Abstract
Nitrated oleic acid (NO2-OA) was first identified in 2003, and after the characterization of its formation and thiol reactivity, it was used as a prototypical molecule to investigate the physiological actions of endogenous nitrated fatty acids (NO2-FA). Based on in vitro observations showing significant activation of cytoprotective and anti-inflammatory signaling responses by NO2-FA, experiments were designed to determine their pharmacological potential. Supported by strong intellectual protection and favorable pharmacokinetic and pharmacodynamic data, 10-NO2-OA (CXA-10) underwent pharmaceutical development as a drug to treat fibrotic and inflammatory diseases. NO2-FA are at the intersection of three unconventional drug candidate classes that include 1) fatty acids, 2) metabolic intermediates and 3) electrophilic molecules. These three groups use different scaffolds for drug development, are characterized by broad activities and are individually gaining traction as alternatives to mono-target drug therapies. In particular, NO2-FA share key characteristics with currently approved pharmacological agents regarding reactivity, distribution, and mechanism of action. This review first presents the characteristics, liabilities, and opportunities that these different drug candidate classes display, and then discusses these issues in the context of current progress in the preclinical and clinical development of NO2-FA as drugs. Lessons learned from the novel approaches presented herein were considered early on during development to structurally define and improve NO2-FA and their disease targets.
Keywords: Nitroalkene, nitro-fatty acid, electrophiles, clinical trial, preclinical development, bardoxolone-me, dimethyl fumarate, Nrf2, Nf-kB, fibrosis, inflammation
NO2-FA therapy at the intersection of other successful pharmacological approaches.
Three drug classes and their intrinsic pharmacological characteristics share commonalities with NO2-FA. Within this framework, the properties of CXA-10, a specific regioisomer of NO2-OA (10-nitro-octadec-9-enoic acid) is presently being clinically evaluated (Fig 1A) and is discussed herein.
Fig. 1.
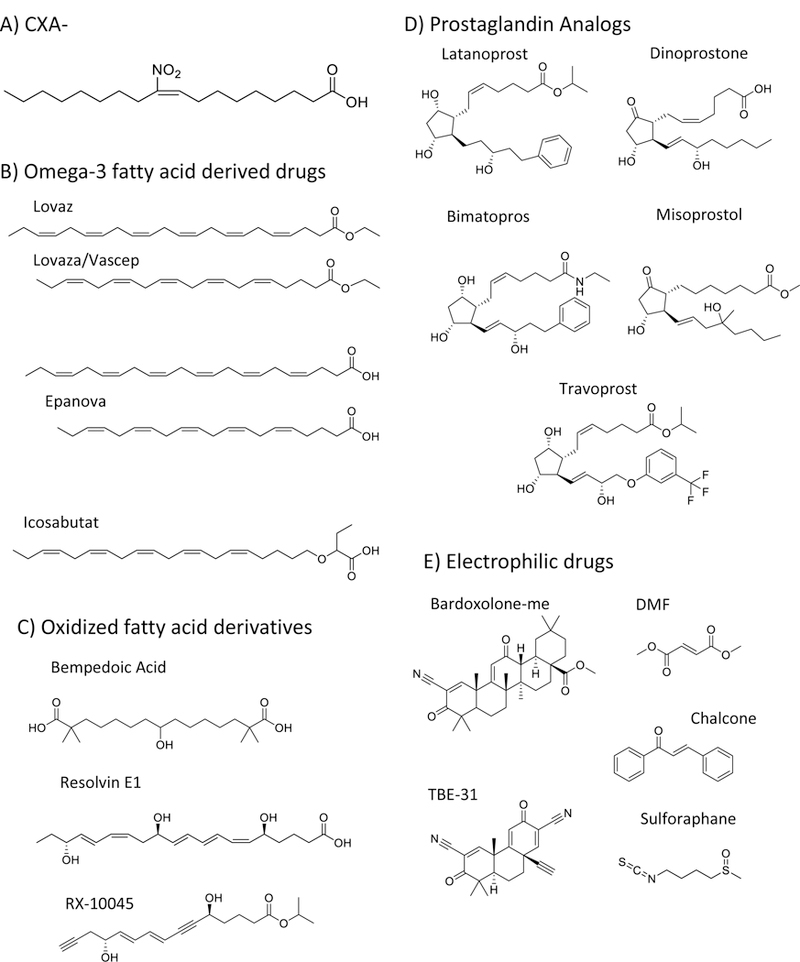
Chemical structures of CXA-10 and other related, clinically used, drugs that share functional, structural and signaling features.
1.1. Fatty acids as metabolic and anti-inflammatory drugs.
Fatty acid-based pharmacological approaches can be broadly divided into omega-3 fatty acids, modified fatty acids, and oxidized fatty acids.
Dietary omega-3 fatty acids as drugs.
Omega-3 fatty acid and fish oil supplementation have shown protective effects in some but not all large clinical trials related to cardiovascular disease (1–5), but were strongly associated with significant reductions in plasma triglyceride levels (6–9). While some trials have failed to demonstrate protective effects (10–14), pre-clinical and clinical data have still motivated pharmaceutical companies to develop treatments based on these molecules. Initially approved by the FDA in 2004, Lovaza (GSK, previously Omacor, Reliant Pharmaceuticals) and later Vascepa (Amarin Pharma) and Epanova (AstraZeneca) are effective at treating hypertriglyceridemic subjects (>500 mg/dL) (Fig 1B)(15,16). These pharmacological benefits are characterized by decreased de novo lipogenesis and increased fatty acid oxidation (17–21). However, it is important to note that large daily doses of omega-3 fatty acids are required, with 4 gr/day being the recommended treatment (16). In terms of delivery, these fatty acids were formulated either as ethyl esters (Lovaza and Vascepa) or as free acids (Epanova), a decision likely based more prominently on intellectual property than on pharmacokinetic reasons, as the absorption of ethyl ester derivatives is actually lower than that of the free acid precursors (22). Nevertheless, the effectiveness of omega-3-based therapies to control severe hypertriglyceridemia underscores the validity and appropriateness of the use of fatty acids as drugs. Perhaps the main liability of omega-3 based supplementation is the relatively high therapeutic dose. Although no major side effects are reported, these drugs are associated with increased incidence of diarrhea, nausea and abdominal discomfort, which greatly impacts treatment compliance.
Beta-oxidation protected fatty acids:
Based on the aforementioned liabilities of omega-3 fatty acid approaches and the experience gathered by inhibitor studies of key enzymes participating in the lipogenic program, a second generation of drugs was developed. Unlike their omega-3 fatty acid predecessors, these derivatives are characterized by structural modifications that inhibit beta-oxidation, the main route for fatty acid catabolism. These modifications extended half-life and permitted the use of significantly lower doses. Two drugs that characterize this group are bempedoic acid and icosabutate (Fig 1C-D). Bempedoic acid, a dicarboxylic fatty acid, recently finished a 12-week, global, pivotal, Phase 3 randomized, double-blind, placebo-controlled, multicenter study that evaluated its efficacy and safety as add-on therapy in patients at risk or with existing atherosclerotic cardiovascular disease. The study revealed a 23 % reduction in LDL-C from baseline (23). Icosabutate, a structurally engineered omega-3 fatty acid resistant to both beta-oxidation and complex lipids incorporation, was developed by Pronova BioPharma and tested as a drug to treat hypertriglyceridemia (Fig 1B)(24–26). Clinical studies demonstrated a significant reduction in triglyceride and cholesterol levels. However, a prolongation of the QT interval observed in a 12-month dog study that ran in parallel to the clinical trial led the FDA to establish an exposure limit and consequently to the termination of the study by the sponsor (25). Nevertheless, both bempedoic and icosabutate (now being evaluated for non-alcoholic steatohepatitis) are still in clinical trials and share a similar pharmacological strategy with regards to the introduction of chemical modifications to prevent fatty acid metabolism and increase their potency.
Oxidized fatty acids:
Oxidized fatty acids such as prostaglandins, thromboxanes, leukotrienes, and prostacyclins are endogenously synthesized by lipoxygenases and cyclooxygenases and have important roles in physiology and pathology. For example, Prostaglandin E2 (dinoprostone) and its synthetic analog misoprostol are effective at dilating the cervix in pregnant women during labor (Fig 1D). Similarly, other prostaglandin analogs such as latanoprost, bimatoprost, misoprostol, and travoprost have shown promise for the treatment of open-angle glaucoma and high pressure in the eye (Fig 1D)(27). Vasoactive prostacyclin analogs, such as epoprostenol, treprostinil, and selexipag, administered as IV, subcutaneous, inhaled and oral formulations, have proven beneficial in the treatment of pulmonary arterial hypertension to reduce pulmonary vascular resistance (28,29). Unfortunately, side effects such as headaches, diarrhea, flushing and both muscle and joint pain can result in significant patient intolerability to the treatment (30,31). Other bioactive lipids, represented by the resolvin family, are proposed to modulate inflammation and adaptive repair mechanisms (32). These molecules have significant pharmacokinetic and development liabilities, including rapid metabolism, a consequent short half-life and a high cost of synthesis. Thus, Phase 1 clinical trials conducted by Resolvyx using the resolvin analog RX-10045 (Fig 1C) focused on dry eye syndrome as a target disease. This approach provided a favorable pharmacological opportunity based on the small doses required (topical eye drops), slower metabolism by this particular tissue compartment and the potent anti-inflammatory properties of resolvins. Nevertheless, the trials were unsuccessful and both the approach and the lead compound were abandoned (33).
This overview of fatty acid-based drug strategies exemplifies the challenges and opportunities in this area, with rapid metabolism (omega-3 fatty acids, resolvins) and cost of synthesis (“resolvins” and prostaglandin analogs) being significant decision drivers. As a result, improved scaffolds have been designed that are now being tested in Phase 2 and 3 clinical trials.
1.2. Use of metabolic intermediates as tissue-protective drugs.
Fumarate is a metabolite of the Krebs cycle, synthesized from succinate by succinate dehydrogenase and metabolized to malate by fumarase. Interestingly, cardiac-specific fumarase knock-out mice exhibit increased cardiac levels of fumarate, upregulated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)-dependent gene expression and protection against ischemia/reperfusion injury (34). Nrf2 is stringently regulated by electrophilic compounds and induces Phase 2 detoxification responses (35). Fumarates play an important role in intermediary metabolism and were recognized early on to have pharmacological value (36). A seminal observation in 1959 by a German pharmacist that suffered from psoriasis led to the development of a fumarate-based dermal treatment for this condition under the name of Fumaderm (37). The pharmacological success of dimethyl fumarate (DMF, Fig 1E) in treating psoriasis, together with its favorable safety profile, led investigators to evaluate this molecule in other conditions characterized by exacerbated immune responses (38). DMF showed significant protection in experimental autoimmune encephalomyelitis (EAE) a preclinical animal model of multiple sclerosis (39,40), prompting the execution of large multi-center phase II and III studies of relapsing-remitting multiple sclerosis (41–43). It is important to recognize that DMF is an electrophilic molecule that exerts many of its pharmacological actions through Michael addition with thiols and activation of Nrf2-dependent gene expression (39). Nevertheless, it has recently been shown that monomethyl fumarate (MMF) also activates the hydroxycarboxylic acid receptor 2 (HCA2, GPR109A, also known as niacin receptor 1 [NIACR1]), a member of the nicotinic acid receptor family. In this regard, MMF is ten times less potent than niacin at activating this receptor, but several orders of magnitude stronger than DMF (44). Importantly, whereas oral administration of DMF protects against EAE, this protection was lost in mice lacking HCA2 expression (45). Moreover, DMF was equally protective against EAE in both wild-type and Nrf2−/− mice, suggesting that the observed beneficial effects of DMF in MS are independent of Nrf2 activation (46).
1.3. Electrophilic small molecules as drugs.
Electrophilicity is the main characteristic of the third class of molecules sharing pharmacological properties with NO2-FA. This is a broad group of structures composed of naturally occurring compounds and their synthetic derivatives containing one or more electron-deficient carbons. Chalcone, flavonoid, isothiocyanate, α−β unsaturated carbonyl and polyphenol-containing molecules all activate Nrf2-dependent gene expression and exert protective effects both in vitro and in preclinical models of disease (Fig 1E)(47–49). Despite protective effects in preclinical model systems, the pharmacological development of naturally-occurring electrophiles and their synthetic homologs is hampered by difficulty in obtaining intellectual property protection. In this regard, the development of bardoxolone-methyl (bardoxolone-me) is a notable exception to these limitations. In this case, the addition of two electrophilic groups to a triterpenoid molecule with otherwise modest Nrf2 activating properties resulted in the generation of a much more potent electrophile (50,51). More specifically, the presence of the cyanoenone moiety allows bardoxolone-me to activate the expression of Nrf2-dependent genes at nanomolar concentrations, with the reactivity of the electrophilic group being a key factor in the potency of engineered triterpenoids. Moreover, small mono, di, and tricyclic structures have been created that retain much of the potency of bardoxolone-me (Fig 1E)(52,53). In this regard, the tricyclic bis(cyano enone) TBE-31 is a strong Nrf2 activator that yielded promising results in preclinical animal models but is yet to be tested in human trials (53).
Despite positive results in a Phase II clinical trial, bardoxolone-me failed a Phase III study aimed at improving renal function in patients with type 2 diabetes and stage 4 chronic kidney disease, with the trial being terminated due to a higher rate of adverse cardiovascular events in the bardoxolone-me group (54). Nonetheless, Reata Pharmaceuticals reported that post-hoc analysis of the trial data demonstrated preservation of kidney function (55). In addition, preliminary data from the follow-up TSUBAKI study showed improved renal function as assessed by inulin clearance (56). As a result, the development strategy for bardoxolone-me has been redirected, with new clinical trials testing efficacy towards the treatment of connective tissue disease-associated pulmonary arterial hypertension (Phase III) and Alport syndrome (Phase II/III) that are now in progress. .
Whereas bardoxolone-me was chemically engineered to strongly increase its electrophilicity, plant-derived sulforaphane is a less reactive electrophile obtained from broccoli sprouts upon enzymatic hydrolysis of glucoraphanin by the enzyme myrosinase (57). Large clinical trials have shown that sulforaphane is a promising therapy for preventing liver carcinogenesis induced by aflatoxin in endemic areas of the province of Qidong in China (58). Aflatoxin is activated in the liver and alkylates cellular components, inducing mutations and cancer development (59). Electrophilic drugs targeting activation of Nrf2 induce the expression of over 100 Phase 2 genes, thus allowing for more efficient detoxification of aflatoxin and protection against carcinogenesis (60,61). In this regard, Evgen Pharma has now successfully stabilized sulforaphane using an α-cyclodextrin-based formulation, a key step towards the evaluation of sulforaphane in clinical trials.
2. Development of nitrated fatty acid as drugs.
Nitrated fatty acids share many of the characteristics of the aforementioned molecules, while at the same time representing a new approach to treat inflammatory and fibrotic diseases. Although NO2-OA is a minor component of the endogenous nitrolipidome, this species offers key advantages for testing in preclinical models. Regarding synthesis, equimolar mixtures of the positional isomers 9- and 10-NO2-OA (NO2-OA) can be obtained by a relatively simple nitroselenylation strategy. In addition, the synthesis of specific regioisomers can be performed by nitro-aldol condensation followed by acetylation and elimination steps. As a result, NO2-OA and the specific isomer CXA-10 were synthesized in sufficiently large quantities to perform pharmacokinetic, metabolic and preclinical experiments. The protective effects obtained in a wide variety of preclinical models of disease propelled the development of nitrated fatty acids as anti-inflammatory and anti-fibrotic drug candidates.
2.1. NO2-FA metabolism as a determinant of pharmacological potency
Consistent with their scaffold, NO2-FA undergo many of the same metabolic transformations as non-nitrated fatty acids. In this regard, studies performed in both rodents and humans demonstrated that NO2-FAs are substrates for omega- and beta-oxidation, as well as for esterification into complex lipids (62–65). Interestingly, many of the products obtained from these reactions retain electrophilic reactivity suggesting that a given NO2-FA can give rise to the formation of an array of secondary species with potentially different compartmentalization and pharmacokinetic profiles. In the case of esterification into complex lipids, the incorporation of electrophilic NO2-FAs into the non-polar environment of a membrane or a lipid droplet prevents these species from reacting with both protein and low molecular weight thiols, thus effectively creating an intracellular NO2-FA reservoir. Furthermore, gut incorporation of orally administered NO2-FAs into lipoproteins could represent a major pathway for their tissue distribution. Although there is ample evidence supporting NO2-FA incorporation into complex lipids in vitro and in vivo, the actual contributions of this stabilized pool to the biological actions of these molecules remain to be established. In contrast to esterification, beta-oxidation of NO2-FA gives rise to the formation of a series of shorter and progressively more polar electrophilic derivatives which, in the case of NO2-OA, can be as short as eight carbons long. In principle, the generation of shorter NO2-FA derivatives should facilitate their reaction with targets in more polar compartments, and thus it is possible that these active metabolites might be responsible for some of the effects ascribed to NO2-OA. In this regard, and although specific NO2-OA beta-oxidation products have not been studied, recent evidence indicates that changes in both the fatty acyl chain length and the position of the nitroalkene group have profound effects on biological activity with shorter alpha chains correlating with stronger Nrf2 induction and shorter omega chains leading to more potent NF-κB inhibition (66).
Besides processing of the fatty acyl chain, NO2-FAs are also subject to phase II metabolism. NO2-FA reduction to corresponding non-electrophilic nitroalkane derivatives by prostaglandin reductase-1 (PtGR-1) is the main pathway for the termination of electrophilicity and downstream signaling actions (67). Notably, PtGR-1 itself is a Nrf2-regulated gene that can be induced by NO2-OA, thus suggesting that chronic administration of this molecule might be faced with increasingly more efficient inactivation reactions. In addition, given the role of PtGR-1 in prostaglandin synthesis, it is possible for NO2-FAs to indirectly modulate eicosanoid-mediated signaling events. In addition to irreversible inhibition by PtGR-1, intracellular NO2-FAs are also conjugated to glutathione and excreted via multidrug resistance-associated proteins (MRPs), which upon further processing by endopeptidases give rise to the formation of cysteine- and N-acetylcysteine adducts (68). Notably, the reversibility of the nitroalkene-thiol adduct allows for the regeneration of free NO2-FA in urine, most likely as a result of the low levels of free thiols in this compartment.
Metabolism is an important determinant of the bioactivity and potency of electrophilic drugs and has been a significant differentiation factor for CXA-10. For example, while DMF is significantly more electrophilic than its proximal metabolite MMF, the former is rapidly degraded in the gastrointestinal tract, with native DMF undetectable in plasma after oral administration (69). Electrophiles are expected to covalently interact with blood protein thiols, adding complexity to PK studies. Sulforaphane derivatives have been shown to react with hemoglobin and albumin, which led to the development of a novel methodological approach to assess isothiocyanate exposure in humans (70). Cysteine 34 (Cys34) in human albumin is the most abundant nucleophile in plasma, with other plasma thiols significantly lower in concentration. Thus, for sulforaphane, blood protein thiols represent a sink and inactivation mechanism. In contrast, CXA-10 follows distribution mechanisms common to fatty acids, associating non-covalently with albumin. This interaction does not involve a reaction with Cys34 but rather a binding to hydrophobic pockets in the protein, thus effectively shielding CXA-10 from inactivation by adventitious thiol reactions in plasma (71). Taken together, it is clear that these so-called “non-specific” reactions and degradation pathways affect drug availability and provide differentiation between classes of electrophilic pharmacological agents.
2.2. Preclinical development of NO2-OA
The preclinical disease model responses to NO2-FA administration has been addressed in the other reviews of this series, thus we will briefly discuss NO2-FA administration and dosing. NO2-OA is an oily substance at room temperature and insoluble in water. Therefore, initial murine model studies utilized intraperitoneal (i.p.) injections of NO2-FA solvated in DMSO and subcutaneous administration via osmotic mini-pumps. While daily i.p. injections posed minimal problems, formulation of NO2-OA for mini-pump delivery was more challenging. To this end, a polyethylene glycol-400 (PEG-400) formulation was devised and used in 14-day mini-pumps, achieving NO2-OA steady-state concentrations of 8–30 nM when 8 mg/kg/day doses were used. In addition, oil-based stabilization of CXA-10 led to the successful development of oral administration schemes later used to redefine dosing in preclinical animal models, pharmacokinetic and pharmacodynamic studies, and toxicological evaluations.
2.3. Clinical development of CXA-10 as a drug candidate
Intellectual property related to NO2-FA composition of matter and methods of use has been licensed by Complexa Inc. (https://www.complexarx.com/) as part of their mission to develop NO2-FA as tissue protective, anti-fibrotic and anti-inflammatory drug candidates. The lead compound for development in acute and chronic settings is CXA-10 (10-NO2-OA, a specific positional isomer of NO2-OA). Although initially focused on acute kidney injury with particular interest in contrast-induced nephropathy, the primary pharmacological targets were later changed to focal segmental glomerulosclerosis and pulmonary arterial hypertension based on promising preclinical studies (72,73).
Intravenous formulation:
Complexa Inc. successfully developed a formulation for intravenous CXA-10 administration that proved effective in treating acute kidney injury related to ischemia/reperfusion in animal models. The favorable preclinical ADME and toxicological data supported an IND submission for Phase Ia and Phase Ib clinical trials. From these trials, it was learned that the IV formulation is well tolerated in humans and that it engaged signaling pathways previously identified in academic studies (74). More specifically, CXA-10 increased Nrf2- and heat shock-dependent gene expression in peripheral blood mononuclear cells, which correlated with kidney-derived urinary exosome biomarkers measured in patients with chronic renal failure (75).
Oral formulation:
Based on the promising pharmacokinetics and bioavailability observed in Phase 1 studies of oral CXA-10, a program was started that focused on the treatment of chronic diseases via the more practical administration of an oral formulation. Radioactive tracing studies using [14C]-CXA-10 given orally to rats showed at least 35% absorption. These studies revealed a biodistribution profile of CXA-10 consistent with lipoprotein-dependent tissue delivery, with CXA-10 and its metabolites predominantly distributed in liver, heart, kidney and adipose tissue. This is of relevance when deciding disease targets, as CXA-10 appears to be the only electrophilic drug candidate in development that is delivered to organs that are characterized by high metabolic rates. Importantly, these tissues are normally exposed to higher levels of respiration-derived oxidants and therefore would likely benefit from increased expression of Nrf2-dependent Phase II genes. In this regard, the high metabolic rates of liver, heart, kidney and adipose tissue point to a central role of mitochondria in the etiology of several pathogenic conditions in these tissues. Of relevance, CXA-10 and NO2-OA are tissue-protective during ischemia and reperfusion in part because of specific interactions with mitochondrial proteins and respiratory complexes, resulting in the regulation of mitochondrial function (76,77).
Biomarkers of CXA-110 signaling pathway engagement in Phase 1 trials of oral formulations:
CXA-10 is being developed as a treatment for chronic inflammatory and metabolic-related diseases such as focal segmental glomerulosclerosis and pulmonary arterial hypertension. Therefore, it was important to demonstrate bioavailability, acceptable pharmacokinetics and pathway engagement in a relevant human population. Two-week dosing of obese individuals with oral CXA-10 formulation not only decreased serum inflammatory biomarkers [monocyte chemotactic protein-1 (MCP-1) and interleukin-6 (IL-6)] but also improved other indices of metabolic dysfunction, by decreasing serum leptin, triglyceride, and cholesterol levels (75). These results are consistent with the observation that Nrf2 plays an important role in metabolic regulation and in particular, lipid metabolism (78). In this regard, pharmacological activation of Nrf2 by TBE-31 resulted in significant protection in non-alcoholic liver steatosis models, an effect that was absent in Nrf2−/− mice (79). Moreover, Nrf2 deletion results in a marked exacerbation of liver steatosis in animals challenged with a methionine- and choline-deficient diet (80). The successful outcomes in a variety of models are rationalized through the pleiotropy of effects that NO2-OA and CXA-10 have exerted in the different models.
Conclusions
Historically considered as liabilities, electrophilic compounds are gaining traction as viable candidates for drug development (81). In fact, in 2011 three out of the ten top drugs by sales volume and about 30 % of drugs in the market were covalent inhibitors that modify their target proteins (82–84). As such, the translation of CXA-10 into clinical therapeutics is based on the protective effects observed in several animal models of inflammatory and metabolic stress studied by different laboratories over the past 15 years. Both the structure and reactivity play a role in the beneficial outcomes and lack of adverse effects reported for CXA-10. With regards to these characteristics, similarities are shared among different classes of pharmacological agents. A unique quality of CXA-10, as opposed to other electrophilic species currently in the drug development pipeline is that CXA-10 gains access to remote tissues after incorporation into lipoproteins as glycerol esters. This protected hydrophobic compartment provides an ability to transport the active drug to target tissues while limiting Michael addition reactions with adventitious nucleophiles in the vascular compartment. The activation of Nrf2 and heat shock factor-regulated gene expression and the inhibition of NF-κB-dependent expression of pro-inflammatory cytokines after CXA-10 oral administration reinforces the potential value of CXA-10 as a treatment for chronic inflammatory/fibrotic diseases. These are often areas with clear unmet clinical needs in which traditional approaches have failed to deliver improvement (75). Data supporting this vision should become available within the next two years as data from ongoing Phase 2 trials becomes available.
Highlights.
Nitrated fatty acids (NO2-FA) share structural and signaling features with other clinically used drugs
Systemic NO2-FA tissue delivery as triglycerides and non-covalently bound albumin complexes provides chemical stabilization
Private development of the lead compound CXA-10 (10-NO2-OA) was based on solid academic biochemical and preclinical work.
Oral and intravenous formulations were developed for human use by Complexa Inc.
Formulations demonstrated tolerability, safety and predicted pathway engagement in relevant populations.
Ongoing Phase II clinical trials will determine the therapeutic potential of CXA-10 in areas of pulmonary and renal disease
Acknowledgments
The authors acknowledge the following funding sources: NIH R37HL058115, P01-HL103455, R01-HL132550 (BAF), K01-HL133331 (DAV), and R01-GM125944, R01-DK112854, American Heart Association (17GRN33660955) (FJS).
Abbreviations:
- NO2-OA
nitro-oleic acid
- NO2-FA
nitro-fatty acid
- CXA-10
10-nitro-oleic acid (10-nitro-octadec-9-enoic acid)
- DMF
dimethyl fumarate
- MMF
(monomethylfumarate)
- NF-κB
nuclear factor κB
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- HSF-1
heat shock factor 1
- LDL-C
low density lipoprotein cholesterol
- (PtGR-1)
prostaglandin reductase-1
- (PEG-400)
polyethylene glycol-400
- (MCP-1)
monocyte chemotactic protein-1
Footnotes
Declaration of interest
BAF, DAV, DKJ and FJS acknowledge financial interest in Complexa Inc.
References
- 1.De Caterina R (2011) n-3 fatty acids in cardiovascular disease. N Engl J Med 364, 2439–2450 [DOI] [PubMed] [Google Scholar]
- 2.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. (2008) Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372, 1223–1230 [DOI] [PubMed] [Google Scholar]
- 3.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. (2002) Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 105, 1897–1903 [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098 [DOI] [PubMed] [Google Scholar]
- 5.(1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 354, 447–455 [PubMed] [Google Scholar]
- 6.Maki KC, Orloff DG, Nicholls SJ, Dunbar RL, Roth EM, Curcio D, et al. (2013) A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther 35, 1400–1411 e1401–1403 [DOI] [PubMed] [Google Scholar]
- 7.Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, et al. (2007) Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther 29, 1354–1367 [DOI] [PubMed] [Google Scholar]
- 8.Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, and Soni PN (2011) Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol 108, 682–690 [DOI] [PubMed] [Google Scholar]
- 9.Brinton EA, and Mason RP (2017) Prescription omega-3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids Health Dis 16, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Investigators OT, Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, et al. (2012) n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 367, 309–318 [DOI] [PubMed] [Google Scholar]
- 11.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, and Elisaf MS (2012) Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 308, 1024–1033 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell F (2012) Diabetes: basal insulin and n-3 fatty acids--no effect on cardiovascular outcomes. Nat Rev Endocrinol 8, 446. [DOI] [PubMed] [Google Scholar]
- 13.Kromhout D, Giltay EJ, Geleijnse JM, and Alpha Omega Trial G (2010) n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 363, 2015–2026 [DOI] [PubMed] [Google Scholar]
- 14.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. (2010) OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 122, 2152–2159 [DOI] [PubMed] [Google Scholar]
- 15.Weintraub HS (2014) Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad Med 126, 7–18 [DOI] [PubMed] [Google Scholar]
- 16.Bradberry JC, and Hilleman DE (2013) Overview of omega-3 Fatty Acid therapies. P T 38, 681–691 [PMC free article] [PubMed] [Google Scholar]
- 17.Borkman M, Chisholm DJ, Furler SM, Storlien LH, Kraegen EW, Simons LA, et al. (1989) Effects of fish oil supplementation on glucose and lipid metabolism in NIDDM. Diabetes 38, 1314–1319 [DOI] [PubMed] [Google Scholar]
- 18.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Ampah SB, Saville BR, et al. (2014) Fish oil supplementation ameliorates fructose-induced hypertriglyceridemia and insulin resistance in adult male rhesus macaques. J Nutr 144, 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris WS, and Bulchandani D (2006) Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol 17, 387–393 [DOI] [PubMed] [Google Scholar]
- 20.Dagnelie PC, Rietveld T, Swart GR, Stijnen T, and van den Berg JW (1994) Effect of dietary fish oil on blood levels of free fatty acids, ketone bodies and triacylglycerol in humans. Lipids 29, 41–45 [DOI] [PubMed] [Google Scholar]
- 21.Khan S, Minihane AM, Talmud PJ, Wright JW, Murphy MC, Williams CM, et al. (2002) Dietary long-chain n-3 PUFAs increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. J Lipid Res 43, 979–985 [PubMed] [Google Scholar]
- 22.Kataoka Y, Uno K, Puri R, and Nicholls SJ (2013) Epanova((R)) and hypertriglyceridemia: pharmacological mechanisms and clinical efficacy. Future Cardiol 9, 177–186 [DOI] [PubMed] [Google Scholar]
- 23.Esperion. (2018) Esperion Announces Positive Top-Line Results from First Pivotal Phase 3 Study of Bempedoic Acid (http://investor.esperion.com/releasedetail.cfm?ReleaseID=1059993).
- 24.Riesen WF (2016) Icosabutate, a More Potent Form of Omega 3 Fatty Acids, Shows Promise in Lowering Triglycerides. Cardiology 135, 1–2 [DOI] [PubMed] [Google Scholar]
- 25.Kastelein JJ, Hallen J, Vige R, Fraser DA, Zhou R, Hustvedt SO, et al. (2016) Icosabutate, a Structurally Engineered Fatty Acid, Improves the Cardiovascular Risk Profile in Statin-Treated Patients with Residual Hypertriglyceridemia. Cardiology 135, 3–12 [DOI] [PubMed] [Google Scholar]
- 26.Bays HE, Hallen J, Vige R, Fraser D, Zhou R, Hustvedt SO, et al. (2016) Icosabutate for the treatment of very high triglycerides: A placebo-controlled, randomized, double-blind, 12-week clinical trial. J Clin Lipidol 10, 181–191 e181–182 [DOI] [PubMed] [Google Scholar]
- 27.Klimko PG, and Sharif NA (2018) Discovery, characterization, and clinical utility of prostaglandin agonists for the treatment of glaucoma. Br J Pharmacol [DOI] [PMC free article] [PubMed]
- 28.McLaughlin VV, and Palevsky HI (2013) Parenteral and inhaled prostanoid therapy in the treatment of pulmonary arterial hypertension. Clin Chest Med 34, 825–840 [DOI] [PubMed] [Google Scholar]
- 29.Taichman DB, Ornelas J, Chung L, Klinger JR, Lewis S, Mandel J, et al. (2014) Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest 146, 449–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, et al. (2013) Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 127, 624–633 [DOI] [PubMed] [Google Scholar]
- 31.O’Connell C, Amar D, Boucly A, Savale L, Jais X, Chaumais MC, et al. (2016) Comparative Safety and Tolerability of Prostacyclins in Pulmonary Hypertension. Drug Saf 39, 287–294 [DOI] [PubMed] [Google Scholar]
- 32.Serhan CN, Chiang N, and Dalli J (2017) New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med, 1–17 [DOI] [PMC free article] [PubMed]
- 33.Hesselink JM, Chiosi F, and Costagliola C (2016) Resolvins and aliamides: lipid autacoids in ophthalmology - what promise do they hold? Drug Des Devel Ther 10, 3133–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, et al. (2012) Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab 15, 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sihvola V, and Levonen AL (2017) Keap1 as the redox sensor of the antioxidant response. Arch Biochem Biophys 617, 94–100 [DOI] [PubMed] [Google Scholar]
- 36.W. S (1959) Heilung von Psoriasis vulgaris. Med Monatschr 13, 103–104 [PubMed] [Google Scholar]
- 37.Nieboer C, de Hoop D, van Loenen AC, Langendijk PN, and van Dijk E (1989) Systemic therapy with fumaric acid derivates: new possibilities in the treatment of psoriasis. J Am Acad Dermatol 20, 601–608 [DOI] [PubMed] [Google Scholar]
- 38.Reich K, Thaci D, Mrowietz U, Kamps A, Neureither M, and Luger T (2009) Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis--a retrospective study (FUTURE). J Dtsch Dermatol Ges 7, 603–611 [DOI] [PubMed] [Google Scholar]
- 39.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, et al. (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–692 [DOI] [PubMed] [Google Scholar]
- 40.Schilling S, Goelz S, Linker R, Luehder F, and Gold R (2006) Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol 145, 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kappos L, Giovannoni G, Gold R, Phillips JT, Arnold DL, Hotermans C, et al. (2015) Time course of clinical and neuroradiological effects of delayed-release dimethyl fumarate in multiple sclerosis. Eur J Neurol 22, 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. (2012) Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 43.Gold R, Phillips JT, Havrdova E, Bar-Or A, Kappos L, Kim N, et al. (2015) Delayed-Release Dimethyl Fumarate and Pregnancy: Preclinical Studies and Pregnancy Outcomes from Clinical Trials and Postmarketing Experience. Neurol Ther 4, 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang H, Lu JY, Zheng X, Yang Y, and Reagan JD (2008) The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist. Biochem Biophys Res Commun 375, 562–565 [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, et al. (2014) Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest 124, 2188–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, et al. (2016) Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A 113, 4777–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar H, Kim IS, More SV, Kim BW, and Choi DK (2014) Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep 31, 109–139 [DOI] [PubMed] [Google Scholar]
- 48.Wu RP, Hayashi T, Cottam HB, Jin G, Yao S, Wu CC, et al. (2010) Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 107, 7479–7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, and Forman HJ (2009) Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health 25, 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liby KT, and Sporn MB (2012) Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev 64, 972–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, and Gribble GW (2011) New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod 74, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Zheng S, Higgins M, Morra RP Jr., Mendis AT, Chien CW, et al. (2015) New Monocyclic, Bicyclic, and Tricyclic Ethynylcyanodienones as Activators of the Keap1/Nrf2/ARE Pathway and Inhibitors of Inducible Nitric Oxide Synthase. J Med Chem 58, 4738–4748 [DOI] [PubMed] [Google Scholar]
- 53.Honda T, Yoshizawa H, Sundararajan C, David E, Lajoie MJ, Favaloro FG Jr., et al. (2011) Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents. J Med Chem 54, 1762–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. (2013) Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369, 2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chin MP, Bakris GL, Block GA, Chertow GM, Goldsberry A, Inker LA, et al. (2018) Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am J Nephrol 47, 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nangaku M, Shimazaki R, and Akizawa T (2017) Bardoxolone Methyl Improved GFR Measured by Standard Inulin Clearance: The TSUBAKI Study. in Kidney Week 2017, New Orleans, LA. [Google Scholar]
- 57.Palliyaguru DL, Yuan JM, Kensler TW, and Fahey JW (2018) Isothiocyanates: Translating the Power of Plants to People. Mol Nutr Food Res, e1700965 [DOI] [PMC free article] [PubMed]
- 58.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, et al. (2014) Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila) 7, 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kensler TW, Roebuck BD, Wogan GN, and Groopman JD (2011) Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci 120 Suppl 1, S28–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, et al. (2012) Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis 33, 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinkova-Kostova AT, Fahey JW, Kostov RV, and Kensler TW (2017) KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci Technol 69, 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salvatore SR, Vitturi DA, Fazzari M, Jorkasky DK, and Schopfer FJ (2017) Evaluation of 10-Nitro Oleic Acid Bio-Elimination in Rats and Humans. Sci Rep 7, 39900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, et al. (2009) Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem 284, 1461–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fazzari M, Khoo N, Woodcock SR, Li L, Freeman BA, and Schopfer FJ (2015) Generation and esterification of electrophilic fatty acid nitroalkenes in triacylglycerides. Free Radic Biol Med 87, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fazzari M, Khoo NK, Woodcock SR, Jorkasky DK, Li L, Schopfer FJ, et al. (2017) Nitro-fatty acid pharmacokinetics in the adipose tissue compartment. J Lipid Res 58, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khoo NKH, Li L, Salvatore SR, Schopfer FJ, and Freeman BA (2018) Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-kappaB signaling:A medicinal chemistry investigation of structure-function relationships. Sci Rep 8, 2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vitturi DA, Chen CS, Woodcock SR, Salvatore SR, Bonacci G, Koenitzer JR, et al. (2013) Modulation of nitro-fatty acid signaling: prostaglandin reductase-1 is a nitroalkene reductase. J Biol Chem 288, 25626–25637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salvatore SR, Vitturi DA, Baker PR, Bonacci G, Koenitzer JR, Woodcock SR, et al. (2013) Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. J Lipid Res 54, 1998–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Litjens NH, Burggraaf J, van Strijen E, van Gulpen C, Mattie H, Schoemaker RC, et al. (2004) Pharmacokinetics of oral fumarates in healthy subjects. Br J Clin Pharmacol 58, 429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar A, and Sabbioni G (2010) New biomarkers for monitoring the levels of isothiocyanates in humans. Chem Res Toxicol 23, 756–765 [DOI] [PubMed] [Google Scholar]
- 71.Turell L, Vitturi DA, Coitiño EL, Lebrato L, Moller MN, Sagasti C, et al. (2016) The Chemical Basis of Thiol Addition to Nitro-Conjugated Linoleic Acid, a Protective Cell-Signaling Lipid. Journal of Biological Chemistry [DOI] [PMC free article] [PubMed]
- 72.Kelley EE, Baust J, Bonacci G, Golin-Bisello F, Devlin JE, St Croix CM, et al. (2014) Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc Res 101, 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jobbagy S, and Tan RJ (2018) Nitrolipids in kidney physiology and disease. Nitric Oxide [DOI] [PMC free article] [PubMed]
- 74.Complexa. (2016) Complexa Announces Successful Completion of Four Phase 1 Studies of CXA-10 and Planned Phase 2 Initiation in Multiple Orphan Indications (https://www.complexarx.com/nov12).
- 75.Chieffo C, Botbyl J, Perry K, Blok TM, and Jorkasky DK (2016) Use of An Obese Population in Phase 1 to Evaluate the Pharmacology of Oral CXA-10, an Endogenous, Nitrofatty Acid Signaling Agent. in 2016 Annual Meeting of the American College of Clinical Pharmacology, Bethesda, MD [Google Scholar]
- 76.Nadtochiy SM, Baker PR, Freeman BA, and Brookes PS (2009) Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res 82, 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koenitzer JR, Bonacci G, Woodcock SR, Chen CS, Cantu-Medellin N, Kelley EE, et al. (2016) Fatty acid nitroalkenes induce resistance to ischemic cardiac injury by modulating mitochondrial respiration at complex II. Redox Biol 8, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitteringham NR, Abdullah A, Walsh J, Randle L, Jenkins RE, Sison R, et al. (2010) Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteomics 73, 1612–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma RS, Harrison DJ, Kisielewski D, Cassidy DM, McNeilly AD, Gallagher JR, et al. (2018) Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell Mol Gastroenterol Hepatol 5, 367–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, et al. (2010) Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med 48, 357–371 [DOI] [PubMed] [Google Scholar]
- 81.Bauer RA (2015) Covalent inhibitors in drug discovery: from accidental discoveries to avoided liabilities and designed therapies. Drug Discov Today 20, 1061–1073 [DOI] [PubMed] [Google Scholar]
- 82.Singh J, Petter RC, Baillie TA, and Whitty A (2011) The resurgence of covalent drugs. Nat Rev Drug Discov 10, 307–317 [DOI] [PubMed] [Google Scholar]
- 83.De Cesco S, Kurian J, Dufresne C, Mittermaier AK, and Moitessier N (2017) Covalent inhibitors design and discovery. Eur J Med Chem 138, 96–114 [DOI] [PubMed] [Google Scholar]
- 84.Robertson JG (2005) Mechanistic basis of enzyme-targeted drugs. Biochemistry 44, 5561–5571 [DOI] [PubMed] [Google Scholar]


