Abstract
Objective:
Neovascularization of the aortic wall may be associated with aortic dissection (AD). Aortic wall endothelial CD31 deposition together with chronic inflammation indicates angiogenesis that may lead to tissue disruption. We studied the presence of neovascularization of the ascending aortic wall by characterizing CD31 positive endothelial cells.
Methods:
Aortic wall routine histology and immunohistochemistry for CD31, T- and B-lymphocytes, plasma cells, macrophages, endothelial cells, smooth muscle cells, and cell proliferation were performed on 35 selected patients who underwent surgery for the ascending aorta, and the samples were grouped according to the presence of AD.
Results:
Three subjects with Marfan syndrome were excluded from the study. A total of 14 out of 32 patients had AD. A total of 18 patients were operated on due to dilatation only. Chronic inflammation of the adventitia (p=0.003), media (p=0.001), and intima (p=0.005) was increased in AD. Neovascularization was predominant in the outer third medial layer in AD (p=0.037), corresponding to the site of aortic wall disruption. A receiver operating characteristic curve analysis showed that neovascularization was associated with AD (AUC 0.750; SE 0.092; p=0.022; 95% CI 0.570–0.930).
Conclusion:
Endothelial immunohistochemistry confirms neovascularization of the outer third medial layer during AD. Aortic wall remodeling including neovascularization characterizes AD. Chronic inflammation and neovascularization of the dilated ascending aorta suggest susceptibility for AD.
Keywords: neovascularization, ascending aortic dissection, chronic inflammation, CD31
Introduction
The main goal for surgery of the dilated ascending aorta is to prevent aortic dissection (AD) and rupture (1, 2). AD consists of an aortic wall tear in a tangential fashion and represents the ultimate rupture due to aortic wall weakness. Pathophysiologically, AD and aortic rupture are interrelated and are manifested by the anatomical site of the aortic tear (2). Despite the sudden occurrence of the aortic tear, the ascending aorta may have undergone a chronic remodeling phase of tissue weakening, including aortic wall hypoxia, hypertension, and chronic inflammation. Although a borderline of a 5.5 cm diameter of the ascending aorta is regarded as the threshold in enhancing the risk for AD (2), there is increasing evidence that aortas with an even smaller diameter may lead to AD (3). The decision for the extension of resection of the aorta during surgery is challenging, as one would aim at preventing AD after surgery.
The perioperative evaluation of the resected aortic wall during surgery for ascending aorta may reveal susceptibility for AD necessitating further extension of surgery. Most AD occurs in the outer third of the media close to the adventitia (4). This site is characterized by vasa vasorum that participates in the nutrition of the aortic wall (4). The significance of endothelial activation of vasa vasorum in aortic pathogenesis is under discussion (5). Arterial neovascularization may be regulated by chronic inflammation, suggesting that hypoxia alone is not leading to tissue remodeling (6). Recent experimental studies suggest that the regulation of angiogenesis is dependent on endothelial activation (7).
We studied the vascular reactivity of the aortic wall by characterizing the angiogenic histology of the ascending aorta as expressed by CD31. We hypothesized that chronic inflammatory remodeling of the ascending aorta is associated with dilatation of the aortic wall, and neovascularization of the ascending aortic media may determine the fate of the dilated aortic wall. Using an extensive immunohistochemical analysis and detection of CD31-positive endothelial cells of the medial layer, we evaluated whether neovascularization is associated with AD.
Methods
Study protocol and surgery
After an institutional review board approval, the need for informed consent was waived. The ascending aortic wall resection of 35 consecutive patients undergoing surgery for ascending aorta was obtained and processed for histology. An ascending aortic aneurysm was preoperatively confirmed and evaluated with computed tomography (CT). According to our institutional policy, aortic aneurysm included an aortic diameter wider than 5.5 cm or aortic growth greater than 1 cm in a year. This definition was adjusted to the presence of Marfan syndrome, gender, patient size, and symptoms, including AD according to the Yale Center criteria (2). Surgery was performed between December 2009 and August 2014, and cases of ascending aortas including AD processed for histology were enrolled. Three patients with Marfan syndrome were excluded. There were 14 patients with acute AD including onset of symptoms that lasted less than 7 days.
The decision on the extension of resection and surgical technique was at the discretion of the operating surgeon. When an aortic aneurysm including the sinotubular junction (STJ) was estimated as the reason for aortic regurgitation, STJ was tailored for a suitable graft in a supracoronary fashion. Whenever dilatation included the aorta root, a radical resection of the dilated ascending aorta together with the root and the aortic valve was performed. The graft size was estimated by the principal surgeon. The entry tears were located in the middle portion of the ascending aorta according to pre-operative CT and intraoperative assessment. Since the surgical procedure was performed upon surgical decision, the sample was procured from the middle of the resected diseased area of the ascending aorta at the vicinity of STJ.
Histology and immunohistochemistry
Two to five blocks of resected ascending aorta were embedded in paraffin, cut to 4-mm-thick segments and stained with hematoxylin and eosin, Verhoeff-van Gieson, Elastase-van Gieson, and Periodic Acid-Schiff. A representative 1-cm-long piece of ascending aortic wall corresponding to all different staining was evaluated systematically for all resected samples procured during surgery.
Aortic wall histology and immunohistochemistry were performed using Ventana Lifesciences Benchmark XT Staining module for leukocytes, T- and B-lymphocytes, plasma cells, macrophages, smooth muscle cells, cell proliferation, elastase, and van Gieson staining. The samples were further investigated for presence and locality of neovascularity within the aortic wall; capillaries with endothelial cells were evaluated using a polyclonal rabbit antibody for CD31 (dilution 1:2500) (DakoCymation). Ventana Lifesciences Antibody Dilution Buffer was utilized for dilution media. The heights of different layers (adventitia, media, and intima) were calculated for each sample. Inflammatory cells, the intensity of inflammation, cell proliferation, medial degeneration, intima cellularity, and thickness were estimated as previously described and expressed as point score units (PSU) in the three aortic wall layers accordingly (8). Briefly, inflammation was graded as none, mild, moderate, or severe (0, 1, 2, or 3). Medial degeneration was graded as patchy, moderate, or severe again on a scale of 0–3. Intima cellularity and thickness were estimated according to an arbitrary scale from 0–3, where 0 indicated normal intima with a single endothelial cell layer; 1, intima cellularity and thickness less than 25% as compared with the media; 2, intima cellularity and thickness more than 25% but less than 50% as compared with the media; 3, intensive intima cellularity and thickness more than 50% as compared with the media.
Quantification of medial neovascularization
Platelet endothelial cell adhesion molecule 1 (PECAM-1), also known as CD31 (cluster of differentiation 31), is expressed in high amounts in endothelial cell junctions (9). As CD31 may be expressed by leukocytes and platelets (10), angiogenesis was defined by accounting CD31-positivity, including only capillary-like morphology with a continuous uninterrupted endothelial monolayer within the media layer. For local quantification of CD31-positivity, we categorized the media into three equal parts; the inner media consisting of the innermost media adjacent to the intima, the outer media adjacent to the adventitia, and the middle media between these two media layers, respectively. The total number of positively stained CD31 new vessels was counted per square millimeter in four arbitrarily selected areas, which showed the most increased density of capillary-like morphology (hot spots).
Follow-up protocol
Documentation of mortality and morbidity was available for all the patients. For the included study patients, follow-up consisted of physical examination and echocardiography at 3 months after surgery, and on-demand thereafter including CT.
Statistical analysis
CD31-positive staining was predominantly found in the media, at the border of the adventitia including formation of small vessels (Fig. 1). To seek clinical relevance associated with immunohistochemistry, the patients were divided into two groups in accordance with the histologically confirmed presence of AD. Although histopathology confirms AD, indices of inflammation, hemorrhage, or fibrosis do not facilitate the temporal diagnosis of AD (11). The patients were categorized in keeping with the presence of dissection (AD+) and dilatation only (AD-). All study patients were followed for a period of 3 months. Quantitative variables are listed as the mean and standard error of the mean. Categorical variables are stated as count and percentage. Statistical analysis was performed with the SPSS version 22.0. The Mann-Whitney U test was used for continuous variables, and the chi-squared test for categorical analysis. The association of CD31 with AD was assessed by the receiver operating characteristic curve (ROC) analysis. P-values less than 0.05 were considered statistically relevant.
Figure 1.
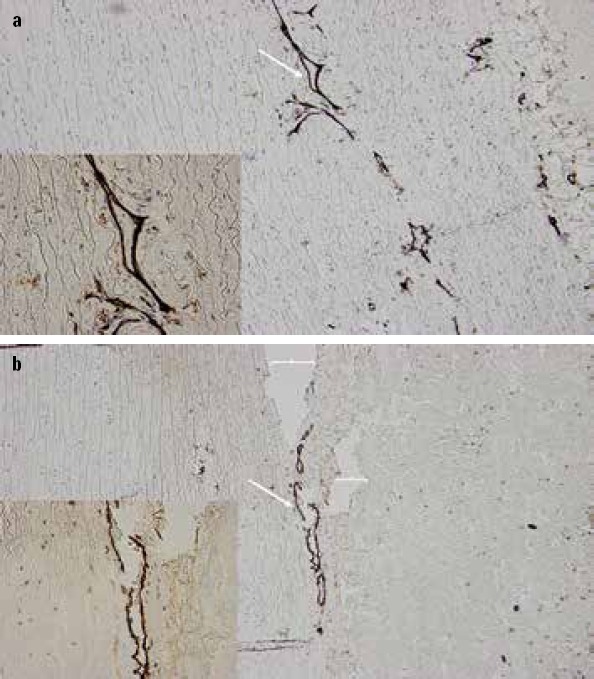
Representative immunohistochemistry (x20) for CD31 of the ascending aorta. Note the CD31 positivity (white arrow) in the outer third medial layer of the ascending aorta suggesting susceptibility for aortic dissection in (a) The onset of dissection (white brackets) in b at the site of CD31 positivity (white arrow). Inlets (x40) at the bottom left corner show the site of interests in detail.
Results
Demographics
Eighteen patients had ascending AD, while 14 out of 32 patients were operated for acute AD (Table 1). The mean age was 64±2 years. Hypertension and coronary artery disease were equally distributed among both groups. One patient without AD had unspecified vasculitis of the aortic wall. Four patients with AD and eight without AD had aortic valve insufficiency. Eleven out of 18 patients without AD had aortic valve stenosis, including five patients with combined aortic valve disease, in contrast to only one aortic valve stenosis in a patient with AD. The mean aortic diameter was 58±2 mm for all patients.
Table 1.
Patient demographics
| All patients | AD+ | AD– | P-value | |
|---|---|---|---|---|
| Number of patients | 32 | 14 | 18 | |
| Age (years) | 64±2 | 69±2 | 59±3 | 0.010 |
| Male, n | 22 (69%) | 8 (57%) | 14 (78%) | 0.267 |
| Hypertension, n | 13 (41%) | 6 (43%) | 7 (39%) | 1 |
| Diabetes, n | 1 (3%) | 1 (7%) | 0 | 0.438 |
| Hypercholesterolemia, n | 3 (9%) | 0 | 3 (17%) | 0.238 |
| Vasculitis, n | 1 (3%) | 0 | 1 (6%) | 1 |
| Arthritis, n | 3 (9%) | 3 (22%) | 0 | 0.073 |
| Asthma, n | 2 (6%) | 1 (7%) | 1 (6%) | 1 |
| Myocardial coronary artery disease, infarction, n | 7 (22%) | 3 (22%) | 4 (22%) | 1 |
| Previous cardiothoracic operation | ||||
| Coronary artery bypass surgery, n | 2 (6%) | 2 (15%) | 0 | 0.183 |
| Correction of aortic coarctation, n | 1 (3%) | 0 | 1 (6%) | 1 |
| Correction of abdominal aorta aneurysm | 1 (3%) | 1 (7%) | 0 | 0.438 |
| Mid-ascending aorta diameter, mm | 58±2 | 59±3 | 57±3 | 0.323 |
| 2-cusp aortic valve, n (%) | 8 (25%) | 1 (7%) | 7 (39%) | 0.053 |
| Aortic valve insufficiency | ||||
| Moderate to severe, n | 12 (38%) | 4 (29%) | 8 (45%) | 0.471 |
| Aortic valve stenosis | ||||
| Moderate to severe, n | 12 (38%) | 1 (7%) | 11* (61%) | 0.003 |
includes five patients with combined aortic valve disease, P=0.001
AD - aortic dissection
Operative technique
In patients with AD, surgery included either a Bentall-type operation with an aortic valve prosthesis, or replacement of the ascending aorta only (Table 2). In eight patients without AD, the aortic root was not operated on. A mechanical or biologic valve was replaced together with a prosthesis encompassing the ascending aorta distally from the sinotubular junction in five patients without AD. In three patients without AD and without aortic valve disease, only the ascending aorta was replaced.
Table 2.
Operative details according to surgical evaluation of extension of diseased aorta
| All Patients | AD+ | AD– | P-value | |
|---|---|---|---|---|
| 32 | 14 | 18 | ||
| Graft replacement of root and ascending aorta | ||||
| Mechanical conduit | 9 (28%) | 3 (22%) | 6 (33%) | 0.694 |
| Biological conduit | 8 (25%) | 4 (29%) | 4 (22%) | 0.703 |
| Graft replacement of ascending aorta | ||||
| Mechanical valve+prosthesis | 2 (7%) | 0 | 2 (11%) | 0.492 |
| Biological valve+prosthesis | 3 (10%) | 0 | 3* (17%) | 0.238 |
| Prosthesis | 10 (32%) | 7 (50%) | 3 (17%) | 0.062 |
| Additional procedure | ||||
| Coronary artery bypass surgery | 4 (13%) | 2 (15%) | 2 (11%) | 1 |
includes aortoplasty
AD - aortic dissection
Perioperative findings, histology, and immunohistochemistry
Histology revealed three cases of aortitis, of which two had AD+ (Tables 3 and 4). The intensity of chronic adventitial, medial, and intimal inflammation was increased in AD+ as compared with AD− (2.2±0.3 vs. 1.3±0.2, p=0.03, 1.4±0.3 vs. 0.3±0.1, p<0.001, and 1.6±0.3 vs. 0.7±0.2, p=0.005, respectively). The media showed increased cell proliferation in AD+ as compared with AD− (1.5±0.3 vs. 0.4±0.2, p=0.002). An increased number of macrophages and T-cells of the intima were found in AD+ as compared to AD− (1.9±0.2 vs. 1.2±0.2 p=0.032 and 1.4±0.2 vs. 0.6±0.2, p=0.006, respectively). The outer third layer of the media at the vicinity of the adventitia expressed an increased number of cytoplasmic CD31-positivity in AD+ as compared with AD− (5.1±1.1 vs. 2.4±0.7, p=0.037), and corresponded to the site of AD tear (Fig. 1).
Table 3.
Histology and quantitative immunohistochemistry
| Mean grade of staining | ||||
|---|---|---|---|---|
| mm mm | All Patients | AD+ | AD– | P-value |
| Adventitia | ||||
| T-cells | 1.4±0.2 | 1.8±0.3 | 1.1±0.2 | 0.077 |
| B-cells | 1.0±0.2 | 1.2±0.4 | 1.0±0.2 | 0.791 |
| Macrophages | 1.8±0.2 | 2.2±0.2 | 1.5±0.2 | 0.068 |
| Plasma cells | 0.6±0.2 | 0.9±0.3 | 0.6±0.2 | 0.201 |
| Inflammation | 1.6±0.2 | 2.2±0.3 | 1.3±0.2 | 0.003 |
| Proliferation | 1.5±0.2 | 1.6±0.2 | 1.3±0.4 | 0.561 |
| Media | ||||
| T-cells | 0.6±0.2 | 0.8±0.3 | 0.5±0.2 | 0.476 |
| B-cells | 0.2±0.1 | 0.3±0.2 | 0.1±0.6 | 0.175 |
| Macrophages | 1.2±0.2 | 1.5±0.3 | 1.0±0.3 | 0.207 |
| Plasma cells | 0.4±0.2 | 0.3±0.2 | 0.5±0.4 | 0.424 |
| Inflammation | 0.8 ± 0.2 | 1.4±0.3 | 0.3±0.1 | 0.001 |
| Proliferation | 0.9±0.2 | 1.5±0.3 | 0.4±0.2 | 0.002 |
| Degeneration | 1.5±0.2 | 1.7±0.3 | 1.4±0.3 | 0.466 |
| Elastase | 1.6±0.2 | 1.6±0.2 | 1.6±0.3 | 0.968 |
| Intima | ||||
| T-cells | 1.0±0.2 | 1.4±0.2 | 0.6±0.2 | 0.006 |
| B-cells | 0.1±0.1 | 0.3±0.2 | 0 | 0.072 |
| Macrophages | 1.5±0.2 | 1.9±0.2 | 1.2±0.2 | 0.032 |
| Plasma cells | 0.7±0.2 | 0.7±0.2 | 0.5±0.4 | 0.622 |
| Inflammation | 1.1±0.2 | 1.6±0.3 | 0.7±0.2 | 0.005 |
| Proliferation | 0.9±0.2 | 1.0±0.2 | 0.5±0.4 | 0.206 |
| Thickness | 2.0±0.3 | 1.9±0.3 | 2.1±0.4 | 0.706 |
| Cellularity | 1.6±0.2 | 1.7±0.2 | 1.3±0.2 | 0.328 |
Mean grade of staining expressed as point score units/mm2
AD - aortic dissection
Table 4.
Quantitative immunohistochemistry for CD31 according to location of staining
| Mean grade of staining | All patients | AD+ | AD– | P-value |
|---|---|---|---|---|
| Media | ||||
| Outer layer | 3.5±0.7 | 5.1±1.1 | 2.4±0.7 | 0.037 |
| Middle layer | 1.7±0.3 | 2.1±0.6 | 1.4±0.4 | 0.258 |
| Inner layer | 0.9±0.2 | 0.7±0.3 | 1.1±0.4 | 0.714 |
Mean grade of staining expressed as point score units/mm2
AD - aortic dissection
ROC analysis and outcome
A ROC analysis showed that the local endothelial activity was associated with AD (AUC 0.750; SE 0.092; p=0.022; 95% CI 0.570–0.930, Fig. 2). Two patients with AD died shortly after surgery: a 55-year old preoperatively unconscious patient and an 88-year old that experienced AD rupture before the onset of hypothermia. Two patients without AD died within 1 week of surgery, due to cerebral infarction caused by hypotension and cerebral emboli at surgery.
Figure 2.
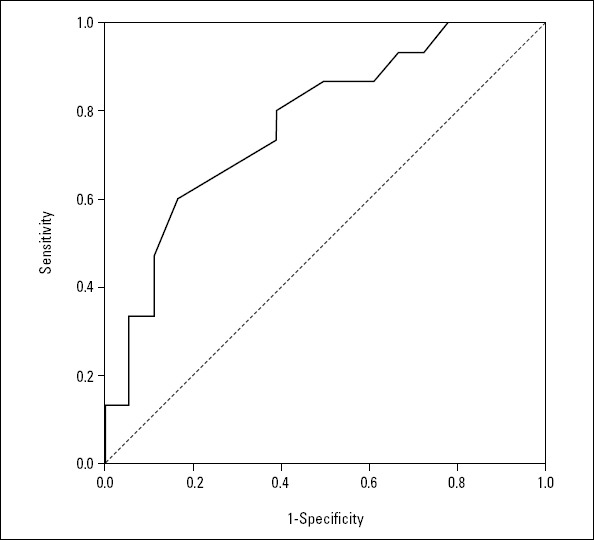
Receiver operating characteristic curve analysis shows that local neovascularization of the aortic wall is associated with AD (AUC 0.750; SE 0.092; P=0.022; 95% CI 0.570–0.930)
Discussion
Based on this study, local neovascularization of the outer medial layer indicates active remodeling of the aortic wall associated with AD; histological characteristics of the ascending aortic wall may be investigated to reveal endothelial activation of newly formed capillaries of the media layer according to the CD31 positivity. Together with neovascularization, an increased number of proliferative cells and macrophages is strongly suggestive of chronic inflammation in patients with AD.
Dilatation of the ascending aorta alone does not undeniably lead to AD. Hypertension and a family history for dilatation of the aorta may increase the risk for dilatation per se, but AD seems to occur in some instances quasi-unexpectedly, while the aortic diameter has not reached the threshold value of 5.5 cm2. According to a large referral center, AD was missed up to 38% of cases on initial evaluation and first established in 28% of patients only at postmortem examination (12). While traditional CT and echocardiography may not provide an accurate estimation of the risk for AD, molecular imaging has emerged as a plausible and promising option (13). Risk stratification of AD may benefit from understanding the heterogeneous pathogenesis of the inflammatory process and angiogenesis during aortic remodeling. Imaging modalities, such as positron emission tomography, single photon emission CT, and magnetic resonance imaging, with the aid of tracing chronic inflammation and neovascularization, are intensively studied for clinical translation (13).
The clinician craves for an applicable means to diagnose accurately an aorta prone for AD. This study emphasizes the importance of investigating both chronic inflammation and neovascularization that together may form a trigger for AD. This message importantly adds to the clinical transition of imaging modalities that are based on tracing chronic inflammation and neovascularization (13). An aortic site characterized by microvessel formation and inflammation during progression of ascending aortic dilatation (14) enhances awareness for the need of early surgical intervention to prevent AD.
The onset of AD includes vertical rupture of the aortic wall often at the junction of the media and adventitia (15). This aortic wall site suggests that the hypoxic environment of the media layer may attract chronic inflammatory components and eventually lead to angiogenesis and tissue tear. Endothelial cells form the inner lining of newly formed capillaries and render the media–adventitia border susceptible to the onset of AD. Inflammation alone without angiogenesis may not suffice to initiate AD. There are sparse data on the impact of CD31 immunohistochemistry on AD. Dysfunction of the microcirculation in the outer media layer of the aorta may suggest ischemia and malnutrition of the aortic wall, thus increasing the risk for AD (4). The impact of ischemia along with intimal hyperplasia and hypertension is not demonstrated in our study among the patients. It is important to distinguish CD31-stained endothelial cells from macrophages, since proinflammatory macrophages have experimentally been shown to influence the aortic wall during AD (16).
Neovascularization is a key factor to the pathophysiology of various arterial diseases, such as atherosclerosis (17), vasculitis (6), intracranial artery aneurysm (18), and abdominal aortic aneurysm (5). Neovascularization barely seems to be a consequence of hypoxia alone, but it involves mechanisms introduced by immunology and subsequent chronic inflammation (5, 6, 19). According to a previous experimental model (20), decreased circulation of vasa vasorum, which often occurs in arterial hypertension (17), may increase the stiffness of the outer media of the aorta, but an immunological activation of the aortic wall seems prerequisite to initiate an active tear, which leads to AD (17, 19, 21, 22). Chronic inflammation of all aortic layers was present in our study, together with neovascularization and AD. Comparably, acute plaque rupture of the atherosclerotic coronary artery (23) or the formation of microhemorrhages within intracranial artery aneurysms (18) may represent disease entities initiated by the activation of neovessels (19). It is tempting to suggest that CD31 immunohistochemistry may reveal a weak aortic wall site prone to AD.
Study limitations
This is a study investigating the association of increased aortic neovascularization in a fairly low number of patients with or without AD. Unfortunately, the rate of expansion of the non-repaired segments of the aorta was not evaluated to correlate with immunohistochemistry, partly due to the small number of patients. As a paradigm of comparable tissue preparation in this study, we did not systematically investigate aortas obtained from autopsied cases. Only less than a third of the patients had bicuspid aortic valve disease, and it is beyond our scope to discuss the plausible interaction of specific heterogenic aortic valve diseases in the development of AD. Increased chronic aortic wall inflammation was associated with AD together with neovascularization, but specific immunological parameters such as complement activation remain to be elucidated.
Conclusion
Heterogeneity of the progression of aortic dilatation to AD is expected (14). Interacting with chronic inflammation and associated neovascularization may impact against the development of AD. Taken together, we suggest that CD31 immunohistochemistry adds to the understanding of the remodeling characteristics of the ascending aorta, although further studies are clearly recommended.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – I.K., T.P., A.M.; Design – E.N., V.P., T.P., A.M.; Supervision – E.N., T.P., A.M.; Fundings – I.K., T.P., A.M.; Materials – E.N., V.P., I.K., A.M.; Data collection &/or processing – E.N., V.P., A.M.; Analysis &/or interpretation – E.N., I.K., T.P., A.M.; Literature search – E.N., V.P., A.M.; Writing – I.K., T.P., A.M.; Critical review – E.N., V.P., I.K., T.P., A.M.
References
- 1.Narayan P, Rogers CA, Davies I, Angelini GD, Bryan AJ. Type A aortic dissection: has surgical outcome improved with time? J Thorac Cardiovasc Surg. 2008;136:1172–7. doi: 10.1016/j.jtcvs.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 2.Elefteriades JA. Thoracic aortic aneurysm: reading the enemy's playbook. World J Surg. 2008;32:366–74. doi: 10.1007/s00268-007-9398-3. [DOI] [PubMed] [Google Scholar]
- 3.Trimarchi S, Jonker FHW, Hutchison S, Isselbacher EM, Pape LA, Patel HJ, et al. Descending aortic diameter of 5.5 cm or greater is not accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg. 2011;142:e101–7. doi: 10.1016/j.jtcvs.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Osada H, Kyogoku M, Ishidou M, Morishima M, Nakajima H. Aortic dissection in the outer third of the media: what is the role of the vasa vasorum in the triggering process? Eur J Cardiothorac Surg. 2013;43:e82–8. doi: 10.1093/ejcts/ezs640. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MM, Jones L, Nasim A, Sayers RD, Bell PR. Angiogenesis in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1996;11:464–9. doi: 10.1016/s1078-5884(96)80183-3. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser M, Younge B, Björnsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–74. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moraes F, Paye J, Mac Gabhann F, Zhuang ZW, Zhang J, Lanahan AA, et al. Endothelial cell-dependent regulation of arteriogenesis. Circ Res. 2013;113:1076–86. doi: 10.1161/CIRCRESAHA.113.301340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levula M, Paavonen T, Valo T, Pelto-Huikko M, Laaksonen R, Kahonen M, et al. A disintegrin and metalloprotease-8 and -15 and susceptibility for ascending aortic dissection. Scand J Clin Lab Invest. 2011;71:515–22. doi: 10.3109/00365513.2011.591939. [DOI] [PubMed] [Google Scholar]
- 9.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J Histochem Cytochem. 2004;52:87–101. doi: 10.1177/002215540405200109. [DOI] [PubMed] [Google Scholar]
- 10.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–23. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 11.Peterss S, Mansour AM, Ross JA, Vaitkeviciute I, Charilaou P, Dumfarth J, et al. Changing pathology of the thoracic aorta from acute to chronic dissection: literature review and insights. J Am Coll Cardiol. 2016;68:1054–65. doi: 10.1016/j.jacc.2016.05.091. [DOI] [PubMed] [Google Scholar]
- 12.Spittell PC, Spittell Jr JA, Joyce JW, Tajik AJ, Edwards WD, Schaff HV, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990) Mayo Clin Proc. 1993;68:642–51. doi: 10.1016/s0025-6196(12)60599-0. [DOI] [PubMed] [Google Scholar]
- 13.Golestani R, Sadeghi MM. Emergence of molecular imaging of aortic aneurysm;implications for risk stratification and management. J Nucl Cardiol. 2014;21:251–67. doi: 10.1007/s12350-013-9845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirsch EW, Radu NC, Gervais M, Allaire E, Loisance DY. Heterogeneity in the remodeling of aneurysms of the ascending aorta with tricuspid aortic valves. J Thorac Cardiovasc Surg. 2006;132:1010–6. doi: 10.1016/j.jtcvs.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 15.Schmitto JD, Popov AF, Coskun KO, Friedrich M, Sossalla S, Didilis V, et al. Morphological investigations of type A aortic dissection. Ann Thorac Cardiovasc Surg. 2010;16:331–4. [PubMed] [Google Scholar]
- 16.Andreata F, Syvannarath V, Clement M, Delbosc S, Guedj K, Fornasa G, et al. Macrophage CD31 signaling in dissecting aortic aneurysm. J Am Coll Cardiol. 2018;72:45–57. doi: 10.1016/j.jacc.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Marcus ML, Heistad DD, Armstrong ML, Abboud FM. Effects of chronic hypertension on vasa vasorum in the thoracic aorta. Cardiovasc Res. 1985;19:777–81. doi: 10.1093/cvr/19.12.777. [DOI] [PubMed] [Google Scholar]
- 18.Ollikainen E, Tulamo R, Frösen J, Lehti S, Honkanen P, Hernesniemi J, et al. Mast cells, neovascularization, and microhemorrhages are associated with saccular intracranial artery aneurysm wall remodeling. J Neuropathol Exp Neurol. 2014;73:855–64. doi: 10.1097/NEN.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 19.Del Porto F, di Giola C, Tritapepe L, Ferri L, Leopizzi M, Nofroni I, et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology. 2014;127:123–9. doi: 10.1159/000355253. [DOI] [PubMed] [Google Scholar]
- 20.Angouras D, Sokolis DP, Dosios T, Kostomitsopoulos N, Boudoulas H, Skalkeas G, et al. Effect of impaired vasa vasorum flow on the structure and mechanics of the thoracic aorta: implications for the pathogenesis of aortic dissection. Eur J Cardiothorac Surg. 2000;17:468–73. doi: 10.1016/s1010-7940(00)00382-1. [DOI] [PubMed] [Google Scholar]
- 21.He R, Guo D-C, Estrera A, Safi HJ, Huynh TT, Yin Z, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissection. J Thorac Cardiovasc Surg. 2006;131:671–8. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Liao M-F, Tian L, Zou SL, Lu QS, Bao JM, et al. Overexpression of interleukin-1b and interferon-g in type I thoracic aortic dissections and ascending thoracic aortic aneurysms: possible correlation with matrix metalloproteinase-9 expression and apoptosis of aortic media cells. Eur J Cardiothorac Surg. 2011;40:17–22. doi: 10.1016/j.ejcts.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, et al. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–91. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]


