Abstract
Background
There have been few treatment advances for patients with glioblastoma (GBM) despite increasing scientific understanding of the disease. While factors such as intrinsic tumor biology and drug delivery are challenges to developing efficacious therapies, it is unclear whether the current clinical trial landscape is optimally evaluating new therapies and biomarkers.
Methods
We queried ClinicalTrials.gov for interventional clinical trials for patients with GBM initiated between January 2005 and December 2016 and abstracted data regarding phase, status, start and end dates, testing locations, endpoints, experimental interventions, sample size, clinical presentation/indication, and design to better understand the clinical trials landscape.
Results
Only approximately 8%–11% of patients with newly diagnosed GBM enroll on clinical trials with a similar estimate for all patients with GBM. Trial duration was similar across phases with median time to completion between 3 and 4 years. While 93% of clinical trials were in phases I–II, 26% of the overall clinical trial patient population was enrolled on phase III studies. Of the 8 completed phase III trials, only 1 reported positive results. Although 58% of the phase III trials were supported by phase II data with a similar endpoint, only 25% of these phase II trials were randomized.
Conclusions
The clinical trials landscape for GBM is characterized by long development times, inadequate dissemination of information, suboptimal go/no-go decision making, and low patient participation.
Keywords: clinical trials, glioblastoma, regulatory science
Importance of the study
This study serves to highlight and characterize the inefficiencies associated with the fragmented and poorly coordinated landscape of studies for developing new therapies and biomarkers for patients with GBM. Specific findings suggest possible areas of improvement.
Glioblastoma (GBM) is one of the most aggressive cancers, with poor overall survival (OS) and few effective therapies.1 Even as the understanding of the molecular underpinnings of GBM has increased substantially over the past decade, there has been minimal development of new drugs that leverage this information. Clearly, the therapeutic development ecosystem for GBM is not producing optimal results.
There are many possible reasons for the lack of progress in developing new therapies for GBM, but there is little evidence to suggest what the main drivers are. Complex biology, the presence of a blood–brain barrier, lack of sufficient investment, and others have all been cited.1 While improvement in the way that patients with GBM feel, function, and survive depends most directly on the discovery of efficacious new therapies, the system for testing and developing such therapies is also a critical element.
An effective system for developing new therapies and biomarkers for GBM would provide ample opportunities for patients to participate on clinical trials (particularly given the poor outcomes), generate and disseminate valuable information from each patient, stop development of ineffective therapies early, understand why such therapies failed, and provide an efficient pathway to approval for effective therapies. Here we present the landscape of clinical trials for GBM between 2005 and 2016 and highlight trends in trial characteristics at different stages of development to understand whether such general goals are being accomplished.
Materials and Methods
Data Acquisition
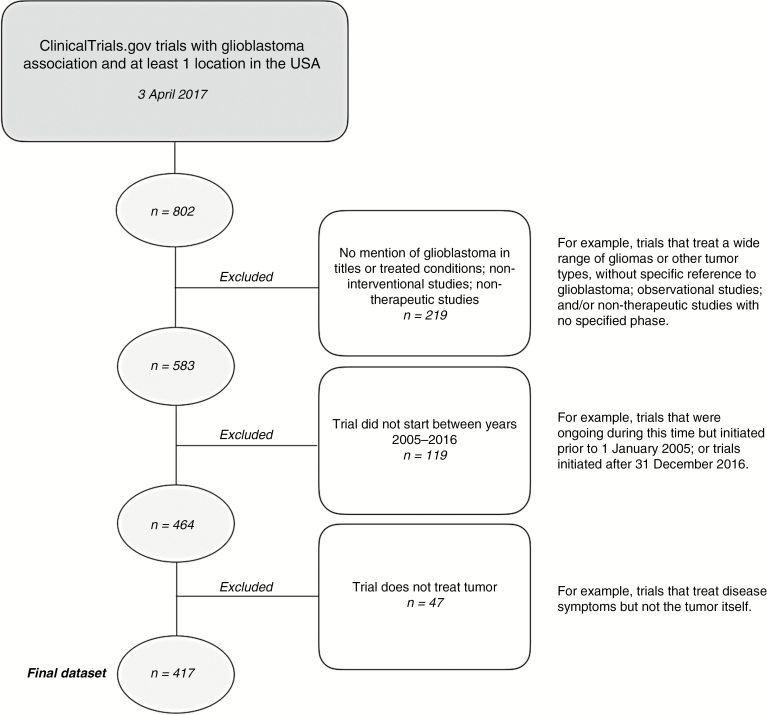
On April 3, 2017, we queried ClinicalTrials.gov using the R package rclinicaltrials2 for clinical trials including GBM with testing locations in the US and start dates from January 1, 2005 to December 31, 2016. Following an elimination schema similar to Cihoric et al,3 we removed non-interventional studies and clinical trials that list GBM neither in the title nor as a primary condition. Thirteen studies that did not list trial phase were included in the dataset after being determined by one investigator (B.M.A.) to be therapeutic trials. Forty-seven trials were further determined to be non-therapeutic despite being listed as “Interventional” and were excluded (Fig. 1).
Fig. 1.
Therapeutic clinical trial identification and selection.
From this set, we collected information about trial characteristics including: phase, status (enrolling, closed, etc), start and end dates, number of testing locations, primary endpoint(s), experimental interventions, sample size, clinical presentation/indication, and design. Using the ClinicalTrials.gov Archives site, we obtained the longitudinal history of change for all trials. In accordance with Section 801 in the Food and Drug Administration Amendments Act of 2007,4 trial duration was defined by the study start date (onset of the enrollment) and the primary completion date. Trial duration was computed only for trials where completion dates were confirmed by the principal investigator. If a confirmed primary completion date was not available, the final completion date was used instead. Endpoints were classified as based on OS, progression-free survival (PFS), tumor response, safety and toxicity, or pharmacokinetics/pharmacodynamics (PK/PD).
Association with Publications
Starting in 2005, the National Library of Medicine (NLM) began to include the ClinicalTrials.gov registry number (NCTID) in the MEDLINE record when the number is published as part of the original paper. ClinicalTrials.gov contains links to publications in PubMed either through this mechanism or when the study team manually provides a publication link.5 After retrieving the list of these associated publications, review by 2 investigators (A.M.V., R.R.) identified publications reporting on primary results. If a publication on primary results from a clinical trial in our dataset did not contain the NCTID or was not input manually, the publication would not have been detected by our search.
Estimation of Percentage of GBM Patients Enrolled on Therapeutic Clinical Trials
We estimated the percentage of patients enrolled (PPE) on clinical trials for both newly diagnosed (ND) and total (ND and recurrent) patients. We queried the ClinicalTrials.gov Archives site for longitudinal changes in trial status to estimate recruitment periods for all trials that opened or were ongoing during the period between 2008 and 2012 (251 studies). We derived the periods between the dates on which the trial status changed from “Recruiting” to “Active, not recruiting,” “No longer recruiting,” “Suspended,” or “Completed.” We then assumed a constant recruitment rate and calculated the number of patients recruited to the trial each month. We assumed equal distribution across sites and then estimated the total number of patients enrolled for US testing sites only. A lower bound estimate for the PPE for ND patients used trials that recruited only ND patients for the years 2008–2012 in the numerator and divided by the estimated average incidence of GBM for those years.6 The upper bound was calculated similarly but included trials that recruited both ND and recurrent patients in the numerator. To estimate PPE for total patients, we used the total number of patients enrolled on all clinical trials as the numerator and GBM prevalence7 as the denominator. PPE for total patients is prone to overestimation due to the possibility of a single patient enrolling on multiple trials.
Results
General Trial and Patient Characteristics
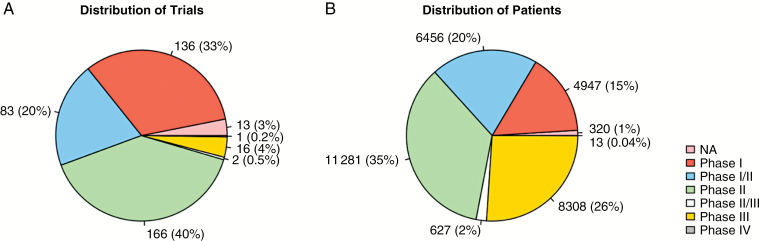
Table 1 shows trial characteristics by phase. There were approximately twice as many clinical trials for patients with recurrent GBM (271 trials) as there were for patients presenting with a new diagnosis (136 trials). Ten trials recruited both indications or did not specify. Trial duration was available for 70 phase I trials, 45 phase I/II trials, 107 phase II trials, and 11 phase III trials. One phase II/III trial was ongoing, another had been suspended, and 1 phase IV trial was terminated but the confirmed termination date was not available. Median trial duration including and not including terminated studies was 37 and 40 months in phase I, 47 and 55 months in phase I/II, 32 and 39 months in phase II, and 38 and 47 months in phase III, respectively. Most of the trials were early stage: 33% were phase I, 20% were phase I/II, and 40% were phase II (Table 1, Fig. 2a). The number of patients allocated for each phase was more evenly distributed, however (Fig. 2b). For example, phase III trials accounted for approximately 26% of the enrollments but comprised only 4% of trials.
Table 1 .
Clinical trials in GBM as found in ClinicalTrials.gov (2005–2016) as of April 3, 2017
| NA | Phase I | Phase I/II | Phase II | Phase II/III | Phase III | Phase IV | Total | |
|---|---|---|---|---|---|---|---|---|
| Trials | ||||||||
| Number of trials | 13 (3%) | 136 (33%) | 83 (20%) | 166 (40%) | 2 (0.5%) | 16 (4%) | 1 (0.2%) | 417 (100%) |
| Trial status* | ||||||||
| Completed | 2 | 52 | 17 | 72 | – | 8 | – | 151 (36%) |
| Active, not recruiting | 4 | 23 | 23 | 31 | 1 | 4 | – | 86 (21%) |
| Recruiting | 7 | 43 | 25 | 32 | – | 3 | – | 110 (26%) |
| Not yet recruiting | – | 1 | – | 2 | – | – | – | 3 (0.7%) |
| Enrolling by invitation | – | – | 1 | – | – | – | – | 1 (0.2%) |
| Suspended | – | 2 | 1 | – | 1 | – | – | 4 (1%) |
| Terminated | – | 12 | 10 | 21 | – | 1 | 1 | 45 (11%) |
| Withdrawn | – | 1 | 1 | 2 | – | – | – | 4 (1%) |
| Unknown status | – | 2 | 5 | 6 | – | – | – | 13 (3%) |
| GBM classification | ||||||||
| Newly diagnosed | 3 | 39 | 29 | 54 | 1 | 10 | – | 136 (33%) |
| Recurrent | 10 | 91 | 53 | 110 | 1 | 5 | 1 | 271 (65%) |
| Both | – | 5 | 1 | 2 | – | 1 | – | 9 (2%) |
| Not specified | – | 1 | – | – | – | – | – | 1 (0.2%) |
| Results provided** | ||||||||
| Results provided | – | 1 | 22 | 67 | – | 6 | – | 96 (23%) |
| Publication provided**† | ||||||||
| Publication provided | 2 | 22 | 15 | 39 | 2 | 11 | 1 | 92 (22%) |
| Results publication provided | 1 | 9 | 8 | 26 | – | 8 | – | 52 (12%) |
*See Supplementary Table S1 for definitions provided by ClinicalTrials.gov.
**In ClinicalTrials.gov.
†Provided publications need not be reporting on trial results. We distinguish between all publications provided (92 trials), and those that report trial results for primary outcomes (52 trials).
Fig. 2.
Distribution of trials (A) and patients (B) among phases.
Forty-five trials were terminated early. Reason for termination was available on ClinicalTrials.gov for 38 (84%) terminated studies. Recorded as reasons for termination were lack of accrual (19 trials), lack of funding (6 trials), futility (6 trials), drug availability (3 trials), administrative reasons pertaining to protocol (eg, principal investigator left the institution in 5 trials), and safety (1 trial). Median trial duration for terminated studies (all phases) was 31 months (range 11 to 50 mo) for ND and 31 months (range 14 to 71 mo) for recurrent trials.
Based on the number of patients accrued to trials between 2008 and 2012 and the incidence of GBM over the same time period, we estimated that approximately 8%–11% of newly diagnosed GBM patients enrolled on therapeutic clinical trials. In 2010 there were approximately 2035 total enrollments on clinical trials, representing an estimated 10.2% of the annual prevalence (19972 GBM cases) in the US.8 As stated above, this analysis does not account for individual patients enrolling on more than one trial.
Reporting of Clinical Trial Results on ClinicalTrials.gov
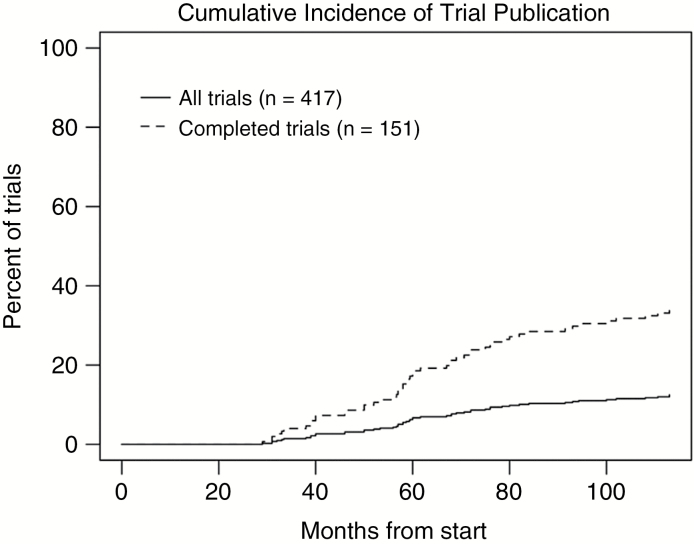
Only 96 (23%) trials provided results in ClinicalTrials.gov, of which only 37 linked to publications reporting results (Table 1). Overall, only 52 trials had linked publications on the primary outcome (Table 1), representing 12% of all trials and 34% of completed trials even after 100 months post trial initiation (Fig. 3). Of the 52 published studies, median time from trial initiation to publication date and from trial completion to publication date for published trials was 60 months and 20 months, respectively. Supplementary Fig. S1 shows the timeline of these trials from trial start to publication of results.
Fig. 3.
Cumulative incidence of linked publications for primary clinical trial results.
Phase III Trials
All trials classified as phase III (16 trials) were designed as randomized controlled trials (RCTs). The average observed sample sizes for ND and recurrent phase III trials (obtained from 8 trials that were not terminated) were 804 and 352, respectively (Supplementary Fig. S2). Average observed enrollment exceeded average planned sample size (804 vs 683 ND; 352 vs 311 recurrent); only 1 of 8 trials fell short of its target enrollment. There is a notable difference in both average planned and observed sample size between ND and recurrent trials with ND trials enrolling more patients. Ten trials (63%) use OS as the primary outcome, 4 (25%) use PFS, and 2 (13%) use OS and PFS as co-primary endpoints (Table 3). Of the phase III studies in the time period, 8 (50%) had been completed with only 1 (NovoTTF; NCT00916409) reporting positive results. Given the overall low rates of success in phase III, we searched ClinicalTrials.gov for the earliest preceding phase II trials using the same drug and indication to better understand the data used to make phase III go/no-go decisions (Table 3). Twelve (75%) phase III studies had antecedent phase II studies in the same indication (ND vs recurrent). Of these phase II studies, 6 (50%) used OS as a primary endpoint and 4 (33%) used PFS. Seven (58%) used the same endpoint as the corresponding phase III study. Nine (75%) were single-arm studies with no control. Using either the anticipated trial end date for ongoing trials or the actual completion date for phase III trials that had been completed, the time from the beginning of phase II through the end of phase III averaged 7.2 years (range 4.3 to 9.9 y).
Table 3.
Details of phase III trials from 2005–2016 and associated phase II trials
| Phase IIII NCTID | Indication | Phase III Status | Phase III Completion Date** | N (III) | Endpoint (III) | Preceding Phase II? | Phase II NCTID | N (II) | Endpoint (II) | Randomized? | Start of Phase II to End of Phase III (years) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP 12009 | NCT00761280 | Recurrent | Terminated | 2012–02 | 27 | OS | Yes | NCT00431561 | 141 | ORR | Yes | 8.83 |
| Bevacizumab | NCT00943826 | Newly diagnosed | Completed | 2012–03 | 921 | OS/PFS | Yes | NCT01013285 | 70* | OS | No | 5.75 |
| Bevacizumab | NCT00884741 | Newly diagnosed | Active, not recruiting | 2013–03 | 637 | OS/PFS | Yes | NCT01013285 | 70* | OS | No | 6.75 |
| Cediranib | NCT00777153 | Recurrent | Completed | 2010–04 | 423 | PFS | Yes | NCT00305656 | 31 | PFS | No | 4.25 |
| Cilengitide | NCT00689221 | Newly diagnosed | Completed | 2012–11 | 545 | OS | Yes | NCT00085254 | 112 | OS | No | 7.58 |
| DCVax† | NCT00045968 | Newly diagnosed | Active, not recruiting | 2016–11* | 348* | OS | Yes | NCT00045968 | 240 | PFS | Yes | 9.92 |
| ddTMZ | NCT00304031 | Newly diagnosed | Completed | 2011–02 | 1173 | OS | No | – | – | – | – | – |
| Enzastaurin | NCT00295815 | Recurrent | Completed | 2007–08 | 397 | PFS | Yes | NCT00190723 | 120* | Anti-tumor activity | No | 4.83 |
| ICT-107 | NCT02546102 | Newly diagnosed | Recruiting | 2019–12* | 414* | OS | Yes | NCT01280552 | 124 | OS | Yes | 8.92 |
| Intraoperative RT | NCT02685605 | Newly diagnosed | Recruiting | 2020–06* | 314* | PFS | Yes | NCT02104882 | 12 | MTD | No | 6.25 |
| Nivolumab | NCT02017717 | Recurrent | Active, not recruiting | 2017–02 | 626 | OS | No | – | – | – | – | – |
| Nivolumab | NCT02617589 | Newly diagnosed | Recruiting | 2019–03* | 550* | OS | No | – | – | – | – | – |
| NovoTTF | NCT00916409 | Newly diagnosed | Completed | 2016–12 | 700* | PFS | No | – | – | – | – | – |
| NovoTTF | NCT00379470 | Recurrent | Completed | 2009–11 | 236 | OS | Yes | Not USA (PMC1886002) | 10 | PFS/OS | No | – |
| Rindopepimut | NCT01480479 | Newly diagnosed | Completed | 2016–11 | 745 | OS | Yes | NCT00458601 | 82 | PFS | No | 9.25 |
| VB-111 | NCT02511405 | Recurrent | Active, not recruiting | 2017–12* | 252* | OS | Yes | NCT01260506 | 75* | OS | No | 7.00 |
† Originally a phase II study; FDA approved protocol change to phase III.
* Planned.
** Primary completion date.
Phase II Trials
Phase II made up the largest proportion of trials (40%). The majority of both ND (74%) and recurrent (90%) phase II trials were not designed as RCTs (Table 2). Only 5 (6%) single institution phase II trials were RCTs. The average observed phase II sample size (excluding those that were terminated or withdrawn) for RCTs and non-RCTs was 150 and 53, respectively, for ND trials and 131 (RCT) and 50 (non-RCT) for recurrent GBM trials (Supplementary Fig. S2). Comparison of planned and observed sample size was available for 86 trials. Thirty-four (10 ND, 24 recurrent) had lower observed sample size than planned; reasons for the discrepancies were not provided in ClinicalTrials.gov. Table 2 also shows the distribution of primary endpoints designated in phase II. Thirty-seven trials (22%) specified more than one primary endpoint. Most phase II trials (59%) had PFS as a primary endpoint. Only 7 trials (4%) included a PK/PD outcome measure. Endpoints that fall into the “Other” category included measures of feasibility, quality of life, and neurocognitive function.
Table 2.
Phase II primary endpoints
| RCT | Non-RCT | Total | |
|---|---|---|---|
| Progression-free survival | 13 (8%) | 85 (51%) | 98 (59%) |
| Overall survival | 11 (7%) | 34 (20%) | 45 (27%) |
| Safety/toxicity/MTD | 7 (4%) | 19 (11%) | 26 (16%) |
| Imaging/response | 3 (2%) | 28 (17%) | 31 (19%) |
| PK/PD | 2 (1%) | 5 (3%) | 7 (4%) |
| Other | 4 (2%) | 4 (2%) | |
| Total | 25 (15%) | 141 (85%) | 166 (100%) |
Phase I and I/II
There were 136 phase I trials (39 ND, 91 recurrent) and 83 phase I/II trials (29 ND, 53 recurrent). Of these trials, 186 included a safety/toxicity endpoint and 142 of these were dose escalation studies. Twenty-two of the phase I and 6 of the phase I/II trials included a PK/PD endpoint.
Of the 68 phase I and I/II ND trials, 58 included temozolomide (TMZ) in the experimental regimen, 40 of which were dose-escalation. Additionally, there were 14 dose-escalation studies in combination with TMZ in the recurrent setting.
Discussion
Improvement in outcomes for patients with GBM will result from groundbreaking science and translational medicine. At the same time, the environment for developing such breakthroughs could be optimized. A strategically planned therapeutic development system for GBM would allow any willing patient to enroll on a clinical trial, learn as much as possible from each patient’s experience, widely disseminate that information quickly, stop the development of ineffective therapies early to limit patient exposure and allow more questions to be answered, understand why such therapies failed, and provide an efficient pathway to approval for truly effective therapies. The current “system” of disparate, independent clinical trials appears to fall short on several of these dimensions.
A Significant Minority of GBM Patients Enroll on Clinical Trials
Understanding whether a new therapy works, in what (potentially biomarker-defined) population, and in what combination or sequence, relies on generating a large amount of clinical data. For a relatively rare disease like GBM, a high rate of clinical trial participation is required to provide such data. But only a significant minority of patients with GBM enroll on clinical trials. While patient preferences are not accounted for, having only 8%–11% of GBM patients on clinical trials seems particularly low given the overall poor outcomes. This range assumes an equal recruitment across all sites (no site-specific enrollment data were available) and so would be an overestimate if international trials accrued a majority of patients outside the US on average. Murthy et al estimated a related percentage (3%) of patients enrolling on oncology trials, though it should be noted that this study specifically looked at patients enrolled on NCI sponsored trials.9
Factors that might lead to a mismatch of patient demand and trial supply could be overly stringent eligibility criteria,10 geographic maldistribution, and difficulty initiating clinical trials due to expense and bureaucracy. Even if trials are available, patients might not be able to find them due to lack of consumer-friendly information. Finally, encouraging industry to maintain a robust pipeline of new therapies for GBM is critical. If enrollment on clinical trials were doubled or tripled, there would need to be sufficient novel therapies available for clinical testing to support the additional patients.
A High Proportion of GBM Trial Patients Were on Mostly Negative Phase III Trials
The clinical trials system does not discontinue development of underperforming therapies early in development, leading to maldistribution of patients among trials. While the number of phase III trials was relatively small, the proportionally larger sample sizes meant phase III accounted for 26% of the entire clinical trial population. A low rate of success in phase III means many patients are exposed to ineffective therapies and there is an enormous waste of resources that may be better employed elsewhere. One reason for the high rate of phase III failure may be overreliance on phase II designs that do not generate enough useful information to make go/no-go decisions. Use of different endpoints (eg, PFS in phase II, OS in phase III) when the relationship between endpoints for a given therapy is unknown may be problematic.11,12 Only 7 of 16 phase III trials in our data had a prior phase II trial using the same endpoint in the same disease setting. Even if concordant endpoints are used, lack of randomization and comparison to a historical control is another possible source of error that can result in overestimation of treatment effect.13 An overwhelming majority (85%) of phase II trials in our data were not RCTs. It is our opinion that this is the major driver of phase III failures. There is also a possibility that single-arm studies and use of discordant endpoints may underestimate treatment effects, which may lead to erroneous discontinuation of development of promising therapies. A more rational estimate of phase III success probability prior to initiation, based on available data and in the context of prior results, would aid sponsors in decision making and serve as a guide to patients and their physicians who are considering enrolling on such trials.
While single-arm phase II trials may not provide optimal data when the endpoint is OS or PFS (as there are many sources of variation unrelated to treatment), other endpoints like objective response rate (ORR) or those based on PD may be more directly attributable to the therapy. Additionally, when therapies do fail, there are rarely enough data in the overall development pathway and limited incentives to understand why. Only a small minority of phase I and II studies used PD endpoints. If early phase signals are sought with limited patients, understanding whether the drug got to the tumor and whether it affected a necessary (if not sufficient) PD endpoint would be a better target than comparisons of survival data with historical controls. Such an endpoint would have less variability explained by factors other than the therapy and if later-stage trials failed, there would be some supportive data to understand why.
Long Development Times
For therapies that do complete phase I testing, development time from phase II through the end of phase III was long, averaging 7.2 years. The amount of time required to develop a clinical trial concept, socialize it among potential collaborators, write a protocol, and obtain approvals through various groups and institutional review boards is significant and may lead to downtime between trials. Additionally, clinical trial sites take time to ramp up accrual and this process must be repeated for each trial, reducing overall efficiency. One solution to these inefficiencies is to conduct more trials on master protocols1,14–18 which frontload the logistical delays and maximize ongoing accrual rates by lowering the bar for new therapeutic evaluation.
There are also a notable number of phase I and phase I/II trials testing experimental therapies in combination with TMZ. Most drug development programs don’t prospectively plan trials for combinations with TMZ, so this represents an additional hurdle specific to therapeutic development for GBM. This additional step of combining a drug that already has a recommended phase II dose (as a monotherapy or with other agents) with TMZ adds to the overall development time. A potential solution is the possibility of starting development only in ND patients with unmethylated tumors and omitting TMZ, a strategy that is gaining broader acceptance.1,19–22 Phase I testing in ND methylated patients with TMZ could be done in parallel. And if the phase I results were safe, combining with TMZ in phase II would follow. On the other hand, if the experimental therapy was found to be unsafe in combination with TMZ, the results of testing in ND unmethylated could help decide whether it was worth considering replacing TMZ in the methylated population for head-to-head testing.
Lack of Quickly and Widely Disseminated Results
Reporting of the primary results of clinical trials enables better research and analysis throughout the therapeutic development system, whereas delay or absence of publication can lead to biased estimates of outcomes and treatment effects. Additionally, reporting of data from trials could be considered an obligation to the patients who enrolled on those trials and engaged in clinical research. Since 2005, clinical trial registration has been a requirement from the International Committee of Medical Journal Editors (ICMJE) and as of 2007, reporting of results on ClinicalTrials.gov is a requirement. Even so, data abstracted from the results portion generally consist of broad summary statistics rather than in-depth data or analysis, and compliance with reporting requirements is low.23 Additionally, linkages to publications for the trials in our dataset through either manual input to ClinicalTrials.gov or referencing the NCTID in the publication were sparse. This is not uncommon—one study of almost 9000 interventional clinical trials phase II or greater on ClinicalTrials.gov completed between 2006 and 2009 showed that only 72% had a structured link to any results publication and only 27% had any results deposited on the site,24 and another study of registered oncology trials showed similar results.25 The reliance on the automated links has known limitations26 but even studies that use both automated and manual methods show that publication of trial results remains limited.26 While additional published results might be found by extensive manual extraction, an absence of automatic links through registries complicates the search and limits the ability to synthesize information across trials and disseminate information widely. Difficulties in synthesizing trial data and information create hurdles for investigators, but also for patients seeking to understand the status of research and development in their disease. Remedying this requires a platform to provide thorough, easily accessible, and understandable data that are relevant to patients and useful for investigators, including results of negative trials. Reporting negative results is particularly necessary to understand what failed and why, so that such designs and/or drugs may not be repeated or tested similarly in the future. Such data can inform subgroup analyses, tuning of adaptive trials,27,28 and evaluation of treatment-biomarker interactions.29,30
ND versus Recurrent Trial Sample Size
It was also interesting to note that phase III trials for ND patients had sample sizes that were on average larger than those for recurrent patients. If one assumes that the number of events in ND disease may be similar to those in recurrent disease but occur over a longer time frame, it begs the question as to whether the additional enrollment in ND is a strategic substitute for waiting additional time for data to mature. Indeed, the median time to completion for ND phase III trials was 50 months versus 19 months for recurrent trials. Enrolling more patients in ND trials might speed results for the one therapy in question but may not be the overall optimal allocation of patients from a patient or systemic perspective, especially when several drugs are in development. Another potential reason for increased sample size in the ND population is the longer survival post progression, which could theoretically “dilute” the drug signal, which traditionally has been thought to act most directly on PFS time while patients are receiving the drug.31 This would not likely impact the power calculation of a trial using a landmark value, however. Nonetheless, development of effective surrogate endpoints and subsequent approval based on such endpoints could reduce sample size requirements under the modeling framework proposed by Broglio and Berry.31 Finally, large sample size also may result from trials designed to detect relatively small clinical benefits. As the original methods and power calculations are not required by ClinicalTrials.gov, it is unclear how much this plays a role. Preferences regarding the appropriate effect size to test in clinical trials may be another area where an individual sponsor differs from the community writ large, however.
Limitations
Limitations of this study include its largely descriptive nature, as it was not intended to comment on the scientific value of individual trials or results. Data may be limited by the use of ClinicalTrials.gov, as described by Cihoric et al.3 Some trials may have been registered incorrectly and data may be out-of-date and/or incomplete, including but not limited to trial start and end dates and enrollment. The calculation for the percent of patients enrolled on trials is estimated without patient-level data, and is meant to provide a ballpark estimate of patient participation, as no such value currently exists. Site-level enrollment data and data regarding the prospective trial methodologies were not available. A limitation of our phase III analysis is that therapeutic development is infrequently a linear process of serial phases; identification of the antecedent phase II data to support go/no-go decisions relied on simplified assumptions of the decision-making process. Finally, while we described the clinical trials landscape for GBM, potential causal relations could not be addressed. Financial resource requirements for investigator initiated trials could be analyzed as a possible factor limiting early-phase trial initiation and, along with academic incentives, the use of single-arm designs in phase II, for example.
Conclusions
Clinical testing of new treatments for GBM is characterized by long development times and suboptimal decision making, resulting in too many patients being exposed to ineffective therapies and requiring large commitments of financial resources. There appears to be a mismatch in supply and demand for patients to enroll on trials, with only a small portion of patients enrolling. Finally, dissemination of information generated by clinical trials is not consistent and direct linkage to the clinical trial registry is sparse. These results perhaps should not be surprising as there is no overarching patient-centered strategy for therapeutic development. There is no “system”; instead, the clinical trials cataloged here resulted organically from an ecosystem focused on the therapies rather than the diseases. Industry is entirely organized around therapies, and academic incentives frequently promote individual rather than common objectives. Breaking down silos as they relate to data sharing has been a common recommendation of expert oncology panels,32 but more general efforts to develop strategic collective action may be needed to create a more efficient, patient-centered therapeutic development system for GBM. This could take the form of direct government-sponsored organization through the National Cancer Institute, government or nongovernment sponsored platform trials under master protocols as highlighted by the FDA,33 and strategies for information dissemination to empower patients directly.
Supplementary material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by a Burroughs Wellcome Innovations in Regulatory Science Award.
Conflict of interest statement
P.Y.W. reports grants, personal fees and nonfinancial support from Agios and Novartis, nonfinancial support from Angiochem, GlaxoSmithKline, ImmunoCellular Therapeutics, VBI Vaccines, and Karyopharm and personal fees and nonfinancial support from AstraZeneca, Genentech/Roche, and Vascular Biogenics, grants and nonfinancial support from Merck, and nonfinancial support from Oncoceutics, Sanofi Aventis, personal fees from Cavion, INSYS Therapeutics, Monteris, from Novogen, Regeneron Pharmaceuticals, and Tocagen. T.F.C. reports consulting fees from: Roche/Genentech, VBL, Merck, BMS, Pfizer, Agios, Novogen, Boston Biomedical, MedQIA, Tocagen, Cortice Biosciences, Novocure, NewGen, Oxigene, Wellcome Trust, Sunovion Pharmaceuticals, Abbvie, Celgene, Lilly; and reports equity in Notable Labs. B.M.A. is the PI of INSIGhT, and adaptive platform trial supported by Eli Lilly, Celgene, and Puma and is the President and CEO of Global Coalition for Adaptive Research.
Supplementary Material
Acknowledgment
This study has not been previously presented.
References
- 1. Alexander B, Cloughesy T. Adult glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. [DOI] [PubMed] [Google Scholar]
- 2. Sachs M. An Interface to the Clinicaltrials.Gov API. 2017. https://github.com/sachsmc/rclinicaltrials. [Google Scholar]
- 3. Cihoric N, Tsikkinis A, Minniti G. Current status and perspectives of interventional clinical trials for glioblastoma—analysis of ClinicalTrials.gov. Radiat Oncol. 2017;12(1). doi:10.1186/s13014-016-0740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. 110th Congress. Food and Drug Administration Amendments Act of 2007 2007. https://www.gpo.gov/fdsys/pkg/PLAW-110publ85/html/PLAW-110publ85.htm.
- 5. Home—ClinicalTrials.gov https://www.clinicaltrials.gov/. Accessed October 31, 2017.
- 6. Ostrom QT, Gittleman H, Fulop J et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang AS, Ostrom QT, Kruchko C, Rogers L, Peereboom DM, Barnholtz-Sloan JS. Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010. Neuro Oncol. 2017;19(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang AS, Ostrom QT, Kruchko C, Rogers L, Peereboom DM, Barnholtz-Sloan JS. Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010. Neuro Oncol. 2017;19(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 10. Kim ES, Bruinooge SS, Roberts S et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35(33):3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexander BM, Trippa L. Progression-free survival: too much risk, not enough reward?Neuro Oncol. 2014;16(5):615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trippa L, Wen PY, Parmigiani G, Berry DA, Alexander BM. Combining progression-free survival and overall survival as a novel composite endpoint for glioblastoma trials. Neuro Oncol. 2015;17(8):1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grossman SA, Schreck KC, Ballman K, Alexander B. Point/counterpoint: randomized versus single-arm phase II clinical trials for patients with newly diagnosed glioblastoma. Neuro Oncol. 2017;19(4):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexander BM, Wen PY, Trippa L et al. Biomarker-based adaptive trials for patients with glioblastoma—lessons from I-SPY 2. Neuro Oncol. 2013;15(8):972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alexander BM, Galanis E, Yung WK et al. Brain Malignancy Steering Committee clinical trials planning workshop: report from the Targeted Therapies Working Group. Neuro Oncol. 2015;17(2):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexander BM, Ba S, Berger MS et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res. 2017; doi:10.1158/1078-0432.CCR-17-0764. [DOI] [PubMed] [Google Scholar]
- 17. Ventz S, Alexander BM, Parmigiani G, Gelber RD, Trippa L. Designing clinical trials that accept new arms: an example in metastatic breast cancer. J Clin Oncol. 2017;35(27):3160–3168. [DOI] [PubMed] [Google Scholar]
- 18. Ventz S, Cellamare M, Parmigiani G, Trippa L. Adding experimental arms to platform clinical trials: randomization procedures and interim analyses. Biostatistics. doi:10.1093/biostatistics/kxx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wick W, Steinbach JP, Platten M et al. Enzastaurin before and concomitant with radiation therapy, followed by enzastaurin maintenance therapy, in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation. Neuro Oncol. 2013;15(10):1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hegi M, Stupp R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter—still a dilemma?Neuro Oncol. 2015;17(11):1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah N, Schroeder B, Cobbs C. MGMT methylation in glioblastoma: tale of the tail. Neuro Oncol. 2015;17(1):167–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wick W, Weller M, van den Bent M et al. MGMT testing—the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372–385. [DOI] [PubMed] [Google Scholar]
- 23. Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372(11):1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huser V, Cimino JJ. Linking ClinicalTrials.gov and PubMed to track results of interventional human clinical trials. PLoS ONE. 2013;8(7):e68409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramsey S, Scoggins J. Commentary: Practicing on the tip of an information iceberg? Evidence of underpublication of registered Clinical Trials in Oncology. The Oncologist. 2008;13(9):925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bashir R, Bourgeois FT, Dunn AG. A systematic review of the processes used to link clinical trial registrations to their published results. Syst Rev. 2017;6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trippa L, Lee EQ, Wen PY et al. Bayesian adaptive randomized trial design for patients with recurrent glioblastoma. J Clin Oncol. 2012;30(26):3258–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wason JMS, Trippa L. A comparison of Bayesian adaptive randomization and multi-stage designs for multi-arm clinical trials. Stat Med. 2014;33(13):2206–2221. [DOI] [PubMed] [Google Scholar]
- 29. Xu Y, Trippa L, Müller P, Ji Y. Subgroup-based adaptive (SUBA) designs for multi-arm biomarker trials. Stat Biosci. 2016;8(1):159–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trippa L, Alexander BM. Bayesian baskets: a novel design for biomarker-based clinical trials. J Clin Oncol. 2017;35(6):681–687. [DOI] [PubMed] [Google Scholar]
- 31. Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaffee EM, Dang CV, Agus DB et al. Future cancer research priorities in the USA: a Lancet Oncology Commission. Lancet Oncol. 18(11):e653–e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.