Abstract
Background
Germline mutations of suppressor of fused homolog (SUFU) predispose to sonic hedgehog (SHH) medulloblastoma. Germline SUFU mutations have been reported in nevoid basal cell carcinoma syndrome (NBCCS), but little is known about the cancer risk and clinical spectrum.
Methods
We performed a retrospective review of all patients with medulloblastoma and a germline SUFU mutation in France.
Results
Twenty-two patients from 17 families were identified with medulloblastoma and a germline SUFU mutation (median age at diagnosis: 16.5 mo). Macrocrania was present in 20 patients, but only 5 met the diagnostic criteria for NBCCS. Despite treatment with surgery and chemotherapy, to avoid radiotherapy in all patients except one, the outcome was worse than expected for SHH medulloblastoma, due to the high incidence of local relapses (8/22 patients) and second malignancies (n = 6 in 4/22 patients). The 5-year progression-free survival and overall survival rates were 42% and 66%. Mutations were inherited in 79% of patients, and 34 additional SUFU mutation carriers were identified within 14 families. Medulloblastoma penetrance was incomplete, but higher than in Patched 1 (PTCH1) mutation carriers. Besides medulloblastoma, 19 other tumors were recorded among the 56 SUFU mutation carriers, including basal cell carcinoma (BCC) in 2 patients and meningioma in 3 patients.
Conclusion
Germline SUFU mutations strongly predispose to medulloblastoma in the first years of life, with worse prognosis than usually observed for SHH medulloblastoma. The clinical spectrum differs between SUFU and PTCH1 mutation carriers, and BCC incidence is much lower in SUFU mutation carriers. The optimal treatment of SUFU mutation–associated medulloblastoma has not been defined.
Keywords: germline SUFU mutations, infant, medulloblastoma, predisposition
Importance of the study
Germline SUFU mutations were described in patients with medulloblastoma for the first time in 2002 and are currently a matter of increasing interest. This condition may concern about 20% of all infants with SHH medulloblastoma. However, little is known about the clinical characteristics, risk of cancer, and prognosis of patients with germline SUFU mutations. Similarly, the optimal treatment for SUFU mutation–associated medulloblastoma has not been defined yet. In this manuscript, we report the largest cohort to date (22 patients with medulloblastoma and SUFU germline mutation from 17 families). Systematic molecular classification of the medulloblastoma at diagnosis should increase the discovery rate of patients with SUFU germline mutations. The findings of our report could have a major impact on the management of these patients and on genetic counseling.
Medulloblastomas are a composite group of tumors with distinct cells of origin as well as biological and clinical characteristics. Advances in molecular profiling have allowed the identification of 4 medulloblastoma subgroups: wingless (WNT), sonic hedgehog (SHH), Group 3, and Group 4.1–7 The SHH subgroup is more frequent in young children (<3 y) and in adults, and is commonly associated with the desmoplastic/nodular histological subtypes (>50% of SHH medulloblastoma).2 Genome sequencing of SHH medulloblastoma samples led to the identification of mutually exclusive somatic alterations in the Patched 1 (PTCH1),8,9 suppressor of fused (SUFU),10–13 and Smoothened (SMO)14 genes. Moreover, a large proportion of somatic SUFU mutations are associated with germline mutations (6 of the 8 concerned patients in a large series of 133 patients with medulloblastoma). Conversely, only a small proportion of somatic PTCH1 mutations (3%) are also present in the germline.15
Gorlin syndrome, also known as nevoid basal cell carcinoma syndrome (NBCCS), has been described as a cancer-predisposition syndrome for medulloblastoma associated with PTCH1 germline mutations.16,17 Affected individuals show phenotypic abnormalities and high risk of developing multiple basal cell carcinoma (BCC).18 NBCCS diagnostic criteria have been recently described by Jones et al (see Supplementary Table S1).9,18–20
Germline SUFU mutation has been identified more recently as a genetic condition that predisposes to medulloblastoma,10 particularly to desmoplastic/nodular medulloblastoma15,21 and almost exclusively in children before the age of 3 years. Thus far, a limited number of pediatric patients with medulloblastoma and a germline SUFU mutation have been described: 11 by different authors (Table 1) and 13 by our group.10,12,13,21–27 Moreover, germline SUFU mutations have also been detected in 8 adult patients with NBCCS, but without personal or family history of medulloblastoma.23,24,28 This suggests an overlapping between NBCCS and germline SUFU mutations.
Table 1.
Patients with medulloblastoma and germline SUFU mutation reported in the literature
| Author (date) | Selection Criteria | Sex, Age, mo at MB | Histological Subtype | SUFU Mutation | Inheritance | Clinical Characteristics and Other Malignancies | Family History |
|---|---|---|---|---|---|---|---|
| Taylor (2002) 1 patient |
MB | Male, 48 mo |
Desmoplastic | IVS8 + 1G>A intron 8 |
NA | Severe developmental delay, frontal bossing, prominent jaw, and hypertelorism | No NBCCS, no family history of cancer |
| Taylor (2002) and NG (2005), 2 patients |
MB | NA | Desmoplastic | c.143insA exon 1 |
NA | No physical abnormality. Meningioma in the radiation field | No family history of cancer |
| MB | NA | Desmoplastic | IVS1-1A>T exon 2 | Adopted | No physical abnormality at the clinical examination | NA | |
| Pastorino (2009) 1 patient | NBCCS | 8 mo | MBEN | c.1022 + 1G>A intron 8 |
Inherited from father | Macrocrania, frontal bossing, palmar and plantar pits. No BCC | Father: Macrocrania, bilateral pits on the soles, falx cerebri calcification |
| Slade (2011) 2 patients |
MB | 22 mo | Desmoplastic | c.846insC exon 7 | NA | No NBCCS | |
| MB | 23 mo | Desmoplastic | c.1022 + 1G>A intron 8 |
NA | No NBCCS | ||
| Smith (2014), 3 patients |
NBCCS | Male, 23 mo | Desmoplastic | c.544G>T | Inherited from mother | BCC, falx cerebri calcification, meningioma, and grade 1 astrocytoma | BCC, falx cerebri calcification. Pancreatic and prostatic cancer (47 and 76 y) |
| NBCCS | Male, 18 mo | Desmoplastic | c.550C>T | Inherited from mother | BCC, falx cerebri calcification | BCC, falx cerebri calcification. Ovarian fibroma (34 y) | |
| NBCCS | Female, 24 mo | Desmoplastic | E5-12del | Inherited from mother | BCC, falx cerebri calcification, squamous cell carcinoma cyst, meningioma, and ovarian fibroma | Falx cerebri calcification, pits, skeletal anomaly. Ovarian fibroma (10 y) | |
| Robinson (2015), 1 patient |
MB | Male, 29 mo | Desmoplastic | ||||
| Šoukalová (2016), 1 patient |
MB | 21 mo | Desmoplastic | Inherited from mother | Other brain tumors in the family |
Abbreviation: NA: not available.
The aim of the present study was to describe medulloblastoma outcome in patients with germline SUFU mutations, as well as the clinical features and tumors associated with this genetic abnormality in order to better define the risks associated with these mutations.
Patients and Methods
Patients
We reviewed the clinical files, molecular data, and family history of all patients with medulloblastoma and germline SUFU mutation (n = 22) identified in France by the 2 reference genetics laboratories (Gustave Roussy and Institut Curie). The indication for germline SUFU mutation screening varied according to the center and period. Nevertheless, it was proposed systematically to patients with a medulloblastoma diagnosed before the age of 5 years and to patients with an SHH medulloblastoma and chromosome 10q loss of heterozygosity (LOH) in the tumor. Written informed consent was obtained for the genetic analysis during a genetic counseling consultation, according to good clinical practice guidelines. Blood samples were collected from the probands and all family members who agreed to the genetic testing. The family history of cancer was explored whenever possible. Clinical examination and dermatological screening were offered to all SUFU mutation carriers. A review of the available radiological exams was performed to detect radiological patterns included in the NBCCS criteria (see Supplementary Table S1).
Tumor Analysis
The most representative formalin-fixed paraffin-embedded (FFPE) specimens from all patients with available tumor tissue samples were reviewed. Standard histological preparations—including immunostaining with anti–beta-catenin, anti–Yes-associated protein 1, anti–GRB2 [growth factor receptor bound protein 2]-associated-binding protein 1, and anti-p53 antibodies—were used to establish the medulloblastoma type according to the criteria defined by the 2007 and 2016 World Health Organization (WHO) classifications.29,30 Medulloblastomas were classified into the 4 main molecular subgroups using a 22-gene signature assay and the nanoString nCounter Technology, as previously described.31 RNA was extracted from FFPE tumor tissue samples obtained at diagnosis.
Screening for Germline SUFU Mutations
The SUFU coding exons and their exon/intron boundaries were sequenced using the BigDye terminator sequencing kit (Applied Biosystems) and an ABI Prism 3130xl automatic DNA sequencer with an ABI 3730 analyzer (Applied Biosystems). The primer sequences and PCR conditions are available on demand.
Large rearrangements of the 12 exons of the SUFU gene were analyzed by quantitative PCR using the Quantifast SYBR Green PCR kit (Qiagen) or Agilent Custom CGH + SNP microarray.
Mutation Interpretation
Mutations were confirmed by separate bidirectional sequencing using independent DNA samples from a second blood sample. Rare missense variants were classified as neutral or pathogenic in 3 steps: (i) literature data screening in the 1000-genome database; (ii) analysis of the possible splice consequences; and (iii) evaluation of the Grantham score. The interface software application Alamut version 2.6 (Interactive Biosoftware) was used for this purpose.21
Statistical Analyses
Progression-free survival (PFS), disease-free survival (DFS), and overall survival (OS) were calculated using the Kaplan–Meier method. PFS was defined as the time from the date of diagnosis until the date of disease progression or first relapse or last contact. DFS was defined as the time from the date of diagnosis until the date of disease progression, first relapse, death from any cause (including death due to treatment-related toxicity), or last contact. OS was defined as the time from the date of diagnosis until death from any cause or last contact.
Results
Medulloblastoma Characteristics and Outcome
Between 1985 and 2015, a germline SUFU mutation was detected in 22 pediatric patients with medulloblastoma (MB+), who belonged to 17 families. Thirteen of these patients have already been described.12,21 Patients and tumor characteristics are listed in Table 2. All children were younger than 3 years (first month of life in 2 patients) at the time of the medulloblastoma diagnosis (median age: 16.5 mo [range, 1–34]). All tumors (but for 3) had a desmoplastic histotype or extensive nodularity. Seven tumors could be analyzed (available samples) for expression-based subgrouping and were classified in the SHH subgroup. Immunostaining with anti-p53 antibody was negative in all tumors with available tissue samples (n = 11). TP53 mutation screening was negative in all 3 patients who had a germline analysis.
Table 2.
Medulloblastoma characteristics, treatment modalities, and outcome of patients with SUFU mutations and medulloblastoma
| Medulloblastoma | First-line Treatment | First Relapse | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex, Age at Diagnosis, mo | Histopathologic Diagnosis | Molecular Diagnosis | Tumor spread | Quality of Surgical Resection | Other Treatment(s) | Pattern (time to relapse after the diagnosis, mo) | Treatment After Relapse | Other Events | Follow- up, y | Status |
| 1 | F, 17 | DNMB | NA | Local | Complete | CC (BBSFOP*) | Local (23 mo) | HDC (misulban-Tt) + focal RT | 27.7 | Alive in CR2 | |
| 2.A | M, 1 | MBEN | NA | Local | Biopsy | CC (1 VPC) | 0.04 | Died because of PD without CR | |||
| 2.B | M, 3 | MBEN | NA | Local | Partial | No | 0.03 | Died because of postoperative complication without CR | |||
| 3 | M, 9 | MBEN | NA | Local | Complete | CC (CCG-9921**, then BBSFOP*) | Local (15 mo) | HDC (Bu-Tt) + focal RT | Second local relapse and AML | 7.8 | Died because of AML |
| 4 | M, 15 | MBEN | NA | Local | Complete | CC (HIT-SKK ’92***) | Thyroid papillary carcinoma | 10.5 | Alive in CR1 | ||
| 5 | M, 30 | Classical MB with neuronal differentiation | NA | Local | Complete | CSI (55/35 Gy) | Multiple BCCs and meningioma | 31.4 | Alive in CR1 | ||
| 6 | F, 31 | DNMB | SHH | Local | Complete | CC (CCG-9921**) | Local and metastatic (14 mo) | CC (Temiri) | 1.3 | Died because of PD | |
| 7 | M, 16 | DNMB | SHH | Local | Complete | CC (HIT-SKK ’92****) | 6.9 | Alive in CR1 | |||
| 8.A | M,30 | Classical MB | NA | Local | Complete | CC (BBSFOP*) | Local (10 mo) | HDC (Bu-Tt) + focal RT | Metastatic relapse | 14.4 | Alive in CR3 |
| 8.B | M, 18 | Classical MB | NA | Local | Partial | No | 0.01 | Died because of postoperative complication without CR | |||
| 8.C | M, 27 | MBEN | NA | Local | Complete | CC (BBSFOP*) | Local (14 mo) | HDC (Bu-Tt) + focal RT | 4.4 | Alive in CR2 | |
| 9 | M, 11 | MBEN | SHH | Metastatic | Partial | CC (3 VPC) + HDC (Mel, Mel, Bu-Tt) | 0.5 | Died as result of toxicity (VOD), in CR1 | |||
| 10 | M, 8 | DNMB | NA | Local | Partial | CC (BBSFOP*) | 8.0 | Alive in CR1 | |||
| 11 | F, 19 | DNMB | NA | Local | Complete | CC (HIT-SKK ’92***) | 0.8 | Alive in CR1 | |||
| 12.A | F, 9 | MBEN | NA | Local | Complete | 4 VPC + HDC (Bu-Tt) | Sex cord– gonadal stromal tumor and meningioma | 13.1 | Alive in CR1 | ||
| 12.B | F, 34 | DNMB | NA | Local | Complete | CC (BBSFOP*) | Local (7 mo) | HDC (Misulban-Tt) + focal RT | 16.6 | Alive in CR2 | |
| 12.C | F, 19 | DNMB | SHH | Local | Complete | CC (BBSFOP*) | Local and metastatic (9 mo) | HDC (Mel-Mel-Tt) + focal RT | Local and metastatic relapse | 2.9 | Died because of PD |
| 13 | F, 6 | DNMB | NA | Metastatic | Complete | CC (HIT-SKK ’92***) | 2.4 | Alive in CR1 | |||
| 14 | F, 18 | DNMB | SHH | Local | Complete | CC (BBSFOP*) | Local (9 mo) | HDC (Bu-Tt) + focal RT | 16.7 | Alive in CR2 | |
| 15 | M, 1 | MBEN | NA | Local | Partial | CC (VP16) | 1.4 | Died because of PD without CR | |||
| 16 | F, 23 | MBEN | SHH | Local | Complete | CC (HIT-SKK ’92***) | 3.9 | Alive in CR1 | |||
| 17 | 9 | MBEN | SHH | Local | Complete | CC (HIT-SKK ’92***) | 0.44 | Alive in CR1 | |||
Abbreviations: NA: not available, CR1: first complete response, CR2: second complete response, CR3: third complete response, PD: progressive disease; CC: conventional chemotherapy; VP16: etoposide; VPC: etoposide-carboplatin; Tt: thiotepa; Mel: melphalan; Bu: busulfan; CSI: craniospinal irradiation; AML: acute myeloid leukemia; VOD veno-occlusive disease.
*Grill et al. Lancet Oncol. 2005; **Geyer et al. J Clin Oncol Off J Am Soc Clin Oncol. 2005; ***Rutkowski et al. N Engl J Med. 2005.
Treatment varied according to the time of diagnosis and tumor extension. All patients had up-front surgery, at least to obtain one biopsy for histological confirmation of medulloblastoma, followed by chemotherapy. According to the published protocols,32–34 most patients received chemotherapy with the aim of avoiding craniospinal radiotherapy. Only one child received craniospinal radiation therapy as part of the first-line treatment. No patient received specific SHH-targeted inhibitors.
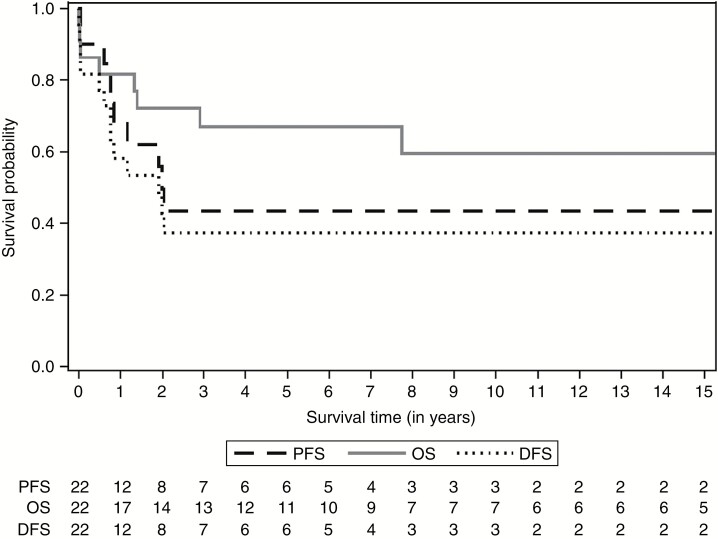
The median follow-up for all patients was 4.9 years (range, 0.01–31). Eight patients presented at least one relapse after diagnosis (median interval: 12 mo [range, 7–24]). Recurrence was local in 6 patients and combined (local and metastatic) in 2 others. A second local relapse was reported in one patient who died. Two infants (2.A and 15) presented an early progression of the disease in the first month after the beginning of the chemotherapy. The 5-year PFS was 43.7% (95% CI: 20.4–64.9%) due to disease progression on therapy and relapses (Fig. 1).
Fig. 1.
Overall survival, disease-free survival, and progression-free survival of children with medulloblastoma and germline SUFU mutation (n = 22).
Eight patients (36%) died after the diagnosis of medulloblastoma (median interval: 0.9 y [range, 0–8]) mostly due to tumor progression (5/8). Two infants died because of hemorrhagic complications just after the initial surgery, and one child due to chemotherapy-related toxicity. The 5-year DFS was 37.5% (95% CI: 17.4–57.7%) (13 events included deaths and relapses) (Fig. 1).
Overall, 14 patients were alive with a median follow-up of 9.2 years (range, 0.8–31) after the diagnosis of medulloblastoma, all in complete remission, including 5 patients in remission after a relapse and treatment including high-dose chemotherapy (HDC) and focal radiation. The 5-year OS was 67.0% (95% CI: 42.9–82.8%) (Fig. 1).
Associated Clinical and Radiological Signs
Five patients (23%) met the NBCCS criteria at the time of the analysis (Table 3). A significant macrocrania (>97th percentile) was observed in all children with available head circumference measurement at the time of diagnosis (n = 20). Moreover, frontal bossing and/or hypertelorism was described in 3 patients. Five patients from all patients with available brain CT scan (n = 16) presented falx cerebri calcification. Multiple melanocytic nevi were reported in 6 of 22 patients. Facial and dental anomalies were described in 4 patients: perinasal skin tag (n = 1), high arched palate (n = 2), and dental agenesis (n = 2 who received HDC, which also could cause dental agenesis). Odontogenic cysts and skeletal anomalies were not observed. Three children had a cognitive deficit worse than expected in patients treated for medulloblastoma in the first years of age.
Table 3.
Evaluation of the NBCCS criteria in patients with a medulloblastoma and germline SUFU mutation
| Sex, Age at MB, mo | Age at Last Follow-up, y | Falx Cerebri Calcification (age at CT scan) | Jaw Cysts or Dental Anomaly | BCC, Pits or Multiple Nevi | Macrocrania | Hypertelorism ± Frontal Bossing | Bifid Rib or Other Feature |
NBCCS
(age at diagnosis) |
|
|---|---|---|---|---|---|---|---|---|---|
| 1 | F, 17 | 28 | NA | No | Nevi (50) | Yes | No | NA, syndactyly of 2/3 toes | No |
| 2.A | M, 1 | 0 | No (1 mo) | NA | No | Yes | No | NA | No |
| 2.B | M, 3 | 0 | NA | NA | No | Yes | No | NA | No |
| 3 | M, 9 | 9 | No (1 y) | No | Nevi (20) | Yes | No | No | No |
| 4 | M, 15 | 11 | NA | High arched palate, dental agenesis | Palmar/plantar pits and nevi | Yes | No | No | Yes (8 y) |
| 5 | M, 30 | 34 | Yes (26 y) | No | BCC and nevi | Yes | No | No | Yes (17.5 y) |
| 6 | F, 31 | 4 | No (4 y) | High arched palate | No | Yes | Yes | No | No |
| 7 | M, 16 | 8 | No (2 y) | No | No | Yes | No | No | No |
| 8.A | M, 30 | 17 | No (4 y) | NA | No | Yes | No | NA | No |
| 8.B | M, 18 | 2 | NA | NA | No | Yes | No | NA | No |
| 8.C | M, 27 | 7 | No (7.5 y) | NA | No | Yes | No | NA | No |
| 9 | M, 11 | 1 | NA | No | No | Yes | No | NA | No |
| 10 | M, 8 | 9 | No (1 y) | NA | No | Yes | Yes | No, perinasal skin tag | No |
| 11 | F, 19 | 2 | Yes (2 y) | No | No | Yes | Yes | No | Yes (2 y) |
| 12.A | F, 9 | 17 | Yes (17 y) | No | No | Yes | No | No | Yes (17 y) |
| 12.B | F, 34 | 19 | NA | NA | Nevi | Yes | No | NA | No |
| 12.C | F, 19 | 4 | Yes, (3 y) | No | No | No | No | No | No |
| 13 | F, 6 | 3 | No (1 y) | No | No | Yes | No | No | No |
| 14 | F, 18 | 18 | Yes (16 y) | Dental agenesis | Nevi | Yes | No | No | Yes (16 y) |
| 15 | M, 1 | 2 | No | NA | No | Yes | No | No | No |
| 16 | F, 23 | 6 | No | No | No | No | No | No | No |
| 17 | M, 9 | 1 | No (1 y) | NA | No | Yes | No | No | No |
Germline SUFU Mutation Screening
All mutations but one were truncating mutations (list in Table 4).
Table 4.
Pathogenic germline SUFU mutations
| No of family | Type of Mutation | International Denomination | Inherited | MB+ | MB− | Mutation Previously Described |
|---|---|---|---|---|---|---|
| 1* | Splice | c.182 + 3A>T | Inherited from father | 1 | 2 | (21) |
| 2* | FS | c.71del p.Pro24Argfs*72 |
Inherited from mother | 2 | 5 | (12) |
| 3* | Splice | c.1297-1G>C | Inherited from father | 1 | 2 | (21) |
| 4 | FS | c.71del p.Pro24Argfs*72 |
Inherited from father | 1 | 1 | |
| 5* | FS | c.294_295dup p.Tyr99Serfs*23 |
NA | 1 | (21) | |
| 6* | Splice | c.318-10del | Inherited from father | 1 | 2 | (21) |
| 7* | Large duplication | Exon 3 duplication c.318-?_454+?dup |
De novo | 1 | 0 | (21,26) |
| 8 | FS | c.567_571delinsT p.Gln189Hisfs*5 |
Inherited from father | 3 | 2 | |
| 9* | FS | c.1149_1150dup p.Cys384Serfs*3 |
NA | 1 | (21) | |
| 10* | MS | c.422T>G p.Met141Arg |
Inherited from father | 1 | 1 | (21) |
| 11 | Large deletion | Exon 9–12 deletion c.1023-?_1455+?del |
NA | 1 | ||
| 12* | FS | c.71dup p.Ala25Glyfs*23 | Inherited from their mothers** | 3 | 18 | (12) |
| 13 | FS | c.1096_1117delinsGAA p.Leu366Glufs*14 |
Inherited from father | 1 | 1 | |
| 14* | NS | c.1123C>T p.Gln375* |
De novo | 1 | 0 | (21) |
| 15 | Splice | c.1022 + 1G>A | NA | 1 | 0 | (13,23) |
| 16 | Splice | c.1022 + 1G>A | NA | 1 | 0 | (13,23) |
| 17 | Large deletion | Exon 3–12 deletion c.318-?_1455+?del |
NA | 1 | 0 | (24) |
Abbreviations: S: splice, FS: frameshift, MS: missense, NS: nonsense, NA: not available.
*Families and SUFU mutations already described by our group. **The 3 children with medulloblastoma are not siblings, but relatives.
Family History
Overall, SUFU mutation inheritance could be tested in 14 of 19 nuclear families (parents and at least one affected child). In 3 patients, it was a de novo mutation, whereas it was inherited in 11 of 14 families (79%), from the father in 7 patients and from the mother in 4.
The familial history of cancer was obtained for all patients. In 3 families (inherited mutation), 2 (2.A and 2.B) and 3 (8.A, 8.B, and 8.C) siblings and 3 cousins (12.A, 12.B, and 12.C) had a medulloblastoma and were all described in this cohort. In 4 other families, 6 siblings, 1 uncle, and 1 nephew of a mutation-transmitting parent died inexplicably in the first years of life, often in a context of vomiting. We cannot exclude that a brain tumor might have been the cause of these early deaths.
SUFU Mutation Carriers and Other Tumors
Overall, 56 SUFU mutation carriers were identified: 22 MB+ and 34 relatives without medulloblastoma (MB−) (Table 4). Among the 14 MB+ who survived more than 2 years (median age at last follow-up: 10.2 y [range, 3.9–33.9]), 4 (27%) developed additional tumors (Table 5). Two patients had a single tumor (acute myeloid leukemia with chromosome 5 abnormality, and a thyroid papillary carcinoma without previous radiotherapy, respectively). One patient had multiple BCC and a meningioma (both in the radiation field), and another patient had a sex cord–gonadal stromal tumor and a meningioma (without previous radiotherapy). At the time of diagnosis of the second malignancy, the median age was 12.1 years (range, 7.7–33.9), and the median time from the medulloblastoma diagnosis was 11.3 years (range, 6.5–31.4).
Table 5.
Tumors observed in SUFU mutation carriers
| Other Tumors in the 22 MB+ Patients | n Patients (pts) | Age at Diagnosis, y |
|---|---|---|
| Acute myeloid leukemia | 1 | 7.8 |
| Thyroid papillary carcinoma | 1 | 7.7 |
| Sex cord–gonadal stromal tumor | 1 | 13.9 |
| Meningioma | 2 (2 pts) | 10.3 and 33.9 |
| Multiple BCC | 1 | 17.5 |
| Other tumors in 7/34 relatives harboring SUFU germline mutations from 3 families | ||
| BCC | 1 | 71 |
| Meningioma | 1 | 48 |
| Carcinoma: | ||
| Breast cancer | 2 (2 pts) | 37 and 71 |
| Bladder cancer | 1 | 73 |
| Sarcomas1 | 4 (2 pts) | 46, 47, 53, and 71 |
| Neurofibroma | 2 (1 pt) | 46 and 47 |
| Multiple colon polyps | 1 | NA |
| Liver adenoma | 1 | NA |
1 No germline TP53 mutation.
The median age of the 34 MB− relatives was 51.3 years. None of them had a previous diagnosis of NBCCS. Overall, 7 of them reported 13 benign or malignant tumors, including one BCC (age at diagnosis: 71 y) (Table 5). The median age of these 7 relatives at the time of diagnosis of the first malignant tumor was 49.5 years.
Discussion
To date, this is the largest reported series of germline SUFU mutation carriers. Our main aim was to describe more precisely the clinical characteristics, the risk of cancer, and the outcome of medulloblastoma associated with a germline SUFU mutation.
SUFU and PTCH1 mutations are the main genetic abnormalities associated with predisposition to SHH medulloblastoma. In PTCH1 mutation carriers, the incidence of medulloblastoma is estimated to be lower than 2%.18,24,35,36 In SUFU mutation carriers, the risk of medulloblastoma is difficult to evaluate due to the small number of families described to date and the difficulty to correct for the ascertainment bias because most families are recruited through a proband with medulloblastoma. In the present study, 14 children with germline SUFU mutation and medulloblastoma belonged to 9 families in which the SUFU mutation was shown to be inherited and that included 34 other mutation carriers without medulloblastoma (MB−) (Table 4). These results confirm our previous findings in a smaller cohort.21 They also suggest that in the case of germline SUFU mutation, medulloblastoma penetrance is incomplete, with a risk of medulloblastoma probably lower than 30%, but still much higher than for PTCH1 mutation carriers.12 Although familial aggregation of medulloblastoma is rare,37,38 in this study we found 7 families with medulloblastoma in several siblings or unexplained deaths in the first years of life. Therefore, we hypothesize that most cases of familial medulloblastoma could be explained by germline SUFU mutations, as already suggested.25,28
As previously described, all medulloblastomas were diagnosed in children younger than 3 years of age and the histological subtype was mainly desmoplastic or with extensive nodularity.10,12,13,21–26 As expected, an involvement of the SHH-driven pathway was shown by immunostaining and with the nanoString nCounter Technology, when it could be explored. We recommend SUFU mutation screening in all children presenting with SHH medulloblastoma before 5 years of age, particularly in the case of chromosome 10q LOH, desmoplastic histotype, or extensive nodularity. If a SUFU somatic alteration is detected, germline mutation analysis should be proposed.
In this study, the 5-year DFS and OS rates were 37.5% and 67.0%, respectively. In order to take into account in this retrospective cohort accrued over 30 years the improvement of supportive care over time leading to reduction of the risk of death due to treatment-related toxicity, we calculated 5-year PFS. The 5-year PFS was 43.7% (95% CI: 20.4–64.9%). This contrasts with the usual good prognosis of SHH medulloblastoma with most current treatment protocols (5-year PFS and OS rates higher than 70% and 80%, respectively).26,34 This was mostly due to the high rate of local relapse. In addition, 4 patients were diagnosed with 6 second malignancies. The spectrum (meningioma, BCC, thyroid carcinoma) was similar to that reported by Tsui et al in a cohort of 376 patients with unselected medulloblastoma/primitive neuroectodermal tumors. In this cohort, the cumulative incidence of second malignancies, 20 years after the first diagnosis, was 12%, which is much lower than the incidence observed in our cohort.39 The best therapeutic strategies for infants with medulloblastoma are currently under discussion, particularly the question of whether patients with a germline SUFU mutation require a specific therapeutic approach. Radiotherapy is probably efficient because only one local relapse in the field of focal radiation occurred among the 8 patients who underwent radiotherapy. However, its use in this genetic context is still debated because of the risk of second malignancies. Therapy with a targeted inhibitor of the SHH pathway, which is clearly involved in the malignant transformation, could offer an alternative therapeutic option to the classic chemoradiotherapy approach for SHH medulloblastoma. Vismodegib (GDC-0449) and sonidegib (LDE-225) inhibit SHH signaling by binding to SMO. They are approved for use in BCC40–43 and have demonstrated good efficacy as monotherapy in a subset of patients with SHH medulloblastoma (prolonged stabilization in 41% of them).27 However, mutations in SHH pathway genes downstream of SMO (SUFU, GLI2, or MYCN) make these tumors intrinsically resistant to SMO targeting drugs.11,15,27,43–45 Indeed, preclinical data confirmed the resistance to targeted SMO inhibition in cells harboring a SUFU mutation.15 Moreover, the only patient with a germline SUFU mutation–associated medulloblastoma and treated with an SHH pathway inhibitor did not experience any response.27 In addition, these agents may induce early growth plate fusion, restricting their use to skeletally mature patients. This highlights the need of a prospective evaluation of the best treatment for these patients with poor prognosis.
Besides patients with medulloblastoma, only a few other SUFU germline mutation carriers have been described so far (Table 1), and their phenotype is still largely unknown. As the clinical phenotypes of SUFU and PTCH1 mutation carriers overlap, SUFU has been described as one of the genes involved in NBCCS. However, clinical manifestations and tumor incidence seem to differ between SUFU and PTCH1 mutation carriers. One of the confounding factors is probably the age at observation, because the established diagnostic criteria for NBCCS are informative when evaluated in adults,18,46 whereas they are often not all present in children until the teenage years.46,47 In our series, 5 patients met the diagnostic criteria for NBCCS (medulloblastoma, macrocrania, and one additional feature). Macrocrania was more often observed in our patients than in patients with NBCCS; however, it could have been associated just with the brain tumor, because macrocrania is described in more than 50% of infants with brain tumors. Three patients had a severe cognitive impairment. A germline SUFU mutation was reported in a patient with severe developmental delay.10 However, given the impact of medulloblastoma treatment on neurocognitive development, it is difficult to clearly associate this feature with the genetic background. Thus, for macrocrania and cognitive deficit, the respective role of the tumor and the predisposition syndrome may be difficult to assess in infants. As previously described in patients with SUFU mutations, no odontogenic cyst and very few pits were observed in our cohort.24 In view of the low incidence of BCC (only 2/56; 3.5%) in our series, the risk of BCC is probably much lower in SUFU mutation carriers than in NBCCS associated with germline PTCH1 mutations. This incidence is also lower than the BCC rate described in patients with a germline SUFU mutation in the context of NBCCS: 7 of 18 patients (39%), among whom 3 with a previous medulloblastoma and 4 without.10,13,22–25 This confirms that the clinical manifestations in germline SUFU mutation carriers vary depending on the recruitment process. Patients recruited based on the diagnosis of medulloblastoma mostly do not show physical abnormalities and very few have a history of BCC.10,13,22,25 Conversely, germline SUFU mutation carriers identified in NBCCS cohorts mostly present the specific NBCCS features, including BCC, except for the jaw cysts.23,24 As patients in our series are still very young, NBCCS features may still appear later, although none of the relatives with germline SUFU mutations had NBCCS or developed a BCC before the age of 30.
We also observed several other cancers in SUFU mutation carriers, suggesting that the cancer risk associated with SUFU mutation might not concern only medulloblastoma during the first years of life. In addition to meningioma (3/56 carriers), already known to be associated with SUFU mutations,48,49 a large tumor spectrum was observed in our series: carcinomas (breast, bladder, and thyroid papillary carcinomas), sarcomas, acute myeloid leukemia, and low-grade tumors (sex cord–gonadal stromal tumor, liver adenoma, and colonic polyps). Few associations of carcinoma or fibroma with germline SUFU mutation have been described so far.24 Because of the large oncological spectrum and the incomplete penetrance of medulloblastoma, genetic counseling, including the question of prenatal diagnosis, is challenging in families with SUFU mutations. Young children with a germline SUFU mutation should be offered a structured follow-up, including regular brain MRI exams during the first years of life.50
Conclusion
The risk of medulloblastoma associated with germline SUFU mutations is still difficult to evaluate but is probably higher than for PTCH1 mutations. The prognosis for these patients is worse than usually observed in SHH medulloblastoma due to the high risk of local relapses. Future international studies exploring the oncogenic pathways involved in each patient could help to improve the early diagnosis of SUFU predisposition syndrome and allow the definition of specific therapeutic guidelines for this group of patients. So far, for early detection of medulloblastoma, frequent MRIs in infants with a pathogenic germline mutation in SUFU are recommended (brain MRI every 4 mo until age 3 and then every 6 mo until age 5). If medulloblastoma occurs, the best therapeutic strategies are currently under discussion for patients with a germline SUFU mutation. Given the worse prognosis, we should consider these as patients with high-risk medulloblastoma. So, chemotherapies should probably be more intensive than those currently used. Taking into account the high risk of local relapse, the good local control after radiation treatment, and the low incidence of BCC in all mutation carriers in this series, radiotherapy should be discussed according to the age of the patient. International databases are needed to describe more comprehensively the spectrum of this predisposition syndrome that has some overlap with NBCCS.
Funding
None declared.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Northcott PA, Korshunov A, Witt Het al. . Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kool M, Korshunov A, Remke Met al. . Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho YJ, Tsherniak A, Tamayo Pet al. . Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor MD, Northcott PA, Korshunov Aet al. . Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson G, Parker M, Kranenburg TAet al. . Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488(7409):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones DT, Jäger N, Kool Met al. . Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramaswamy V, Remke M, Bouffet Eet al. . Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zurawel RH, Allen C, Chiappa Set al. . Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer. 2000;27(1):44–51. [DOI] [PubMed] [Google Scholar]
- 9. Evans G, Burnell L, Campbell R, Gattamaneni HR, Birch J. Congenital anomalies and genetic syndromes in 173 cases of medulloblastoma. Med Pediatr Oncol. 1993;21(6):433–434. [DOI] [PubMed] [Google Scholar]
- 10. Taylor MD, Liu L, Raffel Cet al. . Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31(3):306–310. [DOI] [PubMed] [Google Scholar]
- 11. Lee Y, Kawagoe R, Sasai Ket al. . Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26(44):6442–6447. [DOI] [PubMed] [Google Scholar]
- 12. Brugières L, Pierron G, Chompret Aet al. . Incomplete penetrance of the predisposition to medulloblastoma associated with germ-line SUFU mutations. J Med Genet. 2010;47(2):142–144. [DOI] [PubMed] [Google Scholar]
- 13. Slade I, Murray A, Hanks Set al. . Heterogeneity of familial medulloblastoma and contribution of germline PTCH1 and SUFU mutations to sporadic medulloblastoma. Fam Cancer. 2011;10(2):337–342. [DOI] [PubMed] [Google Scholar]
- 14. Reifenberger J, Wolter M, Weber RGet al. . Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58(9):1798–1803. [PubMed] [Google Scholar]
- 15. Kool M, Jones DT, Jäger Net al. ; ICGC PedBrain Tumor Project . Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hahn H, Wicking C, Zaphiropoulous PGet al. . Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. [DOI] [PubMed] [Google Scholar]
- 17. Johnson RL, Rothman AL, Xie Jet al. . Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272(5268):1668–1671. [DOI] [PubMed] [Google Scholar]
- 18. Kimonis VE, Goldstein AM, Pastakia Bet al. . Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69(3):299–308. [PubMed] [Google Scholar]
- 19. Bree AF, Shah MR; BCNS Colloquium Group . Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS). Am J Med Genet A. 2011;155A(9):2091–2097. [DOI] [PubMed] [Google Scholar]
- 20. Jones EA, Sajid MI, Shenton A, Evans DG. Basal cell carcinomas in gorlin syndrome: a review of 202 patients. J Skin Cancer. 2011;2011:217378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brugières L, Remenieras A, Pierron Get al. . High frequency of germline SUFU mutations in children with desmoplastic/nodular medulloblastoma younger than 3 years of age. J Clin Oncol. 2012;30(17):2087–2093. [DOI] [PubMed] [Google Scholar]
- 22. Ng D, Stavrou T, Liu Let al. . Retrospective family study of childhood medulloblastoma. Am J Med Genet A. 2005;134(4):399–403. [DOI] [PubMed] [Google Scholar]
- 23. Pastorino L, Ghiorzo P, Nasti Set al. . Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A. 2009;149A(7):1539–1543. [DOI] [PubMed] [Google Scholar]
- 24. Smith MJ, Beetz C, Williams SGet al. . Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32(36):4155–4161. [DOI] [PubMed] [Google Scholar]
- 25. Šoukalová J, Vejmělková K, Cermanová Tet al. . Identification of a family with SUFU germline deletion based on a case of desmoplastic medulloblastoma in an infant. Klin Onkol. 2016;29(Suppl 1):S83–S88. [DOI] [PubMed] [Google Scholar]
- 26. Siegfried A, Bertozzi AI, Bourdeaut Fet al. . Clinical, pathological, and molecular data on desmoplastic/nodular medulloblastoma: case studies and a review of the literature. Clin Neuropathol. 2016;35(3):106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robinson GW, Orr BA, Wu Get al. . Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from phase II Pediatric Brain Tumor Consortium studies PBTC-025B and PBTC-032. J Clin Oncol. 2015;33(24):2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mann K, Magee J, Guillaud-Bataille Met al. . Multiple skin hamartomata: a possible novel clinical presentation of SUFU neoplasia syndrome. Fam Cancer. 2015;14(1):151–155. [DOI] [PubMed] [Google Scholar]
- 29. Louis DN, Ohgaki H, Wiestler ODet al. . The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Louis DN, Perry A, Reifenberger Get al. . The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 31. Northcott PA, Shih DJ, Remke Met al. . Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geyer JR, Sposto R, Jennings Met al. ; Children’s Cancer Group . Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 33. Grill J, Sainte-Rose C, Jouvet Aet al. ; French Society of Paediatric Oncology . Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–580. [DOI] [PubMed] [Google Scholar]
- 34. Rutkowski S, Bode U, Deinlein Fet al. . Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. [DOI] [PubMed] [Google Scholar]
- 35. Evans DG, Farndon PA, Burnell LD, Gattamaneni HR, Birch JM. The incidence of Gorlin syndrome in 173 consecutive cases of medulloblastoma. Br J Cancer. 1991;64(5):959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amlashi SF, Riffaud L, Brassier G, Morandi X. Nevoid basal cell carcinoma syndrome: relation with desmoplastic medulloblastoma in infancy. A population-based study and review of the literature. Cancer. 2003;98(3):618–624. [DOI] [PubMed] [Google Scholar]
- 37. Dearlove JV, Fisher PG, Buffler PA. Family history of cancer among children with brain tumors: a critical review. J Pediatr Hematol Oncol. 2008;30(1):8–14. [DOI] [PubMed] [Google Scholar]
- 38. Searles Nielsen S, Mueller BA, Preston-Martin Set al. . Family cancer history and risk of brain tumors in children: results of the SEARCH international brain tumor study. Cancer Causes Control. 2008;19(6):641–648. [DOI] [PubMed] [Google Scholar]
- 39. Tsui K, Gajjar A, Li Cet al. . Subsequent neoplasms in survivors of childhood central nervous system tumors: risk after modern multimodal therapy. Neuro Oncol. 2015;17(3):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rudin CM, Hann CL, Laterra Jet al. . Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rudin CM. Vismodegib. Clin Cancer Res. 2012;18(12):3218–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sekulic A, Von Hoff D. Hedgehog pathway inhibition. Cell. 2016;164(5):831. [DOI] [PubMed] [Google Scholar]
- 43. Gajjar A, Stewart CF, Ellison DWet al. . Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin Cancer Res. 2013;19(22):6305–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kieran MW. Targeted treatment for sonic hedgehog-dependent medulloblastoma. Neuro Oncol. 2014;16(8):1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo H-W. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers. 2016;8(2). doi:10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kimonis VE, Singh KE, Zhong R, Pastakia B, Digiovanna JJ, Bale SJ. Clinical and radiological features in young individuals with nevoid basal cell carcinoma syndrome. Genet Med. 2013;15(1):79–83. [DOI] [PubMed] [Google Scholar]
- 47. Lo Muzio L, Nocini P, Bucci P, Pannone G, Consolo U, Procaccini M. Early diagnosis of nevoid basal cell carcinoma syndrome. J Am Dent Assoc. 1999;130(5):669–674. [DOI] [PubMed] [Google Scholar]
- 48. Kijima C, Miyashita T, Suzuki M, Oka H, Fujii K. Two cases of nevoid basal cell carcinoma syndrome associated with meningioma caused by a PTCH1 or SUFU germline mutation. Fam Cancer. 2012;11(4):565–570. [DOI] [PubMed] [Google Scholar]
- 49. Smith MJ. Germline and somatic mutations in meningiomas. Cancer Genet. 2015;208(4):107–114. [DOI] [PubMed] [Google Scholar]
- 50. Foulkes WD, Kamihara J, Evans DGRet al. . Cancer surveillance in Gorlin syndrome and rhabdoid tumor predisposition syndrome. Clin Cancer Res. 2017;23(12):e62–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.