Abstract
Background
Depending on the level, differentiation state, and tumor stage, reactive nitrogen and oxygen species inhibit or increase cancer growth and tumor initiating cell maintenance. The rate-limiting enzyme in a pathway that can regulate reactive species production but has not been thoroughly investigated in glioblastoma (GBM; grade IV astrocytoma) is guanosine triphosphate (GTP) cyclohydrolase 1 (GCH1). We sought to define the role of GCH1 in the regulation of GBM growth and brain tumor initiating cell (BTIC) maintenance.
Methods
We examined GCH1 mRNA and protein expression in patient-derived xenografts, clinical samples, and glioma gene expression datasets. GCH1 levels were modulated using lentiviral expression systems, and effects on cell growth, self-renewal, reactive species production, and survival in orthotopic patient-derived xenograft models were determined.
Results
GCH1 was expressed in GBMs with elevated but not exclusive RNA and protein levels in BTICs in comparison to non-BTICs. Overexpression of GCH1 in GBM cells increased cell growth in vitro and decreased survival in an intracranial GBM mouse model. In converse experiments, GCH1 knockdown with short hairpin RNA led to GBM cell growth inhibition and reduced self-renewal in association with decreased CD44 expression. GCH1 was critical for controlling reactive species balance, including suppressing reactive oxygen species production, which mediated GCH1 cell growth effects. In silico analyses demonstrated that higher GCH1 levels in glioma patients correlate with higher glioma grade, recurrence, and worse survival.
Conclusions
GCH1 expression in established GBMs is pro-tumorigenic, causing increased growth due, in part, to promotion of BTIC maintenance and suppression of reactive oxygen species.
Keywords: GCH1, glioblastoma, glioma, reactive oxygen species, tumor initiating cell
Importance of the study.
Our study demonstrated for the first time the significant roles of GCH1 in glioma and in the tumor initiating cell fraction. We expand on prior literature indicating inhibition or promotion of tumor growth and BTIC maintenance depending on nitric oxide and reactive oxygen species levels: GCH1 is described as an important regulator of reactive species in glioma, protecting cancer cells from oxidative damage and promoting GBM growth self-renewal. The correlation of GCH1 levels with patient outcomes and the ability of GCH1 targeting to inhibit tumor growth suggest that inhibition of GCH1 and/or its downstream pathways might be beneficial as adjuvant therapy in combination with standards of care to improve glioma patient outcome.
Glioblastoma (GBM; grade IV astrocytoma) is the most common malignant primary brain tumor in adults and rapidly lethal. Median GBM patient survival after diagnosis is only 14 months with standard of care.1 Contributing to our inability to cure this devastating disease is the highly heterogeneous landscape of GBM. Differences in genotype, methylation, microenvironment, and cellular differentiation state all contribute to variances in therapeutic response and patient outcome.2–4 The lack of targeted treatment strategies for effective eradication of different tumor and cell subgroups is thought to contribute to treatment failures. Particularly, brain tumor initiating cells (BTICs) are a subpopulation of GBM cells that possess the vital ability to propagate tumors in immunocompromised mice, even when orthotopically injected at relatively low cell numbers.4–6 Previous studies showed that BTICs share certain similarities with neural stem cells, including the expression of stem cell markers, such as cluster of differentiation (CD)133 and CD44, and the capabilities for self-renewal and multiple lineage differentiation.4–6 Importantly, BTICs are resistant to conventional therapies, making them appealing targets for novel treatment strategies.3,7–9
GBM growth and BTIC maintenance are known to be regulated by free radicals with reactive oxygen or nitrogen, such as superoxide and nitric oxide (NO), respectively. Reactive oxygen species (ROS) and NO can mediate either pro- or anti-tumorigenic effects depending on concentration, duration, and cell state.10–12 For example, ROS have been suggested to both increase13 and decrease14,15 BTIC maintenance and tumorigenic potential, while NO produced by nitric oxide synthase 2 (NOS2) in BTICs and NOS3 in the tumor endothelium were both shown to have pro-tumorigenic and BTIC-maintaining effects.7,16 Increasing ROS often sensitizes BTICs to radio- and chemotherapy,14,15 but the ratio of the types of ROS present is likely critical for mediating therapeutic response.17 NO induced by irradiation promotes BTIC maintenance and therapeutic resistance,18 but earlier studies sought to sensitize GBM cells to irradiation through elevation of NO to nonphysiologic levels with NO donors.19 Thus, although the majority of studies suggest an anti-tumorigenic effect of elevated ROS and a pro-tumorigenic effect for endogenous NO, context-dependent differences continue to increase our understanding of the contribution of ROS and NO to GBM and BTICs.
Guanosine triphosphate cyclohydrolase I (GCH1) is known to contribute to the production of reactive species, but its role in cancer is not well characterized. GCH1 is the first, rate-limiting enzyme in a biosynthetic pathway producing tetrahydrobiopterin (BH4).20 BH4 is a required cofactor for NOS to generate NO, and, when GCH1 activity is decreased, NOS can become uncoupled, leading to superoxide production. Targeting this pathway has been suggested for cancer pain management,21 but very few studies have addressed the expression, activity, or function of the GCH1 pathway in cancer. One report demonstrated GCH1 expression in colon and skin cancer,22 and rat C6 glioma cells expressed GCH1 with cytokine stimulation.23 However, the levels or role of GCH1 in human gliomas or tumor initiating cells from any cancer have not been previously determined to our knowledge.
In this study, we sought to determine the expression and role of GCH1 in GBM and the BTIC fraction. We found that GCH1 was elevated in GBM patient sections in comparison to normal brains and that GCH1 was increased in but not limited to BTICs in comparison to non-BTICs isolated from the same xenograft. Our studies demonstrate that GCH1 promotes GBM growth in vivo and correlates with increasing glioma grade and poor glioma patient prognosis. Our report defines an important and novel role for GCH1 as a pro-tumorigenic factor in GBM, including through the promotion of BTIC maintenance.
Materials and Methods
Cells and GBM Patient-Derived Xenografts
GBM patient-derived xenografts (PDX) were acquired from the Brain Tumor Core Facility (University of Alabama at Birmingham [UAB]) and as a kind gift from Dr. Darrel Bigner at Duke University. Xenografts, BTICs, and non-BTICs were propagated as previously described.7,24,25 Immortalized human astrocytes were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum, epidermal growth factor (EGF), and N2 NeuroPlex. Immortalized neural progenitors ReNcell VM were purchased from Lonza and cultured in the BTIC conditions above.
Extraction of mRNA, Generation of cDNA, and Quantitative PCR
Total mRNA from cells was harvested using the Illustra RNAspin mini kit (GE Healthcare) and synthesized into cDNA using the iScript cDNA Synthesis kit (BioRad). Quantitative PCR was performed on the generated cDNA with the SsoAdvanced Universal SYBR Green Supermix (BioRad). The relative expression of GCH1 was measured using a primer pair that recognizes GCH1 cDNA (GGACTTGCTTGTTAGGAAGATAACC and ATGATGAGATGGTGATTGTGAAGGA). Expressions of other genes were measured using predesigned PrimePCR PCR primers (BioRad). The data were analyzed and normalized against actin beta expression to determine relative expression of target genes.
Western Blotting and Antibodies
Cells were collected and lysed in M-PER (Mammalian Protein Extraction Reagent, Thermo Fisher Scientific). Protein concentration was determined with a bicinchoninic acid assay (Thermo Fisher Scientific). Protein lysate was denatured with Laemmli sample buffer (BioRad) 94°C, electrophoresed on Mini-Protean Precast Gels (BioRad), and then transferred to polyvinylidene difluoride membranes (BioRad). Primary antibodies for western blot were mouse anti–human-GCH1 (Clone: 4A12, Abnova) and mouse anti–α-tubulin (Sigma).
Gene Expression and Knockdown
Viral particles were produced by transient cotransfection of psPAX2, pCMV-VSVG, and a lentiviral vector into CSC293T cells as previously described.26 More information on plasmids is available in the Supplementary materials.
Measurement of Cell Growth
Cell numbers were determined indirectly using CellTiter-Glo 2.0 Assay (Promega) according to the manufacturer’s instructions.
Neurosphere Formation Assay
The serially diluted cell suspensions were plated in 96-well plates and grown for 14 days to allow neurosphere formation. The percentage of wells that contain neurospheres was determined by counting under a light microscope. The Extreme Limiting Dilution Analysis tool27 was used to analyze and visualize the data.
Nitrite Measurement
Nitrite analysis of the cellular supernatant was measured by high performance liquid chromatography (ENO20, Eicom), and all values were normalized to total protein as previously described.28,29
H2O2 Measurement
H2O2 levels were measured using the ROS-Glo H2O2 kit (Promega) following the manufacturer’s manual, and all values were adjusted to cell titer measured using CellTiter-Glo 2.0 Assay (Promega).
Intracranial Tumor Model
Cells were counted and equal numbers of viable cells suspended in phosphate buffered saline (PBS). The cells were then intracranially injected into athymic nude mice. Mice were monitored for the development of neurologic signs daily. Mice were euthanized when they developed neurologic signs or at experimental endpoints indicated in figure legends. Data were analyzed using GraphPad Prism 6 software, employing the log-rank (Mantel–Cox) test to compare survival curves (P-values determine whether the survival distributions are statistically significantly different). Animal studies were approved by the Institutional Animal Care and Use Committee of UAB.
Histology
Collected tissues were fixed with 10% neutral buffered formalin (Fisher), dehydrated with 30% sucrose in PBS solution, and preserved in 70% denatured ethanol. Paraffin blocks and staining were completed by the UAB Neuroscience Molecular Detection Core. Immunohistochemical staining was performed using the same antibodies indicated above for Western blotting.
In Silico Analysis
Publicly available clinical datasets were retrieved from The Cancer Genome Atlas (TCGA) and GlioVis.30 Correlations between GCH1 expression in patients and different clinical parameters, including but not limited to tumor grades and subtypes, were established.
Statistical Analysis
Data were processed in spreadsheets using Excel (Microsoft) and analyzed using GraphPad Prism 6 software. Data shown are representative of, or mean ± SD of, at least 3 independent experiments. P-values were calculated as described in the figure legends with either Student’s t-test or log-rank analysis (see above).
Results
GCH1 Is Expressed in GBM and Elevated in but Not Exclusive to Brain Tumor Initiating Cells
To determine genes involved in reactive species signaling that had not been previously characterized in GBM and were differentially expressed in the BTIC fraction, we performed quantitative real-time polymerase chain reaction (qRT-PCR) with an array of 80 genes implicated in control of reactive nitrogen and oxygen species levels or signaling. Differences in mRNA expression between xenograft-derived BTICs and non-BTICs determined GCH1 to be one of the top 5 genes with elevated expression in BTICs (Supplementary Fig. S1A, B). Additional qRT-PCR assays for GCH1 determined that GCH1 was expressed in GBM cells isolated from PDX encompassing all GBM molecular subtypes and that expression was usually higher than that found in immortalized but nontumorigenic neural progenitors (Fig. 1A). Furthermore, GCH1 was significantly elevated in BTICs in comparison to non-BTICs in the majority of GBM xenografts tested (Fig. 1B, Supplementary Fig. S1C). The presence of the BTIC-maintaining growth factors EGF and fibroblast growth factor (FGF) did cause a statistically significant increase in the expression of GCH1 in comparison to the absence of EGF and FGF (Supplementary Fig. S1D), but the fold change was not equivalent to that observed in the same cells in BTICs in comparison to non-BTICs (<1.4 vs >5; Fig. 1B, Supplementary Fig. S1C). These data suggest that media conditions can alter GCH1 levels but do not fully account for the differences in GCH1 expression observed with changes in differentiation state. In contrast to the results with GCH1, we found no significant differences in mRNA expression of 6-pyruvoyltetrahydropterin synthase (PTS) and sepiapterin reductase (SPR), other enzymes in the GCH/BH4 pathway (Supplementary Fig. S1E). Western blotting for total GCH1 confirmed GCH1 expression in both BTICs and non-BTICs with increased GCH1 protein expression in the BTIC fraction across multiple GBM xenolines (Fig. 1C). GCH1 protein levels decreased in BTICs upon exposure to the differentiating agent fetal bovine serum (Fig. 1D), further validating a correlation between GCH1 and the GBM differentiation state. Immunostaining of tumor sections confirmed in vivo expression of GCH1 in both PDX models and patient biopsies (Fig. 1E, Supplementary Fig. S2). Importantly, patient sections demonstrated elevated GCH1 expression in GBM cells in comparison to the surrounding normal tissue as well as greater heterogeneity in GCH1 expression (Fig. 1E). Data from the Human Protein Atlas also showed minimal GCH1 expression in normal brain with GCH1 expression increasing with glioma grade (Fig. 1F, Supplementary Fig. S3).
Fig. 1 .
GCH1 is expressed in GBM and elevated in tumor initiating cells. (A) GCH1 mRNA level was measured in BTICs from the indicated GBM xenolines (red) and immortalized neural progenitor cells (black) using qRT-PCR (n = 7). (B) GCH1 mRNA levels in BTICs were compared with non-BTICs from GBM xenolines (n = 7); *P < 0.05. (C and D) Western blot analyses of GCH1 expression in BTICs versus non-BTICs from GBM xenolines (C) and in non-BTICs at multiples time points since cultured in non-BTIC condition (D). Numbers show relative expression of GCH1, normalized to tubulin expression, in comparison to the sample with lowest GCH1 expression. Quantification was done using ImageJ. (E) Immunohistochemistry of GCH1 in human GBM xenografts (GBM PDX D456, representative of n = 5) and in GBM patient specimens. Scorings of staining and more samples are available in the Supplementary material. Scale bars represent 0.1 mm. (F) Analysis of GCH1 expression in normal brain and tumor tissue with quantification provided by The Human Protein Atlas at http://www.proteinatlas.org (representative images, n = 3 for cerebellum, cerebral cortex and lateral ventricle, n = 4 for low grade, and n = 7 for high-grade glioma). Complete sets of samples and their respective scorings are available in the Supplementary material. For all graphs, error bars represent standard deviations.
Modulation of GCH1 Expression Regulates Glioblastoma Cell Growth In Vitro
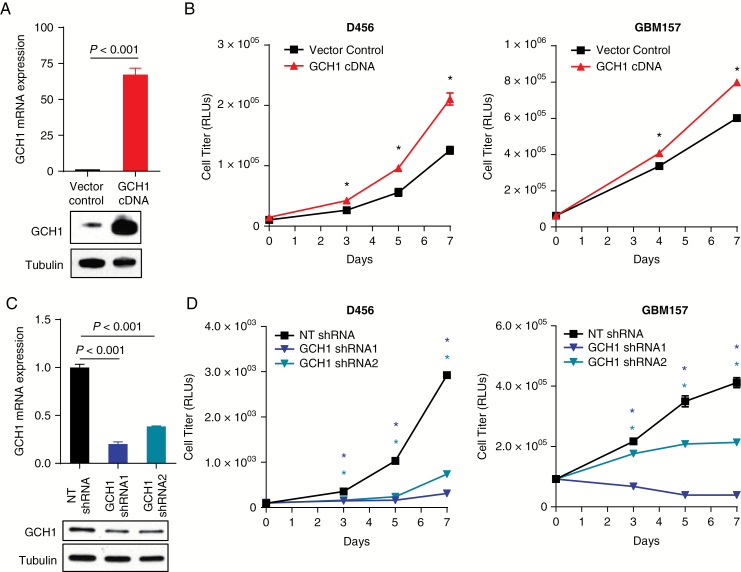
To investigate the impact of GCH1 on BTICs, we utilized a lentiviral system to generate cells expressing GCH1 cDNA and 2 different GCH1 short hairpin (sh)RNAs (schematic in Supplementary Fig. S4A). Successful infection in the cDNA overexpression system was evidenced by resistance to blasticidin S as well as fluorescence (data not shown). Overexpression of GCH1 was confirmed at the mRNA level using qRT-PCR and at the protein level using immunoblotting (Fig. 2A, Supplementary Fig. S2B). The human D456 and GBM157 cells and the mouse GL261 glioma cells overexpressing GCH1 gained an in vitro growth advantage over vector control (Fig. 2B, Supplementary Fig. S4C). Effects of GCH1 overexpression in immortalized but nontumorigenic human astrocytes (NHA hTERT E7) were more modest (Supplementary Fig. S4C). In converse experiments, we successfully reduced GCH1 expression at both mRNA and protein levels in BTICs using constitutively expressed shRNAs (Fig. 2C). Consistent with the overexpression results, GCH1 knockdown significantly reduced GBM xenoline growth in vitro (Fig. 2D). This effect was readily observed in BTICs cultured as spheres or on geltrex (data not shown). These data suggest that GCH1 elevation positively affects in vitro growth rates of both immortalized stromal cells and GBM cells but that GBM cells have more potent growth induction by GCH1 overexpression.
Fig. 2 .
Modulation of GCH1 level affects cell growth in vitro. (A) Analyses by qRT-PCR and Western blot demonstrating overexpression of GCH1 using a lentiviral system in D456 cells. (B) Growth of human D456 and GBM157 GBM cells, measured with tshe Promega CellTiter-Glo 2.0 assay; *P < 0.001 with t-test comparison of vector to GCH1 cDNA. (C) Analyses by qRT-PCR and western blot confirmed knockdown of GCH1 using 2 distinct shRNAs directed against GCH1 in comparison to a nontargeting control (NT shRNA) in D456 cells. (D) Growth of human GBM cells D456 and GBM157, measured with Promega CellTiter-Glo 2.0 assay; *P < 0.001 with t-test comparison of nontargeting control shRNA (NT shRNA) to both GCH1 shRNAs. (E) Representative images of D456 cells with shRNAs at day 7. For all graphs, error bars represent standard deviations.
GCH1 Regulates CD44 Expression and BTIC Maintenance
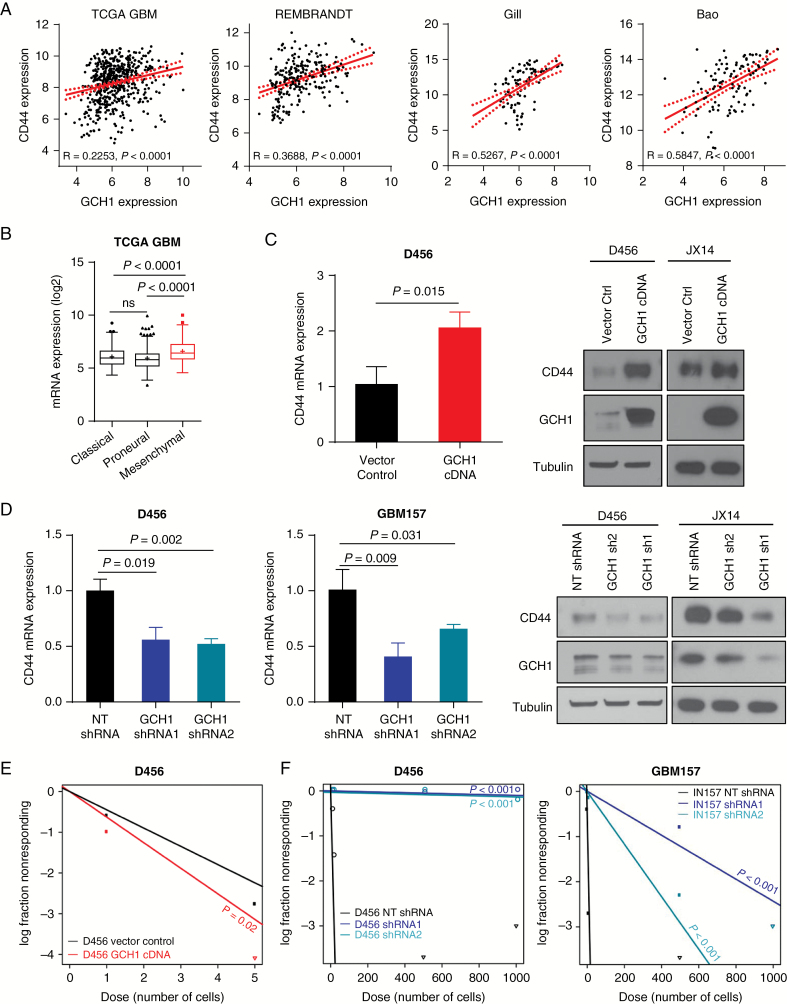
To assess the potential effects of GCH1 on BTIC marker expression, we performed in silico analyses across multiple datasets (Fig. 3A, Supplementary Fig. S5). The results established a positive correlation between the BTIC marker CD44 and GCH1 expression in patient gliomas (Fig. 3A, Supplementary Fig. S5A). Consistent with this finding, we also noted an elevation of GCH1 in the mesenchymal subset of both human (Fig. 3B, Supplementary Fig. S6A) and mouse (Supplementary Fig. S6B) gliomas. Analysis by qRT-PCR demonstrated significant increases in CD44 levels in GBM cells with GCH1 elevation (Fig. 3C). An increase in CD44 protein expression was confirmed via western blot with the extent of the increase being variable depending on the xenograft (Fig. 3C). Similarly, CD44 mRNA and protein decreased when GCH1 was genetically targeted (Fig. 3D). Significant but less extensive changes in levels of the BTIC marker CD133 (PROM1) and the differentiation marker glial fibrillary acidic protein were also observed with GCH1 modulation (Supplementary Fig. S5B, C). To determine if this change in BTIC marker expression correlated with a phenotypic shift, we evaluated the consequences of GCH1 level changes in the in vitro limiting dilution assay. We found a significant increase in neurosphere formation potential in D456 cells with GCH1 overexpression (Fig. 3E). Furthermore, our data demonstrated that GCH1 knockdown significantly decreased neurosphere formation in vitro compared with a nontargeting control (Fig. 3F). Together these data indicate that GCH1 promotes BTIC self-renewal and is associated with CD44 expression.
Fig. 3 .
Modulation of GCH1 level affects BTIC maintenance in human GBM cells. (A) In silico analyses showing correlations between GCH1 and the BTIC marker CD44 in human glioma gene expression datasets. Spearman rho value (R) for each correlation is shown. (B) In silico analysis showing GCH1 expression in 3 GBM molecular subtypes in the GBM datasets from TCGA. Note that the mesenchymal subtype (red) is marked for its increased CD44 expression. P-value shown with t-test comparison. (C) Analyses by qRT-PCR and western blot of CD44 expression in D456 cells with GCH1 overexpression in comparison to the vector control. P-value shown with t-test comparison. (D) Analyses by qRT-PCR and western blot of CD44 expression in human GBM xenolines with GCH1 knockdown. P-value shown with t-test comparison between pair of indicated samples. (E) Comparison of BTIC frequencies in D456 with GCH1 overexpression measured using in vitro limiting dilution sphere formation assay. P-value shown with extreme limiting dilution analysis (ELDA). (F) Comparisons of BTIC frequencies in D456 and GBM157 with GCH1 knockdown measured using in vitro limiting dilution sphere formation assay. P-value shown with ELDA analysis. For all bar graphs, error bars represent standard deviations.
GCH1 Is a Critical Regulator of Reactive Species in GBM Cells
Considering GCH1 can impact NOS function, we next investigated the effects of GCH1 modulation on NO levels in GBM PDX cells. Overexpression of GCH1 caused a nonsignificant increase in NO levels (Supplementary Fig. S7A and data not shown), suggesting that expression of enzymes downstream of GCH1 could have become rate limiting when GCH1 was overexpressed. This would be consistent with another study in which interferon gamma–induced GCH1 activity failed to increase NO level significantly due to the rate-limiting effect of the downstream enzymes PTS and SPR.31 However, we did observe elevated BH4 production in GCH1-overexpressing cells, demonstrating that GCH1 overexpression was sufficient to alter levels of this NOS cofactor in GBM cells (Supplementary Fig. S7B). GCH1 knockdown also significantly decreased NO production in BTICs (Supplementary Fig. S7C) with a moderate overall change, demonstrating that GCH1 does play a role in reactive nitrogen species levels in GBM.
As decreased GCH1 activity may also lead to NOS uncoupling and superoxide production, we next evaluated ROS production levels in BTICs with GCH1 modulation. Measuring levels of hydrogen peroxide because it is a more stable ROS and is a product of reactions of other ROS, including superoxide, we found that GCH1 overexpression significantly decreased the levels of ROS more than 2-fold (Fig. 4A). The extent of antioxidant effects of GCH1 in astrocytes was marginal in comparison to that in GBM cells, again suggesting that astrocytes are less affected by changes in GCH1 expression (Supplementary Fig. S7D). In experiments where GCH1 levels were decreased with shRNA, ROS levels significantly increased (Fig. 4B). To more directly link the GCH1 growth advantage to suppression of ROS levels, we treated GBM cells with hydrogen peroxide and found decreased levels of the proliferative marker phospho-histone H3 in vector but not GCH1-overexpressing cells (Supplementary Fig. S7E). We also found that daily treatment with a cell-permeable form of the antioxidant catalase was capable of rescuing the growth inhibitory effects of GCH1 knockdown in the short term (Supplementary Fig. S7F).
Fig. 4 .
Modulation of GCH1 levels in GBM cells affects oxidative stress in vitro. (A) Relative ROS levels in BTICs isolated from D456 xenografts with GCH1 overexpression using Promega ROS-Glo assay (normalized to vector control). P-value shown with t-test comparison. (B) Relative ROS levels in BTICs isolated from GBM D456 and GBM157 with GCH1 knockdown using Promega ROS-Glo assay (normalized to vector control). P-value shown with t-test comparison. (C) In silico analyses showing correlations between GCH1 and mRNA expression of PARK7 in human glioma gene expression datasets. Spearman rho value for each correlation is shown. (D) Analyses by qRT-PCR and western blot showing expression of the oxidative stress sensing molecule PARK7. P-value shown with t-test comparison. (E) Representative immunohistochemical analyses showing expression of the antioxidant protein PARK7 in D456 xenografts with and without GCH1 overexpression. (F) Growth of D456 and JX14 with GBM cells GCH1 shRNA was partially rescued by PARK7 cDNA as measured with the Promega CellTiter-Glo 2.0 assay; P-value shown with t-test comparison. Error bars represent standard deviations.
To investigate whether GCH1 effects on ROS levels in BTICs could be due to contributions from other antioxidant-related pathways, we also performed proteomic analysis of cells with GCH1 overexpression. Our results suggested that elevation of GCH1 significantly increased levels of several proteins that reduce and/or detect oxidative stress in multiple compartments of the cell, including the mitochondria (Supplementary Fig. S8A). The relationships between GCH1 and the identified proteins were further confirmed in our in silico analyses showing a positive correlation at the mRNA level across datasets (Fig. 4C, Supplementary Fig. S8B, C), including for Parkinsonism associated protein 7 (PARK7/DJ-1) (Fig. 4C, Supplementary Fig. S8C), an oxidative stress sensor implicated in glioma growth and invasion.32 PARK7 mRNA and protein were confirmed to be significantly but relatively moderately induced in vitro in GCH1-overexpressing cells with more robust elevation in vivo (Fig. 4E, Supplementary Figure S8D). Importantly, elevation of PARK7 partially rescued the growth inhibitory effects of loss of GCH1 (Fig. 4F). As this rescue was not as substantial as that observed with catalase, PARK7 is not likely to be the sole downstream target of GCH1. However, when we assessed patient outcomes in the context of both GCH1 and PARK7, GCH1lo/PARK7lo patients did have the best outcome (Supplementary Fig. S9A). Even though survival was still predominantly dictated by GCH1 levels, PARK7 levels do increase with glioma grade (Supplementary Fig. S9B) but were consistent between all GBM subtypes (Supplementary Fig. S9C), in contrast to GCH1 expression, which was higher in the mesenchymal subtype. Together, our data suggest that GCH1 may affect different biological processes in GBM via multiple downstream pathways, including through ROS level regulation. Furthermore, GCH1 may have a more global impact on ROS detection and production in GBM than previously understood.
GCH1 Increases the Growth of GBM In Vivo and Targeting GCH1 Decreases Tumor Initiating Potential
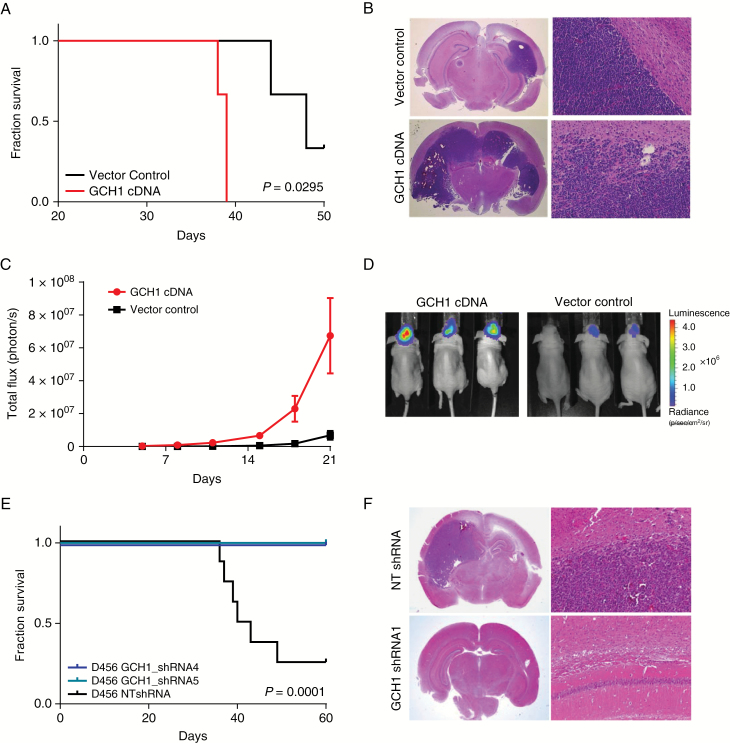
We next sought to investigate the potential effects of GCH1 modulation on the in vivo tumorigenicity of GBM cells. First, we intracranially injected D456 GBM cells expressing either the vector control or overexpressing GCH1 into the forebrains of athymic nude mice at different cell numbers and monitored animals daily for the development of neurologic signs. Consistent with the effect suggested by the in vitro data, animal survival was significantly decreased in mice injected with GBM PDX cells overexpressing GCH1 (Fig. 5A, Supplementary Fig. S10A, Supplementary Table S1). The presence of tumors was confirmed by histological staining of the harvested brains (Fig. 5B). To additionally visualize tumor growth, cells were engineered to express green fluorescent protein and luciferase and were subsequently infected with control or GCH1 cDNA. Monitoring bioluminescence over time, we confirmed that GCH1 elevation in GBMs increased tumor growth (Fig. 5C, D). Targeting of GCH1 also potently inhibited BTIC tumorigenic potential in vivo, as mice injected with GCH1 shRNA–expressing cells did not develop neurologic signs over the course of the experiment (Fig. 5E, F, Supplementary Fig. S10B, Supplementary Table S1). In contrast, animals injected with GBM cells expressing the nontargeting control developed tumors within 40 days (Fig. 5E, F, Supplementary Fig. S10B). These results were consistent with injection of either 500 (Fig. 5A, E, Supplementary Table S1) or 5000 cells per animal (Supplementary Fig. S10A, B). Thus, our data demonstrate a potent pro-tumorigenic role for GCH1 in GBM.
Fig. 5 .
Modulation of GCH1 levels in GBM cells affects in vivo survival. (A) Kaplan‒Meier plot showing survival of mice injected orthotopically with 500 cells from the human GBM xenoline D456 with or without GCH1 overexpression (n = 3). Log-rank (Mantel‒Cox) test was employed to calculate P-values. (B) Representative histological images of tumors from panel A; hematoxylin and eosin (H&E) staining, objective 1.25x (left), 20x (right). (C) Tumor growth of orthotopic human D456 xenografts with and without GCH1 overexpression (n = 5), measured by bioluminescent imaging. (D) Representative images of mice in two treatment groups from panel C. (E) Kaplan‒Meier plot showing survival of mice injected orthotopically with 500 cells from the human GBM xenoline D456 with GCH1 knockdown or a non-targeting control (n = 8). (F) Representative histological images of tumors from panel D; H&E staining, objective 1.25x (left), 20x (right).
GCH1 Expression Correlates with Worse Glioma Diagnosis and Prognosis
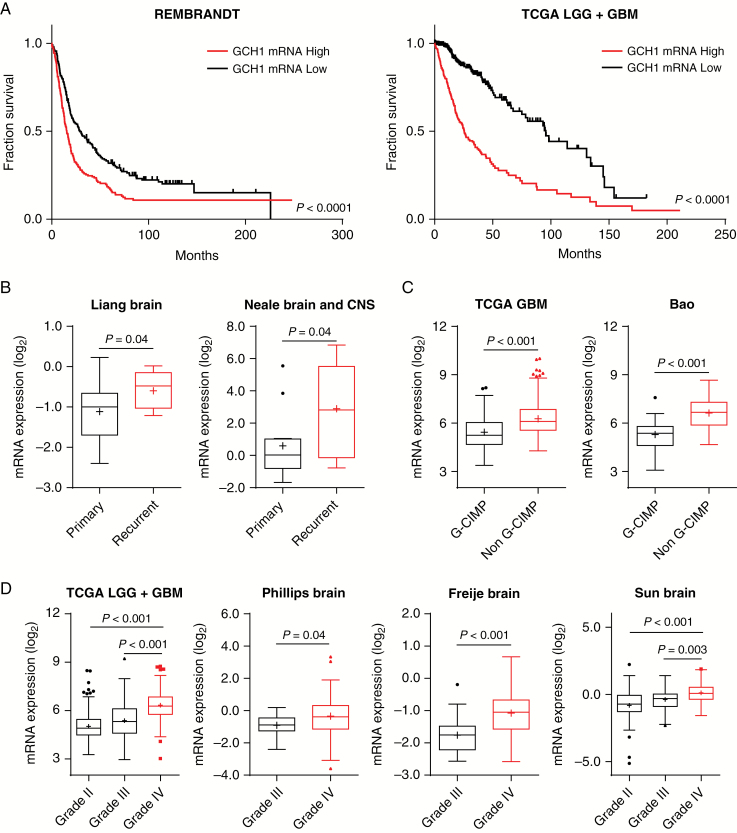
Considering the xenograft survival data and our prior data demonstrating GCH1 expression in GBM patient sections (Figure 1E, F), we further evaluated GCH1 significance for glioma patient outcomes. We performed in silico analyses on publicly available gene expression datasets to assess the clinical significance of differential GCH1 expression. Data from the Repository for Molecular Brain Neoplasia Data (REMBRANDT) and TCGA showed that expression of GCH1 in glioma negatively correlated with patient survival. Patients with high GCH1 mRNA levels in comparison to median have significantly worse prognosis (Fig. 6A). Assessment of data from the Liang and Neale datasets (retrieved using Oncomine platform33) also demonstrated that GCH1 expression was increased in recurrent compared with primary brain tumors (Fig. 6B). In comparison to the glioma cytosine-phosphate-guanine island methylator phenotype (G-CIMP), GCH1 expression is higher with the non–G-CIMP subtype (Fig. 6C and data not shown), which has been associated with poor patient prognosis.34GCH1 expression is also elevated with increasing glioma grade, with highest expression in GBM as evidenced by TCGA and the Phillips, Frejie, and Sun datasets (Fig. 6D). GCH1 expression as an independent predictor of patient survival cannot be definitely derived from retrospective data. However, these data strongly suggest that elevated GCH1 level in human glioma patients is a negative prognostic factor and implicates GCH1 in glioma progression.
Fig. 6 .
GCH1 expression correlates with higher glioma grade and worse prognosis. (A) Kaplan‒Meier survival plots depicting survival of glioma patients with different GCH1 expression levels in tumors. Top and bottom quartiles, log-rank (Mantel‒Cox) test was employed to calculate P-values. (B‒D) GCH1 mRNA expression in patient tumors from different datasets, grouped by recurrence status (B), G-CIMP status (C), and histological glioma grades (D). P-value shown with t-test comparison between pair of indicated samples.
Discussion
Here we demonstrate that GCH1 is elevated in GBM, where it promotes cell survival and BTIC maintenance due in part to regulation of CD44 expression and reduction of oxidative stress through a mechanism partially regulated by PARK7. Targeting of GCH1 resulted in decreased BTIC growth and self-renewal in vitro and tumorigenic potential in vivo, demonstrating a key role for GCH1 in the BTIC fraction. However, GCH1 is not restricted to BTICs, suggesting that pathways explored here are likely relevant for other tumor cell subsets. As GCH1 initiates the pathway which produces the NOS cofactor BH4 and decreased GCH1 activity can lead to superoxide production, our findings are consistent with previous reports showing pro-tumorigenic NO and anti-tumorigenic ROS roles in BTICs,7 as well as in other cancer types.11,35,36 Our study is the first of which we are aware to define a role for GCH1 in tumor initiating cells, particularly BTICs.
We found that reactive species balance is altered via modulation of GCH1 levels, where cells with higher GCH1 expression were able to effectively reduce oxidative stress. Studies have established that cancer cells must endure higher oxidative stress than normal cells as a result of their malignant transformation, hence upregulation of antioxidant pathways is vital for their survival.37,38 A study on neural stem cells, which have been proposed as potential glioma cells of origin,39 also suggested that, not unlike NO in BTICs, the effects of ROS on self-renewal properties of those cells were also dependent on its levels: a moderate elevated level would act as second messenger to promote proliferative and self-renewing properties, while a low level led to quiescence, and an excessive amount may cause toxicity.40 Studies in cancers, including GBM and leukemia, also revealed a similar requirement for redox control mechanisms in cancer cells.41,42 Combinations between ROS-generating agents and other compounds targeting proteasomes, epigenetic modifications, and DNA integrity have been proposed as therapeutic strategies with improved antitumor efficacy.15,43 The differential expression of GCH1 between glioma tumors and normal tissue, together with the less substantial effects in astrocytes compared with tumor cells, suggests that glioma cells may be dependent on the GCH1 pathway for redox control. This indicates the potential for adjuvant therapies targeting GCH1 in combination with standard treatments, particularly irradiation, which also causes cellular damage by generating free radicals.
Our study demonstrated GCH1-mediated BTIC maintenance as evidenced by GCH1 effects on tumor cell self-renewal and tumorigenic potential. However, it is important to note the distinctions between the molecular mechanisms that support the malignant characteristics of BTICs in an established tumor and those that drive de novo transformation.44 As GCH1 effects on nontransformed astrocyte growth were minimal and GCH1 could potentially protect from reactive species–induced damage, GCH1 may not have a key role in the initial transformation events that generate tumor cells. However, GCH1 may contribute to increased glioma grade once the cancer is established, with GCH1 being co-opted by tumors to support their propagation through BTIC maintenance.
Although we have explored the role of GCH1 in BTIC maintenance through reactive species balance, GCH1-dependent mechanisms contributing to GBM growth are likely multifaceted. Beyond the production of NO and superoxide,45,46 GCH1-mediated BH4 production is required for production of some neurotransmitters, including L-DOPA. Dopamine receptor antagonists have shown significant efficacy against the growth of GBM cells in vitro, suggesting that there must be a GBM cell intrinsic pathway for dopamine signaling which would be expected to depend on GCH1. Dopamine receptor antagonists also inhibit the growth of orthotopic tumors when used in combinatorial approaches,47 suggesting the benefit of dopamine signaling inhibition in GBM.48 Furthermore, the potential for GCH1 elevation to damage neurons and contribute to cancer-induced pain provides another potential benefit for GCH1 inhibition in GBM.21,49 Therefore, further studies are needed to better understand the underlying mechanisms of GCH1-mediated tumor initiating cell maintenance and promotion of tumor growth.
Supplementary material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the National Institutes of Health (R21NS096531, R01 NS104339 to A.B.H.); UAB Brain Tumor SPORE Career Development award to A.B.H.; pilot award from the UAB-HudsonAlpha Center for Genomic Medicine; startup funds to A.B.H. from the Department of Cell, Developmental and Integrative Biology, the Comprehensive Cancer Center, the Civitan International Research Center for Glial Biology in Medicine, the Center for Free Radical Biology, and the Neuro-Oncology Brain SPORE; P01HL69999 to J.S.S.; F31DK111067 to R.S.S.; 1UL1TR001417-01 to S.J.C.; P30 NS47466, R21NS082888 to M.S.G., UAB Comprehensive Cancer Center Agency, National Cancer Institute (NCI), project # P30CA013148 to J.M.
Conflict of interest statement
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
We appreciate the assistance of Marion Spell at the UAB Flow Cytometry Facility. We sincerely appreciate the helpful discussions with and advice of Dr. Victor Darley-Usmar.
References
- 1. Hottinger AF, Stupp R, Homicsko K. Standards of care and novel approaches in the management of glioblastoma multiforme. Chin J Cancer. 2014;33(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verhaak RG, Hoadley KA, Purdom E et al. ; Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piccirillo SG, Spiteri I, Sottoriva A et al. Contributions to drug resistance in glioblastoma derived from malignant cells in the sub-ependymal zone. Cancer Res. 2015;75(1):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh SK, Clarke ID, Terasaki M et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 5. Galli R, Binda E, Orfanelli U et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. [DOI] [PubMed] [Google Scholar]
- 6. Lee J, Kotliarova S, Kotliarov Y et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. [DOI] [PubMed] [Google Scholar]
- 7. Eyler CE, Wu Q, Yan K et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146(1): 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laks DR, Visnyei K, Kornblum HI. Brain tumor stem cells as therapeutic targets in models of glioma. Yonsei Med J. 2010;51(5):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bayin NS, Modrek AS, Placantonakis DG. Glioblastoma stem cells: molecular characteristics and therapeutic implications. World J Stem Cells. 2014;6(2):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ridnour LA, Thomas DD, Switzer C et al. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide. 2008;19(2):73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tran AN, Boyd NH, Walker K, Hjelmeland AB. NOS expression and NO function in glioma and implications for patient therapies. Antioxid Redox Signal. 2017;26(17):986–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hjelmeland A, Zhang J. Metabolic, autophagic, and mitophagic activities in cancer initiation and progression. Biomed J. 2016;39(2):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monticone M, Taherian R, Stigliani S et al. NAC, tiron and trolox impair survival of cell cultures containing glioblastoma tumorigenic initiating cells by inhibition of cell cycle progression. PLoS One. 2014;9(2):e90085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang W, Shen Y, Wei J, Liu F. MicroRNA-153/Nrf-2/GPx1 pathway regulates radiosensitivity and stemness of glioma stem cells via reactive oxygen species. Oncotarget. 2015;6(26):22006–22027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singer E, Judkins J, Salomonis N et al. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charles N, Ozawa T, Squatrito M et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6(2):141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koh LW-H, Koh GR-H, Ng FS-L et al. A distinct reactive oxygen species profile confers chemoresistance in glioma-propagating cells and associates with patient survival outcome. Antioxid Redox Signal. 2013;19(18):2261–2279. [DOI] [PubMed] [Google Scholar]
- 18. Kim RK, Suh Y, Cui YH et al. Fractionated radiation-induced nitric oxide promotes expansion of glioma stem-like cells. Cancer Sci. 2013;104(9):1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurimoto M, Endo S, Hirashima Y, Hamada H, Ogiichi T, Takaku A. Growth inhibition and radiosensitization of cultured glioma cells by nitric oxide generating agents. J Neurooncol. 1999;42(1):35–44. [DOI] [PubMed] [Google Scholar]
- 20. Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 21. Pickert G, Myrczek T, Rückert S et al. Inhibition of GTP cyclohydrolase reduces cancer pain in mice and enhances analgesic effects of morphine. J Mol Med (Berl). 2012;90(12):1473–1486. [DOI] [PubMed] [Google Scholar]
- 22. Pickert G, Lim HY, Weigert A et al. Inhibition of GTP cyclohydrolase attenuates tumor growth by reducing angiogenesis and M2-like polarization of tumor associated macrophages. Int J Cancer. 2013;132(3):591–604. [DOI] [PubMed] [Google Scholar]
- 23. D’Sa C, Hirayama K, West A, Hahn M, Zhu M, Kapatos G. Tetrahydrobiopterin biosynthesis in C6 glioma cells: induction of GTP cyclohydrolase I gene expression by lipopolysaccharide and cytokine treatment. Brain Res Mol Brain Res. 1996;41(1-2):105–110. [DOI] [PubMed] [Google Scholar]
- 24. Laks DR, Crisman TJ, Shih MY et al. Large-scale assessment of the gliomasphere model system. Neuro Oncol. 2016;18(10):1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panosyan EH, Laks DR, Masterman-Smith M et al. Clinical outcome in pediatric glial and embryonal brain tumors correlates with in vitro multi-passageable neurosphere formation. Pediatr Blood Cancer. 2010;55(4):644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker K, Hjelmeland A. Method for efficient transduction of cancer stem cells. J Cancer Stem Cell Res. 2014;2:e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1-2):70–78. [DOI] [PubMed] [Google Scholar]
- 28. Foster JM, Carmines PK, Pollock JS. PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol. 2009;297(2):F471–F480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin interacts with NOS1 in the renal collecting duct and activates NO production. FASEB J. 2011;25(1 suppl):1041.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowman RL, Wang Q, Carro A, Verhaak RG, Squatrito M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro Oncol. 2017;19(1):139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Werner ER, Werner-Felmayer G, Fuchs D et al. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem. 1990;265(6):3189–3192. [PubMed] [Google Scholar]
- 32. Jin S, Dai Y, Li C, Fang X, Han H, Wang D. MicroRNA-544 inhibits glioma proliferation, invasion and migration but induces cell apoptosis by targeting PARK7. Am J Transl Res. 2016;8(4):1826–1837. [PMC free article] [PubMed] [Google Scholar]
- 33. Rhodes DR, Yu J, Shanker K et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noushmehr H, Weisenberger DJ, Diefes K et al. ; Cancer Genome Atlas Research Network Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levesque MC, Misukonis MA, O’Loughlin CW et al. IL-4 and interferon gamma regulate expression of inducible nitric oxide synthase in chronic lymphocytic leukemia cells. Leukemia. 2003;17(2):442–450. [DOI] [PubMed] [Google Scholar]
- 36. Ambs S, Merriam WG, Bennett WP et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58(2):334–341. [PubMed] [Google Scholar]
- 37. Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–947. [DOI] [PubMed] [Google Scholar]
- 38. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. [DOI] [PubMed] [Google Scholar]
- 39. Modrek AS, Bayin NS, Placantonakis DG. Brain stem cells as the cell of origin in glioma. World J Stem Cells. 2014;6(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Le Belle JE, Orozco NM, Paucar AA et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8(1):59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwin ME, Rivera-Del Valle N, Chandra J. Redox control of leukemia: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2013;18(11):1349–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agnihotri S, Golbourn B, Huang X et al. PINK1 is a negative regulator of growth and the Warburg effect in glioblastoma. Cancer Res. 2016;76(16):4708–4719. [DOI] [PubMed] [Google Scholar]
- 43. Miller CP, Singh MM, Rivera-Del Valle N, Manton CA, Chandra J. Therapeutic strategies to enhance the anticancer efficacy of histone deacetylase inhibitors. J Biomed Biotechnol. 2011;2011:514261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Visvader JE. Cells of origin in cancer. Nature. 2011;469(7330):314–322. [DOI] [PubMed] [Google Scholar]
- 45. Gross SS, Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992;267(36):25722–25729. [PubMed] [Google Scholar]
- 46. Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab. 2002;3(2):159–173. [DOI] [PubMed] [Google Scholar]
- 47. Li J, Zhu S, Kozono D et al. Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget. 2014;5(4):882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dolma S, Selvadurai HJ, Lan X et al. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29(6):859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Campbell CM, Edwards RR, Carmona C et al. Polymorphisms in the GTP cyclohydrolase gene (GCH1) are associated with ratings of capsaicin pain. Pain. 2009;141(1-2):114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.