Abstract
Rheumatoid arthritis is a chronic autoimmune disease affecting typically synovial joints and leading to progressive articular damage, disability, and reduced quality of life. Despite better recent therapeutic strategies improving long-term outcomes, RA is associated with a high rate of comorbidities, infections, malignancies, and cardiovascular disease (CVD). Remarkably, some well-known pathogenic proinflammatory mediators in RA, such as interleukin-1β (IL-1β) and tumor necrosis factor (TNF), may play a pivotal role in the development of CVD. Interestingly, different preclinical and clinical studies have suggested that biologic agents commonly used to treat RA patients may be effective in improving CVD. In this context, the contribution of adipocytokines has been suggested. Adipocytokines are pleiotropic molecules, mainly released by white adipose tissue and immune cells. Adipocytokines modulate the function of different tissues and cells, and in addition to energy homeostasis and metabolism, amplify inflammation, immune response, and tissue damage. Adipocytokines may contribute to the proinflammatory state in RA patients and development of bone damage. Furthermore, they could be associated with the occurrence of CVD. In this study, we reviewed available evidence about adipocytokines in RA, because of their involvement in disease activity, associated CVD, and possible biomarkers of prognosis and treatment outcome and because of their potential as a possible new therapeutic target.
1. Introduction
Rheumatoid arthritis is a chronic autoimmune disease affecting typically synovial joints and leading to progressive articular damage, disability, and reduced quality of life [1–4]. RA is associated with an increased rate of comorbidities, including infections, malignancies, and cardiovascular disease (CVD), leading to the excess of mortality experienced by these patients [5–7]. Remarkably, a close association between RA and accelerated atherosclerosis has been highlighted, due to the interaction between traditional cardiovascular (CV) risk factors and proinflammatory pathways [8–11]. Furthermore, the fact that traditional CV risk factors are underdiagnosed and undertreated may increase the atherosclerotic process [12, 13]. In addition, some well-known pathogenic proinflammatory mediators in RA, such as interleukin-1β (IL-1β) and tumor necrosis factor (TNF), may play a pivotal role in the development of CVD [14–16]. In fact, common pathogenic inflammatory pathways between the atherosclerotic process and rheumatic diseases have been shown [16–18]. Different reports have suggested that biologic DMARDs, commonly used to treat RA patients, may be effective in improving CV comorbidities [19, 20]. In this context, the contribution of adipocytokines has been suggested [21]. Adipocytokines are pleiotropic molecules, mainly released by white adipose tissue and by immune cells [21, 22]. Adipocytokines modulate the function of different tissues and cells, amplifying inflammation, immune response, and tissue damage [21]. During RA, adipocytokines could contribute to the proinflammatory state, develop bone damage, and accelerate concomitant atherosclerosis [22–25].
In this study, we reviewed available evidence about adipocytokines in RA, because of their involvement in disease activity, associated CVD, and possible biomarkers of prognosis and treatment outcome and because of their potential as possible new therapeutic targets.
2. Methods
We designed a narrative review aimed at providing an overview about leptin, adiponectin, resistin, and visfatin in RA, because of their involvement in disease activity, associated cardiometabolic diseases, and possible biomarkers of prognosis and treatment outcome and because of their potential as possible new therapeutic targets. We performed an analysis of available evidence linking the same molecule to joint damage and cardiometabolic comorbidities, in order to discuss previous studies but also to provide a rationale for further researches. MEDLINE (via PubMed) was searched and the bibliography of relevant articles was also hand searched for identification of other potentially suitable studies.
3. Adipocytokines in RA: Generality, Pathogenic Mechanisms, and Changing Pattern to Treatment
3.1. Leptin
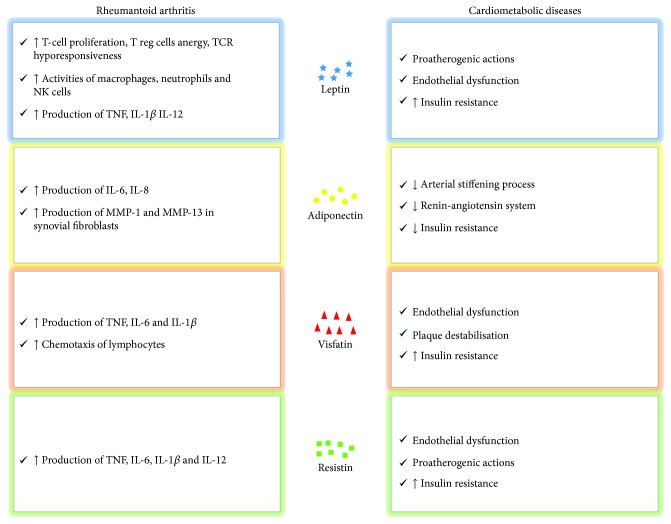
Leptin is a 16 kDa nonglycosylated adipocytokine with a long-helix structure and it is one of the most common adipocyte-derived molecules [26]. Leptin shows different biological actions deriving from an activation of OB-Rb long-form isoform receptors, which are encoded by the diabetes (db) gene [27]. Acting on hypothalamic nuclei, leptin decreases food intake and increases energy consumption, via induction of anorexigenic factors and suppression of orexigenic neuropeptides [28]. Furthermore, leptin is involved in both innate and adaptive immune responses being its production influenced by proinflammatory mediators [21–23]. Specifically, this adipocytokine exerts proinflammatory activities upregulating the production of TNF, IL-6, IL-1β, and IL-12, which, in turn, increase the expression of leptin in adipose tissue [21, 28, 29]. Leptin modulates the activity of innate immune cells by (i) enhancing the phagocytic activity of monocytes/macrophages; (ii) stimulating chemotaxis and release of reactive oxygen species by neutrophils; and (iii) promoting NK cell differentiation, proliferation, activation, and cytotoxicity [27, 30–32]. Concerning the effects on adaptive immune cells, leptin is able to (i) stimulate proliferation of naïve T lymphocytes and activate B cells; (ii) shift the T-cell cytokine production towards a Th1 phenotype, increasing the production of IFN-γ and IL-2; and (iii) induce regulatory T-cell anergy and T-cell receptor-reduced responsiveness [33–35]. As shown by a recent meta-analysis, circulating leptin levels are significantly higher in RA patients compared with controls [36]. Furthermore, it has been reported that obese RA patients showed an increased production of leptin according to ACPA positivity, thus suggesting that leptin could favour the humoral response against citrullinated proteins [37]. In addition, Olam et al. assessed the ratio between serum leptin levels and the synovial fluid [38]. Synovial/serum leptin ratio was significantly higher in RA patients and correlated with disease duration, disease activity, proinflammatory cytokines, and acute phase reactants [38]. However, conflicting results are also available in the literature and future studies are needed to elucidate the pathogenic role of leptin in RA [39, 40]. In fact, although this adipocytokine is considered to be proinflammatory, it has also been reported to be associated with reduced radiographic joint damage and this effect could be related to the anabolic effects of leptin [39, 40].
Recently, many studies assessed the effects of biologic DMARDs on leptin in RA, considering a relevant issue the changing pattern of this molecule after treatments [41–43]. RA patients treated by TNFi were investigated for leptin levels, assessing serum levels before and after such treatment [42, 43]. Interestingly, leptin levels did not change, suggesting that the beneficial effect of TNFi therapy on CVD outcomes in RA could not be mediated by a reduction of leptin [44, 45]. In fact, no significant modification was observed assessing leptin levels during therapy with adalimumab, etanercept, and infliximab [41–45]. However, these studies should be cautiously interpreted because the number of enrolled patients was relatively small.
3.2. Adiponectin
Adiponectin is a 244-residue protein, also known as GBP28, apM1, Acrp30, or AdipoQ, and it is mainly synthesised by adipose tissue [46]. This adipocytokine increases fatty acid oxidation and glucose uptake in the muscle and reduces the synthesis of glucose in the liver, acting via 2 receptors, AdipoR1 and AdipoR2, found in skeletal muscle and liver, respectively [47]. Ablation of the adiponectin gene has a dramatic effect in knockout mice on a high-fat/high-sucrose diet, inducing insulin resistance and lipid accumulation in muscles [46, 47]. Mirroring animal models, adiponectin levels are lower in obese patients and higher in patients losing weight [48, 49]. On the contrary, adiponectin and its receptors increase during physical activities [50]. Furthermore, the secretion of adiponectin is inhibited by proinflammatory cytokines, suggesting that inflammation may contribute to hypoadiponectinemia in insulin resistance and obesity [51].
In rheumatic diseases, adiponectin could act as a proinflammatory mediator in joints and it could be involved in matrix degradation [52, 53]. During RA, adiponectin and AdipoR1 expressions were higher in the synovial fluids and synovial tissues of patients compared with those of controls [54]. In this study, many cells derived from RA synovial fluids and tissues, including synovial fibroblasts, showed adiponectin, adipoR1, and adipoR2. Interestingly, the addition of adiponectin to cultures of synovial fibroblasts increased the production of proinflammatory cytokines, such as IL-6 and IL-8 [54]. The stimulation with adiponectin also contributed to the production of metalloproteinases, such as MMP-1 and MMP-13, by RA synovial fibroblasts [55]. Furthermore, adiponectin could synergise with IL-1β thus increasing the production of proinflammatory mediators by RA synovial fibroblasts [56, 57]. Adiponectin aggravated bone erosions by promoting osteopontin production in RA synovial tissue, suggesting that adiponectin induced the expression of osteopontin, which in turn recruited osteoclasts [58]. Recently, the effects of adiponectin were assessed on adipose mesenchymal stem cells (ASCs) derived from the infrapatellar fat pad of RA patients [59]. ASCs were stimulated with both low molecular weight (LMW) and high/middle molecular weight (HMW/MMW) adiponectin isoforms. The authors observed that the secretion of proinflammatory mediators was upregulated by HMW/MMW adiponectin, but not by LMW adiponectin. In addition, they observed that the stimulation with HMW/MMW adiponectin reduced the proproliferative effects of ASC-derived soluble factors on RA synovial fibroblasts [59]. Taking together these results, it is possible to suggest a proinflammatory and joint destructive role of adiponectin in RA [55–59].
3.3. Visfatin
Visfatin is a protein of 471 amino acids and 52 kDa, and it is produced by the liver, bone marrow, muscle, macrophages, and visceral adipose tissue [60, 61]. This adipocytokine is increased in obesity [61]. Visfatin is regulated by proinflammatory cytokines and, in turn, it induces chemotaxis and the production of inflammatory cytokines, such as IL-1β, IL-6, and TNF, in lymphocytes from obese patients, suggesting involvement in the obesity proinflammatory milieu [62]. Furthermore, the proinflammatory actions of visfatin have been observed in experimental models of arthritis, in which the high levels of visfatin were proposed to modulate the proinflammatory process and the joint destruction [63, 64].
During RA, serum visfatin levels were higher in patients and correlated with radiographic joint damage [65–67]. Despite the association with radiographic outcome, the correlation with disease activity has shown conflicting results. In fact, the association with disease activity reported in some studies has been not confirmed in others [66–68]. The relatively small sample size and different experimental conditions could partially explain these results. Similarly, the analysis of results derived from clinical studies evaluating the changing pattern of visfatin after treatment with TNFi showed conflicting results. Serum visfatin levels were analysed in RA patients, who were differently treated (i) after 16 weeks of adalimumab treatment, (ii) after 2 weeks of high-dose prednisolone, and (iii) after 22 weeks of treatment with a combination regimen with tapered high-dose prednisolone and synthetic DMARD. Treatment with adalimumab was associated with a reduction in visfatin levels, whereas in other groups of patients, opposing effects on visfatin levels were observed [42]. On the contrary, other authors showed that visfatin levels did not change after the administration of infliximab [68].
3.4. Resistin
Resistin is a 12.5 kDa protein included in the resistin-like molecule (RELM) family, and it is mainly produced by nonadipocyte resident inflammatory cells, mainly macrophages [69–71]. Resistin increases with obesity and promotes insulin resistance, suggesting a possible link between obesity and diabetes [72–74].
Although a significant difference was not found in serum resistin levels between patients and controls, a pathogenic role for resistin has been suggested in RA [75, 76]. In fact, the intra-articular injection of recombinant resistin in the knee joints of murine models induced arthritis and increased the production of several proinflammatory cytokines, such as an increased expression of several proinflammatory cytokines including IL-1β, IL-6, IL-12, and TNF [76]. Furthermore, higher levels of this adipocytokine were observed in synovial fluid samples from RA patients and were correlated with disease activity and joint damage [77]. These data could suggest the production and the contribution of resistin in the inflamed joint, despite the lack of correlation with inflammatory markers in peripheral blood [76, 77].
Concerning the changing pattern after treatment, TNFi reduced serum resistin levels [42, 78]. After the administration of infliximab, the serum resistin levels significantly decreased in RA patients. In this cohort, resistin levels also correlated with inflammatory markers thus suggesting a possible role in the RA inflammatory process [78].
4. Adipocytokines and Cardiometabolic Diseases in RA
RA patients characteristically experience an increased risk of CVD derived from the synergy between traditional CV risk factors and inflammation [7–10]. In this context, the role of adipocytokines has been suggested as a possible link between adiposity, inflammation, and cardiometabolic diseases (Figure 1) [79, 80]. A previous study was performed to evaluate whether adipocytokines could affect insulin resistance and coronary atherosclerosis in RA patients [81]. In this study, the authors assessed the coronary calcium score, homeostatic model assessment for insulin resistance (HOMA-IR), and serum adipocytokine (leptin, adiponectin, resistin, and visfatin) levels in 169 RA patients. To date, high leptin levels correlated with HOMA-IR, even after adjustment for possible clinical confounders, age, gender, BMI, traditional CV risk factors, and inflammatory mediators. On the contrary, visfatin, adiponectin, and resistin showed no association with the HOMA-IR index. No association was retrieved between the coronary calcium score and assessed adipocytokines [81]. More recently, adipocytokines were further investigated as a link between inflammation, insulin resistance, and atherosclerosis in RA, being associated with pathogenic mechanisms of these diseases (Figure 2) [82]. A study evaluated HOMA-IR, intima-media thickness (IMT), carotid artery (CCA) resistive index (RI), and carotid plaques in 192 RA patients. These data were correlated with levels of adiponectin, leptin, and resistin. The authors observed that leptin and leptin : adiponectin (L : A) ratio were correlated with HOMA-IR and with CCA-RI after adjustment for CV risk factors, suggesting a possible independent role of leptin in predicting CVD in RA [82]. Although these correlations were not observed in another experience [83], it is possible to speculate that leptin is associated with insulin resistance in RA. Multiple lines of evidence showed the influence of leptin in the metabolism of glucose and pathogenesis of insulin resistance and diabetes [84, 85]. Insulin resistance in diabetic leptin receptor-deficient or genetic leptin-deficient animal models could not be fully attributed for their obesity and hyperphagia; the restriction in caloric intake failed to improve or recover the sensitivity of insulin in these models [86]. Furthermore, leptin administration in these models reduced plasma insulin and blood glucose levels [87]. In addition, leptin could influence glucose metabolism via the modulation of glucagon by α-cells of pancreas [88]. Furthermore, leptin could provide a functional link between obesity and CVD [88]. The link between fat mass and atherogenesis is confirmed by the findings in animal models of obesity [89, 90]. Leptin levels were associated with endothelial dysfunction proatherogenic actions, enhancing oxidative stress in endothelial cells, smooth muscle cell proliferation, and vascular calcification [90].
Figure 1.
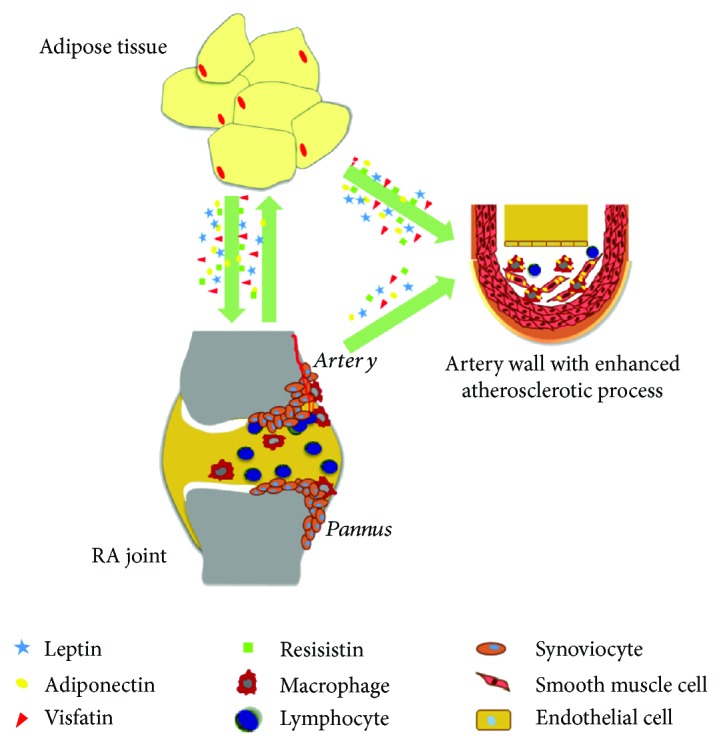
Schematic role of adipocytokines on the relationship among adipose tissue, rheumatoid arthritis, and atherosclerotic process.
Figure 2.
Pathogenic mechanisms of adipocytokines in rheumatoid arthritis and cardiometabolic diseases. Abbreviations: T Reg cells—T regulatory cells; TCR—T-cell receptor; NK cells—natural killer cells; TNF—tumor necrosis factor; IL—interleukin; MMP—metalloproteinase.
Concerning adiponectin, the correlation between total and HMW adiponectin concentrations, cardiometabolic risk, and surrogate markers of enhanced early atherogenesis was performed in 210 RA patients [91]. Total and HMW adiponectin concentrations correlated with high systolic, diastolic, and mean blood pressure and HDL cholesterol concentrations, low total HDL cholesterol ratios and triglyceride concentrations, and triglyceride-HDL cholesterol ratios and glucose concentrations [91]. These results mirrored what was observed in a lipoatrophy mouse model with adiponectin deficiency [92]. In these models, the replacement of adiponectin improved insulin resistance, fatty acid oxidation, and energy consumption, leading to a reduction of triglyceride levels in muscle and liver tissue [92, 93]. Furthermore, wild-type mice which received a high-fat diet showed a reduction in adiponectin levels and the replacement of adiponectin improved this diet-induced hypertriglyceridemia [94, 95]. To date, the possible role of adiponectin in modulating the homeostasis of blood pressure has been suggested [96]. In a cross-sectional study assessing patients with high blood pressure, high serum adiponectin levels were correlated with low procollagen type I carboxy-terminal propeptide circulating levels, a molecule reported to be associated with the arterial stiffening process [97]. Furthermore, adiponectin showed the ability to increase the gene expression and to activate the endothelial nitric oxide synthase by activation of AMPK [98]. Finally, it has been reported that adiponectin inhibited the deleterious effect of the renin-angiotensin system on the vascular system [99].
The potential impact of visfatin was assessed on CVD in 232 RA patients [100]. Visfatin concentrations were related to increased diastolic blood pressure and presence of diabetes [100]. In this context, it has been reported that visfatin could represent a proinflammatory cytokine influenced by insulin and/or insulin sensitivity via the NF-κB and JNK pathways [101, 102]. The role of visfatin was investigated in the impairment of the insulin pathway by TNF activity in adipocytes. In that study, the authors showed that visfatin was involved in TNF-mediated insulin resistance in adipocytes, via the NAD(+)/Sirt1/PTP1B pathway [103]. Furthermore, heterozygous mice with a mutation in the visfatin gene had higher levels of plasma glucose, impaired glucose tolerance, and reduced glucose-stimulated insulin secretion when compared with controls [104]. In addition, high visfatin levels could mediate vascular damage by inducing the expression of adhesion molecules via oxidative stress-dependent NF-κB activation, thus leading to endothelial inflammation and plaque destabilisation [105]. However, conflicting results are available concerning the role of visfatin [106, 107], thus future studies are needed to entirely clarify its role in cardiometabolic diseases.
Finally, the role of resistin has been proposed in cardiometabolic diseases. Of note, a certain degree of crosstalk between resistin and other adipokines has been reported [108, 109]. In fact, the expression on endothelial cells of VCAM-1 and ICAM-1 by resistin is counteracted by adiponectin [108]. A further link between leptin and resistin has also been proposed, and the expression of resistin was shown to be suppressed by leptin administration in animal models with subsequently decreased glucose and insulin levels [109]. In addition, the pathogenic role of resistin in atherogenesis has been proposed [110]. The secretion of resistin from atheroma-derived macrophages was suggested because of the colocalization of resistin and CD68 in the staining of human aneurysms and the higher mRNA resistin expression in cultured macrophages than in controls [111].
5. Adipocytokines as Future Possible Therapeutic Targets
In the last decades, long-term outcomes of RA have remarkably improved by using synthetic and biological DMARDs [112–114] and, presently, multiple lines of evidence assessed the best therapeutic strategy of concomitant diseases [115, 116]. In this context, it has been proposed that the inhibition of some cytokines may extend beyond the inflamed joints thus targeting, at the same time, associated comorbidities and improving the management of these patients [115–117]. Taking together these observations, it could be possible to speculate whether targeting adipocytokines may be effective in RA and comorbidities. Presently, antagonists of leptin have been developed to treat metabolic disorders. It should be tested if they could also have anti-inflammatory activities in vivo [118–120]. Interestingly, a monoclonal antibody against the leptin receptor was shown to block human TNF production by monocytes acting as an antagonist [121]. Recently, an orally active adiponectin receptor agonist improved insulin resistance and glucose intolerance in mice [122]. Considering that adiponectin showed anti-inflammatory properties, it could be speculated that adiponectin or adiponectin receptor agonists could be promising targets for the development of therapeutic drugs to treat insulin-resistant states and possible inflammatory states [123].
6. Conclusions
RA is a chronic autoimmune disease with increased mortality, due mainly to CVD. Adipocytokines are shown to be of importance in the pathogenesis of RA and associated comorbidities. Future studies are needed to identify the new mechanisms of action of adipocytokines and to elucidate if these molecules could be new possible therapeutic targets, thus improving the management of RA patients.
Acknowledgments
The authors thank Mrs. Federica Sensini for her technical assistance.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.McInnes I. B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet. 2017;389(10086):2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 2.Giacomelli R., Gorla R., Trotta F., et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology (Oxford, England) 2015;54(5):792–797. doi: 10.1093/rheumatology/keu398. [DOI] [PubMed] [Google Scholar]
- 3.Ruscitti P., Cipriani P., Carubbi F., et al. The role of IL-1β in the bone loss during rheumatic diseases. Mediators of Inflammation. 2015;2015:10. doi: 10.1155/2015/782382.782382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barile A., Arrigoni F., Bruno F., et al. Computed tomography and MR imaging in rheumatoid arthritis. Radiologic Clinics of North America. 2017;55(5):997–1007. doi: 10.1016/j.rcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Cipriani P., Berardicurti O., Masedu F., et al. Biologic therapies and infections in the daily practice of three Italian rheumatologic units: a prospective, observational study. Clinical Rheumatology. 2017;36(2):251–260. doi: 10.1007/s10067-016-3444-1. [DOI] [PubMed] [Google Scholar]
- 6.Ursini F., Russo E., Mauro D., et al. Complement C3 and fatty liver disease in rheumatoid arthritis patients: a cross-sectional study. European Journal of Clinical Investigation. 2017;47(10):728–735. doi: 10.1111/eci.12798. [DOI] [PubMed] [Google Scholar]
- 7.Ruscitti P., Cipriani P., Masedu F., et al. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS One. 2017;12(1, article e0170108) doi: 10.1371/journal.pone.0170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruscitti P., Ursini F., Cipriani P., et al. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: A 1-year, single-centre, longitudinal study. PLoS One. 2017;12(7, article e0181203) doi: 10.1371/journal.pone.0181203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruscitti P., Ursini F., Cipriani P., et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: results from a cross-sectional study. Medicine. 2017;96(34, article e7896) doi: 10.1097/MD.0000000000007896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruscitti P., Margiotta D. P. E., Macaluso F., et al. Subclinical atherosclerosis and history of cardiovascular events in Italian patients with rheumatoid arthritis: results from a cross-sectional, multicenter GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Medicine. 2017;96(42, article e8180) doi: 10.1097/MD.0000000000008180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou K., Xiao F. K., Li H. Y., et al. Risk of cardiovascular disease in Chinese patients with rheumatoid arthritis: a cross-sectional study based on hospital medical records in 10 years. PLoS One. 2017;12(7, article e0180376) doi: 10.1371/journal.pone.0180376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ursini F., D’Angelo S., Russo E., et al. Serum complement C3 strongly correlates with whole-body insulin sensitivity in rheumatoid arthritis. Clinical and Experimental Rheumatology. 2017;35(1):18–23. [PubMed] [Google Scholar]
- 13.Ursini F., D’Angelo S., Russo E., et al. Complement C3 is the strongest predictor of whole-body insulin sensitivity in psoriatic arthritis. PLoS One. 2016;11(9, article e0163464) doi: 10.1371/journal.pone.0163464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arts E. E. A., Fransen J., den Broeder A. A., Popa C. D., van Riel P. L. C. M. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Annals of the Rheumatic Diseases. 2015;74(6):998–1003. doi: 10.1136/annrheumdis-2013-204531. [DOI] [PubMed] [Google Scholar]
- 15.Ruscitti P., Cipriani P., Liakouli V., et al. The emerging role of IL-1 inhibition in patients affected by rheumatoid arthritis and diabetes. Reviews on Recent Clinical Trials. 2018;13(3):210–214. doi: 10.2174/1574887113666180314102651. [DOI] [PubMed] [Google Scholar]
- 16.Popa C., Netea M. G., van Riel P. L. C. M., van der Meer J. W. M., Stalenhoef A. F. H. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. Journal of Lipid Research. 2007;48(4):751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Ruscitti P., Cipriani P., di Benedetto P., et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3 (NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clinical and Experimental Immunology. 2015;182(1):35–44. doi: 10.1111/cei.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K. S., Kronbichler A., Eisenhut M., Lee K. H., Shin J. I. Cardiovascular involvement in systemic rheumatic diseases: an integrated view for the treating physicians. Autoimmunity Reviews. 2018;17(3):201–214. doi: 10.1016/j.autrev.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Suciu C. F., Prete M., Ruscitti P., Favoino E., Giacomelli R., Perosa F. Oxidized low density lipoproteins: the bridge between atherosclerosis and autoimmunity. Possible implications in accelerated atherosclerosis and for immune intervention in autoimmune rheumatic disorders. Autoimmunity Reviews. 2018;17(4):366–375. doi: 10.1016/j.autrev.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Ursini F., Leporini C., Bene F., et al. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: a systematic review and meta-analysis. Scientific Reports. 2017;7(1):p. 5346. doi: 10.1038/s41598-017-05759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abella V., Scotece M., Conde J., et al. Adipokines, metabolic syndrome and rheumatic diseases. Journal of Immunology Research. 2014;2014:14. doi: 10.1155/2014/343746.343746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keustermans G., van der Heijden L. B., Boer B., et al. Differential adipokine receptor expression on circulating leukocyte subsets in lean and obese children. PLoS One. 2017;12(10, article e0187068) doi: 10.1371/journal.pone.0187068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann E., Junker S., Schett G., Frommer K., Müller-Ladner U. Adipokines in bone disease. Nature Reviews Rheumatology. 2016;12(5):296–302. doi: 10.1038/nrrheum.2016.49. [DOI] [PubMed] [Google Scholar]
- 24.Scotece M., Conde J., Gómez R., et al. Role of adipokines in atherosclerosis: interferences with cardiovascular complications in rheumatic diseases. Mediators of Inflammation. 2012;2012:14. doi: 10.1155/2012/125458.125458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lago F., Gómez R., Conde J., Scotece M., Gómez-Reino J. J., Gualillo O. Cardiometabolic comorbidities and rheumatic diseases: focus on the role of fat mass and adipokines. Arthritis Care & Research. 2011;63(8):1083–1090. doi: 10.1002/acr.20488. [DOI] [PubMed] [Google Scholar]
- 26.Toussirot É., Michel F., Binda D., Dumoulin G. The role of leptin in the pathophysiology of rheumatoid arthritis. Life Sciences. 2015;140:29–36. doi: 10.1016/j.lfs.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Abella V., Scotece M., Conde J., et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nature Reviews Rheumatology. 2017;13(2):100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 28.Ahima R. S., Prabakaran D., Mantzoros C., et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 29.Gajewski M., Gajewska J., Rzodkiewicz P., Wojtecka-Łukasik E. Influence of exogenous leptin on redox homeostasis in neutrophils and lymphocytes cultured in synovial fluid isolated from patients with rheumatoid arthritis. Reumatologia. 2016;54(3):103–107. doi: 10.5114/reum.2016.61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos-Alvarez J., Goberna R., Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cellular Immunology. 1999;194(1):6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 31.Zarkesh-Esfahani H., Pockley A. G., Wu Z., Hellewell P. G., Weetman A. P., Ross R. J. M. Leptin indirectly activates human neutrophils via induction of TNF-α. Journal of Immunology. 2004;172(3):1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]
- 32.Kiguchi N., Maeda T., Kobayashi Y., Fukazawa Y., Kishioka S. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochemical and Biophysical Research Communications. 2009;384(3):311–315. doi: 10.1016/j.bbrc.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 33.Lord G. M., Matarese G., Howard J. K., Baker R. J., Bloom S. R., Lechler R. I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 34.Martín-Romero C., Santos-Alvarez J., Goberna R., Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cellular Immunology. 2000;199(1):15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Margalet V., Martín-Romero C., Santos-Alvarez J., Goberna R., Najib S., Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clinical and Experimental Immunology. 2003;133(1):11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y. H., Bae S.-C. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: a meta-analysis. Zeitschrift für Rheumatologie. 2016;75(10):1021–1027. doi: 10.1007/s00393-016-0050-1. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Bañuelos E., Navarro-Hernández R. E., Corona-Meraz F., et al. Serum leptin and serum leptin/serum leptin receptor ratio imbalance in obese rheumatoid arthritis patients positive for anti-cyclic citrullinated peptide antibodies. Arthritis Research & Therapy. 2015;17(1):p. 335. doi: 10.1186/s13075-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olama S. M., Senna M. K., Elarman M. Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatology International. 2012;32(3):683–690. doi: 10.1007/s00296-010-1698-5. [DOI] [PubMed] [Google Scholar]
- 39.Najafizadeh S. R., Farahmand G., Roudsari A. T., et al. Absence of a positive correlation between CRP and leptin in rheumatoid arthritis. Heliyon. 2016;2(12, article e00205) doi: 10.1016/j.heliyon.2016.e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oner S. Y., Volkan O., Oner C., Mengi A., Direskeneli H., Tasan D. A. Serum leptin levels do not correlate with disease activity in rheumatoid arthritis. Acta Reumatológica Portuguesa. 2015;40(1):50–54. [PubMed] [Google Scholar]
- 41.Tian G., Liang J. N., Wang Z. Y., Zhou D. Emerging role of leptin in rheumatoid arthritis. Clinical and Experimental Immunology. 2014;177(3):557–570. doi: 10.1111/cei.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klaasen R., Herenius M. M. J., Wijbrandts C. A., et al. Treatment-specific changes in circulating adipocytokines: a comparison between tumour necrosis factor blockade and glucocorticoid treatment for rheumatoid arthritis. Annals of the Rheumatic Diseases. 2012;71(9):1510–1516. doi: 10.1136/annrheumdis-2011-200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derdemezis C. S., Filippatos T. D., Voulgari P. V., Tselepis A. D., Drosos A. A., Kiortsis D. N. Effects of a 6-month infliximab treatment on plasma levels of leptin and adiponectin in patients with rheumatoid arthritis. Fundamental & Clinical Pharmacology. 2009;23(5):595–600. doi: 10.1111/j.1472-8206.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Gay M. A., Garcia-Unzueta M. T., Berja A., et al. Anti-TNF alpha therapy does not modulate leptin in patients with severe rheumatoid arthritis. Clinical and Experimental Rheumatology. 2009;27(2):222–228. [PubMed] [Google Scholar]
- 45.Sattar N., McCarey D. W., Capell H., McInnes I. B. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108(24):2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 46.Oh D. K., Ciaraldi T., Henry R. R. Adiponectin in health and disease. Diabetes, Obesity & Metabolism. 2007;9(3):282–289. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 47.Iwabu M., Okada-Iwabu M., Yamauchi T., Kadowaki T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging and Mechanisms of Diseases. 2015;1(1, article 15013) doi: 10.1038/npjamd.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadowaki T., Yamauchi T., Waki H., Iwabu M., Okada-Iwabu M., Nakamura M. Adiponectin, adiponectin receptors, and epigenetic regulation of adipogenesis. Cold Spring Harbor Symposia on Quantitative Biology. 2011;76:257–265. doi: 10.1101/sqb.2012.76.010587. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi T., Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metabolism. 2013;17(2):185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Blüher M., Bullen J. W., Jr, Lee J. H., et al. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. The Journal of Clinical Endocrinology & Metabolism. 2006;91(6):2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 51.Weyer C., Funahashi T., Tanaka S., et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. The Journal of Clinical Endocrinology and Metabolism. 2001;86(5):1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 52.Schäffler A., Ehling A., Neumann E., et al. Adipocytokines in synovial fluid. Journal of the American Medical Association. 2003;290(13):1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 53.Ozgen M., Koca S. S., Dagli N., Balin M., Ustundag B., Isik A. Serum adiponectin and vaspin levels in rheumatoid arthritis. Archives of Medical Research. 2010;41(6):457–463. doi: 10.1016/j.arcmed.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Tan W., Wang F., Zhang M., Guo D., Zhang Q., He S. High adiponectin and adiponectin receptor 1 expression in synovial fluids and synovial tissues of patients with rheumatoid arthritis. Seminars in Arthritis and Rheumatism. 2009;38(6):420–427. doi: 10.1016/j.semarthrit.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Choi H. M., Lee Y. A., Lee S. H., et al. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Research & Therapy. 2009;11(6, article R161) doi: 10.1186/ar2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y. A., Choi H. M., Lee S. H., et al. Synergy between adiponectin and interleukin-1β on the expression of interleukin-6, interleukin-8, and cyclooxygenase-2 in fibroblast-like synoviocytes. Experimental & Molecular Medicine. 2012;44(7):440–447. doi: 10.3858/emm.2012.44.7.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frommer K. W., Schäffler A., Büchler C., et al. Adiponectin isoforms: a potential therapeutic target in rheumatoid arthritis? Annals of the Rheumatic Diseases. 2012;71(10):1724–1732. doi: 10.1136/annrheumdis-2011-200924. [DOI] [PubMed] [Google Scholar]
- 58.Qian J., Xu L., Sun X., et al. Adiponectin aggravates bone erosion by promoting osteopontin production in synovial tissue of rheumatoid arthritis. Arthritis Research & Therapy. 2018;20(1):p. 26. doi: 10.1186/s13075-018-1526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skalska U., Kontny E. Adiponectin isoforms and leptin impact on rheumatoid adipose mesenchymal stem cells function. Stem Cells International. 2016;2016:6. doi: 10.1155/2016/6532860.6532860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curat C. A., Wegner V., Sengenès C., et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49(4):744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 61.Xie H., Tang S. Y., Luo X. H., et al. Insulin-like effects of visfatin on human osteoblasts. Calcified Tissue International. 2007;80(3):201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 62.Chang Y. C., Chang T. J., Lee W. J., Chuang L. M. The relationship of visfatin/pre-B-cell colony-enhancing factor/nicotinamide phosphoribosyltransferase in adipose tissue with inflammation, insulin resistance, and plasma lipids. Metabolism. 2010;59(1):93–99. doi: 10.1016/j.metabol.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Olszanecka-Glinianowicz M., Kocełak P., Nylec M., Chudek J., Zahorska-Markiewicz B. Circulating visfatin level and visfatin/insulin ratio in obese women with metabolic syndrome. Archives of Medical Science. 2012;8(2):214–218. doi: 10.5114/aoms.2012.28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Luis D. A., Aller R., Gonzalez Sagrado M., Conde R., Izaola O., de la Fuente B. Serum visfatin levels and metabolic syndrome criteria in obese female subjects. Diabetes/Metabolism Research and Reviews. 2013;29(7):576–581. doi: 10.1002/dmrr.2430. [DOI] [PubMed] [Google Scholar]
- 65.Nourbakhsh M., Nourbakhsh M., Gholinejad Z., Razzaghy-Azar M. Visfatin in obese children and adolescents and its association with insulin resistance and metabolic syndrome. Scandinavian Journal of Clinical and Laboratory Investigation. 2015;75(2):183–188. doi: 10.3109/00365513.2014.1003594. [DOI] [PubMed] [Google Scholar]
- 66.Senolt L., Kryštůfková O., Hulejová H., et al. The level of serum visfatin (PBEF) is associated with total number of B cells in patients with rheumatoid arthritis and decreases following B cell depletion therapy. Cytokine. 2011;55(1):116–121. doi: 10.1016/j.cyto.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Klein-Wieringa I. R., van der Linden M. P. M., Knevel R., et al. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis and Rheumatism. 2011;63(9):2567–2574. doi: 10.1002/art.30449. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez-Gay M. A., Vazquez-Rodriguez T. R., Garcia-Unzueta M. T., et al. Visfatin is not associated with inflammation or metabolic syndrome in patients with severe rheumatoid arthritis undergoing anti-TNF-alpha therapy. Clinical and Experimental Rheumatology. 2010;28(1):56–62. [PubMed] [Google Scholar]
- 69.Patel L., Buckels A. C., Kinghorn I. J., et al. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochemical and Biophysical Research Communications. 2003;300(2):472–476. doi: 10.1016/S0006-291X(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 70.Fain J. N., Cheema P. S., Bahouth S. W., Lloyd Hiler M. Resistin release by human adipose tissue explants in primary culture. Biochemical and Biophysical Research Communications. 2003;300(3):674–678. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 71.Tarkowski A., Bjersing J., Shestakov A., Bokarewa M. I. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. Journal of Cellular and Molecular Medicine. 2010;14(6B):1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steppan C. M., Bailey S. T., Bhat S., et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 73.Lazar M. A. Resistin- and obesity-associated metabolic diseases. Hormone and Metabolic Research. 2007;39(10):710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- 74.Gharibeh M. Y., al Tawallbeh G. M., Abboud M. M., Radaideh A., Alhader A. A., Khabour O. F. Correlation of plasma resistin with obesity and insulin resistance in type 2 diabetic patients. Diabetes & Metabolism. 2010;36(6):443–449. doi: 10.1016/j.diabet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Yoshino T., Kusunoki N., Tanaka N., et al. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Internal Medicine. 2011;50(4):269–275. doi: 10.2169/internalmedicine.50.4306. [DOI] [PubMed] [Google Scholar]
- 76.Bokarewa M., Nagaev I., Dahlberg L., Smith U., Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. Journal of Immunology. 2005;174(9):5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 77.Senolt L., Housa D., Vernerova Z., et al. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Annals of the Rheumatic Diseases. 2006;66(4):458–463. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzalez-Gay M. A., Garcia-Unzueta M. T., Gonzalez-Juanatey C., et al. Anti-TNF-alpha therapy modulates resistin in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology. 2008;26(2):311–316. [PubMed] [Google Scholar]
- 79.van Halm V. P., Peters M. J. L., Voskuyl A. E., et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE investigation. Annals of the Rheumatic Diseases. 2009;68(9):1395–1400. doi: 10.1136/ard.2008.094151. [DOI] [PubMed] [Google Scholar]
- 80.Peters M. J. L., van Halm V. P., Voskuyl A. E., et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis and Rheumatism. 2009;61(11):1571–1579. doi: 10.1002/art.24836. [DOI] [PubMed] [Google Scholar]
- 81.Rho Y. H., Chung C. P., Solus J. F., et al. Adipocytokines, insulin resistance, and coronary atherosclerosis in rheumatoid arthritis. Arthritis & Rheumatism. 2010;62(5):1259–1264. doi: 10.1002/art.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang Y., Park H. J., Kang M. I., et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Research & Therapy. 2013;15(6, article R194) doi: 10.1186/ar4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dessein P. H., Norton G. R., Woodiwiss A. J., Tsang L., Solomon A. Age impacts on the independent relationships of leptin with cardiometabolic risk and surrogate markers of enhanced early atherogenesis in black and white patients with rheumatoid arthritis: a cross-sectional study. Rheumatology International. 2014;34(3):329–339. doi: 10.1007/s00296-013-2933-7. [DOI] [PubMed] [Google Scholar]
- 84.Amitani M., Asakawa A., Amitani H., Inui A. The role of leptin in the control of insulin-glucose axis. Frontiers in Neuroscience. 2013;7 doi: 10.3389/fnins.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park H. K., Ahima R. S. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’souza A. M., Johnson J. D., Clee S. M., Kieffer T. J. Suppressing hyperinsulinemia prevents obesity but causes rapid onset of diabetes in leptin-deficient Lepob/ob mice. Molecular Metabolism. 2016;5(11):1103–1112. doi: 10.1016/j.molmet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morton G. J., Schwartz M. W. Leptin and the central nervous system control of glucose metabolism. Physiological Reviews. 2011;91(2):389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rehman K., Akash M. S. H., Alina Z. Leptin: a new therapeutic target for treatment of diabetes mellitus. Journal of Cellular Biochemistry. 2018;119(7):5016–5027. doi: 10.1002/jcb.26580. [DOI] [PubMed] [Google Scholar]
- 89.Yamagishi S. I., Edelstein D., du X. L., Kaneda Y., Guzmán M., Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. The Journal of Biological Chemistry. 2001;276(27):25096–25100. doi: 10.1074/jbc.m007383200. [DOI] [PubMed] [Google Scholar]
- 90.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189(1):47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Dessein P. H., Woodiwiss A. J., Norton G. R., Tsang L., Solomon A. Independent associations of total and high molecular weight adiponectin with cardiometabolic risk and surrogate markers of enhanced early atherogenesis in black and white patients with rheumatoid arthritis: a cross-sectional study. Arthritis Research & Therapy. 2013;15(5, article R128) doi: 10.1186/ar4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.von Frankenberg A. D., Reis A. F., Gerchman F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: a literature review. Archives of Endocrinology and Metabolism. 2017;61(6):614–622. doi: 10.1590/2359-3997000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamauchi T., Kamon J., Waki H., et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 94.Berg A. H., Combs T. P., Du X., Brownlee M., Scherer P. E. The adipocyte secreted protein Acrp30 enhances hepatic insulin action. Nature Medicine. 2001;7(8):947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 95.Combs T. P., Berg A. H., Obici S., Scherer P. E., Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. The Journal of Clinical Investigation. 2001;108(12):1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu A., Vanhoutte P. M. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. American Journal of Physiology Heart and Circulatory Physiology. 2012;302(6):H1231–H1240. doi: 10.1152/ajpheart.00765.2011. [DOI] [PubMed] [Google Scholar]
- 97.Tsai W. C., Lin C. C., Chen J. Y., et al. Association of adiponectin with procollagen type I carboxyterminal propeptide in non-diabetic essential hypertension. Blood Pressure. 2009;17(4):233–238. doi: 10.1080/08037050802308895. [DOI] [PubMed] [Google Scholar]
- 98.Vaiopoulos A. G., Marinou K., Christodoulides C., Koutsilieris M. The role of adiponectin in human vascular physiology. International Journal of Cardiology. 2012;155(2):188–193. doi: 10.1016/j.ijcard.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 99.van Stijn C. M. W., Kim J., Barish G. D., Tietge U. J. F., Tangirala R. K. Adiponectin expression protects against angiotensin II-mediated inflammation and accelerated atherosclerosis. PLoS One. 2014;9(1, article e86404) doi: 10.1371/journal.pone.0086404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robinson C., Tsang L., Solomon A., et al. Nesfatin-1 and visfatin expression is associated with reduced atherosclerotic disease risk in patients with rheumatoid arthritis. Peptides. 2018;102:31–37. doi: 10.1016/j.peptides.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 101.McGee K. C., Harte A. L., da Silva N. F., et al. Visfatin is regulated by rosiglitazone in type 2 diabetes mellitus and influenced by NFκB and JNK in human abdominal subcutaneous adipocytes. PLoS One. 2011;6(6, article e20287) doi: 10.1371/journal.pone.0020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kieswich J., Sayers S. R., Silvestre M. F., Harwood S. M., Yaqoob M. M., Caton P. W. Monomeric eNAMPT in the development of experimental diabetes in mice: a potential target for type 2 diabetes treatment. Diabetologia. 2016;59(11):2477–2486. doi: 10.1007/s00125-016-4076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gouranton E., Romier B., Marcotorchino J., et al. Visfatin is involved in TNFα-mediated insulin resistance via an NAD(+)/Sirt1/PTP1B pathway in 3T3-L1 adipocytes. Adipocytes. 2014;3(3):180–189. doi: 10.4161/adip.28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grunfeld C. Leptin and the immunosuppression of malnutrition. The Journal of Clinical Endocrinology and Metabolism. 2002;87(7):3038–3039. doi: 10.1210/jcem.87.7.8778. [DOI] [PubMed] [Google Scholar]
- 105.Mattu H. S., Randeva H. S. Role of adipokines in cardiovascular disease. The Journal of Endocrinology. 2013;216(1):T17–T36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 106.Lim S. Y., Davidson S. M., Paramanathan A. J., Smith C. C. T., Yellon D. M., Hausenloy D. J. The novel adipocytokine visfatin exerts direct cardioprotective effects. Journal of Cellular and Molecular Medicine. 2008;12(4):1395–1403. doi: 10.1111/j.1582-4934.2008.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hausenloy D. J., Yellon D. M. Cardioprotective growth factors. Cardiovascular Research. 2009;83(2):179–194. doi: 10.1093/cvr/cvp062. [DOI] [PubMed] [Google Scholar]
- 108.Kawanami D., Maemura K., Takeda N., et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochemical and Biophysical Research Communications. 2004;314(2):415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 109.Rajala M. W., Qi Y., Patel H. R., et al. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53(7):1671–1679. doi: 10.2337/diabetes.53.7.1671. [DOI] [PubMed] [Google Scholar]
- 110.Cohen G., Hörl W. H. Resistin as a cardiovascular and atherosclerotic risk factor and uremic toxin. Seminars in Dialysis. 2009;22(4):373–377. doi: 10.1111/j.1525-139X.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 111.Jung H., Park K., Cho Y., et al. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovascular Research. 2006;69(1):76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 112.Giacomelli R., Ruscitti P., Bombardieri S., et al. What could we learn from the sub-analysis of a single nation cohort in a worldwide study? Lessons from the results observed in the Italian cohort of the GO-MORE trial. Clinical and Experimental Rheumatology. 2017;35(4):623–629. [PubMed] [Google Scholar]
- 113.Cipriani P., Ruscitti P., Carubbi F., Liakouli V., Giacomelli R. Methotrexate in rheumatoid arthritis: optimizing therapy among different formulations. Current and emerging paradigms. Clinical Therapeutics. 2014;36(3):427–435. doi: 10.1016/j.clinthera.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 114.Cipriani P., Ruscitti P., Carubbi F., Liakouli V., Giacomelli R. Methotrexate: an old new drug in autoimmune disease. Expert Review of Clinical Immunology. 2014;10(11):1519–1530. doi: 10.1586/1744666x.2014.962996. [DOI] [PubMed] [Google Scholar]
- 115.Giacomelli R., Afeltra A., Alunno A., et al. International consensus: what else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren’s syndrome)? Autoimmunity Reviews. 2017;16(9):911–924. doi: 10.1016/j.autrev.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 116.Giacomelli R., Ruscitti P., Alvaro S., et al. IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Review of Clinical Immunology. 2016;12(8):849–855. doi: 10.1586/1744666X.2016.1168293. [DOI] [PubMed] [Google Scholar]
- 117.Ruscitti P., Cipriani P., Cantarini L., et al. Efficacy of inhibition of IL-1 in patients with rheumatoid arthritis and type 2 diabetes mellitus: two case reports and review of the literature. Journal of Medical Case Reports. 2015;9(1):p. 123. doi: 10.1186/s13256-015-0603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gertler A., Elinav E. Novel superactive leptin antagonists and their potential therapeutic applications. Current Pharmaceutical Design. 2014;20(4):659–665. doi: 10.2174/13816128113199990014. [DOI] [PubMed] [Google Scholar]
- 119.Shpilman M., Niv-Spector L., Katz M., et al. Development and characterization of high affinity leptins and leptin antagonists. The Journal of Biological Chemistry. 2011;286(6):4429–4442. doi: 10.1074/jbc.M110.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Solomon G., Atkins A., Shahar R., Gertler A., Monsonego-Ornan E. Effect of peripherally administered leptin antagonist on whole body metabolism and bone microarchitecture and biomechanical properties in the mouse. American Journal of Physiology Endocrinology and Metabolism. 2014;306(1):E14–E27. doi: 10.1152/ajpendo.00155.2013. [DOI] [PubMed] [Google Scholar]
- 121.Fazeli M., Zarkesh-Esfahani H., Wu Z., et al. Identification of a monoclonal antibody against the leptin receptor that acts as an antagonist and blocks human monocyte and T cell activation. Journal of Immunological Methods. 2006;312(1-2):190–200. doi: 10.1016/j.jim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 122.Okada-Iwabu M., Yamauchi T., Iwabu M., et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503(7477):493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 123.Andrade-Oliveira V., Câmara N. O. S., Moraes-Vieira P. M. Adipokines as drug targets in diabetes and underlying disturbances. Journal of Diabetes Research. 2015;2015:11. doi: 10.1155/2015/681612.681612 [DOI] [PMC free article] [PubMed] [Google Scholar]