Abstract
Objective:
The aim of the present study was to identify morphological and pathophysiological factors associated with long-term patency of grafts used in coronary artery bypass grafting (CABG).
Methods:
A total of 127 patients who underwent CABG between 2000 and 2006 and presented for computed tomography evaluation of graft patency at 139.78±36.64 months post-CABG were analyzed. Patients received 340 grafts (2.68 grafts/patient), 399 distal anastomoses (3.14 anastomoses/patient), 220 (55.14%) performed using arterial grafts, and 179 (44.86%) using saphenous vein grafts (SVGs).
Results:
Graft patency varied according to vessel type and coronary territory. Overall graft patency was 90.16% for the left internal thoracic artery (LITA), 75.55% for the right internal thoracic artery (RITA), 79.25% for the radial artery (RA), and 74.3% for the SVG. The maximum patency rate was obtained with the RA (80.65%) for the right coronary territory, RITA (92.86%) for the anterolateral territory, and SVG (82.54%) for the circumflex territory. The LITA.left anterior descending artery graft occluded in 13 (7.93%) cases, 7 due to competitive flow. The influence of graft length on patency rates after indexing to height was not significant. The target vessel degree of stenosis influenced arterial graft patency rates with an occlusion odds ratio (OR) of 3.02 when anastomosed to target vessels with <90% stenosis. Target vessel caliber also influenced patency rates with occlusion ORs of 2.63 for SVGs and 2.31 for arterial grafts when anastomosed to .1.5 mm target vessels.
Conclusion:
Morphological parameters, such as graft type, target territory, target vessel caliber, and degree of stenosis, are important factors conditioning long-term graft patency.
Introduction
Coronary artery bypass grafting (CABG) is nowadays one of the most frequent surgical interventions in Europe (between 18 and 91/100,000 inhabitants) (1). According to both European (2) and American (3) societies’ guidelines, it is associated with an increase in quality of life and survival in patients with unprotected left main (or equivalent) and multivessel disease, but the optimal grafting technique has not been established.
Early CABG interventions were performed almost entirely using aorta-to-coronary saphenous vein grafts (SVGs), but angiographic follow-up studies revealed a late attrition rate of 2%–5% per year after surgery related to intrinsic pathological changes in grafts (4, 5). Despite their anatomical imperfection, venous grafts are easy to harvest and use, even for inexperienced surgeons. Compared with the veins, the internal thoracic arteries [left internal thoracic artery (LITA) or right internal thoracic artery (RITA)] have an extremely low attrition rate with very good long-term patency rates (96.4% >15 years) (6). The anatomical imperfection of the veins and the incapacity of performing a complete revascularization using only ITAs led to the pursuit of additional grafts [the most commonly used being a. radialis (RA), followed by a. gastroepiploica dextra, a. epigastrica inferior, a. splenica, a. ulnaris, a. subscapularis, a. gastrica sinistra, and a. circumflexa femoris lateralis] and imagining new operative techniques in order to obtain a complete revascularization (5-6 distal anastomoses) using a limited number of grafts (7).
Although the literature is unanimous in stating that graft patency is an important factor conditioning CABG long-term prognosis, there is no consensus regarding the optimal grafting technique in terms of graft type, harvesting, preparation, features, configuration, or anastomoses.
The purpose of the current study was to identify morphological and pathophysiological factors associated with long-term patency of grafts used in CABG.
Methods
Patient population and surgical technique
Demographic, clinical, echocardiographic, and angiographic data on patients undergoing CABG at our institute have been retrospectively collected and introduced into a database since 2000, together with intraoperative parameters (extracorporeal circulation type and time, aortic cross-clamp time, CABG technique, number and type of grafts, and associated procedures) and postoperative data (intensive care unit parameters, complications, and mortality within 30 days). The CABG technique varied according to the practice at the time of surgery from total venous to total arterial revascularization and from one graft–one anastomosis to composite and sequential grafting.
Patient follow-up
In 2016, the status of all patients (394) who received CABG between 2000 and 2006 and were discharged from the hospital was verified through the National Health Insurance House database, and there were 269 identified survivors (68.27%). All survivors were recalled for a coronary computed tomography angiography (CCTA) evaluation of graft patency through an invitation letter or phone call. One hundred twenty-seven patients agreed to the examination, presented no contraindications to CCTA graft patency assessment, and had good quality examinations that allowed quantifying all grafts. Patients were evaluated after a mean postoperative interval of 139.78±36.64 months.
None of the patients had myocardial infarction within 30 days suggestive of early graft failure.
Postoperative long-term medical treatment consisted of beta blockers, statins, and enteric-coated aspirin in all cases. Patients who benefited from an RA graft received a calcium channel blocker (amlodipine) for the first 3 months to prevent spasm. Treatment was adjusted according to blood pressure, left ventricular ejection fraction, and comorbidities.
CCTA evaluation
All CCTA evaluations were performed using a second generation 2*128-slices dual source multidetector CT scanner (Siemens Somatom Definition Flash) with the following scan parameters: 100 or 120 kV tube voltage, 128×0.6 mm collimation, and 280 ms gantry rotation time. Optimal reconstructions were performed (0.75 mm slice thickness) and submitted to the Syngo.via workstation (Siemens Medical Solutions, Germany) for image analysis.
Image analysis
All CT examinations were evaluated twice by the same radiologist for the following parameters: graft type and status (confronted with the operative protocol), target coronary artery (confronted with the operative protocol), graft length, graft caliber, and target vessel caliber. Occluded grafts were traced based on density differences relative to the adjacent mediastinal or epicardial fat translated by a light gray color in the case of occluded grafts and dark gray or black color for mediastinal/epicardial fat (Fig. 1).
Figure 1.
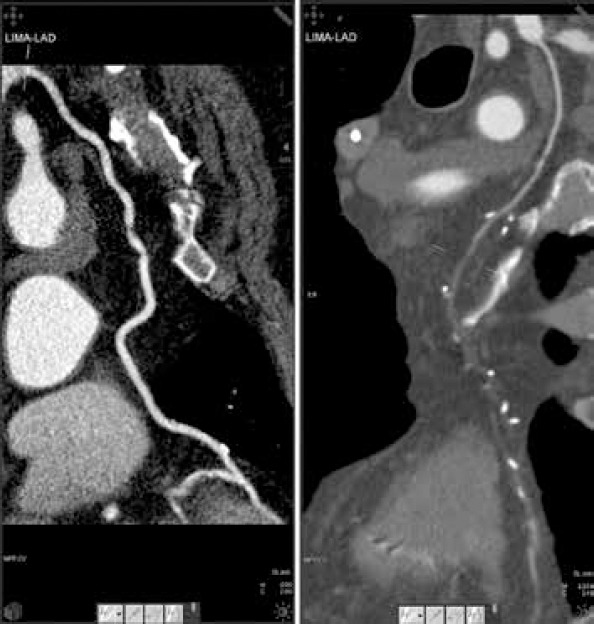
Patent (left) and occluded (right) LITA–LAD graft
Statistical analysis
Continuous variables are expressed as mean±standard deviation. Categorical variables are expressed as percentages. Groups were compared using the chi-square test for categorical variables and the Student’s t-test and Wilcoxon– Mann-Whitney U test for continuous variables based on their distribution. Normality was verified using the Kolmogorov–Smirnov and the Shapiro–Wilk W tests. Logistic regression was employed for testing the association between categorical and continuous variables previously identified as affecting graft patency at univariable analysis with a p value <0.05. A receiver operating characteristic (ROC) curve was used for identification of the discrimination threshold (cut-off) value for continuous variables. Statistical analyses were performed using IBM SPSS Statistics 24 for Mac OS X.
The study was approved by the Ethics Committee of the “Grigore T. Popa” University of Medicine and Pharmacy (Iasi, Romania) and the “Prof. Dr. George I.M. Georgescu” Cardiovascular Diseases Institute (Iasi, Romania).
Results
Baseline characteristics
The preoperative, operative, and postoperative data of 127 patients are summarized in Tables 1-3.
Table 1.
Preoperative data
| Variable | Value (127 patients) | Percentage (%) |
|---|---|---|
| Mean age (years)±SD | 67.54±8.84 | - |
| ≤65 years | 44 | 34.65% |
| >65 years | 83 | 65.35% |
| Female sex | 19 | 14.96% |
| Family history | 41 | 32.28% |
| Smoking | 49 | 38.58% |
| Diabetes mellitus | 28 | 22.05% |
| Dyslipidemia | 97 | 76.38% |
| MAD | 19 | 14.96% |
| AHT | 77 | 60.63% |
| COPD | 8 | 6.30% |
| NYHA II heart failure | 18 | 14.17% |
| NYHA III–IV heart failure | 24 | 18.90% |
| Prior AMI | 65 | 51.18% |
| Arrhythmias | 23 | 18.11% |
| Mean LVEF (%) | 53.81±10.77 | - |
| No. of affected | 2.86±1.24 | - |
| coronary arteries | ||
| Diffuse disease | 29 | 22.83% |
| Three vessel disease | 71 | 55.91% |
MAD - multisite artery disease; AHT - arterial hypertension; COPD - chronic obstructive pulmonary disease; NYHA - New York Heart Association; AMI - acute myocardial infarction; LVEF - left ventricular ejection fraction
Table 2.
Surgical data
| Variable | Value (127 patients) | Percentage (%) |
|---|---|---|
| Emergency surgery | 3 | 2.36% |
| Associated interventions | 13 | 10.24% |
| ACC time (min)±SD | 90.01±61.35 | |
| ECC time (min)±SD | 136.82±64.13 | |
| Mean no. of | 2.68±0.94 | |
| grafts/patient | ||
| Mean no. of arterial | 1.64±1.20 | |
| grafts/patient | ||
| Mean no. of venous | 1.52±0.79 | |
| grafts/patient | ||
| Mean no. of distal | 3.14±1 | |
| anastomoses/patient | ||
| Conventional CABG | 79 | 62.20% |
| (at least 1 SVG) | ||
| TAR | 38 | 29.92% |
| Single graft | 5 | 3.94% |
| Total venous | 5 | 3.94% |
| IABP usage | 1 | 0.79% |
| Complete revascularization | 102 | 80.31% |
ACC - aortic cross-clamp; ECC - extracorporeal circulation; SVG - saphenous vein graft; TAR - total arterial revascularization; IABP - intra-aortic balloon pump.
Associated interventions: valve surgery, atrial fibrillation ablation, ascending aorta replacement, and left ventricular aneurysm repair
Table 3.
Postoperative data (initial 30 days)
| Variable | Value (127 patients) | Percentage (%) |
|---|---|---|
| Reintervention for hemorrhage or sternal dehiscence | 10 | 7.87% |
| Acute renal failure | 2 | 1.57% |
| Arrhythmia | 31 | 24.41% |
| Neurological complications | 2 | 1.57% |
| Deep sternal wound infection | 2 | 1.57% |
| Other infections (urinary tract, pneumonia) | 3 | 2.36% |
| Digestive complications (ileus, Clostridium difficile infection) | 4 | 3.15% |
Graft patency assessment
One hundred twenty-seven patients presented a total number of 340 grafts (2.68 grafts/patient), 399 distal anastomoses (3.14 anastomoses/patient), 220 (55.14%) performed using arterial grafts (122 LITA, 53 RA, and 45 RITA), and 179 (44.86%) using SVGs. Overall graft patency at 10 to 16 years after the surgical intervention was 90.16% for the LITA, 75.55% for the RITA, 79.25% for the RA, and 74.3% for the SVG. Without considering the LITA anastomosed to the left anterior descending artery (LAD) and the coronary territory, there was no statistically significant association between graft type and long-term patency.
The influence of the graft length on patency rates was analyzed after indexing its value to the height of the patient (151-190 cm). The analysis was performed for each graft-target vessel configuration, but the limited number of cases for certain configurations did not allow statistical testing. In the case of grafts with sufficient number of cases (LITA–LAD, RA–posterior descending artery (PDA), SVG–diagonal, SVG–marginal oblique artery (MO), SVG–PDA, and SVG–right coronary artery (RCA)), no statistically significant difference was recorded (Table 4).
Table 4.
Graft patency according to length
| Graft | Patent | Occluded | P |
|---|---|---|---|
| LITA–LAD | 0.117 | 0.119 | 0.818 |
| RA–PDA | 0.844 | 0.824 | 0.541 |
| RA–diagonal | 0.066 | - | |
| RA–MO | 0.089 | - | |
| RA–MO (Y) | 0.071 | 0.064 | - |
| RA–RCA | 0.0867 | 0.072 | - |
| RA–IB (Y) | 0.024 | 0.028 | - |
| RITA–diagonal (Y) | 0.040 | 0.031 | - |
| RITA–MO (Y) | 0.065 | 0.074 | - |
| RITA–PL (Y) | 0.095 | 0.074 | - |
| RITA–RCA | 0.113 | 0.106 | - |
| RITA–IB (Y) | 0.046 | 0.024 | - |
| SVG–diagonal | 0.62 | 0.74 | 0.680 |
| SVG–LAD | 0.082 | - | - |
| SVG–MO | 0.83 | 0.75 | 0.933 |
| SVG–PDA | 0.09 | 0.079 | 0.533 |
| SVG–PL | 0.095 | 0.064 | - |
| SVG–RCA | 0.0736 | 0.0732 | 0.637 |
| SVG–IB | 0.067 | 0.052 | - |
LITA - left internal thoracic artery; LAD - left anterior descending artery; Recognizer; MO - marginal obtuse artery; RCA - right coronary artery; IB - intermediate branch; RITA - right internal thoracic artery; SVG - saphenous vein graft; PDA - posterior descending artery; PL - posterolateral artery
Graft patency varied according to coronary territory. Specifically, the RITA occlusion rate was 3/8 (37.5%) for the RCA territory, 7/23 (30.43%) for the circumflex (CX) territory, and 1/14 (7.14%) for the anterolateral territory. The RA occlusion rate was 6/31 (19.35%) for the RCA territory, 4/16 for the CX territory (25%), and 1/6 (16.67%) for the anterolateral territory. The SVG occlusion rate was 24/68 (35.29%) for the RCA territory, 11/63 (17.46%) for the CX territory, and 11/48 (22.92%) for the anterolateral territory. The maximum patency rate was obtained with the RA for the RCA territory, RITA for the anterolateral territory, and SVG for the CX territory.
The LAD was analyzed separately from other target vessels. It was revascularized using in situ LITAs in 118/122 (96.72%) cases, SVGs in 3/122 (2.46%) cases, and in situ RITAs in 1 (0.82%) case. No SVG–LAD or RITA–LAD occlusion was identified. The LITA–LAD graft was occluded in 12 (9.83%) cases, with 7 cases presenting identifiable potential causes of competitive flow (concomitant grafting of diagonal arteries with no interposed segment with ≥75% stenosis–2 cases, grafting to an LAD with <75% stenosis–4 cases, and subsequent percutaneous left main stenting–1 case) (Fig. 1).
Target vessel status was recorded considering stenosis severity and vessel caliber. In the case of SVGs, there was no statistically significant difference in stenosis severity between patent (mean stenosis 90.5%) and occluded grafts (mean stenosis 90.62%) (p=0.607). For arterial grafts, the difference was significant (p=0.005), and target vessel stenosis was 91.22% for patent grafts compared with 78.52% for occluded ones. Using the area under the curve of ROC, a stenosis of 90% as a threshold value with an 80% sensitivity and specificity in affirming graft occlusion was determined. Logistic regression identified an occlusion odds ratio (OR) of 3.02 for arterial grafts anastomosed to target vessels with <90% stenosis (95% CI 1.321–6.902, p<0.001).
In order to analyze graft patency according to target vessel caliber, coronary arteries were divided into two groups depending on the caliber immediately downstream from anastomosis given the spatial resolution of CCTA: ≤1.5 mm and >1.5 mm. For the SVG, 43.33% of grafts anastomosed to ≤1.5 mm target vessels were occluded compared with 22.15% of those anastomosed to >1.5 mm target vessels (p=0.001) (Fig. 2).
Figure 2.
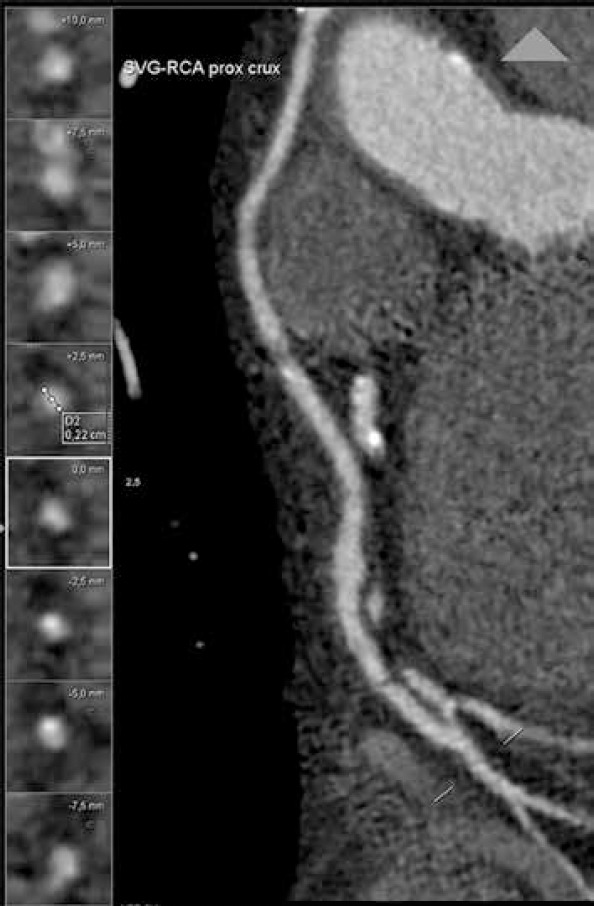
RCA diameter downstream from an SVG–RCA anastomosis
For arterial grafts, the result was similar with a 30.77% occlusion rate for grafts anastomosed to ≤1.5 mm target vessels versus 15.03% in the case of >1.5 mm target vessels (p=0.008). Logistic regression yielded a 2.63 occlusion OR for SVGs (95% CI 1.32–2.98, p=0.0041) and 2.31 occlusion OR for arterial grafts (95% CI 1.53–3.19, p=0.0001) anastomosed to ≤1.5 mm target vessels.
Discussion
Over the decades, graft patency has been assessed for individual grafts alone and not for CABG as an entity per se while the dilemma of designing an optimal grafting technique in terms of graft type, harvesting, preparation, features, configuration, or anastomoses remained unsolved despite the increase in worldwide research. Literature review allowed us to classify the factors influencing graft patency into morphological (vessel type, graft length, and caliber) (7, 8), pathophysiological (competitive flow through the native coronary artery and graft degenerative changes) (8, 9), and surgical (technical expertise, graft harvesting and preparation, grafting design, and anastomosis technique) (10-12). This single center study analyzed long-term graft patency according to morphological and pathophysiological factors.
Graft type
In 2001, Taggard et al. (13) performed a meta-analysis on 15,962 patients and identified a 10-year patency rate of 90%–95% for ITAs while 75% of SVGs presented stenotic or occlusive lesions. Benedetto et al. (14) analyzed nine randomized trials including 1620 angiographic controls at 1–7.7 years after CABG and quantified four times higher occlusion risk for the SVG than that for the RITA and three times for the RA. For patients >70 years old, Habib et al. (15) proved on a study group of 2120 cases an increased survival rate of 5 and 10 years for the ITA–RA association (85.1% and 70.9%, respectively) compared with the ITA–SVG association (70.6% and 50.5%, respectively). In our case, the highest patency rate was obtained with LITAs (90.16%), followed by RAs (79.25%), RITAs (75.55%), and SVGs (74.3%). The SVG is particularly sensitive to dysfunction by thrombosis in the first month (due to focal destruction of the venous endothelium while harvesting), intimal hyperplasia between 1 month and 1 year, and atherosclerotic lesions after 1 year. Necropsic studies identified the presence of atherosclerotic lesions in the SVG starting from the first postoperative year (16). These lesions rarely generate significant stenosis in the first 3 postoperative years and are responsible for clinical symptoms after 5 years. Arterial grafts are resistant to atherosclerotic lesions, but the RA is a muscular artery prone to spasm in the case of inadequate harvesting and competitive flow. Fibro-intimal hyperplasia as an isolated process occurs particularly in ITA grafts (17).
Graft length
Compared with graft type, graft length-related patency was not analyzed by extensive studies. According to small series (18) and case presentations (19), a short, tensed graft is predisposed to spasm in the case of arteries and to flattening in the case of veins with hypoperfusion of the grafted territory compared with a long graft with excessive length that is predisposed to transection and kinking. Graft length could prove insufficient secondary to imprecise estimation of the cardiac volume or of the graft itself, peripheral localization of the target vessel, anatomical features of the graft (high ITA bifurcation), harvesting or manipulation errors (destruction of a graft segment), and pulmonary hyperinflation (emphysema). Several techniques could be applied if a length deficit is discovered during surgery: composite anastomosis, elongation with a venous segment, using a skeletonized instead of a pedicled graft, right atrial plication (18), and grafting the CX territory via the transverse sinus (Fig. 3). In our case, the limited number of grafts for each configuration allowed statistical analysis only for LITA–LAD, RA–PDA, SVG–diagonal, SVG–MO, SVG–PDA, and SVG–RCA configurations with no significant difference between patent and occluded grafts. Patent grafts presented minimal curvatures, allowing graft accommodation to respiratory movements and cardiac distension.
Figure 3.
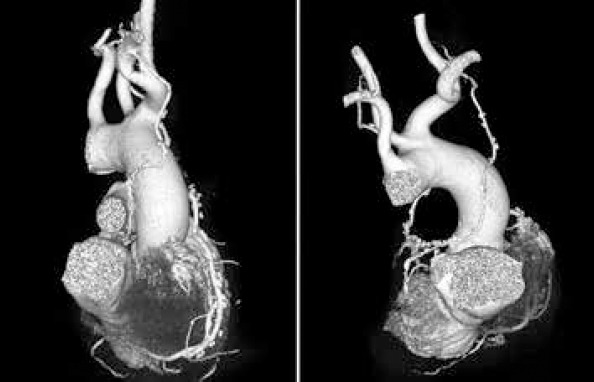
RITA passage through the transverse sinus to reach a marginal obtuse artery
Coronary territory
In our study, the patency of both arterial and venous grafts was influenced by the coronary territory. The maximum patency rate was obtained with the RAs for the RCA territory, RITAs for the anterolateral territory, and SVGs for the CX territory. A series of studies (20, 21) reported lower patency rates for the RA anastomosed to vessels from the right coronary territory than those from the anterolateral territory. In 2015, Parissis et al. (22) performed a meta-analysis of 44 studies reporting graft patencies for the right coronary territory and concluded that both the RA and the RITA patency rates are superior to the SVG patency for the right coronary territory contrary to our study that proves the supremacy of the RA (80.65% patency rate) in front of the SVG (64.71%) but not of the in situ RITA (62.5%).
Target vessel status
According to Poiseuille’s law, vessel resistance is proportional to the length and inversely related to the fourth power of the inner diameter of the conduit. Competitive flow between a graft and the target vessel occurs when the conductance of the graft is similar to the latter. In this case, both the graft and the target vessel contribute to the perfusion of the distal vascular bed in variable proportions depending on the hemodynamic condition that may induce vasodilation or vasoconstriction. Ding et al. (23) analyzed the impact of competitive flow on wall shear stress and confirmed that graft flow is conditioned by native vessel flow. The amplitude of competitive flow is determined not only by the degree of stenosis of the target vessel but also by the volume of myocardial tissue perfused by the grafted artery. Our study proves a higher susceptibility to competitive flow in the case of arterial grafts, with a mean target vessel stenosis of 91.22% for patent graft compared with 78.52% for occluded grafts and an occlusion OR of 3.02 for arterial grafts anastomosed to target vessels with <90% stenosis. The SVGs were not sensitive to target vessel degree of stenosis. The SVGs are anastomosed directly to the aorta being subsequently perfused at higher pressures compared with free arterial grafts anastomosed in a Y/T manner to the LITA and have a thinner media that does not allow lumen diameter adjustment to metabolic needs. These two aspects could explain the lack of a statistically significant difference between the target vessel degree of stenosis in the case of patent SVGs versus occluded SVGs. Ding et al. (23) analyzed the impact of competitive flow on a computerized model of an ITA graft (4.6 mm diameter) anastomosed to the LAD (4.5 mm diameter) under an anastomosis angle of 45°. Simulations were performed for stenosis severities of 30%, 50%, 75%, and 100% and showed that the higher the degree of LAD stenosis was, the lower the mean velocity in the proximal LAD artery was with the opposite occurring for the ITA. In the initial and terminal part of the cardiac cycle, a reverse flow was detected in the graft for 30% and 50% LAD stenosis. The phenomena decreased in amplitude for 75% stenosis and disappeared in 100% stenosis. Competitive flow may occur secondary to residual flow through a non-critical stenosis or due to retrograde flow through coro-coronary auto-anastomoses (collateral circulation) as proven by Maniar et al. (21). Compared with competitive flow through a non-critical stenosis, the one induced by collateral branches diminishes gradually until complete extinction as collateral branches close if the graft perfuses efficiently the target vessel. A LITA–LAD graft can also occlude secondary to competitive flow generated by a grafted diagonal artery if no significant (≥75%) stenosis is interposed between the origin of the grafted diagonal artery and the LITA–LAD anastomosis according to Kawamura et al. (24). On the contrary, a well-perfused CX by a grafted MO is unable to generate competitive flow for the LITA–LAD graft. The difference between the two cases is represented by the length of the interposed segment, allowing flow attenuation in the second case. Simulations performed by Kute et al. (25) demonstrated that the flow condition in the proximal artery is an important determinant of the hemodynamics at the distal end-to-side anastomosis. Since hemodynamic forces affect the response of vascular endothelial cells, the flow situation in the proximal artery may affect intimal hyperplasia formation and, therefore, long-term graft patency.
Another target vessel parameter affecting long-term graft patency is the caliber. A target vessel ≤1.5 mm is associated with both arterial and venous graft occlusion. The first research investigating the relationship between graft patency and target vessel caliber was performed by Roth in 1979 and identified a 90% patency rate of 1 year for SVGs anastomosed to coronary arteries with a >1.5 mm diameter and a 65% patency rate in the case of anastomosis to ≤1.5 mm coronary arteries (26). The study was resumed and extended by Goldman et al. (27) in 2004 who identified a 10-year patency rate of 88% for SVGs and 100% for ITAs anastomosed to coronary arteries with a diameter >2 mm compared with 55% for SVGs and 82% for ITAs anastomosed to ≤2 mm target vessels. An increased caliber of the target vessel is associated with a better runoff and graft flow, thus explaining higher patency rates.
Study limitations
The study was limited by the small sample, an extensive, multicenter study being necessary to confirm the findings. In addition, CCTA is a method limited by its spatial resolution (0.5–0.625 mm), inferior to standard X-ray coronary angiography (0.2 mm), and a 3.8% discrepancy compared with the latter (28). Thus, CCTA measurements may overestimate diameters compared with conventional angiography. This potential limitation does not bias the results of the study as it is homogenous, affecting all vessels.
Conclusion
Morphological parameters, such as graft type, target territory, target vessel caliber, and degree of stenosis, are important factors conditioning long-term graft patency. A large target vessel with a severe stenosis has a good runoff and is associated with higher long-term patency rates, especially if located in the left coronary territory. The right coronary territory has particular morphological features yielding to lower patency rates compared with the left coronary territory, the radial artery offering the best long-term patency rates in this case.
Footnotes
Funding: The scientific research was financed by the “Grigore T. Popa” University of Medicine and Pharmacy (Iasi, Romania) under the contract no. 29031/28.12.2016.
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – G.T., C.F.; Design – R.O.C.; Supervision – G.T.; Materials – R.O.C.; Data collection &/or processing – M.M.L.C., M.C.; Analysis &/or interpretation – R.O.C.; Literature search – G.T., C.F.; Writing – R.O.C., C.F.; Critical review – M.E., M.M.L.C., M.C.
References
- 1.Eurostat-Surgical operations and procedures statistics. [(cited 2017 August 31st)]. Available from: http://ec.europa.eu/eurostat/statistics-explained/index.php/Surgical_operations_and_procedures_statistics .
- 2.European Society of Cardiology: ESC/EACTS Guidelines in Myocardial Revascularisation. 2014. [(cited 2017 August 31st)]. Available from: http://eurheartj.oxfordjournals.org/content/ehj/35/37/2541.full.pdf .
- 3.Coronary Revascularization Writing Group. Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA, et al. American College of Cardiology Foundation;American College of Cardiology Foundation Appropriate Use Criteria Task Force;Society for Cardiovascular Angiography and Interventions;Society of Thoracic Surgeons;American Association of Thoracic Surgery;American Heart Association;American Society of Nuclear Cardiology;Society of Cardiovascular Computed Tomography. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Thorac Cardiovasc Surg. 2012;143:780–803. doi: 10.1016/j.jtcvs.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 4.Patil CV, Nikolsky E, Boulos M, Grenadier E, Beyar R. Multivessel coronary artery disease: current revascularization strategies. Eur Heart J. 2001;22:1183–97. doi: 10.1053/euhj.2000.2497. [DOI] [PubMed] [Google Scholar]
- 5.Sabik JF., 3rd Understanding saphenous vein graft patency. Circulation. 2011;124:273–5. doi: 10.1161/CIRCULATIONAHA.111.039842. [DOI] [PubMed] [Google Scholar]
- 6.Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004;77:93–101. doi: 10.1016/s0003-4975(03)01331-6. [DOI] [PubMed] [Google Scholar]
- 7.Aydin S, Aydin S, Nesimi Eren M, Sahin I, Yilmaz M, Kalayci M, et al. The cardiovascular system and the biochemistry of grafts used in heart surgery. SpringerPlus. 2013;2:612. doi: 10.1186/2193-1801-2-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porto I, Gaudino M, De Maria GL, Di Vito L, Vergallo R, Bruno P, et al. Long-term morphofunctional remodeling of internal thoracic artery grafts: a frequency-domain optical coherence tomography study. Circ Cardiovasc Interv. 2013;6:269–76. doi: 10.1161/CIRCINTERVENTIONS.113.000200. [DOI] [PubMed] [Google Scholar]
- 9.Melby SJ, Saint LL, Balsara K, Itoh A, Lawton JS, Maniar H, et al. Complete Coronary Revascularization Improves Survival in Octogenarians. Ann Thorac Surg. 2016;102:505–11. doi: 10.1016/j.athoracsur.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 10.Carranza CL, Ballegaard M, Werner MU, Hasbak P, Kjær A, Kofoed KF, et al. Endoscopic versus open radial artery harvest and mammario-radial versus aorto-radial grafting in patients undergoing coronary artery bypass surgery: protocol for the 2×2 factorial designed randomised NEO trial. Trials. 2014;15:135. doi: 10.1186/1745-6215-15-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghista DN, Kabinejadian F. Coronary artery bypass grafting hemodynamics and anastomosis design: a biomedical engineering review. Biomed Eng Online. 2013;12:129. doi: 10.1186/1475-925X-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Xie B, Gu C, Gao M, Zhang F, Wang J, et al. Distal end side-to-side anastomoses of sequential vein graft to small target coronary arteries improve intraoperative graft flow. BMC Cardiovasc Disord. 2014;14:65. doi: 10.1186/1471-2261-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–5. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 14.Benedetto U, Raja SG, Albanese A, Amrani M, Biondi-Zoccai G, Frati G. Searching for the second best graft for coronary artery bypass surgery: a network meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2015;47:59–65. doi: 10.1093/ejcts/ezu111. [DOI] [PubMed] [Google Scholar]
- 15.Habib RH, Schwann TA, Engoren M. Late effects of radial artery versus saphenous vein grafting in patients aged 70 years or older. Ann Thorac Surg. 2012;94:1478–84. doi: 10.1016/j.athoracsur.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Hess CN, Lopes RD, Gibson CM, Hager R, Wojdyla DM, Englum BR, et al. Saphenous Vein Graft Failure after Coronary Artery Bypass Surgery: Insights from PREVENT IV. Circulation. 2014;130:1445–51. doi: 10.1161/CIRCULATIONAHA.113.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otsuka F, Yahagi K, Sakakura K, Virmani R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann Cardiothorac Surg. 2013;2:519–26. doi: 10.3978/j.issn.2225-319X.2013.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voucharas C, Bisbos A, Zandes N. Plication of the right atrium in order to confront a right coronary artery under tension graft. Updates Surg. 2011;63:209–11. doi: 10.1007/s13304-011-0082-7. [DOI] [PubMed] [Google Scholar]
- 19.Morritt DG, Shah SS, Morritt AN, Kaul P. Acute transection of the left internal mammary artery remote from the anastomosis following coronary artery bypass surgery. Interact CardioVasc Thorac Surg. 2004;3:653–5. doi: 10.1016/j.icvts.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Jung SH, Song H, Choo SJ, Je HG, Chung CH, Kang JW, et al. Comparison of radial artery patency according to proximal anastomosis site: Direct aorta to radial artery anastomosis is superior to radial artery composite grafting. J Thorac Cardiovasc Surg. 2009;138:76–83. doi: 10.1016/j.jtcvs.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Maniar HS, Sundt TM, Barner HB, Prasad SM, Peterson L, Absi T, et al. Effect of target stenosis and location on radial artery graft patency. J Thorac Cardiovasc Surg. 2002;123:45–52. doi: 10.1067/mtc.2002.118686. [DOI] [PubMed] [Google Scholar]
- 22.Parissis H, Ramesh BC, Al-Alao B. Which is the best graft for the right coronary artery? Asian Cardiovasc Thorac Ann. 2014;23:100–13. doi: 10.1177/0218492314523766. [DOI] [PubMed] [Google Scholar]
- 23.Ding J, Liu Y, Wang F, Bai F. Impact of Competitive Flow on Hemodynamics in Coronary Surgery: Numerical Study of ITA-LAD Model. Comput Math Methods Med. 2012;2012:356187. doi: 10.1155/2012/356187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamura M, Nakajima H, Kobayashi J, Funatsu T, Otsuka Y, Yagihara T, et al. Patency rate of the internal thoracic artery to the left anterior descending artery bypass is reduced by competitive flow from the concomitant saphenous vein graft in the left coronary artery. Eur J Cardiothorac Surg. 2008;34:833–8. doi: 10.1016/j.ejcts.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Kute SM, Vorp DA. The effect of proximal artery flow on the hemodynamics at the distal anastomosis of a vascular bypass graft: Computational study. J Biomech Eng. 2001;123:277–83. doi: 10.1115/1.1374203. [DOI] [PubMed] [Google Scholar]
- 26.Roth JA, Cukingnan RA, Brown BG, Gocka E, Carey JS. Factors influencing patency of saphenous vein grafts. Ann Thorac Surg. 1979;28:176–83. doi: 10.1016/s0003-4975(10)63777-0. [DOI] [PubMed] [Google Scholar]
- 27.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. VA Cooperative Study Group #207/297/364. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–56. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Kwag HJ, Yoo SM, Yoo JY, Chae IH, Choi DJ, et al. Discrepancies between coronary CT angiography and invasive coronary angiography with focus on culprit lesions which cause future cardiac events. Eur Radiol. 2018;28:1356–64. doi: 10.1007/s00330-017-5095-2. [DOI] [PubMed] [Google Scholar]


