Abstract
As the number of implanted left ventricular assist devices (LVADs) used increases, the frequency of chronic complications encountered also increases. The pause of blood flow in the outflow graft is a rare, but fatal, complication. The aim of this article was to present the case of a patient in whom HeartWare outflow graft occlusion was removed by balloon angioplasty and to examine the treatment modalities of HeartWare outflow graft occlusions that have been percutaneously performed to date. The literature was searched for percutaneous interventions on outflow grafts of the left ventricular assist devices. The results of six patients who underwent interventions on outflow grafts were analyzed. Three of six patients with HeartWare outflow graft stenosis were treated with covered stents, while the remaining three were treated with bare metal stents. All procedures were applied successfully. Percutaneous interventions can be performed with appropriate equipment in patients with HeartWare outflow graft stenosis or total occlusion.
Keywords: heart failure, ventricular assist device, outflow graft, balloon angioplasty
Introduction
Left ventricular support systems are being increasingly used for treating heart failure in the terminal period. An important fatal complication of these devices is the thrombus-related malfunction of the device even though patients receive continuous anticoagulant treatment while under support and reach the desired anticoagulation levels [Each year, 4 to 9% of patients who have a left ventricular assist device (LVAD)]. Obstruction, which can cause device malfunction, can be grouped as pre-device, intra-device and post-device. Pre-device obstruction is used to describe conditions associated with large plugs obstructing the inflow cannula, while post-device obstruction is used to define malfunctions associated with plugs in the outflow graft (1). In this article, we aimed to present the case of a patient with outflow graft occlusion and who was percutaneously treated under emergency conditions and to evaluate the outcome of outflow graft occlusions percutaneously treated by examining the results of six similar cases in the literature.
Case Report
A 47-year-old male with a HeartWare ventricular assist device (HVAD; HeartWare, Inc., Framingham, MA, USA) implanted for dilated cardiomyopathy three years previously presented for undergoing evaluation of low-flow alarms. His past history was unremarkable. At the time of HVAD implantation, he was at Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) level 2. Since the time of implantation, he was maintained on warfarin and was intermittently maintained on aspirin. His INR usually remained in the therapeutic range since implantation. He was at home and made a call to an LVAD coordinator because of low-flow alarm. He was living 303 km away and was immediately transferred to our center by an air ambulance. His waveform history showed no HVAD flow. His measured flow rate was −0.1 Lpm (Fig. 1). The patient had moderate dyspnea that did not require mechanical ventilation. Hemodynamic monitoring revealed pulsatile blood pressure of 80/50 mm Hg. His central venous pressure was 12 mm Hg. Lactate levels were in the normal range, and blood gas analysis did not demonstrate hypoxia. The HVAD was heard during auscultation and was operating at 2500 rpm. Blood tests showed normal renal and liver function, and his INR was 3.5. Transthoracic echocardiography revealed a dilated left ventricle with an ejection fraction of 15%. There were no signs of inflow or left ventricular thrombus. Outflow cannula obstruction was suspected, and multislice thorax CT was performed. CT showed total occlusion in the outflow cannula. The outflow graft was also attached to the sternum and sternal wire sutures (Fig. 2). The patient underwent discussion with the heart failure team with interventional cardiology and percutaneous intervention planned because of the high risk of mortality and morbidity of a probable surgical treatment. After obtaining written informed consent from the patient and relatives, the patient was taken to an intervention laboratory.
Figure 1.
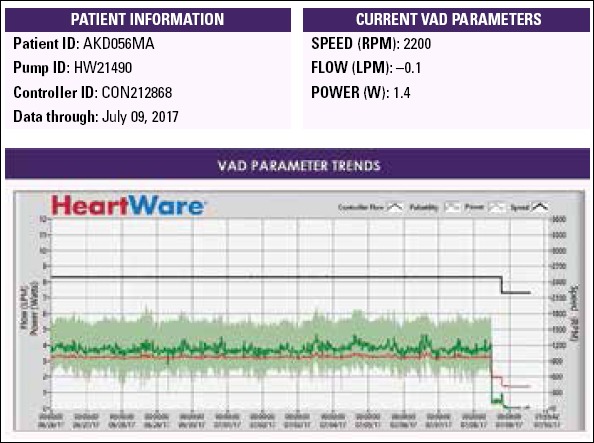
HVAD parameters and flow pulsatility over the last 14 days
Figure 2.
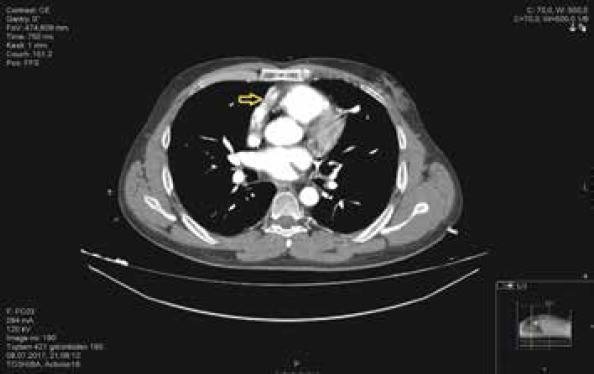
Multislice CT view; a totally occluded outflow graft is seen and is adherent to sternal sutures (arrow)
A 6 F introducer was placed at the right femoral artery by the Seldinger technique. A 0.38-inch guide wire and a 6-F right Judkins coronary catheter were placed together to the ostium of the outflow graft. In the angiograms, stenosis was detected from the ostium of the outflow graft to the middle part (Fig. 3). A 6-F right coronary catheter with a 0.38-inch guide wire was advanced to the middle portion of the outflow graft. Then, a Nitrex 0.35×300-cm guidewire was replaced with the 0.38-inch guidewire. The right coronary catheter was removed by leaving the guide wire in the outflow graft. First, an EverCross 8×40-mm peripheral balloon was advanced over the guidewire, and successive predilatations were performed at 8 ATM pressures for 2 min from the ostium to the middle portion of the aortic cannula (Fig. 4). After balloon dilatation, HVAD was turned on under bilateral carotid compression, but no flow was observed. Then, in the angiograms taken, severe stenosis was detected at the junction of the graft with the device, and the same procedure was performed in this region. The delivered radiopaque material reached the left ventricular cavity, and the device was restarted under bilateral carotid compression; the flow rates gradually increased and reached their normal values. In control angiograms, there was no narrowing along the cannula and full lumen clearance was observed. The process was terminated after observing that the values associated with device flow returned to normal.
Figure 3.
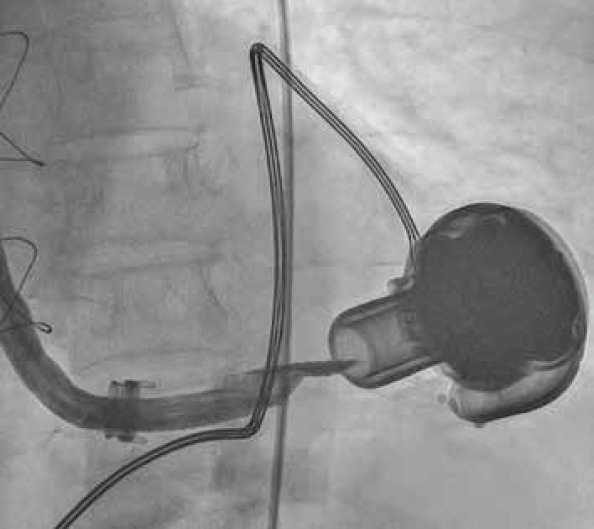
Angiogram; stenosis was detected from the ostium of the outflow graft to the middle part
Figure 4.
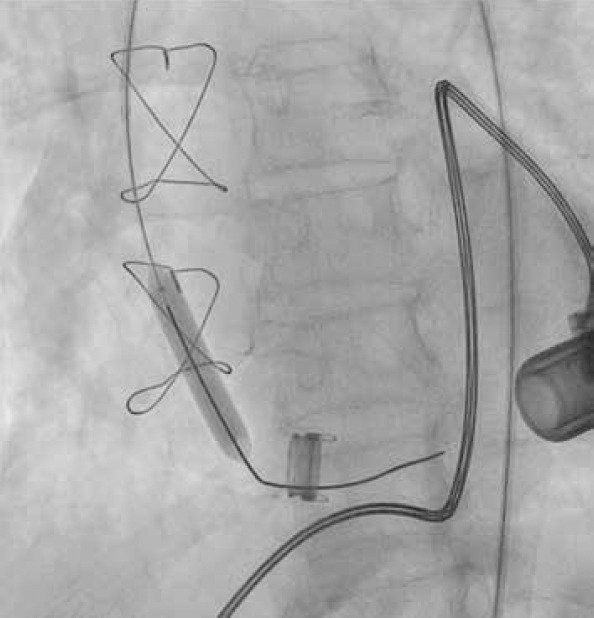
Angiogram; balloon dilatations
The patient was taken to the intensive care unit (ICU) after the procedure. He was hemodynamically stable. At 3 h post operatively, the patient complained of paresthesia and severe pain in both lower extremities. Duplex US showed a thrombotic occlusion in the distal abdominal aorta and iliac bifurcation. The patient then underwent embolectomy. Under local anesthesia, both common femoral arteries were explored. There was no antegrade flow in both sides. After heparinization and bilateral distal clamping of the femoral arteries, proximal embolectomy was performed with 5F Fogarty catheter. Fibrinous embolic material was taken from both sides, and antegrade flow was maintained. The embolic material was whitish pink and gelatinous in some places (Fig. 5). Retrograde flow was normal; arteriotomies were closed, and the patient was taken to the ICU. Heparin infusion was continued for 24 h, and ACT values were around 200 s. The patient was discharged from the ICU after 24 h and was discharged from hospital after an uneventful 3-day period. Warfarin and the ASA regimen were not changed on discharge. First mounth CT, showed the open outflow graft (Fig. 6).
Figure 5.
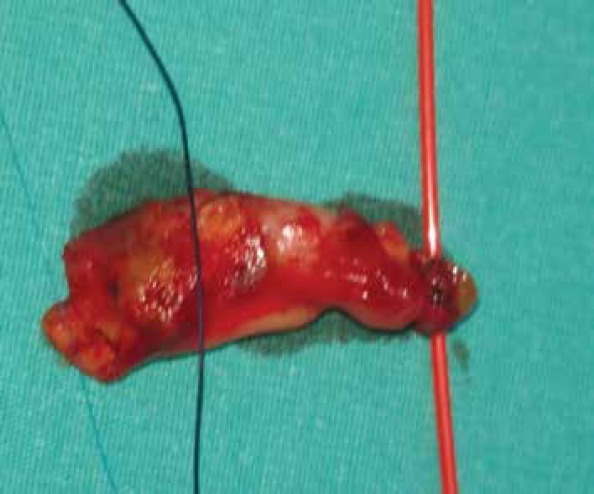
Embolectomy material
Figure 6.
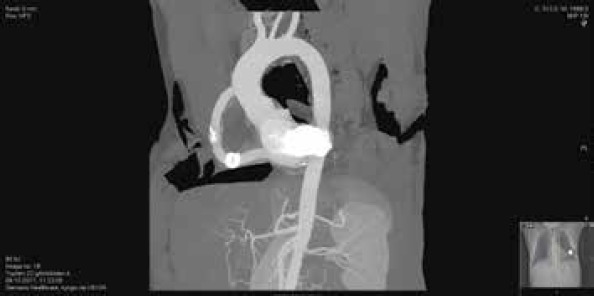
First-month CT view (outflow graft is seen)
Discussion
PubMed and Web of Science databases were searched using the following keywords: “ LVAD, outflow graft thrombosis”; “stenting, outflow graft thrombosis”; “outflow graft obstruction, stenting”; “Lvad, stenting”; “Lvad, balloon angioplasty”; and “outflow graft thrombosis, balloon angioplasty. As a result of the searches, a total of 13 case reports in which outflow graft occlusions were treated percutaneously were found. Totally, 7 of 13 cases were HeartMate II patients and excluded. There were six different cases in the literature, except for our case, in which mechanical intervention was performed for outflow grafts in patients with HVADs (2-5). The summary of these cases is given in Table 1.
Table 1.
Interventions to stenotic outflow grafts: a summary
| Reference | Pre-Interventional flow and power (L/W) | Pre-Interventional rate (RPM) | Post-Interventional flow and power (L/W) | Post-Interventional rate (RPM) | Intervention | Cerebral protection | Device On/Off | Stenosis severity |
|---|---|---|---|---|---|---|---|---|
| 2 | 2.2/4.9 | 3600 | 5/6 | 3200 | Covered stent implantation | None | Off | Unknown |
| 3 | 2.5/Unknown | 2900 | 4/Unknown | 2350 | Covered stent implantation | None | On | 90% |
| 4 | 1/2 | 2700 | 6/4.9 | 2700 | Bare-metal stent implantation | None | On | Nearly occluded |
| 4 | 3/2.5 | 3000 | 6/5.9 | 3000 | Bare-metal stent implantation | None | On | Nearly occluded |
| 4 | 2/1 | 2500 | 4/3 | 2500 | Bare- metal stent implantation | Balloon blockade | On | occluded |
| 5 | 8/Unknown | Unknown | Unknown | Unknown | Covered stent implantation | Balloon blockade + embolic filter | Off | Unknown |
| * | 0 | 2500 | 3.7/2.9 | 2500 | Balloon dilatation only | Manual carotid occlusion | Off | Total occlusion |
L - Liters; W - Watts; RPM - Revolutions per minute
Our patient
There are not many cases in the literature to determine the treatment approach of outflow graft occlusions of HVAD cases. To date, successful outcomes have been reported for six patients with HVADs who have outflow graft occlusions. Summarized information of these six patients is presented in Table 1. After all procedures, normal flow rates were obtained and there was no thromboembolic complication.
One of the largest series of LVAD malfunctions related to flow interruption is by the Berlin Heart Center team. Three types of LVAD-related blood flow obstruction were identified, and an algorithm was developed for optimal diagnosis and treatment. In only 4% of 652 LVAD-implanted patients, flow reduction due to outflow graft occlusion was detected. The team emphasized that the first choice of treatment for outflow graft stenosis is mechanical intervention under cerebral protection (1).
Device parameters must be well analyzed to be able to distinguish between obstructions that reduce the flow of the device. Outflow graft occlusion or stenosis is suspected when the device is operating at the same rate (rpm), but drops in power and flow rates. The increase in power and flow rates of the device should be considered as a problem inside the device (4). There were similar changes in the parameters in our case and five other cases (2-4). In contrast, an increase in flow rates was reported in another patient (5). In this case, there could an intra-device thrombus. It should not be forgotten that the thrombotic process can be presented as, both pre-, intra-, or post-device. During post-device occlusions occur, the device’s power consumption and reduction in flow rate are spread over the time period of occlusion. In case of sudden occlusion, the flow may suddenly change even though there is no change in any parameter beforehand.
In our case, HVAD flow completely stopped, unlike other outflow graft occlusions treated percutaneously. From this aspect, to the best of our knowledge, this is the first case in the literature. For this reason, our case is a clinical entity that requires quicker and more immediate intervention than other cases.
Thrombolytics are good agents for pre- and in-device thrombosis. Our first approach in the treatment of pre- or in-device thrombosis is to administered systemic or in-device thrombolytic therapy with a transfemoral catheter (6). However, the literature does not include sufficient data on the efficacy of thrombolytic therapy in outflow graft occlusions. In one case, lytic therapy was attempted before stent implantation; however, it failed (5). In our post-device thrombotic case, which required immediate correction, we believed that lytic treatment would not be able to reach the target and that we could easily catheterize and solve the occlucion with a simple stent or stent graft implantation considering that it could be a new-onset thrombus. Unfortunately, when we catheterized the outflow graft, we found that the graft was almost completely filled with chronic fibrinous material. We were convinced that before total obstruction, the development of the stenosis of the outflow graft has occurred over a long period of time. Interestingly, no device parameters were changed to show that it is a chronic process. Normally, even if we planned to implant a stent graft to the area where it was obstructed, we believed that it would not be successful with almost the entire outflow graft being blocked by fibrinous material. The other cases showed us that in outflow graft occlusions, both metal and graft-coated stents can be successfully used. Our case is different from other cases in the literature due to the lack of stent or stent graft implantation during outflow graft occlusion removal.
The riskiest part of placing a catheter in a thrombosed outflow graft is the risk of causing a systemic thromboembolism. Embolisms that may be present in the lower extremities can be easily treated, so the risk of emboli can be considered. However, cerebrovascular embolisms should be avoided as they can be life threatening. While interventions are made for thrombosed outflow grafts, carotid filters or balloon dilatation in carotids can be used for cerebral protection; their use has been successfully demonstrated (6, 7). These methods were used in two cases, while cerebral protection was not performed in four cases. There was no embolic event in any case.
When we decided to apply multiballoon dilatations into the conduit, we believed that the thrombus or fibrous tissue could be embolized. We told the patient and relatives that cerebrovascular or mesenteric embolies might be catastrophic. Aortoiliac embolies were one of the best scenarios that we could come up with. Surgical intervention that can be planned was not accepted by the relatives of the patient due to high risk of mortality. With the general situation of the patient rapidly deteriorating, we had to take this risk. We were unable to perform cerebral protection due to the absence of cerebral filters and the absence of balloons of appropriate diameters. We attempted manual carotid compression, which has also been previously attempted when percutaneous outflow graft intervention is required (7). Our case differs from other cases as it resulted with an embolism.
Another point to note when intervening an outflow graft is that the device will be turned off or on. While the device was turned off in two patients, it was turned on in four. We chose to close the device because of the high risk of embolisms. Although there was no flow in the outflow graft, we did not run the device until the last moment, believing that the flow would suddenly start during the intervention. We believe that it is acceptable to work with a running device when the stenosis is localized to a small area, but we believe that it should be turned off if lesions are longer than the stent graft. After balloon dilatations in the outflow graft, we restarted the device two times. After observing that the delivered radiopaque material reached the left ventricular cavity, when we started the device second time, the flow rates gradually increased. Balloon dilatations performed at this time may have allowed the thrombotic material to separate from the graft. When we restarted the device, we performed the washout maneuver that was recommended by the Berlin Heart Center for inflow cannula occlusion, during which thromboembolic materials moved to the lower extremities. We believe that it is beneficial to apply pressure to the bilateral carotids while restarting the device.
Conclusion
Percutaneous intervention for the removal of outflow graft occlusions may be preferred due to the fact that it is fast, feasible, and cheaper than surgical management. To date, all percutaneous interventions have been successful in removing outflow graft stenosis. We believe that the first choice of treatment for eliminating total occlusion in HVAD outflow grafts in the removal of partial stenosis is percutaneous intervention. We strongly recommend that manual carotid compression be performed when it is not possible to perform carotid protection during treatment. We believe that the risk of embolism that may occur in the lower extremities during the procedure is acceptable.
Limitations of this case report
We believe that cerebral protection should definitely be performed, but we were unable to perform it as we did not have the appropriate equipment for an emergency.
Limitations of this article
The numbers provided in the table may not reflect exact data as they were obtained from the analysis of graphs in case presentations. As we analyzed the results of a small number of cases, reviews with more cases can provide a better idea about percutaneous interventions to HVAD outflow grafts.
Video 1
Video showing that the outflow graft is open and that the opaque material reaches the LV cavity.
Acknowledgements
We would like to thank Volkan Alanya for his technical support in this case and all other HVAD cases. We would like to express our gratitude to the VAD coordinator Fatma Temel who has adopted all VAD patients as a family.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – C.K., R.E.A.; Design – C.K., R.E.A.; Supervision – C.K., R.E.A., Ö.B.; Fundings – None; Materials – None; Data collection &/or processing - C.K., R.E.A., Ö.B.; Analysis &/or interpretation - R.E.A., Ö.B.; Literature search – C.K., R.E.A., Ö.B.; Writing – R.E.A., Ö.B.; Critical review – Ö.B.
References
- 1.Scandroglio AM, Kaufmann F, Pieri M, Kretzschmar A, Müller M, Pergantis P, et al. Diagnosis and Treatment Algorithm for Blood Flow Obstructions in Patients With Left Ventricular Assist Device. J Am Coll Cardiol. 2016;67:2758–68. doi: 10.1016/j.jacc.2016.03.573. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad FS, Sauer AJ, Ricciardi MJ. Endovascular repair of ventricular assist device outflow cannula stenosis. Catheter Cardiovasc Interv. 2016 Nov 17; doi: 10.1002/ccd.26852. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Retzer EM, Tannenbaum SA, Fedson SE, Kim GH, Sayer GT, Paul JD, et al. Successful percutaneous trans-catheter treatment of left ventricular assist device outflow graft stenosis with a covered stent. ESC Heart Fail. 2015;2:100–2. doi: 10.1002/ehf2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiedemann D, Schlöglhofer T, Haberl T, Riebandt J, Dimitrov K, Schima H, et al. Interventional Treatment of LVAD Outflow Graft Stenosis by Introduction of Bare Metal Stents. ASAIO J. 2018;64:e3–e7. doi: 10.1097/MAT.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 5.Hanke JS, ElSherbini A, Rojas SV, Avsar M, Shrestha M, Schmitto JD. Aortic Outflow Graft Stenting in Patient With Left Ventricular Assist Device Outflow Graft Thrombosis. Artif Organs. 2016;40:414–6. doi: 10.1111/aor.12569. [DOI] [PubMed] [Google Scholar]
- 6.Köksel U, Erbasan O, Bayezid Ö, Kemaloğlu C, Özçobanoğlu S, Gölbaşı I, et al. Thrombosis in Continuous Flow Left Ventricular Assist Devices:Our Clinical Experience With Medical and Surgical Management. Transplant Proc. 2016;48:2162–7. doi: 10.1016/j.transproceed.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Abraham J, Remick JD, Caulfield T, Puhlman M, Evenson K, Ott G, et al. Left ventricular assist device outflow cannula obstruction treated with percutaneous endovascular stenting. Circ Heart Fail. 2015;8:229–30. doi: 10.1161/CIRCHEARTFAILURE.114.001891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


