that about half of surgery- and radiation-refractory meningiomas, including most WHO grade III tumors, demonstrate multinodular metastatic relapses that are not amenable to GTR of the whole tumor burden despite possible GTR of the growing nodule.23 The notion of GTR itself may be reconsidered in such cases and should therefore be carefully discussed. Although postoperative radiotherapy has become the mainstay of treatment following GTR, there are only a few retrospective studies demonstrating its benefit on survival in patients with anaplastic meningioma.24,25 As a majority (91%) of patients underwent either radiotherapy or radiosurgery in our series, we were not able to discuss this matter.
Several retrospective series have demonstrated that de novo anaplastic meningiomas present with better outcome than secondary tumors and should be considered as 2 distinct clinical subgroups. In recent series, there was a slightly increased frequency of de novo cases compared with secondary cases, which was not the case in our cohort (Table 1). This question could remain unanswered, as there are many selection biases due to the retrospective nature of the study and the lack of exhaustiveness of the accrual. Moreover, it is possible that de novo anaplastic meningiomas could correspond to the anaplastic transformation of a previously undiagnosed lower-grade meningioma. Nonetheless, there is a distinct clinical feature already reported by others, which is the difference in sex repartition. While de novo cases are more frequent in women, secondary anaplastic tumors predominate in men. Following a recent monocentric study,15 our work confirms the better prognosis of patients with de novo anaplastic meningiomas diagnosed on histological anaplasia only. This result on the prognostic value of histological anaplasia alone is consistent with the study of Vaubel and colleagues, who demonstrated that most meningiomas that have rhabdoid features and lack other features of malignancy (WHO grade I or II) are not as aggressive as rhabdoid meningiomas with independent histological features consistent with WHO grade III.12 As 6 out of the 7 patients with histological anaplasia alone did not have postoperative radiotherapy, the prognosis of this subgroup of anaplastic meningiomas might be even better with postoperative radiotherapy.
Regarding secondary anaplastic meningiomas, to our knowledge our study is the first to address the question of the natural history of those patients before anaplastic transformation. We demonstrate that patients follow variable clinical courses before being diagnosed with an anaplastic meningioma and that this pre-malignant period defines the global prognosis of the disease. We should also keep in mind that this heterogeneity in terms of recurrence numbers might also be a bias in statistical evaluation. This variability may also reflect varying underlying molecular pathways of malignant progression. The poor prognosis of secondary anaplastic meningiomas after anaplastic transformation reinforces the need for early detection of meningiomas with progression potential based on molecular testing in order to develop specific therapeutic strategies. In this regard, the definition of methylation subgroups could provide more precise prediction of clinical behavior than the WHO classification and grading system.26 Recently, 2 papers have highlighted the prognostic value of methylation subgroups among meningiomas.26,27 Particularly, Sahm et al demonstrated that anaplastic meningiomas, while predominantly mapping to the high risk methylation subgroup (31 [47%] of 66 cases), also had a substantial fraction in intermediate risk subgroups (35 [53%] of 66 cases). As these methylation subgroups represent a precise prediction tool of clinical behavior, they should in future studies be compared with histological parameters described here.
Apart from recent works underlining the role of TERT promoter mutation in meningioma progression,28 another recent study by Sahm et al demonstrated that TERT promoter mutation was statistically significantly associated with shorter time to progression in all WHO grades. They even considered assigning grade IV to the specific subgroup of TERT-mutated WHO grade III meningiomas due to their particularly poor prognosis but lacked OS data. Here we present the largest cohort of anaplastic meningiomas with TERT promoter mutation analysis and OS data. The proportion of TERT-mutated anaplastic meningiomas is lower in our cohort compared with Sahm et al (14% vs 20%). We did not find any statistically significant association between TERT promoter mutations and reduced PFS or OS in the whole cohort. However, we were able to demonstrate that TERT promoter mutation was a prognostic marker of unfavorable PFS in the secondary anaplastic meningioma subgroup, as demonstrated previously by Sahm et al. Despite limitations due to the low number of mutant cases, we believe TERT promoter mutation analysis may not be of clinical interest in de novo WHO grade III meningiomas and should not therefore be systematically assessed in those cases. Nonetheless, we acknowledge that TERT promoter mutation analysis remains clinically relevant in low-grade meningiomas and there may be an association with prognosis in grade I meningiomas with larger studies needed.17 Although TERT promoter mutations have previously been described as statistically significantly associated with secondary anaplastic meningiomas,17,29 in this study mutations were slightly more frequent in de novo compared with secondary cases. In addition, our analysis demonstrated that the rate of TERT promoter methylation was high in anaplastic meningiomas, in line with a previous study.19 We also show for the first time that patients with meningiomas without TERT promoter methylation have a statistically significantly better OS compared with methylated patients. This result should be viewed with caution due to the low number of unmethylated cases and the confounding factors, but we would recommend further studies to address this question more precisely.
In conclusion, our work provides a study of available clinical and histomolecular prognostic factors in anaplastic meningiomas. While slightly undermining the value of TERT promoter mutation analysis in this specific subgroup of meningiomas, we stress the importance of mitotic count analysis in de novo anaplastic meningiomas and the impact of pre-anaplastic clinical course in secondary anaplastic tumors. We therefore believe that future molecular studies should focus on those particular clinical features for analysis in order to discover specific molecular pathways attached to each subgroup of anaplastic meningiomas. In secondary anaplastic meningiomas, attention should be paid to the detection of TERT mutations and the molecular portrait of pre-anaplastic tumors, especially using methylation subgroup clusterization.26 On the other hand, in de novo anaplastic meningioma, TERT mutational status seems not to be of clinical relevance, while we emphasize here the importance of the extent of resection in this specific subgroup.
Funding
None.
Conflict of interest statement
The authors declare that they have no personal conflicts of interest and no institutional financial interest in any drugs, materials, or devices described in this manuscript.
Acknowledgments
We would like to thank Luc Bauchet and French Brain Tumor Data Base for help in patient accrual and the Tumor Banks at Pitié-Salpêtrière Hospital, Beaujon Hospital, Bordeaux and Marseille (CRB-TBM BB-0033-00097/APHM tumor bank AC-2013-1786). This manuscript is not currently under consideration, in press, or published elsewhere, and is truthful original work without fabrication, fraud, or plagiarism.
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. . CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rogers L, Barani I, Chamberlain M et al. . Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aghi MK, Carter BS, Cosgrove GR et al. . Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64(1):56–60; discussion 60. [DOI] [PubMed] [Google Scholar]
- 4. Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 2015;17(8):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aizer AA, Bi WL, Kandola MS et al. . Extent of resection and overall survival for patients with atypical and malignant meningioma: extent of resection and recurrence in meningioma. Cancer. 2015;121(24):4376–4381. [DOI] [PubMed] [Google Scholar]
- 6. Rosenberg LA, Prayson RA, Lee J et al. . Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiat Oncol Biol Phys. 2009;74(2):427–432. [DOI] [PubMed] [Google Scholar]
- 7. Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization grade III meningiomas. J Neurosurg. 2010;113(2):202–209. [DOI] [PubMed] [Google Scholar]
- 8. Moliterno J, Cope WP, Vartanian ED et al. . Survival in patients treated for anaplastic meningioma. J Neurosurg. 2015;123(1):23–30. [DOI] [PubMed] [Google Scholar]
- 9. Cao X, Hao S, Wu Z et al. . Survival rates, prognostic factors and treatment of anaplastic meningiomas. J Clin Neurosci. 2015;22(5):828–833. [DOI] [PubMed] [Google Scholar]
- 10. Zhu H, Xie Q, Zhou Y et al. . Analysis of prognostic factors and treatment of anaplastic meningioma in China. J Clin Neurosci. 2015;22(4):690–695. [DOI] [PubMed] [Google Scholar]
- 11. Louis DN, Perry A, Reifenberger G et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 12. Vaubel RA, Chen SG, Raleigh DR et al. . Meningiomas with rhabdoid features lacking other histologic features of malignancy: a study of 44 cases and review of the literature. J Neuropathol Exp Neurol. 2016;75(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krayenbühl N, Pravdenkova S, Al-Mefty O. De novo versus transformed atypical and anaplastic meningiomas: comparisons of clinical course, cytogenetics, cytokinetics, and outcome. Neurosurgery. 2007;61(3):495–503; discussion 503. [DOI] [PubMed] [Google Scholar]
- 14. Zhao P, Hu M, Zhao M, Ren X, Jiang Z. Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg Rev. 2015;38(1):101–107; discussion 107. [DOI] [PubMed] [Google Scholar]
- 15. Champeaux C, Jecko V. World Health Organization grade III meningiomas. A retrospective study for outcome and prognostic factors assessment. Neurochirurgie. 2016;62(4):203–208. [DOI] [PubMed] [Google Scholar]
- 16. Kleihues P, Louis DN, Scheithauer BW et al. . The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225; discussion 226. [DOI] [PubMed] [Google Scholar]
- 17. Sahm F, Schrimpf D, Olar A et al. . TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5):djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rigau V, Zouaoui S, Mathieu-Daudé H et al. ; Société Française de Neuropathologie (SFNP), Société Française de Neurochirurgie (SFNC); Club de Neuro-Oncologie of the Société Française de Neurochirurgie (CNO-SFNC); Association des Neuro-Oncologues d’Expression Française (ANOCEF) French brain tumor database: 5-year histological results on 25 756 cases: FBTDB of histological results 2004–2008. Brain Pathol. 2011;21(6):633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fürtjes G, Köchling M, Peetz-Dienhart S et al. . hTERT promoter methylation in meningiomas and central nervous hemangiopericytomas. J Neurooncol. 2016;130(1):79–87. [DOI] [PubMed] [Google Scholar]
- 20. Balasubramanian SK, Sharma M, Silva D et al. . Longitudinal experience with WHO grade III (anaplastic) meningiomas at a single institution. J Neurooncol. 2017;131(3):555–563. [DOI] [PubMed] [Google Scholar]
- 21. Dziuk TW, Woo S, Butler EB et al. . Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37(2):177–188. [DOI] [PubMed] [Google Scholar]
- 22. Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86(5):793–800. [DOI] [PubMed] [Google Scholar]
- 23. Peyre M, Zanello M, Mokhtari K et al. . Patterns of relapse and growth kinetics of surgery- and radiation-refractory meningiomas. J Neurooncol. 2015;123(1):151–160. [DOI] [PubMed] [Google Scholar]
- 24. Durand A, Labrousse F, Jouvet A et al. . WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol. 2009;95(3):367–375. [DOI] [PubMed] [Google Scholar]
- 25. Sun SQ, Hawasli AH, Huang J, Chicoine MR, Kim AH. An evidence-based treatment algorithm for the management of WHO grade II and III meningiomas. Neurosurg Focus. 2015;38(3):E3. [DOI] [PubMed] [Google Scholar]
- 26. Sahm F, Schrimpf D, Stichel D et al. . DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 27. Olar A, Wani KM, Wilson CD et al. . Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017;133(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abedalthagafi MS, Bi WL, Merrill PH et al. . ARID1A and TERT promoter mutations in dedifferentiated meningioma. Cancer Genet. 2015;208(6):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]