Abstract
AIM
To evaluate viral hepatitis knowledge among individuals from different resource areas and health conditions to identify possible gaps.
METHODS
A cross-sectional, descriptive study was carried out among 447 individuals from five distinct populations in Brazil: Southeast Viral Hepatitis Ambulatory (n = 100), South (n = 89) and Northeast (n = 114) Health Center, Southeast (n = 77) and Northeast (n = 67) low resource areas. All individuals answered a questionnaire assessing sociodemographic characteristics and viral hepatitis awareness. The perception was scored based on the average number of correct answers of all participants and categorized as “low” (0-28 correct answers) or “desirable” (29-46 correct answers). Associations between sociodemographic characteristics and perception were also evaluated.
RESULTS
A low level of knowledge was observed in individuals from Northeast Health Center, Northeast and Southeast low resource areas while desirable knowledge was observed in individuals from Viral Hepatitis Ambulatory and South Health Center. According to sociodemographic characteristics, desirable scores were more common among those with secondary education (47.1%), those who declared themselves as white (46.3%), and those who lived in houses with three individuals (25.5%). Multivariate analysis showed an association between viral hepatitis perception and type of population.
CONCLUSION
The results demonstrated high level of knowledge among study participants from health clinics from the Southeast region of Brazil and the importance of education programs in increasing the level of knowledge in low resource areas.
Keywords: Viral hepatitis, Knowledge, Perception, Urban population, Brazil
Core tip: This study evaluated viral hepatitis knowledge among individuals from five different resource areas and health conditions in Brazil. Participants responded to a questionnaire and the perception was scored as “low” or “desirable”. Individuals from Northeast Health Center and Northeast and Southeast low resource areas exhibited low perception, while Southeast and South Health Center exhibited a desirable perception. A positive association was observed between perception and education level, race, number of individuals living in the same house and population type. The results showed the importance of prevention campaigns, especially among individuals living in low resource areas.
INTRODUCTION
Hepatitis is the name given to liver inflammation resulting from autoimmune disease, excessive consumption of alcohol or drugs, bacteria and viruses. Viral hepatitis is a group of viruses (hepatitis A, B, C, D, E, G known as HAV, HBV, HCV, HDV, HEV, HGV) that are etiologically and epidemiologically distinct[1-3].
Ingestion of contaminated food or water transmits HAV and HEV; in this fashion, washing food and hands and treating water are methods of prevention. On the other hand, HBV, HCV and HDV can be transmitted by contact with infected bodily fluids (transfusion of blood or blood products, or invasive medical procedures), unsafe sexual practices, or from transmission from mother to child. Prevention of HBV, HCV and HDV is made by blood and organ donor selection, using disposable or sterilized materials and the use of condoms in sexual intercourse[1,4-6].
There are vaccines to prevent HAV and HBV that are safe and effective; one vaccine for HEV is commercialized only in China, but there are no Federal Drug Administration-approved vaccines for HCV and HDV[7]. The clinical course of hepatitis viruses can be acute and chronic for HBV, HCV, HDV and HEV. The clinical manifestations of hepatitis can be absent or appear when the disease is advanced, with cirrhosis or liver cancer[1,2]. Viral hepatitis laboratory diagnosis is performed through the detection of specific antigens, antibodies and viral genome, mainly by enzyme immunoassays and molecular assays such as the polymerase chain reaction[8].
HBV and HCV occur chronically in 257 and 71 million people respectively, causing more than 1.2 million deaths annually[2]. Approximately 15 million people are infected with HDV[2]. Annually, there are an estimated 126 million new cases of HAV and 3.3 million new cases of HEV[2,9,10]. In 2016, 61297 deaths were related to viral hepatitis in Brazil. HEV prevalence in Brazil varies from 2% to 29%[11-15].
The evaluation of knowledge is assessed to verify how far community knowledge corresponds to biomedical concepts[5]. Some factors, such as education, health literacy, family income, age, and access to information, could be associated with gaps in knowledge[16,17].
Around the world studies have been conducted in order to evaluate viral hepatitis perception among health professionals and students, viral hepatitis patients or other risk groups[17-21]. There are still few reports regarding viral hepatitis knowledge in low resource areas[5,22-24]. In view of these gaps, the aim of the present study is to evaluate the viral hepatitis knowledge among individuals from different resource areas and health conditions in Brazil to identify possible gaps and help authorities in the development of prevention and education programs.
MATERIALS AND METHODS
Population studied
This was a cross-sectional study conducted from March 2015 to November 2015, wherein a minimum sample size of 50 participants per group was defined. A nonprobability sampling method with consecutive sampling was used in which every subject meeting the criteria of inclusion was selected until the required sample size was achieved in this setting.
Individuals were previously informed about the study and participant eligibility criteria were: Both genders, more than 18 years of age, free from psychoactive drug use, agreement to inclusion, and signed, informed consent. The local ethical committee approved the study (CAEE 38846914.5.0000.5248).
The final sample was made up of a total of 447 questionnaires about hepatitis knowledge obtained from five groups belonging to different geographic regions in Brazil, as follows: (1) Southeast Viral Hepatitis Ambulatory, comprising 100 individuals living in the Rio de Janeiro state, both in nearby cities and in different districts of the city of Rio de Janeiro, who were referred to the outpatient clinic. These individuals included not only those with acute, chronic or suspected cases of viral hepatitis but also those accompanying patients to the Brazilian Referral center for viral hepatitis diagnosis. The recruitment was performed prior to medical consultation. The Rio de Janeiro state is situated in the Southeast region of Brazil, with a human development Index (HDI) of 0.761[25]; (2) South Health Center, comprising 89 individuals residing in the city of Curitiba (Paraná State) that were recruited in the Guidance and Monitoring Center prior to medical consultation. This center performs anonymous testing for hepatitis, syphilis and human immunodeficiency virus. Curitiba is situated in the South region of Brazil with an estimated population of 1908359, an HDI of 0.823, and poverty rate of 31.71%[25]; (3) Northeast Health Center, comprising 114 individuals resident in the city of Fortaleza (Ceará State) and who were users of the Brazilian Unified Health System seeking care in Medical Care Center integrated to the University of Fortaleza. Recruitment was carried out prior to medical consultation. Fortaleza is situated in the Northeast region of Brazil, with an estimated population of 2627482, an HDI of 0.754, and a poverty rate of 43.17%[25]; (4) Southeast low resource areas, comprising 77 individuals living in low resource communities from the Southeast region of Brazil (Complex of Manguinhos district of Rio de Janeiro city). Interviewers visited residents in their homes and only applied the questionnaires to those who agreed to participate. Rio de Janeiro city is situated in the Southeast region of Brazil, with an estimated population of 6520266, an HDI of 0.799, and a poverty rate of 23.85%. Manguinhos complex exhibited the fifth worst HDI (0.726) among the 126 neighborhood groups in the city of Rio de Janeiro, and the average family income in population was below a minimum Brazilian income[25]; and (5) Northeast low resource areas, comprising 67 individuals resident in a low-resource community from the Northeast Region of Brazil (Nossa Senhora de Nazaré city, Piauí State). This city had approximately 5000 inhabitants and residents had a low income. Interviewers visited residents in their homes and only applied the questionnaires to those who agreed to participate. Nossa Senhora de Nazaré is situated in the Northeast region of Brazil, with an estimated population of 4786, an HDI of 0.586, and a poverty rate of 56.6%[25].
To assess knowledge scores, five populations were further aggregated into three groups, which were categorized as Southeast Viral Hepatitis Ambulatory (n = 100), medical centers (n =203, South and Northeast) and under-privileged communities (n = 144, Southeast and Northeast low resource areas).
Data collection instrument
The questionnaire was composed of two parts: (1) Social-demographic characteristics; and (2) viral hepatitis perception. Social-demographic characteristics included gender, age, education level, race, monthly family income, marital status and number of people in-house. Monthly family income was considered in relation to the Brazilian minimum salary and classified as “low” (< US $276.00 approximately), “intermediate” (US $276.00 to $828.00 approximately) or “high” (> US $828.00 approximately).
Viral hepatitis perception was assessed by the participants’ understanding of the proposed questions. The questionnaire was composed of nine groups of questions covering aspects about viral hepatitis including general information (questions 1 to 4), transmission (question 5), prevention (question 6), clinical manifestations (question 7), risk factors (question 8), and complications (questions 9). All questions except items 3 and 4 had subdivisions (i.e. 1a, 1b, 1c and 1d); thus, a total of 46 answers could be correctly pointed out. Additionally, in items 3, 4, 5 and 6, individuals were asked to report which type of hepatitis virus related to their response.
The initial version of the questionnaire was structured in the Brazilian Portuguese language and developed from a questionnaire applied in a previous study[24] and through literature review about knowledge in viral hepatitis[5,16,17]. The questionnaire was then piloted with 30 respondents for its acceptability and consistency, including 15 self-administered and 15 interviewed. From the self-administered questionnaire, three of them had many unfilled questions, and one of them entirely unfilled. The questionnaire was modified after the pilot study and the interview format was chosen for data collection. After this evaluation, the questionnaire was made available for data collection. Data from the pilot study was not included in the final analysis. Participants were interviewed face-to-face in a confidential setting. At the end of the interview, the correct answers were shown to each volunteer.
Score of knowledge
The viral hepatitis perception score was created based on the average of correct answers of all participants’ responses (28.7). The perception was divided in two scores: “low” (0-28 correct answers) and “desirable” (29-46 correct answers). Associations between sociodemographic characteristics and perception were also evaluated.
Statistical analysis
Descriptive statistics were generated for the responses and the chi-squared test for independence or for trend and Kruskal-Wallis test were used to compare categorical and continuous variables respectively, among the perception score groups. The variables that were associated with perception score categories were inserted into the logistic regression model, using a forward stepwise method. The 95%CIs of the estimated odds ratios were also calculated, and a P-value was calculated using the Statistical Package for the Social Sciences (SPSS for Windows, release 20.0; IBM Corp., Armonk, NY, United States).
RESULTS
Demographic characteristics
Most of the participants were female (269/60.2%), aged over 40 years (254/56.8%), had secondary education (186/41.6%), received intermediate monthly family income (250/55.9%), and declared themselves as non-white (225/50.3%), married (224/50.1%) and living in houses with three individuals (128/28.6%). Only marital status was not significantly different between the five populations (P = 0.909) (Table 1).
Table 1.
Participants' sociodemographic characteristics of studies, n (%)
| Item | Total, 447 | Southeast viral hepatitis ambulatory, 100 | South health center, 89 | Northeast health center, 114 | Southeast low resource areas, 77 | Northeast low resource areas, 67 | P value |
| Gender | |||||||
| Female | 269 (60.2) | 55 (55.0) | 33 (37.1) | 76 (66.7) | 51 (66.2) | 54 (80.6) | 0.000 |
| Male | 178 (39.8) | 45 (45.0) | 56 (62.9) | 38 (33.3) | 26 (33.8) | 13 (19.4) | |
| Age groups by yr | |||||||
| ≤ 40 | 193 (43.2) | 29 (29.0) | 28 (31.5) | 68 (59.6) | 27 (35.1) | 41 (61.2) | 0.000 |
| > 40 | 254 (56.8) | 71 (71.0) | 61 (68.5) | 46 (40.4) | 50 (64.9) | 26 (38.8) | |
| Education | |||||||
| Illiterate | 136 (30.4) | 28 (28.0) | 11 (12.4) | 27 (23.7) | 38 (49.3) | 32 (47.8) | 0.000 |
| Primary school | 66 (14.8) | 16 (16.0) | 12 (13.5) | 15 (13.2) | 13 (16.9) | 10 (14.9) | |
| Secondary school | 186 (41.6) | 42 (42.0) | 48 (53.9) | 51 (44.7) | 25 (32.5) | 20 (29.8) | |
| College | 59 (13.2) | 14 (14.0) | 18 (20.2) | 21 (18.4) | 1 (1.3) | 5 (7.5) | |
| Family income | |||||||
| Low | 38 (8.5) | 3 (3.0) | 1 (1.1) | 5 (4.4) | 7 (9.1) | 17 (25.3) | 0.000 |
| Intermediate | 250 (55.9) | 62 (62.0) | 25 (28.1) | 72 (63.2) | 55 (71.4) | 41 (61.2) | |
| High | 145 (32.5) | 35 (35.0) | 61 (68.5) | 34 (29.8) | 11 (14.3) | 4 (6.0) | |
| Race | |||||||
| White | 211 (47.2) | 47 (47.0) | 67 (75.3) | 33 (28.9) | 42 (54.5) | 22 (32.8) | < 0.0001 |
| Non-white | 225 (50.3) | 51 (51.0) | 20 (22.4) | 74 (64.9) | 35 (45.5) | 45 (67.2) | |
| Marital status | |||||||
| Married | 222 (49.7) | 46 (46.0) | 44 (49.4) | 59 (51.8) | 40 (51.9) | 33 (49.3) | 0.909 |
| Unmarried | 224 (50.1) | 54 (54.0) | 45 (50.6) | 55 (48.2) | 36 (46.8) | 34 (50.7) | |
| People in home | |||||||
| 1 | 39 (8.7) | 14 (14.0) | 9 (10,1) | 8 (7.0) | 7 (9.1) | 1 (1.5) | 0.000 |
| 2 | 97 (21.7) | 23 (23.0) | 28 (31.5) | 16 (14.0) | 24 (31.1) | 6 (9.0) | |
| 3 | 128 (28.6) | 34 (34.0) | 23 (25.8) | 30 (26.3) | 24 (31.2) | 17 (25.4) | |
| 4 | 94 (21.0) | 14 (14.0) | 17 (19.1) | 32 (28.1) | 8 (10.4) | 23 (34.3) | |
| 5 | 88 (19.7) | 14 (14.0) | 12 (13.5) | 28 (24.6) | 14 (18.2) | 20 (29.8) | |
Viral hepatitis perception
In the case of most categories, the majority of questions were correctly answered (varying from 56.4% to 77.3%), with the exception of the complications category where only 39.4% were answered correctly. Individuals from Southeast Viral Hepatitis Ambulatory showed the highest number of correct answers in general (66.4%), clinical manifestations (84.7%), complications (46.5%), transmission (81.4%) and prevention (80.6%). Participants from South Health Center showed the highest number of correctly answered questions regarding risk of acquiring hepatitis (68.1%) (data not shown).
Table 2 describes the correct responses towards viral hepatitis knowledge separated by populations. More than 70% of participants recognized that hepatitis is caused by viruses, the existence of HAV, HBV, HCV and the availability of vaccines for hepatitis. Additionally, more than 60% of individuals did not know that hepatitis can be caused by alcohol or medicines and that an individual cannot have the same type of hepatitis more than once, while more than 70% of participants were unaware of the existence of HDV and HEV.
Table 2.
Correct answers regarding viral hepatitis given by individuals from each group evaluated (n = 447) according to general aspects, clinical manifestations, risk of acquiring hepatitis, complications, transmission and prevention, n (%)
| Sentence | Total, n = 447 | Southeast viral hepatitis ambulatory, n = 100 | South health center, n = 89 | Northeast health center, n = 114 | Southeast low resource areas, n = 77 | Northeast low resource areas, n = 67 | P value |
| General aspects | |||||||
| Can hepatitis be caused by viruses | 321 (71.8) | 84 (84.0) | 69 (77.5) | 75 (65.8) | 48 (62.3) | 45 (67.2) | 0.005 |
| Can hepatitis be caused by bacteria | 242 (54.1) | 50 (50.0) | 19 (21.3) | 74 (64.9) | 56 (72.7) | 43 (64.2) | 0.000 |
| Can hepatitis be caused by alcohol | 172 (38.5) | 31 (31.0) | 31 (34.8) | 38 (33.3) | 34 (44.2) | 38 (56.7) | 0.006 |
| Can hepatitis be caused by medicines | 154 (34.5) | 45 (45.0) | 29 (32.6) | 32 (28.1) | 24 (31.2) | 24 (35.8) | 0.110 |
| Does hepatitis A exist | 394 (88.1) | 98 (98.0) | 78 (87.6) | 97 (85.1) | 68 (88.3) | 53 (79.1) | 0.004 |
| Does hepatitis B exist | 410 (91.7) | 99 (99.0) | 88 (98.9) | 95 (83.3) | 73 (94.8) | 55 (82.1) | 0.000 |
| Does hepatitis C exist | 359 (80.3) | 99 (99.0) | 86 (96.6) | 66 (57.9) | 61 (79.2) | 47 (70.1) | 0.000 |
| Does hepatitis D exist | 121 (27.1) | 56 (56.0) | 18 (20.2) | 10 (8.8) | 18 (23.4) | 19 (28.4) | 0.000 |
| Does hepatitis E exist | 92 (20.6) | 40 (40.0) | 15 (16.9) | 7 (6.1) | 14 (18.2) | 16 (23.9) | 0.000 |
| Does a vaccine for hepatitis exist | 376 (84.1) | 91 (91.0) | 78 (87.6) | 97 (85.1) | 58 (75.3) | 52 (77.6) | 0.026 |
| Can you have the same hepatitis more the once | 132 (29.5) | 37 (37.0) | 23 (25.8) | 37 (32.5) | 13 (16.9) | 22 (32.8) | 0.040 |
| Clinical manifestations | |||||||
| No clinical manifestations | 292 (65.3) | 89 (89.0) | 75 (84.3) | 61 (53.5) | 35 (45.5) | 32 (47.8) | 0.000 |
| After years | 311 (69.6) | 81 (81.0) | 75 (84.3) | 61 (53.5) | 47 (61.0) | 47 (70.1) | 0.000 |
| Fever discomfort, nausea | 369 (82.6) | 76 (76.0) | 67 (75.3) | 99 (86.8) | 64 (83.1) | 63 (94.0) | 0.008 |
| Jaundice, pale stools and dark urine | 410 (91.7) | 93 (93.0) | 81 (91.0) | 103 (90.4) | 72 (93.5) | 61 (91.0) | 0.922 |
| People at risk of acquiring hepatitis | |||||||
| People working in laboratory | 235 (52.6) | 61 (61.0) | 39 (43.8) | 67 (58.8) | 38 (49.4) | 30 (44.8) | 0.054 |
| People who work in hospitals | 310 (69.4) | 72 (72.0) | 58 (65.2) | 88 (77.2) | 56 (72.7) | 36 (53.7) | 0.014 |
| Not people who work in rural areas | 157 (35.1) | 36 (36.0) | 41 (46.1) | 46 (40.4) | 18 (23.4) | 16 (23.9) | 0.006 |
| People who work in the beauty areas | 353 (79.0) | 91 (91.0) | 70 (78.7) | 89 (78.1) | 57 (74.0) | 46 (68.7) | 0.007 |
| People who use drugs | 393 (87.9) | 98 (98.0) | 85 (95.5) | 96 (84.2) | 64 (83.1) | 50 (74.6) | 0.000 |
| People who receive tattoos or piercings | 389 (87.0) | 98 (98.0) | 79 (88.8) | 96 (84.2) | 64 (83.1) | 52 (77.6) | 0.001 |
| People who live indoors | 253 (56.6) | 46 (46.0) | 45 (50.6) | 66 (57.9) | 57 (74.0) | 39 (58.2) | 0.004 |
| Not people who work in offices | 299 (66.9) | 19 (19.0) | 68 (76.4) | 28 (24.6) | 26 (33.8) | 31 (46.3) | 0.000 |
| Complications | |||||||
| Cirrhosis | 361 (80.8) | 91 (91.0) | 82 (92.1) | 79 (69.3) | 62 (80.5) | 47 (70.1) | 0.000 |
| Liver cancer | 378 (84.6) | 91 (91.0) | 78 (87.6) | 95 (83.3) | 65 (84.4) | 49 (73.1) | 0.031 |
| There is no loss of body movements | 88 (19.7) | 32 (32.0) | 17 (19.1) | 27 (23.7) | 6 (7.8) | 6 (9.0) | 0.233 |
| There is no loss of blood through the mouth | 65 (14.5) | 17 (17.0) | 18 (20.2) | 16 (14.0) | 8 (10.4) | 6 (9.0) | 0.000 |
| There is no vision loss | 117 (26.2) | 30 (30.0) | 23 (25.8) | 40 (35.1) | 13 (16.9) | 11 (16.4) | 0.016 |
| There is no blood in the stool | 49 (11.0) | 18 (18.0) | 10 (11.2) | 9 (7.9) | 7 (9.1) | 5 (7.5) | 0.121 |
| Transmission | |||||||
| By transfusion and transplantation | 386 (86.4) | 94 (94.0) | 85 (95.5) | 91 (79.8) | 67 (87.0) | 49 (73.1) | 0.000 |
| By sex | 310 (69.4) | 96 (96.0) | 76 (85.4) | 64 (56.1) | 42 (54.5) | 32 (47.8) | 0.000 |
| By water and contaminated vegetables | 318 (71.1) | 88 (88.0) | 49 (55.1) | 79 (69.3) | 60 (77.9) | 42 (62.7) | 0.000 |
| By seafood | 135 (30.2) | 59 (59.0) | 17 (19.1) | 27 (23.7) | 23 (29.9) | 9 (13.4) | 0.000 |
| By tattoo and piercing | 361 (80.8) | 96 (96.0) | 76 (85.4) | 88 (77.2) | 57 (74.0) | 44 (65.7) | 0.000 |
| By cutting instruments | 385 (86.1) | 99 (99.0) | 77 (86.5) | 90 (78.9) | 66 (85.7) | 53 (79.1) | 0.005 |
| By hemodialysis | 280 (62.6) | 74 (74.0) | 58 (65.2) | 60 (52.6) | 53 (68.8) | 35 (52.2) | 0.010 |
| Cannot be by mosquito bite | 221 (49.4) | 58 (58.0) | 49 (55.1) | 60 (52.6) | 31 (40.3) | 23 (34.3) | 0.000 |
| Cannot be by air | 268 (60.0) | 69 (69.0) | 69 (77.5) | 68 (59.6) | 34 (44.2) | 28 (41.8) | 0.000 |
| Prevention | |||||||
| Building cesspools | 324 (72.5) | 78 (78.0) | 49 (55.1) | 89 (78.1) | 63 (81.8) | 45 (67.2) | 0.000 |
| Channeling water | 318 (71.1) | 76 (76.0) | 53 (59.6) | 84 (73.7) | 63 (81.8) | 42 (62.7) | 0.007 |
| Selecting uninfected donors | 363 (81.2) | 90 (90.0) | 71 (79.8) | 105 (92.1) | 58 (75.3) | 39 (58.2) | 0.000 |
| Filtering water and treating drinks | 372 (83.2) | 88 (88.0) | 57 (64.0) | 101 (88.6) | 71 (92.2) | 55 (82.1) | 0.000 |
| Killing mosquitoes does not prevent hepatitis | 189 (42.3) | 53 (53.0) | 41 (46.1) | 41 (36.0) | 33 (42.9) | 21 (31.3) | 0.029 |
| Providing vaccine | 405 (90.6) | 94 (94.0) | 80 (89.9) | 107 (93.9) | 70 (90.9) | 54 (80.6) | 0.030 |
| Using masks does not prevent hepatitis | 210 (47.0) | 69 (69.0) | 57 (64.0) | 46 (40.4) | 26 (33.8) | 12 (17.9) | 0.000 |
| Using condoms | 378 (84.6) | 97 (97.0) | 82 (92.1) | 93 (81.6) | 56 (72.7) | 50 (74.6) | 0.000 |
Clinical manifestations and risk of acquiring hepatitis questions were correctly answered by most individuals. However, work in rural areas as a risk factor in the acquisition of hepatitis was incorrectly answered by more than 60% of participants. Less than 27% of interviewees were able to associate loss of body movements, blood through the mouth, loss of vision and blood in the stool as complications of hepatitis. In addition, more than 50% of participants incorrectly answered questions about transmission by seafood, the absence of transmission by mosquito bite, and modes of prevention, such as killing mosquitoes and using masks.
In general, correct answers were more common in Southeast Viral Hepatitis Ambulatory and less common in Northeast low resource areas. In questions such as “Does hepatitis D exist?”, “Can hepatitis be transmitted by mosquito bite?”, “Can killing mosquitoes prevent viral hepatitis?” and “Does using masks prevent hepatitis?” less than 50% were correctly answered by all participants but more than 50% of such questions were correctly answered in Southeast Viral Hepatitis Ambulatory (Table 2).
Less than 10% of correct answers were observed in questions such as “Do hepatitis D and E exist?” in Northeast Health Center, “Is loss of body movement a complication of hepatitis?” in Southeast and Northeast low resource areas, and “Is blood in the stool a complication of hepatitis?” in Northeast Health Center, Southeast and Northeast low resource areas (Table 2).
In bivariate analysis of answered questions, some were not significant, such as those informing whether hepatitis can be caused by medicines, whether jaundice, pale stools and dark urine are clinical manifestations of hepatitis, whether people working in laboratories are at risk of infection, and whether loss of blood through the mouth or blood in the stool are complications of infection (P = 0.110, P = 0.922, P = 0.054, P = 0.233 and P = 0.121, respectively) (Table 2).
Figure 1 shows the distribution of correct answers in each population; the highest number of correct answered were found in the Southeast Viral Hepatitis Ambulatory group and the lowest number in Northeast low resource areas. Also, it was possible to observe a larger dispersion of correct-answered in Northeast low resource areas.
Figure 1.
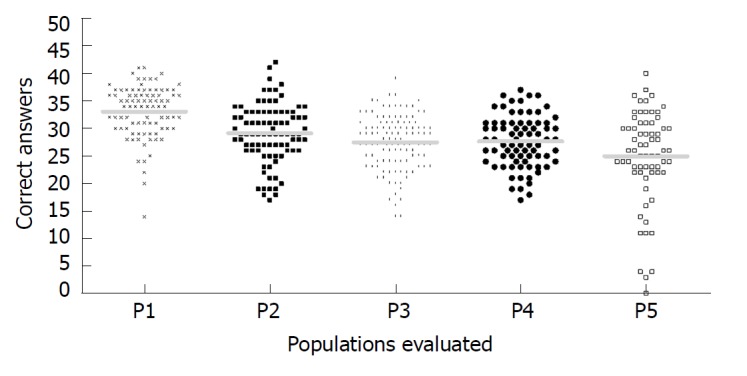
Distribution of correct answers plotted according to each population evaluated. The y-axis represents the number of correct answers. The solid lines represent the average for P1 (Southeast Viral Hepatitis Ambulatory), P2 (South Health Center), P3 (Northeast Health Center), P4 (Southeast low resource areas) and P5 (Northeast low resource areas), which were respectively: 33.1 ± 4.5; 29.1 ± 5.3; 27.5 ± 5.0; 27.6 ± 4.7; and, 25.0 ± 8.5.
In 19 questions, it was necessary to determine which hepatitis type was related to the participant’s response; only in three of them were more than 50% of the participants able to correctly identify at least one of the related hepatitis types. The percentage of incorrect answers (i.e. did not know, did not respond, or did not associate the correct hepatitis type with the question) from these three questions were 14.5% for “Selecting uninfected donors is hepatitis prevention”, 40.3% for “Can hepatitis be transmitted by air?” and 41.2% for “Which hepatitis types have a vaccine?”. For the other questions, the percentage of wrong answers varies from 50.6% (“Can hepatitis be transmitted by hemodialysis?”) to 88.1% (“Can hepatitis be transmitted by seafood?”) (data not shown).
Perception about viral hepatitis
The average of correct answers from all individuals was 28.7 ± 6.1- which was considered as the cut off value in this analysis; in this way, scores from 0 to 28 were considered “low” and scores of 29 to 46 were considered “desirable”. Only Southeast Viral Hepatitis Ambulatory and South Health Center demonstrated a desirable knowledge (30.5 ± 5.0 and 29.5 ± 5.6, respectively) (Table 3).
Table 3.
Sociodemographic characteristics according to knowledge scores for viral hepatitis, n (%)
| Item | Mean of knowledge score (± SD) |
Knowledge score |
Bivariate analysis P value | |
| Low (0-28), n = 192 | Desirable (29-46), n = 255 | |||
| Gender | ||||
| Female | 28.49 ± 6.16 | 120 (62.5) | 149 (58.4) | 0.430 |
| Male | 29.04 ± 6.10 | 72 (37.5) | 106 (41.6) | |
| Age in yr | ||||
| ≤ 40 | 27.6 ± 6.6 | 91 (47.4) | 102 (40.0) | 0.120 |
| > 40 | 27.5 ± 8.5 | 101 (52.6) | 153 (60.0) | |
| Education level | ||||
| Illiterate | 25.4 ± 8.3 | 74 (38.5) | 62 (24.3) | 0.002 |
| Primary school | 28.1 ± 5.1 | 32 (16.7) | 34 (13.3) | |
| Secondary school | 30.9 ± 5.7 | 66 (34.4) | 120 (47.1) | |
| College | 30.8 ± 5.4 | 20 (10.4) | 39 (15.3) | |
| Family income | ||||
| Low | 26.1 ± 6.9 | 21 (10.9) | 17 (6.7) | 0.200 |
| Indeterminate | 29.4 ± 7.0 | 105 (54.7) | 145 (56.9) | |
| High | 30.3 ± 5.5 | 57 (29.7) | 88 (34.5) | |
| Race | ||||
| White | 29.8 ± 7.1 | 79 (41.1) | 132 (51.8) | 0.030 |
| Non-white | 28.2 ± 6.3 | 107 (55.7) | 118 (46.3) | |
| Marital status | ||||
| Married | 28.4 ± 7.1 | 94 (48.9) | 128 (50.2) | 0.840 |
| Unmarried | 27.3 ± 8.0 | 97 (50.5) | 127 (49.8) | |
| Individuals living in the same home | ||||
| 1 | 30.5 ± 5.0 | 11 (5.7) | 28 (11.0) | 0.014 |
| 2 | 29.5 ± 5.6 | 41 (21.4) | 56 (22.0) | |
| 3 | 28.3 ± 6.3 | 63 (32.8) | 65 (25.5) | |
| 4 | 28.8 ± 6.6 | 31 (16.1) | 63 (24.7) | |
| ≥ 5 | 27.6 ± 6.4 | 46 (24.0) | 42 (16.5) | |
| Population | ||||
| Southeast viral hepatitis ambulatory | 33.1 ± 4.5 | 13 (6.8) | 87 (34.1) | < 0.0001 |
| South health center | 29.1 ± 5.3 | 37 (19.3) | 52 (20.4) | |
| Northeast health center | 27.5 ± 5.0 | 58 (30.2) | 56 (22.0) | |
| Southeast low resource areas | 27.6 ± 4.7 | 43 (22.4) | 34 (13.3) | |
| Northeast low resource areas | 25.0 ± 8.5 | 41 (21.3) | 26 (10.2) | |
Regarding the rate of correct answers, 255 (57.0%) individuals scored above average, with 87 (87.0%) from Southeast Viral Hepatitis Ambulatory, 52 (58.4%) from South Health Center, 56 (49.1%) from Northeast Health Center, 34 (44.1%) from Southeast low resource areas, and 26 (39.4%) from Northeast low resource areas.
The caveats of gender, age, marital status and number of people in the home were associated with approximately the same average number of correct answers. The majority of the individuals with both low and desirable scores received an intermediate family income; however, a lower average number of correct answers was observed in individuals who received low family income.
Desirable perception was more common among females (58.4%), subjects aged over 40 years (60.0%), with a secondary education (47.1%), receiving intermediate family income (56.9%), declaring themselves white (51.8%), married (50.2%) and living in houses with three individuals (25.5%), and belonging to Southeast Viral Hepatitis Ambulatory (34.1%) (Table 3).
Perception was associated only with education level, race, individuals living in the same home and populations in bivariate analysis (Table 3). In multivariate analysis, population-type was found to be statistically significant (Table 4).
Table 4.
Final adjusted model of multivariate logistic regression for knowledge scores for viral hepatitis
| Variable |
Knowledge score |
|||
| OR |
95%CI |
P value | ||
| Lower | Upper | |||
| Education level | ||||
| Illiterate | 2.230 | 1.084 | 4.586 | 0.290 |
| Primary school | 1.799 | 0.807 | 4.009 | 0.151 |
| Secondary school | 1.028 | 0.528 | 2.002 | 0.936 |
| College | 1.000 | - | - | - |
| Individuals living in the same home | ||||
| 1 | 1.000 | - | - | - |
| 2 | 1.611 | 0.671 | 3.867 | 0.286 |
| 3 | 2.328 | 0.992 | 5.465 | 0.052 |
| 4 | 0.818 | 0.332 | 2.017 | 0.663 |
| ≥ 5 | 1.832 | 0.748 | 4.486 | 0.185 |
| Population | ||||
| Southeast viral hepatitis ambulatory | 1.000 | - | - | - |
| South health center | 6.154 | 2.900 | 13.058 | 0.000 |
| Northeast health center | 8.617 | 4.177 | 17.777 | 0.000 |
| Southeast low resource areas | 7.491 | 3.508 | 15.994 | 0.000 |
| Northeast low resource areas | 11.262 | 5.007 | 25.327 | 0.000 |
CI: Confidence interval; OR: Odds ratio.
Southeast Viral Hepatitis Ambulatory showed a higher number of desirable scores while underprivileged communities showed a lower number of desirable scores compared to low scores in the same areas. Medical centers also present a larger proportion of desirable scores compared to low scores though this was less pronounced (Figure 2).
Figure 2.
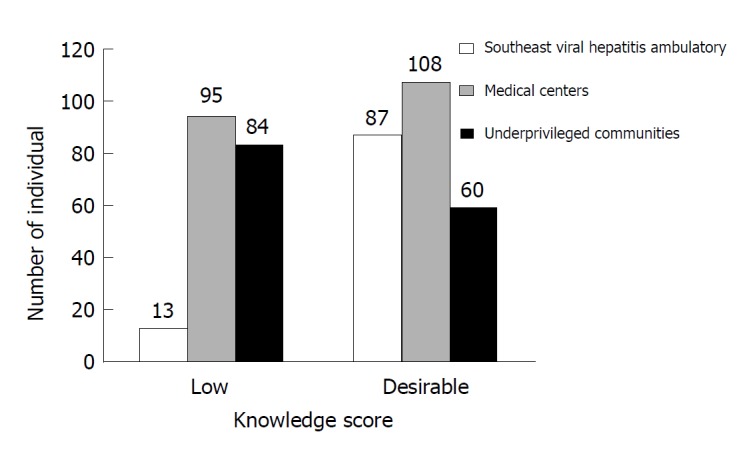
Number of individuals according to knowledge score in each group evaluated.
DISCUSSION
In the present study, knowledge level was scored according to the mean number of correct answers. Individuals from Southeast Viral Hepatitis Ambulatory and South Health Center showed a desirable knowledge in contrast to those recruited at Northeast Health Center, Southeast and Northeast low resource areas. The findings of the current study are in line with previous findings[5,22,24]. However, the study in Egypt noted high baseline knowledge about HCV[23], likely due to the scale of the HCV epidemic in this country.
Complications arising from viral hepatitis was the worst set of questions evaluated in the current study. Although more than 80% of participants can correctly correlate cirrhosis and liver cancer with complications of viral hepatitis, most of them related complications that are not caused by hepatitis. In previous studies between health professionals, more than half of participants answered correctly to the questions about HCV complications[26]. However, an insufficient knowledge regarding HCV complications was observed in a study among health professionals[27]. Clinical manifestations of viral hepatitis were the best set of questions evaluated, contrary to previous observations[28].
In the present study, most individuals recognize the existence of HAV, HBV and HCV and do not recognize the existence of hepatitis D or E. The same finding has previously been observed in Brazil[24]. Another study[28] observed a very weak knowledge regarding the five hepatitis types among medical science students. Transmission and prevention modes were correctly answered in general; this data was also observed among medical and health science students in Ethiopia in the evaluation of HBV knowledge[21]. A large number of individuals do not know that viral hepatitis can be transmitted by seafood, as observed previously[24]. Since HAV and HEV can be transmitted in this way[29,30], the transmission may continue if preventive measures are not taken.
Viral hepatitis transmission by mosquito and forms to prevent it were incorrectly answered by most participants, probably due to the country-wide presence of Dengue virus, the transmission of which is widely understood by the public. Most individuals did not cite transmission by air but, curiously, masks to avoid airborne contamination were cited. These questions highlight the need for raising awareness among the public to reinforce knowledge related to the modes of transmission and prevention.
In present study, population type was the significant demographic factor associated with knowledge level in multivariate analysis, the same as found in other studies[5,24,31]. Contrary to a previous general population study in Brazil[24], monthly family income had no association with knowledge in the present study.
The results obtained in the present study can be used as a data source for the projection of intervention methods in health and public health policies, such as explanatory educational leaflet, educational booklets, lectures in schools, health campaigns, health fairs and others, in order to increase access to information of viral hepatitis and possibly to reduce the number of cases of these infections, especially among individuals from low-resource areas that showed a lower level of knowledge in the present study.
The present study has some limitations. The study did not assess the information regarding the neighborhood of each participant to observe the sociodemographic diversity. The study did not assess the occupation of participants to categorize and compare with studies in specific groups, such as health or beauty professionals. In Viral Hepatitis Ambulatory and in medical centers, it was not asked whether participants had previously consulted and whether they had any prior knowledge about hepatitis.
In conclusion, in general, desirable knowledge was observed among most participants. However, Northeast Health Center and under-privileged communities showed low knowledge. Knowledge levels were associated with education level, race and number of individuals living in the same home. The results of the present study should prove useful for information and prevention campaigns targeted at the general population, especially between neglected communities, in order to reduce the transmission of viral hepatitis.
ARTICLE HIGHLIGHTS
Research background
Viral hepatitis is an important public health problem in the world, causing more than 1 million deaths annually. It is important to evaluate viral hepatitis perception to identify the possible gaps and help public health authorities to create strategies to increase access to information about these infections.
Research motivation
Few studies have been done to evaluate viral hepatitis perception in uninfected individuals, particularly in Latin America.
Research objectives
The main aim of this study was to evaluate the viral hepatitis knowledge among individuals from different resource areas and health conditions in Brazil to identify possible gaps and help authorities in the development of prevention and education programs.
Research methods
This was a cross-sectional study, wherein a questionnaire to evaluate viral hepatitis perception was applied among 447 individuals from five different populations in Brazil (Southeast low resource areas, Northeast low resource areas, South Health Center, Northeast Health Center, Southeast Viral Hepatitis Center). The viral hepatitis perception score was created based on the average of correct answers of all participants’ responses (28.7), and associations between sociodemographic characteristics and perception were also evaluated.
Research results
High perception level about viral hepatitis was observed in Southeast Viral Hepatitis Ambulatory and South Health Center compared to Northeast Health Center, Southeast and Northeast low resource areas. According to sociodemographic characteristics, desirable scores were more common among those with secondary education (47.1%), those who declared themselves as white (46.3%), and those who lived in houses with three individuals (25.5%). Population type was associated with knowledge level in multivariate analysis.
Research conclusions
The study demonstrated a low level of perception about viral hepatitis among individuals from low resource areas. Identifying the knowledge gaps in this group could help to create strategies for increasing access to information and consequently reducing the transmission of these diseases.
Research perspectives
This study demonstrates that it is necessary to improve the access to health information about viral hepatitis, especially among residents of low-resource settings. It is important to conduct a random sampling evaluation of larger numbers of individuals to confirm the results observed. A questionnaire could help to conduct these studies, the same as was used in the present work.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support of CAPES, CNPq and FAPERJ, and the volunteers who agreed to participate in this study.
Footnotes
Institutional review board statement: The study was reviewed and approved by Fiocruz Ethics Committee.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflicts of interest to report.
Data sharing statement: No additional data are available.
STROBE statement: The manuscript was revised according to the STROBE statement.
Manuscript source: Unsolicited manuscript
Peer-review started: April 18, 2018
First decision: May 11, 2018
Article in press: October 8, 2018
Specialty type: Gastroenterology and Hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Gigi E, Pokorska-Spiewak M, Shenoy SM, Nozic D S- Editor: Wang JL L- Editor: Filipodia E- Editor: Bian YN
Contributor Information
Helena Medina Cruz, Laboratory of Viral Hepatitis, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro 21040360, Brazil.
Jakeline Ribeiro Barbosa, Laboratory of Viral Hepatitis, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro 21040360, Brazil; Postgraduate Program in Pathology, Federal University of Ceará, Fortaleza, Ceará 60020181, Brazil.
Jeová Keny Baima Colares, Postgraduate Program in Pathology, Federal University of Ceará, Fortaleza, Ceará 60020181, Brazil; Postgraduate Program in Medical Sciences, University of Fortaleza, Ceará 60430160, Brazil.
Antonio Henrique Almeida de Moraes Neto, Laboratory of Innovations in Therapies, Teaching and Bioproducts, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro 21040360, Brazil.
Maria de Fátima Leal Alencar, Laboratory of Innovations in Therapies, Teaching and Bioproducts, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro 21040360, Brazil.
Francisco Inácio Bastos, Institute of Communication and Scientific Information and Technology for Health, Oswaldo Cruz Foundation, Rio de Janeiro 21040900, Brazil.
Jurema Corrêa da Mota, Institute of Communication and Scientific Information and Technology for Health, Oswaldo Cruz Foundation, Rio de Janeiro 21040900, Brazil.
Filipe Aníbal Carvalho-Costa, Laboratory of Epidemiology and Molecular Systematics, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro 21040900, Brazil.
Claudia Alexandra Pontes Ivantes, Orientation and Counselling Centre, Curitiba, Paraná 80810070, Brazil.
Lia Laura Lewis-Ximenez, Laboratory of Viral Hepatitis, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro 21040360, Brazil.
Livia Melo Villar, Laboratory of Viral Hepatitis, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro 21040360, Brazil. lvillar@ioc.fiocruz.br.
References
- 1.Focaccia R. Tratado de hepatites virais e doenças associadas. 3rd ed. São Paulo: Editora Atheneu; 2013. [Google Scholar]
- 2.World Health Organization. Health topics Hepatitis, 2017. Available From URL: http://www.who.int/topics/hepatitis/en/ [Google Scholar]
- 3.Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. In: Atkinson W, Hamborsky J, Wolfe S, editors. 12th ed. Washington DC: Public Health Foundation; 2012. [Google Scholar]
- 4.Pereira LM, Martelli CM, Merchán-Hamann E, Montarroyos UR, Braga MC, de Lima ML, Cardoso MR, Turchi MD, Costa MA, de Alencar LC, et al. Population-based multicentric survey of hepatitis B infection and risk factor differences among three regions in Brazil. Am J Trop Med Hyg. 2009;81:240–247. [PubMed] [Google Scholar]
- 5.ul Haq N, Hassali MA, Shafie AA, Saleem F, Farooqui M, Aljadhey H. A cross sectional assessment of knowledge, attitude and practice towards Hepatitis B among healthy population of Quetta, Pakistan. BMC Public Health. 2012;12:692. doi: 10.1186/1471-2458-12-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komatsu H, Inui A, Sogo T, Tateno A, Shimokawa R, Fujisawa T. Tears from children with chronic hepatitis B virus (HBV) infection are infectious vehicles of HBV transmission: experimental transmission of HBV by tears, using mice with chimeric human livers. J Infect Dis. 2012;206:478–485. doi: 10.1093/infdis/jis293. [DOI] [PubMed] [Google Scholar]
- 7.Ogholikhan S, Schwarz KB. Hepatitis Vaccines. Vaccines (Basel) 2016;4:E6. doi: 10.3390/vaccines4010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villar LM, Cruz HM, Barbosa JR, Bezerra CS, Portilho MM, Scalioni Lde P. Update on hepatitis B and C virus diagnosis. World J Virol. 2015;4:323–342. doi: 10.5501/wjv.v4.i4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, An J, Tu A, Liang X, Cui F, Zheng H, Tang Y, Liu J, Wang X, Zhang N, et al. Comparison of immune persistence among inactivated and live attenuated hepatitis a vaccines 2 years after a single dose. Hum Vaccin Immunother. 2016;12:2322–2326. doi: 10.1080/21645515.2015.1134069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debing Y, Moradpour D, Neyts J, Gouttenoire J. Update on hepatitis E virology: Implications for clinical practice. J Hepatol. 2016;65:200–212. doi: 10.1016/j.jhep.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Pang L, Alencar FE, Cerutti C Jr, Milhous WK, Andrade AL, Oliveira R, Kanesa-Thasan N, MaCarthy PO, Hoke CH Jr. Short report: hepatitis E infection in the Brazilian Amazon. Am J Trop Med Hyg. 1995;52:347–348. doi: 10.4269/ajtmh.1995.52.347. [DOI] [PubMed] [Google Scholar]
- 12.Paraná R, Vitvitski L, Andrade Z, Trepo C, Cotrim H, Bertillon P, Silva F, Silva L, de Oliveira IR, Lyra L. Acute sporadic non-A, non-B hepatitis in Northeastern Brazil: etiology and natural history. Hepatology. 1999;30:289–293. doi: 10.1002/hep.510300143. [DOI] [PubMed] [Google Scholar]
- 13.Trinta KS, Liberto MI, de Paula VS, Yoshida CF, Gaspar AM. Hepatitis E virus infection in selected Brazilian populations. Mem Inst Oswaldo Cruz. 2001;96:25–29. doi: 10.1590/s0074-02762001000100004. [DOI] [PubMed] [Google Scholar]
- 14.Santos DC, Souto FJ, Santos DR, Vitral CL, Gaspar AM. Seroepidemiological markers of enterically transmitted viral hepatitis A and E in individuals living in a community located in the North Area of Rio de Janeiro, RJ, Brazil. Mem Inst Oswaldo Cruz. 2002;97:637–640. doi: 10.1590/s0074-02762002000500007. [DOI] [PubMed] [Google Scholar]
- 15.Lyra AC, Pinho JR, Silva LK, Sousa L, Saraceni CP, Braga EL, Pereira JE, Zarife MA, Reis MG, Lyra LG, et al. HEV, TTV and GBV-C/HGV markers in patients with acute viral hepatitis. Braz J Med Biol Res. 2005;38:767–775. doi: 10.1590/s0100-879x2005000500015. [DOI] [PubMed] [Google Scholar]
- 16.Ataei B, Shirani K, Alavian SM, Ataie M. Evaluation of Knowledge and Practice of Hairdressers in Women’s Beauty Salons in Isfahan About Hepatitis B, Hepatitis C, and AIDS in 2010 and 2011. Hepat Mon. 2013;13:e6215. doi: 10.5812/hepatmon.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu E, Chen X, Guan Z, Cao C, Rao H, Feng B, Chan M, Fu S, Lin A, Wei L, et al. A comparative study of patients’ knowledge about hepatitis C in the United States and in urban and rural China. Hepatol Int. 2015;9:58–66. doi: 10.1007/s12072-014-9559-z. [DOI] [PubMed] [Google Scholar]
- 18.Maniero VC, Goldbach T, Marques APC, Cavaretto LSP, Santos AMO, Villar LM. Evaluation of the knowledge of nursing students about viral hepatitis. J Nurs UFPE on line. 2012;6:831–838. [Google Scholar]
- 19.Villar LM, de Paula VS, de Almeida AJ, do Ó KM, Miguel JC, Lampe E. Knowledge and prevalence of viral hepatitis among beauticians. J Med Virol. 2014;86:1515–1521. doi: 10.1002/jmv.23993. [DOI] [PubMed] [Google Scholar]
- 20.Ganczak M, Dmytrzyk-Daniłów G, Korzeń M, Drozd-Dąbrowska M, Szych Z. Prevalence of HBV Infection and Knowledge of Hepatitis B Among Patients Attending Primary Care Clinics in Poland. J Community Health. 2016;41:635–644. doi: 10.1007/s10900-015-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdela A, Woldu B, Haile K, Mathewos B, Deressa T. Assessment of knowledge, attitudes and practices toward prevention of hepatitis B virus infection among students of medicine and health sciences in Northwest Ethiopia. BMC Res Notes. 2016;9:410. doi: 10.1186/s13104-016-2216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouard C, Gautier A, Saboni L, Jestin C, Semaille C, Beltzer N; KABP France group. Hepatitis B knowledge, perceptions and practices in the French general population: the room for improvement. BMC Public Health. 2013;13:576. doi: 10.1186/1471-2458-13-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemaitelly H, Abu-Raddad LJ, Miller FD. An apparent lack of epidemiologic association between hepatitis C virus knowledge and the prevalence of hepatitis C infection in a national survey in Egypt. PLoS One. 2013;8:e69803. doi: 10.1371/journal.pone.0069803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz HM, de Paula VS, Villar LM. A Cross-Sectional Study of Viral Hepatitis Perception among Residents from Southeast and North Regions of Brazil. Int J Environ Res Public Health. 2018;15:E189. doi: 10.3390/ijerph15020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Instituto Brasileiro de Geografia e Etatística. Portal do IBGE. Available from: https://www.ibge.gov.br/index.php.
- 26.Sood A, Midha V, Awasthi G. Hepatitis C--knowledge & practices among the family physicians. Trop Gastroenterol. 2002;23:198–201. [PubMed] [Google Scholar]
- 27.Joukar F, Mansour-Ghanaei F, Soati F, Meskinkhoda P. Knowledge levels and attitudes of health care professionals toward patients with hepatitis C infection. World J Gastroenterol. 2012;18:2238–2244. doi: 10.3748/wjg.v18.i18.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghahramani F, Mohammadbeigi A, Mohammadsalehi N. A survey of the students’ knowledge about hepatitis in Shiraz University of Medical Sciences. Hepat Mon. 2006;6:59–62. [Google Scholar]
- 29.Polo D, Varela MF, Romalde JL. Detection and quantification of hepatitis A virus and norovirus in Spanish authorized shellfish harvesting areas. Int J Food Microbiol. 2015;193:43–50. doi: 10.1016/j.ijfoodmicro.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Cui W, Sun Y, Xu A, Gao R, Gong L, Zhang L, Jiang M. Hepatitis E seroprevalence and related risk factors among seafood processing workers: a cross-sectional survey in Shandong Province, China. Int J Infect Dis. 2016;49:62–66. doi: 10.1016/j.ijid.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 31.ul Haq N, Hassali MA, Shafie AA, Saleem F, Farooqui M, Haseeb A, Aljadhey H. A cross-sectional assessment of knowledge, attitude and practice among Hepatitis-B patients in Quetta, Pakistan. BMC Public Health. 2013;13:448. doi: 10.1186/1471-2458-13-448. [DOI] [PMC free article] [PubMed] [Google Scholar]


