Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second cause of cancer-related death worldwide. The incidence of HCC is constantly increasing in correlation with the rise in diabetes and obesity, arguing for an urgent need for new developments in the treatment of this lethal cancer. Exosomes are small double-membrane vesicles loaded with distinct cargos, particularly small non-coding RNAs called microRNAs, representative of each donor cell and secreted to affect the features of neighboring cells or recipient cells located further away, like in the case of metastasis. A better understanding of the role of exosomes with a microRNA signature in cancer pathogenesis gave rise to the concept of their use as a non-invasive diagnostic biomarker and in the treatment of cancer, including HCC. In this communication, we review recent works that demonstrate that hepatic stellate cells establish an epigenetic communication with liver cancer cells, which affects their pro-malignant features. If naturally secreted patient-derived exosomes show major limitations concerning their clinical use, bio-engineered exosome mimetics that incorporate controlled components and exhibit no protumoral properties could be promising carriers for the treatment of liver cancers, which is the organ preferentially targeted by systemic injection of exosomes.
Keywords: MicroRNAs, Hepatocellular carcinoma, Targeted therapy, Exosomes
Core tip: Despite the intensive research efforts to identify the molecular events responsible for the emergence of liver cancer, hepatocellular carcinoma (HCC) remains a major health problem worldwide. Thus, the identification of new therapeutic opportunities to counteract the challenging issues linked to HCC heterogeneity and resistance to conventional treatments is a short-term necessity. Over the last few decades, microRNAs have appeared as interesting therapeutic strategies with their pleiotropic inhibitory action, but the use of a delivery system is a requirement for miRNA mimic administration. Exosomes, which are small vesicles naturally produced by immune cells and aberrantly by cancer cells, have recently emerged as a promising vehicle.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the second cause of cancer-related death worldwide for which therapeutic options are very limited. Indeed, because of its heterogeneity, the development of effective therapies against this cancer remains a challenging issue. HCC is considered to be a paradigm of inflammation-associated cancer, since 80% of HCC emerges following vast liver remodeling. Briefly, HCC primarily affects men with cirrhosis due to hepatitis B and C viruses, alcohol abuse, genotoxic exposure and metabolic disorders, and the incidence is increasing due to diabetes and obesity[1]. Efforts in the molecular and genetic profiling of HCC revealed that among the mutational landscape of HCC, the Wnt/β-catenin, p53 and Ras pathways are the most frequently mutated. Other prevalent mutations occur in epigenetic modifiers such as chromatin remodelers and imprinted clusters[2].
Despite these molecular findings, the molecular pathogenesis of HCC is still not fully understood, and novel strategies are urgently needed to cure this lethal disease with high incidence. Over the last decades, other crucial epigenetic regulators, the small non-coding RNAs named microRNAs (miRNAs), have been largely found to be disturbed during hepatocarcinogenesis[3]. Promisingly, due to their large spectrum of action on proliferation, inflammation and metabolism, miRNAs have emerged as robust therapeutic opportunities in various cancers. Regarding liver diseases, miRNA-based therapies have been successfully tested[4,5] - this type of molecule is preferentially delivered to the liver[6]. Despite promising results obtained with miRNA-based therapies, a number of challenges remain to improve the efficiency of this type of treatments. Their limitations are similar to those which have delayed the use of therapies based on small interfering RNAs: Improvement of stability, free or encapsulated administration, problems of specificity, tissue distribution, response persistence and secondary effects.
Recently, new therapeutic candidates for anticancer drug delivery have been proposed that are based on a biological system of transporting active cargos called exosomes. Exosomes are double membrane cell-derived microvesicles defined by a diameter of 30 to 100 nm that contain a great diversity of nucleic acids, protein and lipids - 3408 mRNAs, 2838 miRNAs and 9769 proteins according to the Exocarta database based on 286 studies[7]. Most cells, and particularly immune cells, are physiologically secreting exosomes that originate from multivesicular bodies. Multivesicular body fusion with the plasma membrane is orchestrated by Rab, soluble N-ethylmaleimide-sensitive-factor attachment protein (commonly known as SNAP) and SNAP receptor (commonly known as SNARE) proteins[8]. In response to different activating signals like antigenic, cytokinic or mitogenic stimuli, immune cells are able to increasingly release these small vesicles[9]. Recent studies revealed that disequilibrium in exosome formation and/or delivery contributes to pathological processes leading to immunological disorders and cancers. Indeed, during tumorigenesis, tumor cells aberrantly secrete exosomes to communicate with stromal cells and to modify secondary sites favoring metastasis[10]. In consequence, the detection of exosomal miRNAs in body fluids has appeared as a potent non-invasive diagnosis tool for cancer, including HCC[11], but also as a new therapeutic opportunity.
Exosome-based therapies have emerged over the past decade as an attractive strategy for tissue repair, immune vaccine and against cancer, firstly because of their biocompatibility. Second, their small size facilitates their crossing through biological barriers, notably the blood-brain barrier, and limits their renal clearance. These microvesicles might prove to be suitable for liver disease, especially for liver cancer treatment, since exosomes accumulate in the liver after systemic injection. In particular, exosomes preferentially target the resident macrophages, the Kupffer cells. Interestingly, the uptake of extracellular vesicles by Kupffer cells increases in the case of liver damage[12]. Over the past two years, the Selaru laboratory published two compelling manuscripts studying exosomes carrying miRNAs as a way to dialog between stromal cells, in particular stellate cells, and cancer cells in cholangiocarcinoma (CCA)[13] and in HCC[14]. Both studies revealed the clinical potential of miRNAs loaded in stellate cell-derived exosomes for in vivo delivery in mice and, on a longer-term perspective, CCA or HCC treatment.
STUDY ANALYSIS
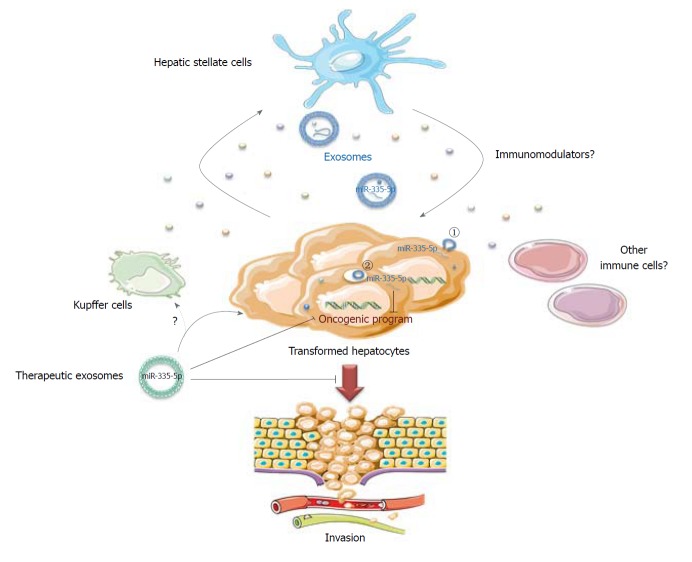
The work from Wang et al[14] focused on miR-335-5p, a microRNA already described as a tumor suppressor in HCC[15] and that is known to gradually decrease in activated hepatic stellate cells (HSCs) during hepatic fibrosis[16]. This miRNA is also reduced in the serum of HCC patients in association with progressive features and is predictive of chemo-embolization response[17]. For their study, the authors used different HCC cell lines either mutated for p53 (HuH-7) or β-catenin (HepG2), or infected with HBV (MHCC97) with low or high metastatic features. They confirmed a global tumor suppressive role of miR-335-5p associated with miR-335-5p loss in all cell lines compared to the LX-2 HSC line[14]. A miR-335-5p mimic inhibited HCC cell proliferation and invasion upon transfection, but also, crucially, when transfected in LX-2 cells seeded in co-culture with HCC cells. This suggests that a miRNA dialog between HCC and stromal cells might be established to favor tumor progression and invasion (Figure 1).
Figure 1.
An exosomal miR-335-5p-based therapy for hepatocellular carcinoma. In the case of hepatocellular carcinoma, miR-335-5p is lost in cancer cells, favoring cell proliferation and invasion. The hepatic stellate cells could counteract these pro-malignant features by secreting exosomes containing nucleic acids and miRNAs, including miR-335-5p, which are captured by HCC cells by a direct fusion with recipient cell membrane (1) or by endocytosis (2). Mimicking this biological process, therapeutic exosomes, either isolated from patients or bioengineered exosome mimetics, loaded with miR-335-5p might slow cell proliferation, promote apoptosis and limit cell invasion. It remains to be determined whether other immune cells could participate in this material transfer and which immunomodulators could regulate this exchange.
To confirm the material transfer via the exosomal route between HSC and HCC cell lines, the authors used an elegant system with two fluorophores coupled to a stop signal based on the Cre-lox strategy (loxP-dsRED-loxP-stop-eGFP) transfected in HCC cells. Using this tool, they demonstrated that LX-2 cells transferred Cre recombinase to neighboring HCC cells, as evidenced by green labeling. An efficient transfer was also observed when exosomes were purified from LX-2 cells and later added to HCC cell culture. In vivo, intra-tumoral injection of LX-2 Cre-positive exosomes to a subcutaneous HCC xenograft also led to GFP signal in the tumors. This means that the construct was efficiently recombined in HCC via exosome capture. Finally, with a view to treatment, Wang et al[14] showed that LX-2 isolated exosomes, extemporaneously enriched with miR-335-5p, reduce HCC growth after intra-tumoral injection every 2 d for 4 wk in MHCC97H cell xenografts.
The intra-tumoral administration was preferred to concentrate exosomes into the tumor mass and to mimic the historically intra-arterial administration of chemotherapeutic agents. Exosome treatment led to overexpression of miR-335-5p by 30-fold in the tumors and modification of its target landscape, resulting in an attenuation of proliferation and an increase in apoptosis. This study confirmed the previous observations performed by the Selaru laboratory showing that LX2 cells secrete miR-195-enriched exosomes, which can communicate with CCA cells and decrease in vivo tumor growth in a CCA rat model after intravenous injections every 2 d[13].
PERSPECTIVES
In conclusion, these proof of concept studies performed in two different models of liver cancer support the existence of an epigenetic dialog between cancer cells and stromal cells driven by exosomes to favor tumor progression. In particular, HSCs play an important part in this dialog, but other non-parenchymal cells like Kupffer cells could also probably participate. They also highlight that HSC-derived exosome manipulation succeeds in restoring a more physiological expression of miRNAs found deregulated in cancer cells in vivo. The restoration of miRNA expression modifies gene expression, and subsequently limits cell proliferation and favors apoptosis. These results, and others generated in various cancer models, support extracellular vesicles as an attractive modality for personalized treatment for liver cancer, especially since this type of particle primarily targeted the liver after systemic injection.
Several studies have used exosomes as a targeted delivery system for chemotherapeutic agents, leading to cancer cell death and promoting a domino effect through the release of secondary cytotoxic vesicles[18]. Additionally, exosomes produced by mesenchymal stem cells have been largely studied in liver disease and found to be modulators of the immune response, favored by their engulfment by resident macrophages. They are also key modulators of oxidative stress and fibrotic processes[19]. Despite all these encouraging features, a number of limitations for their clinical feasibility are currently a brake for using these delivery systems. Indeed, the production of patient-derived exosomes for clinical application appears expensive, time-consuming and complex (preparation method, loading, characterization, etc). Since these biological carriers present pro-tumoral characteristics, the pro-malignant factors have to be preliminarily identified and removed before re-injection. The reproducibility also remains a major barrier, since a previous study suggested that three independent preparations of exosomes from mesenchymal stem cells only shared 20% of their proteome[20].
A promising alternative for extracellular vesicle-based therapeutics is the synthesis of bioengineered exosome mimetics, which could allow the production of exosome preparations suitable for clinical use (sterile, characterized, reproducible)[21]. In conclusion, even if about a hundred clinical trials are currently testing the benefit of exosomes as therapeutic agents, a gold standard method for their isolation and loading has to be approved. A better characterization of their specificity, functionality and safety is required, for which liver cancer, characterized by its pro-inflammatory microenvironment and its refractoriness to conventional treatments, undoubtedly constitutes a model of choice.
Footnotes
Conflict-of-interest statement: The author has no conflict of interest to declare. No financial support.
Manuscript source: Invited manuscript
Peer-review started: August 3, 2018
First decision: August 24, 2018
Article in press: October 10, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bogdanos DP, El Din NGB S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gougelet A, Colnot S. [microRNA: new diagnostic and therapeutic tools in liver disease?] Med Sci (Paris) 2013;29:861–867. doi: 10.1051/medsci/20132910013. [DOI] [PubMed] [Google Scholar]
- 4.Gougelet A, Sartor C, Bachelot L, Godard C, Marchiol C, Renault G, Tores F, Nitschke P, Cavard C, Terris B, et al. Antitumour activity of an inhibitor of miR-34a in liver cancer with β-catenin-mutations. Gut. 2016;65:1024–1034. doi: 10.1136/gutjnl-2014-308969. [DOI] [PubMed] [Google Scholar]
- 5.Shibata C, Otsuka M, Kishikawa T, Ohno M, Yoshikawa T, Takata A, Koike K. Diagnostic and therapeutic application of noncoding RNAs for hepatocellular carcinoma. World J Hepatol. 2015;7:1–6. doi: 10.4254/wjh.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts J, Palma E, Sazani P, Ørum H, Cho M, Kole R. Efficient and persistent splice switching by systemically delivered LNA oligonucleotides in mice. Mol Ther. 2006;14:471–475. doi: 10.1016/j.ymthe.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gougelet A, Colnot S. Hepatocellular carcinoma diagnosis: Circulating microRNAs emerge as robust biomarkers. Clin Res Hepatol Gastroenterol. 2016;40:367–369. doi: 10.1016/j.clinre.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cells Transl Med. 2017;6:1262–1272. doi: 10.1002/sctm.16-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, Mezey E, Gould SJ, Fordjour FK, Meltzer SJ, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology. 2017;65:501–514. doi: 10.1002/hep.28735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Li L, Piontek K, Sakaguchi M, Selaru FM. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67:940–954. doi: 10.1002/hep.29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Li W, Chen C, Pei Y, Long X. MiR-335 acts as a potential tumor suppressor miRNA via downregulating ROCK1 expression in hepatocellular carcinoma. Tumour Biol. 2015;36:6313–6319. doi: 10.1007/s13277-015-3317-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Wu CQ, Zhang ZQ, Yao DK, Zhu L. Loss of expression of miR-335 is implicated in hepatic stellate cell migration and activation. Exp Cell Res. 2011;317:1714–1725. doi: 10.1016/j.yexcr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Cui L, Hu Y, Bai B, Zhang S. Serum miR-335 Level is Associated with the Treatment Response to Trans-Arterial Chemoembolization and Prognosis in Patients with Hepatocellular Carcinoma. Cell Physiol Biochem. 2015;37:276–283. doi: 10.1159/000430352. [DOI] [PubMed] [Google Scholar]
- 18.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 19.Borrelli DA, Yankson K, Shukla N, Vilanilam G, Ticer T, Wolfram J. Extracellular vesicle therapeutics for liver disease. J Control Release. 2018;273:86–98. doi: 10.1016/j.jconrel.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim OY, Lee J, Gho YS. Extracellular vesicle mimetics: Novel alternatives to extracellular vesicle-based theranostics, drug delivery, and vaccines. Semin Cell Dev Biol. 2017;67:74–82. doi: 10.1016/j.semcdb.2016.12.001. [DOI] [PubMed] [Google Scholar]