Abstract
Aging is a physiological decline process. The number of older adults is growing around the world; therefore, the incidence of cognitive impairment, dementia, and other diseases related to aging increases. The main cellular factors that converge in the aging process are mitochondrial dysfunction, antioxidant impairment, inflammation, and immune response decline, among others. In this context, these cellular changes have an influence on the kynurenine pathway (KP), the main route of tryptophan (Trp) catabolism. KP metabolites have been involved in the aging process and neurodegenerative diseases. Although there are changes in the metabolite levels with age, at this time, there is no study that has evaluated cognitive decline as a consequence of Trp catabolism fluctuation in aging. The aim of this study was to evaluate the relation between the changes in Trp catabolism and cognitive impairment associated with age through KP metabolites level alterations in women over 50 years of age. Seventy-seven nondemented women over 50 years old were examined with a standardized cognitive screening evaluation in Spanish language (Neuropsi), Beck anxiety inventory (BAI), and the geriatric depression scale (GDS). Also, serum levels of Trp, kynurenine (Kyn), kynurenic acid (KYNA), and 3-hydroykynurenine (3-HK) and the glutathione ratio (GSH/GSSG) were measured. Results showed a negative correlation between age and Trp levels and a positive correlation between age and KYNA/Trp and 3-HK/Trp ratios. The level of cognitive impairment showed a significant positive association with age and with kynurenine pathway activation and a significant negative correlation with Trp levels. The GSH/GSSG ratio correlated positively with Trp levels and negatively with Kyn/Trp and 3-HK/Trp ratios. The depression score correlated negatively with Trp and positively with the 3-HK/Trp ratio. We concluded that KP activation increases with age and it is strongly associated with the level of cognition performance in nondemented women over 50 years of age.
1. Introduction
Aging is a time-dependent physiological process that is characterized by a progressive loss of physiological integrity, leading to impairment of functions, and an increased vulnerability to death, affecting all higher organisms [1]. The rising life expectancy leads to higher risk in development of age-related diseases, such as cancer and neurodegenerative diseases [2]. Factors that converge during aging are mitochondrial dysfunction, oxidative stress, decline in antioxidant defense, cellular senescence, stem cell exhaustion, alterations of intercellular communication, genomic instability, epigenetic alterations, deregulated nutrient sensing, and chronic low-grade inflammatory state, among others [3–6].
Some of these factors, such as inflammation and redox state alteration, directly influence the Trp catabolism, which also changes with age. Trp is an essential amino acid, which is metabolized mainly through the kynurenine pathway (KP) (~95%) [7]. Trp can be catabolized by Trp 2,3-dioxygenase (TDO) in the liver and by indoleamine 2,3-dioxygenase (IDO) elsewhere to produce kynurenine (Kyn). Kyn can be a substrate for three enzymes: (1) kynurenine aminotransferases to produce kynurenic acid (KYNA), (2) kynureninase to form anthranilic acid (AA), and (3) kynurenine-3-monooxygenase (KMO) to produce 3-hydroxykynurenine (3-HK), which is further hydrolyzed by kynureninase to 3-hydroxyanthranilic acid (3-HANA). 3-HANA is catabolized as a substrate of 3-hydroxyanthranilate 3,4-dioxygenase which produces an unstable intermediate that rapidly can be converted to quinolinic acid (QUIN) by nonenzymatic cyclization or to produce picolinic acid by the 2-amino-3-carboxymuconate semialdehyde decarboxylase. Finally, quinolinate phosphoribosyl transferase catabolizes QUIN to produce NAD+. KP is controlled mainly by TDO and IDO, which are modulated in different ways. TDO is inducible by glucocorticoids, while IDO is activated by proinflammatory cytokines and superoxide [8–10]. The clinical importance of the KP is due to the fact that metabolites with redox and neuroactive properties as 3-HK, 3-HANA, KYNA, and QUIN are formed through it. QUIN is an agonist of NMDAr, while KYNA is an antagonist of NMDAr and can also inhibit noncompetitively α7-nicotinic receptors [11, 12]. It has been observed that brain KYNA level fluctuations impact the cognition [13–21].
There are a few human studies that relate aging with kynurenine pathway components. Pertovaara and coworkers [22] found that the Kyn/Trp ratio is higher in older people than in healthy and younger controls and was able to predict mortality in nonagenarian population. In another study, age was positively associated with kynurenine levels in human serum [23]. It has been shown that picolinic acid concentration in CSF correlates positively with age [24]. Also, KYNA in CSF increased during aging and correlated with high titers of IgG and β2-microglobulin (markers of immune system activation) [25]. Even with this evidence which suggests that activation of KP occurs during aging and knowing that aging is associated with impairment in cognitive information processing, decline in attention, memory, and other cognitive functions, until now, there have been no studies correlating cognitive performance and KP metabolite levels during aging. The purpose of this study was to determine whether cognitive decline associated with age is related to Trp catabolism fluctuations in nondemented women over 50 years of age.
2. Materials and Methods
2.1. Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) and J.T.Baker® (Center Valley, PA, USA), unless otherwise mentioned in the text.
2.2. Ethical Approval
The protocol, previously approved by the institutional committees (reference no. 114/15), was in agreement with the Declaration of Helsinki and local regulations regarding research on human subjects (Reglamento de la Ley General de Salud en Materia de Investigación para la Salud en México). Written informed consents were obtained from all recruited subjects.
2.3. Subject Recruitment Inclusion
The individuals included in the study were 77 adult women, over 50 years old, without evident cognitive impairment, that is, individuals with complete functionality and independent in their basic activities of daily life. No history of neurodegenerative, psychiatric, chronic inflammatory, or autoimmune diseases was present. Also, inclusion criteria required no history of cerebrovascular disease in the previous 6 months and no current use of immunosuppressive or immunomodulatory drugs. Subjects with severe visual or auditory deficit that could affect the cognitive evaluation were excluded. Subjects who fulfilled all the inclusion criteria gave their informed consent to participate in the study.
2.4. Cognitive and Emotional Evaluations
For cognitive status evaluation in subjects, a standardized and validated neuropsychological test battery, in adult population, was used, which also allowed to weight the effect of scholarship and age of subjects (Neuropsychological Assessment in Spanish language, Neuropsi) [26]. This evaluation has reference standards made in the Mexican population being able to identify individuals with normal performance or with different levels of cognitive impairment as mild, moderate, and severe. Likewise, the Beck anxiety inventory (BAI) and the geriatric depression scale (GDS) were applied, in order to rule out that the cognitive alterations were caused by some emotional state disturbance. All evaluations were performed immediately before the peripheral blood collection.
2.5. Sample Blood Collection
Blood samples (5 ml) were collected by vein puncture and allowed to clot, and serum was obtained by centrifugation at 2500 rpm for 20 minutes and stored at −70°C until analysis.
2.6. Serum GSH and GSSG Determinations
Serum reduced glutathione (GSH) and oxidized GSH concentrations were determined using a fluorometric method reported by Senft et al. [27] and adapted by Ramos-Chavez et al. [28]. The method is based in the GSH reaction with o-phthaldialdehyde (OPA) to form a highly stable and fluorescent isoindole derivative. Briefly, 50 μl of serum sample was treated with 150 μl of 5% (w/v) metaphosphoric acid and vigorously mixed. Then, tubes were placed on ice for 15 minutes and centrifuged at 14,000 rpm for 20 minutes at 4°C. 5 μl of supernatant was used for GSH and 30 μl for GSSG determination. For GSSG determination, the first step was to inhibit GSH isoindole derivation using N-ethylmaleimide; subsequently, GSSG is reduced to GSH by dithionite treatment and then derivation with OPA to obtain the isoindole. The fluorescence was measured at 370 nm excitation and 420 nm of emission (FLx800 Multimode Lector BioTex, Houston, Texas, USA). Calibration curves were built for GSH and GSSG, and the concentrations were obtained by interpolation in the standard curve. The results are expressed as μmol/l.
2.7. Kynurenines Determination
Kynurenines were measured by an HPLC method with fluorescence detection for KYNA, Trp, and Kyn, while 3-HK was determined using electrochemical detection [29–32]. The equipment used was a PerkinElmer chromatograph (PerkinElmer, Waltham, MA, USA) coupled to a variable wavelength UV detector (PDA Plus Detector Flexar), a fluorescence detector (model S200), an electrochemical detector (CC-5E LC-4C Amperometric Detector), an automatic delivery pump (Flexar binary LC pump), and an autosampler injector (Flexar LC autosampler). 200 μl of the serum sample was treated with 200 μl of 6% perchloric acid, centrifugated at 14,000 rpm and 4°C. The supernatant was stored at −70°C until analysis.
2.7.1. KYNA and Kyn Determinations
20 μl of the serum supernatant sample or standard solution was injected onto an Eclipse XDB-C18 reverse phase column (5 μm, 4.6 × 150 mm, Agilent, Santa Clara, CA, USA) and isocratically eluted with a mobile phase consisting of 50 mM of sodium acetate, 250 mM of zinc acetate, and 3% of acetonitrile, pH adjusted to 6.2 with glacial acetic acid, at a flow rate of 1 ml/min; Kyn was eluted with the same mobile phase but without acetonitrile. Both metabolites were detected by fluorescence, KYNA at excitation wavelength of 344 nm and emission wavelength of 398 nm and Kyn at excitation wavelength of 368 nm and emission wavelength of 480 nm. The retention time for KYNA was ~7 min and for Kyn was ~10 min.
2.7.2. Trp Analysis
Trp levels were determined using a ZORBAX Eclipse AAA column (3.5 μm, 4.6 × 150 mm, Agilent, Santa Clara, CA, USA) and isocratically eluted with a mobile phase containing 100 mM of zinc acetate and 3% of acetonitrile (pH adjusted to 4.2 with glacial acetic acid) at a flow rate of 1 ml/min. 20 μl of biological sample or standard solutions was injected for Trp determination. Trp was detected by fluorescence (excitation wavelength: 254 nm and emission wavelength: 404 nm). The retention time of Trp was ~5 min.
2.7.3. 3HK Measurement
The 3HK was determined using an electrochemical method described by Heyes and Quearry et al. [29]. Briefly, 3HK was eluted at a constant flow rate of 0.6 ml/min with a mobile phase containing 9% of triethylamine, 0.59% phosphoric acid, 0.27 mM EDTA, and 8.9 mM heptane sulfonic acid; 40 μl of the sample or standard was injected onto an Adsorbosphere Catecholamine C18 reverse phase column (3 μm, 4.6 mm × 100 mm, Fisher Scientific, Hampton, Nuevo Hampshire, USA). The retention time was ~11 min.
2.8. Statistical Analysis
Correlation between each pair of the following variables was assessed with Spearman's rho coefficient: age, cognition, Trp, Kyn/Trp, KYNA/Trp, 3-HK/Trp, depression score, anxiety, and GSH/GSSG. The Mann-Whitney test and T-test were used to compare distributions associated with normal (undetected) and detected cognitive impairment groups; the T-test was obtained using the logarithmic scale. Finally, a logistic regression model was adjusted considering age and Trp levels in the logarithmic scale as covariables, where the dependent variable corresponds to the binary variable associated with cognitive impairment (1 = normal and 0 = CI). The logistic regression model obtained was the following:
| (1) |
3. Results
Demographic and clinical characteristics of the participants are shown in Table 1. The average age was 71.9 years (SD: 11.8), the average number of years of education was 8.4 (SD: 5.0), and only 26% of them had education over 9 years. 77% of them had between 1 and 3 comorbidities (e.g., hypertension, diabetes, dyslipidemia, osteoarthritis, and obesity). Regarding their cognitive performance, 70% of them obtained a normal evaluation, and 30% (n = 23) presented some degree of cognitive impairment (CI). 33% found significant symptoms of depression and 53% with some symptoms of anxiety. Descriptive statistics of analytes is shown in Table 2.
Table 1.
Clinical and demographic features of all individuals (n = 77).
| Age (years) | Mean ± SD | 71.92 ± 11.84 |
| Min, Max | 51.00–97.00 | |
|
| ||
| Education | Mean ± SD | 8.48 ± 5.02 |
| 0–4 years | 11 (14%) | |
| 5–9 years | 46 (60%) | |
| 10–24 years | 20 (26%) | |
|
| ||
| Civil status | Married | 30 (39%) |
| Single | 19 (25%) | |
| Widow | 28 (36%) | |
|
| ||
| Occupation | Paid employment | 47 (61%) |
| Vacant/retired | 30 (39%) | |
|
| ||
| Comorbidities | 0–3 | 59 (77%) |
| 4–6 | 16 (20%) | |
| 7–9 | 2 (3%) | |
|
| ||
| Cognition profile | Mean ± SD | 85.86 ± 20.24 |
| Min, Max | 23.00 ± 119.00 | |
| Normal | 54 (70%) | |
| Mild impairment | 10 (13%) | |
| Moderate impairment | 11 (14%) | |
| Severe impairment | 2 (3%) | |
|
| ||
| GDS (depression), n = 63 | Mean ± SD | 3.44 ± 2.60 |
| Min, Max | 0.0 ± 10.00 | |
| Without depression symptoms | 42 (67%) | |
| With depression symptoms | 21 (33%) | |
|
| ||
| BAI (anxiety) | Mean ± SD | 12.52 ± 10.98 |
| Min, Max | 0.0–42.00 | |
| Minimal | 36 (47%) | |
| Mild | 21 (27%) | |
| Moderate | 14 (18%) | |
| Severe | 6 (8%) | |
GDS: geriatric depression scale; BAI: Beck anxiety inventory.
Table 2.
Descriptive statistics of GSH, GSSG, and KP metabolites for each group (normal and detected cognitive impairment (CI)).
| Min | Percentile 25 | Median | Mean | Percentile 75 | Max | n | |
|---|---|---|---|---|---|---|---|
| GSH (μmol/l) | |||||||
| Total | 53.760 | 151.030 | 196.860 | 209.920 | 236.740 | 766.000 | 79 |
| Normal | 53.760 | 148.710 | 187.210 | 206.580 | 220.590 | 766.000 | 48 |
| CI | 129.700 | 165.100 | 218.800 | 213.900 | 253.300 | 340.400 | 18 |
| GSSG (μmol/l) | |||||||
| Total | 0.090 | 12.650 | 40.180 | 39.700 | 62.800 | 118.780 | 79 |
| Normal | 0.090 | 14.480 | 41.440 | 41.750 | 63.470 | 118.780 | 48 |
| CI | 7.410 | 19.430 | 40.720 | 43.260 | 67.950 | 85.270 | 18 |
| Trp (pmoles/μl) | |||||||
| Total | 2.380 | 11.450 | 22.740 | 26.360 | 37.290 | 87.600 | 82 |
| Normal | 3.401 | 15.802 | 23.370 | 28.208 | 35.173 | 87.599 | 48 |
| CI | 2.380 | 5.017 | 11.472 | 15.384 | 17.506 | 47.816 | 21 |
| KYNA (fmoles/μl) | |||||||
| Total | 0.832 | 2.708 | 4.525 | 10.534 | 6.662 | 209.894 | 79 |
| Normal | 0.832 | 2.514 | 4.465 | 13.325 | 7.191 | 209.894 | 47 |
| CI | 1.694 | 2.768 | 3.731 | 4.595 | 4.908 | 15.561 | 19 |
| 3-HK (pmoles/μl) | |||||||
| Total | 0.000 | 0.025 | 0.036 | 0.046 | 0.053 | 0.376 | 82 |
| Normal | 0.000 | 0.025 | 0.041 | 0.050 | 0.057 | 0.376 | 48 |
| CI | 0.006 | 0.026 | 0.036 | 0.045 | 0.052 | 0.155 | 21 |
| L-Kyn (pmoles/μl) | |||||||
| Total | 0.000 | 3.478 | 12.513 | 20.305 | 22.417 | 283.361 | 80 |
| Normal | 0.015 | 5.636 | 14.697 | 20.158 | 22.820 | 224.934 | 47 |
| CI | 2.174 | 6.396 | 12.513 | 14.127 | 17.542 | 39.094 | 20 |
Pairwise correlations among age, cognition level, Trp levels, and the Kyn/Trp, KYNA/Trp, and 3-HK/Trp ratios as well as depression, anxiety, and GSH/GSSG ratio are showed in Figure 1. As expected, age correlated positively with the cognitive impairment level (four ordinal categories were used: normal, mild, moderate, and severe). Trp levels (pmoles/μl) correlated negatively with age, while Kyn (pmoles/μl)/Trp, KYNA (fmoles/μl)/Trp, and 3-HK (pmoles/μl)/Trp ratios correlated positively with age. Also, the cognitive impairment level was associated negatively with Trp levels and positively with KYNA/Trp and 3-HK/Trp ratios. As it has been described by other groups [23], the depression score correlates positively with anxiety and negatively with the levels of Trp, as was observed in this study. We also found a positive correlation between the depression score and 3-HK/Trp ratio. The GSH/GSSG ratio was determined as a redox status marker and correlated positively with Trp and negatively with Kyn/Trp and 3-HK/Trp ratios.
Figure 1.
The lower triangular matrix contains the scatterplot for each pair of variables in the logarithmic scale. The upper triangular matrix contains Spearman's rank correlation coefficient and its associated p value. In this case, the variable cognition corresponds to an ordinal variable with four categories depending on the level of cognitive impairment: normal, mild, moderate, and severe.
To extend the analysis on the association between Trp catabolism and cognitive impairment, only two groups of subjects were considered. The first one consisted of those subjects who did not present with cognitive impairment, the second, those that presented some level of cognitive impairment. The results of the statistical tests comparing the distributions of these two groups for the serum levels of Trp, Kyn/Trp, KYNA/Trp, and 3-HK/Trp are shown in Table 3. It was found that the levels of Trp were significantly different in women with cognitive impairment in comparison with those without any cognitive impairment; the median of the Trp levels in women with cognitive impairment was around half the median value found of those in women without cognitive impairment (Table 3). Also, Kyn/Trp, KYNA/Trp, and 3-HK/Trp serum ratios were significantly different between women with cognitive impairment and those without any cognitive impairment.
Table 3.
Descriptive statistics of Trp levels and Kyn/Trp, KYNA/Trp, and 3-HK/Trp ratios for each group (normal and detected cognitive impairment (CI)).
| Trp | Kyn/Trp | KYNA/Trp | 3-HK/Trp | |||||
|---|---|---|---|---|---|---|---|---|
| Normal | CI | Normal | CI | Normal | CI | Normal | CI | |
| Descriptive statistics | ||||||||
| Percentile 25 | 15.802 | 5.017 | 0.198 | 0.359 | 0.117 | 0.236 | 0.001 | 0.001 |
| Median | 23.370 | 11.472 | 0.579 | 0.856 | 0.191 | 0.459 | 0.002 | 0.003 |
| Percentile 75 | 35.173 | 17.506 | 1.045 | 3.094 | 0.352 | 0.686 | 0.003 | 0.005 |
| Mean | 28.208 | 15.384 | 0.915 | 2.120 | 0.454 | 0.661 | 0.003 | 0.007 |
| n | 48 | 21 | 47 | 18 | 46 | 19 | 47 | 21 |
| p values | ||||||||
| Mann-Whitney test | 0.0015 | 0.0700 | 0.0280 | 0.0262 | ||||
| T-test | 0.0017 | 0.0350 | 0.0306 | 0.0189 | ||||
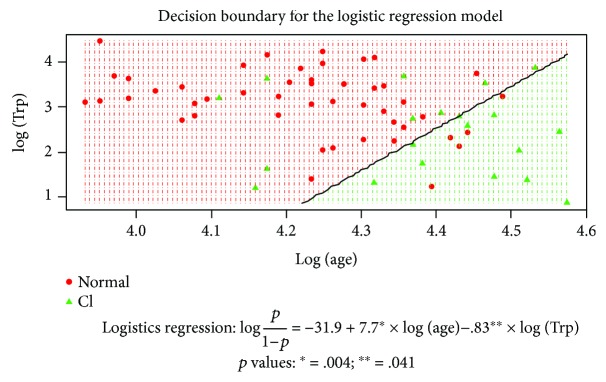
Moreover, to study the correlation between the presence of cognitive impairment and Trp levels, a logistic regression model including age as a covariable was adjusted; as was mentioned before, there was a significant correlation between age and cognitive impairment. The results show that both covariables, age and Trp levels, are significant in the model, and for a given age, it means that at lower Trp levels, there is a greater probability of observing cognitive impairment. Figure 2 shows the decision boundary for the adjusted logistic regression.
Figure 2.
Scatterplot for age and Trp in the logarithmic scale distinguishing between the status of cognitive impairment: detected and normal. The adjusted logistic regression considering these two variables where the dependent variable corresponds to the binary variable associated with cognitive impairment (1: detected and 0: normal) is also shown together with the corresponding p values for the coefficients in the logistic regression model.
4. Discussion
According to our knowledge, this is the first time that Trp metabolites have been correlated with cognitive performance during normal aging in women. Our results suggest an overactivation of the KP during aging since we found a negative correlation between age and Trp levels and a positive influence of age on Kyn/Trp, KYNA/Trp, and 3-HK/Trp ratios; which is supported by the fact that the serum Kyn/Trp ratio is a measure of the beginning of KP activity [33, 34]. These data are consistent with previous studies in which low plasma, serum, and CSF Trp levels and high values of the Kyn/Trp ratio were also observed in elderly people [22, 23, 33, 35–39]. These alterations on the Kyn/Trp ratio may be due to enhanced activity of IDO and/or TDO. Although the major site of Trp conversion into kynurenine is the liver via TDO, it has been shown that liver TDO activity, both holoenzyme and apoenzyme, decreases significantly with age in rats [40], so that if we consider that during aging there is an increased concentration of inflammatory markers, the Kyn/Trp ratio could reflect mainly IDO activity [41]. Supporting this idea, it has been previously described that IL-6 correlates positively with Kyn and the Kyn/Trp ratio in serum of older people [4], suggesting that IDO activity increases with aging [37]. In addition, it has been found that the Kyn/Trp ratio and neopterin concentration (marker associated with inflammation and oxidative stress) were significantly correlated with increased age, while Trp correlated negatively with age in CSF samples from women [33].
Additionally, our results indicated a strong association between KP activation and redox status. The ratio of GSH and GSSG (GSH/GSSG) has been noted as an index of oxidative stress. Specifically, in this study, the GSH/GSSG ratio correlated negatively with KP activation, and as was mentioned before, some metabolites produced through KP have neuroactive and redox properties [10]. Kyn and KYNA have shown antioxidant properties [32, 42], while 3-HK and 3-HANA can also be scavengers in a concentration-dependent way and after their interaction with ROS leads to more toxic compounds inducing cellular death [43–45]. An important point is that GSH levels can be reduced because they can produce an adduct with the 3-HK glucoside, which, at the same time, is a product of 3-HK deamination [46, 47]. Keeping this in mind, the low GSH/GSSG ratio found in this study could be related to the high levels of redox kynurenines.
In this study, the Kyn/Trp, KYNA/Trp, and 3-HK/Trp ratios that are associated with age reflect a greater amount of KP metabolites in the circulation. However, just Trp, Kyn, and 3-HK can cross the blood-brain barrier, either through simple diffusion across the vascular membranes or as a result of active transport via the large neutral amino acid transporter [48–51]. Recently, Hestad and coworkers showed that serum Kyn levels correlated highly with CSF Kyn levels [23]. The alterations of blood KP metabolites can produce significant secondary changes in the levels of kynurenine metabolites in the CNS and consequently impact processes such as cognition, by altering the degree of activation or blockade of NMDAr as well as the α-7 nicotinic receptor [52, 53].
In this context, the 3-HK/Trp ratio is consistent with the increase in downstream metabolites such as 3-HANA, PIC, and QUIN found in human CSF, with age. In rats the increase of QUIN with age has also been described [24, 33, 54]. Moreover, Trp and Kyn that really cross the blood-brain barrier, can produce KYNA by KATs, considered the canonical way. However, there are other mechanisms by which KYNA is produced and that involve the interaction of D- and L-isomers of Trp and Kyn with reactive oxygen species (ROS) [32, 55, 56], which as we know, is an important factor during aging. Fluctuation in KYNA levels leads to behavioral and cognitive changes during aging [25]. According to data obtained in this study, Trp catabolism through KP and the level of cognitive impairment are associated with aging in women over 50 years of age. Experimental studies have shown that increased levels of Kyn are linked with deficits of spatial working memory [17, 57]. The deletion of the major KAT isoenzyme, KAT-II, results in a substantial decrease in the extracellular concentration of KYNA, improving cognitive performance in a range of behavioral tasks which include exploration, object recognition, and passive avoidance learning [20]. Also, it has been demonstrated that Trp depletion affects a variety of cognitive processes in healthy individuals, such as memory and learning skills and long-term memory consolidation, which can be associated with the bioactive kynurenines such as KYNA and QUIN [58, 59].
A strong relationship between KP activation and cognitive impairment is also observed in Alzheimer's disease, which is an age-related neurodegenerative disease. Widner and coworkers found decreased serum Trp levels and increased serum kynurenine levels, and these changes correlated with the level of cognitive decline in Alzheimer's disease patients [60, 61]. Also, plasma Trp concentrations were found to be lower in HIV+ compared with HIV− individuals, and a higher plasma Kyn/Trp ratio was associated with cognitive impairment and major depressive disorder in the overall HIV+ group [62]. Another study found positive correlations between cognitive function tests and lower plasma KYNA levels, and inverse correlations between these tests and increased QUIN levels in Alzheimer's patients [63]. Plasma and the CSF Kyn/Trp ratio were correlated with risk of dementia in Alzheimer's patients [63–65]. Interestingly, it has also been observed that increased KP activation and changes in the levels of kynurenine metabolites correlate negatively with cognitive performance in patients undergoing cardiac surgery, which suggest that Trp catabolism can be a biomarker of non-age-related cognitive impairment [66].
Noteworthy to mention is that in this study, Trp levels correlated negatively with the depression score and positively with the 3-HK/Trp ratio and anxiety in women over 50 years of age. These results suggest the KP activation in depression, which may induce a transient reduction in serotonin synthesis, which may also be associated with depression [67–71]. In our study, we did not take into account serotonin production, considering that the serotonin synthesized in the periphery is unable to cross the blood-brain barrier, whereas the production of brain serotonin is dependent on the amount of circulating Trp [72]. Low Trp intake has been assumed to cause lower brain serotonin levels and to be an important risk factor involved in the onset and course of a variety of affective disorders, including depression [73]. In this context, Suga and coworkers showed an inverse association between Trp intake and depressive symptoms in young women participants (mean age around 18 years old), which suggests that the adequate intake is necessary to prevent depression [74]. A recent study observed that the increased Trp catabolism related to peripheral inflammation is accompanied by marked elevation in brain kynurenine and QUIN levels, and these alterations correlated with depressive symptoms in patients with hepatitis C [75].
Interestingly, our analysis suggests elements to establish that circulating Trp levels are a predictive biomarker and could be used to distinguish women over 50 years of age with some degree of cognitive impairment. However, it is important to take into account that this study was performed in women over 50 years of age; it is therefore necessary to confirm these results in a wider age range and to determine whether this effect is also present in men, in order to establish whether Trp levels are predictive for cognitive impairment in the general population.
5. Conclusion
The identification of novel biomarkers associated with the cognitive impairment that occurs during aging could provide key biological insights to identify an adequate intervention. This study confirms a close relationship between age, the Trp catabolism through KP activation, and cognitive impairment. However, based on our logistic regression model, given the age, Trp levels were the only significant predictor among the KP metabolites. Then, Trp levels can be considered as a useful indicator of cognitive impairment in women over 50 years of age, and these results afford a basis for further investigation in order to design future intervention strategies focused on prevention and treatment of cognitive impairment.
Acknowledgments
This work was supported by CONACYT Grant 262010. LARC received a postdoctoral fellowship from DGAPA-UNAM.
Contributor Information
P. Carrillo Mora, Email: neuropolaco@yahoo.com.mx.
V. Pérez de la Cruz, Email: veped@yahoo.com.mx.
Data Availability
The cognitive and biochemistry data used to support the findings of this study are available with the corresponding author upon request.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Flatt T. A new definition of aging? Frontiers in Genetics. 2012;3:p. 148. doi: 10.3389/fgene.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohn A., Weber D., Jung T., et al. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biology. 2017;11:482–501. doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi C., Valensin S., Bonafe M., et al. The network and the remodeling theories of aging: historical background and new perspectives. Experimental Gerontology. 2000;35(6-7):879–896. doi: 10.1016/S0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- 4.Capuron L., Schroecksnadel S., Feart C., et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biological Psychiatry. 2011;70(2):175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Otin C., Blasco M. A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii R., Canuet L., Aoki Y., et al. Healthy and pathological brain aging: from the perspective of oscillations, functional connectivity, and signal complexity. Neuropsychobiology. 2018;75(4):151–161. doi: 10.1159/000486870. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez-Ortega D., González-Esquivel D., Pineda B., Ríos C., Pérez-de la Cruz V. Role of kynurenine pathway in aging. In: Mittal S., editor. Targeting the Broadly Pathogenic Kynurenine Pathway. Springer, Cham; 2015. [DOI] [Google Scholar]
- 8.Ball H. J., Jusof F. F., Bakmiwewa S. M., Hunt N. H., Yuasa H. J. Tryptophan-catabolizing enzymes – party of three. Frontiers in Immunology. 2014;5:p. 485. doi: 10.3389/fimmu.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y. Indoleamine 2,3-dioxygenase--a new antioxidant enzyme. Materia Medica Polona. 1989;21(3):244–250. [PubMed] [Google Scholar]
- 10.Gonzalez Esquivel D., Ramirez-Ortega D., Pineda B., Castro N., Rios C., Perez de la Cruz V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology. 2017;112, Part B:331–345. doi: 10.1016/j.neuropharm.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Albuquerque E. X., Alkondon M., Pereira E. F., et al. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. The Journal of Pharmacology and Experimental Therapeutics. 1997;280(3):1117–1136. [PubMed] [Google Scholar]
- 12.Hilmas C., Pereira E. F. R., Alkondon M., Rassoulpour A., Schwarcz R., Albuquerque E. X. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. The Journal of Neuroscience. 2001;21(19):7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander K. S., Wu H. Q., Schwarcz R., Bruno J. P. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology. 2012;220(3):627–637. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hightower B. J., Rodefer J. S. Investigation of kynurenic acid as a possible biomarker for cognitive impairment associated with schizophrenia. Society for Neuroscience Abstract. 2011;36:p. 163.19. [Google Scholar]
- 15.Pocivavsek A., Wu H. Q., Potter M. C., Elmer G. I., Pellicciari R., Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36(11):2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chess A. C., Landers A. M., Bucci D. J. L-Kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behavioural Brain Research. 2009;201(2):325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Chess A. C., Simoni M. K., Alling T. E., Bucci D. J. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophrenia Bulletin. 2007;33(3):797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vunck S. A., Supe K., Schwarcz R., Bruno J. P. Working memory deficit in adult rats after acute elevation of brain kynurenic acid are alleviated by co-administration of the alpha7 nicotinic positive modulator galantamine. Society for Neuroscience Abstract. 2014;38:p. 255.09. [Google Scholar]
- 19.Phenis D., Vunck S. A., Schwarcz R., Bruno J. P. Acute elevations of brain kynurenic acid induce working memory deficits: relative contributions of α7 nicotinic and NMDA receptor activity. Society for Neuroscience Abstract. 2014;51:p. 10/T17. [Google Scholar]
- 20.Potter M. C., Elmer G. I., Bergeron R., et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35(8):1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pocivavsek A., Baratta A. M., Mong J. A., Viechweg S. S. Acute kynurenine challenge disrupts sleep-wake architecture and impairs contextual memory in adult rats. Sleep. 2017;40(11) doi: 10.1093/sleep/zsx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pertovaara M., Raitala A., Lehtimaki T., et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mechanisms of Ageing and Development. 2006;127(5):497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Hestad K. A., Engedal K., Whist J. E., Farup P. G. The relationships among tryptophan, kynurenine, indoleamine 2,3-dioxygenase, depression, and neuropsychological performance. Frontiers in Psychology. 2017;8:p. 1561. doi: 10.3389/fpsyg.2017.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coggan S. E., Smythe G. A., Bilgin A., Grant R. S. Age and circadian influences on picolinic acid concentrations in human cerebrospinal fluid. Journal of Neurochemistry. 2009;108(5):1220–1225. doi: 10.1111/j.1471-4159.2009.05868.x. [DOI] [PubMed] [Google Scholar]
- 25.Kepplinger B., Baran H., Kainz A., Ferraz-Leite H., Newcombe J., Kalina P. Age-related increase of kynurenic acid in human cerebrospinal fluid - IgG and beta2-microglobulin changes. Neurosignals. 2005;14(3):126–135. doi: 10.1159/000086295. [DOI] [PubMed] [Google Scholar]
- 26.Ostrosky-Sols F., Dvila G., Ortiz X., et al. Determination of normative criteria and validation of the SKT for use in Spanish-speaking populations. International Psychogeriatrics. 1999;11(2):171–180. doi: 10.1017/S1041610299005724. [DOI] [PubMed] [Google Scholar]
- 27.Senft A. P., Dalton T. P., Shertzer H. G. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Analytical Biochemistry. 2000;280(1):80–86. doi: 10.1006/abio.2000.4498. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Chávez L. A., Rendón-López C. R. R., Zepeda A., Silva-Adaya D., Del Razo L. M., Gonsebatt M. E. Neurological effects of inorganic arsenic exposure: altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Frontiers in Cellular Neuroscience. 2015;9, article 21 doi: 10.3389/fncel.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heyes M. P., Quearry B. J. Quantification of 3-hydroxykynurenine in brain by high-performance liquid chromatography and electrochemical detection. Journal of Chromatography. 1988;428:340–344. doi: 10.1016/S0378-4347(00)83925-0. [DOI] [PubMed] [Google Scholar]
- 30.Widner B., Werner E. R., Schennach H., Fuchs D. An HPLC method to determine tryptophan and kynurenine in serum simultaneously. Advances in Experimental Medicine and Biology. 1999;467:827–832. doi: 10.1007/978-1-4615-4709-9_105. [DOI] [PubMed] [Google Scholar]
- 31.Perez-de la Cruz V., Amori L., Sathyasaikumar K. V., et al. Enzymatic transamination of D-kynurenine generates kynurenic acid in rat and human brain. Journal of Neurochemistry. 2012;120(6):1026–1035. doi: 10.1111/j.1471-4159.2012.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco Ayala T., Lugo Huitron R., Carmona Aparicio L., et al. Alternative kynurenic acid synthesis routes studied in the rat cerebellum. Frontiers in Cellular Neuroscience. 2015;9, article 178 doi: 10.3389/fncel.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bie J., Guest J., Guillemin G. J., Grant R. Central kynurenine pathway shift with age in women. Journal of Neurochemistry. 2016;136(5):995–1003. doi: 10.1111/jnc.13496. [DOI] [PubMed] [Google Scholar]
- 34.Schrocksnadel K., Wirleitner B., Winkler C., Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clinica Chimica Acta. 2006;364(1-2):82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Adachi Y., Shimodaira Y., Nakamura H., et al. Low plasma tryptophan is associated with olfactory function in healthy elderly community dwellers in Japan. BMC Geriatrics. 2017;17(1):p. 239. doi: 10.1186/s12877-017-0639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theofylaktopoulou D., Midttun Ø., Ulvik A., et al. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clinical and Experimental Immunology. 2013;173(1):121–130. doi: 10.1111/cei.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frick B., Schroecksnadel K., Neurauter G., Leblhuber F., Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clinical Biochemistry. 2004;37(8):684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Truscott R. J. W., Elderfield A. J. Relationship between serum tryptophan and tryptophan metabolite levels after tryptophan ingestion in normal subjects and age-related cataract patients. Clinical Science. 1995;89(6):591–599. doi: 10.1042/cs0890591. [DOI] [PubMed] [Google Scholar]
- 39.Demling J., Langer K., Mehr M. Q. Age dependence of large neutral amino acid levels in plasma. In: Filippini G. A., Costa C. V. L., Bertazzo A., editors. Recent Advances in Tryptophan Research. Advances in Experimental Medicine and Biology, Vol 398. Boston, MA, USA: Springer; 1996. pp. 579–582. [DOI] [PubMed] [Google Scholar]
- 40.Comai S., Costa C. V. L., Ragazzi E., Bertazzo A., Allegri G. The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clinica Chimica Acta. 2005;360(1-2):67–80. doi: 10.1016/j.cccn.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Capuron L., Moranis A., Combe N., et al. Vitamin E status and quality of life in the elderly: influence of inflammatory processes. The British Journal of Nutrition. 2009;102(10):1390–1394. doi: 10.1017/S0007114509990493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lugo-Huitrón R., Blanco-Ayala T., Ugalde-Muñiz P., et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicology and Teratology. 2011;33(5):538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez-Ortega D., Ramiro-Salazar A., Gonzalez-Esquivel D., Rios C., Pineda B., Perez de la Cruz V. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid enhance the toxicity induced by copper in rat astrocyte culture. Oxidative Medicine and Cellular Longevity. 2017;2017:12. doi: 10.1155/2017/2371895.2371895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giles G. I., Collins C. A., Stone T. W., Jacob C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochemical and Biophysical Research Communications. 2003;300(3):719–724. doi: 10.1016/S0006-291X(02)02917-0. [DOI] [PubMed] [Google Scholar]
- 45.Vazquez S., Garner B., Sheil M. M., Truscott R. J. W. Characterisation of the major autoxidation products of 3-hydroxykynurenine under physiological conditions. Free Radical Research. 2000;32(1):11–23. doi: 10.1080/10715760000300021. [DOI] [PubMed] [Google Scholar]
- 46.Van Heyningen R. Fluorescent derivatives of 3-hydroxy-L-kynurenine in the lens of man, the baboon and the grey squirrel. The Biochemical Journal. 1971;123(4):30P–31P. doi: 10.1042/bj1230030p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garner B., Vazquez S., Griffith R., Lindner R. A., Carver J. A., Truscott R. J. W. Identification of glutathionyl-3-hydroxykynurenine glucoside as a novel fluorophore associated with aging of the human lens. The Journal of Biological Chemistry. 1999;274(30):20847–20854. doi: 10.1074/jbc.274.30.20847. [DOI] [PubMed] [Google Scholar]
- 48.Speciale C., Hares K., Schwarcz R., Brookes N. High-affinity uptake of L-kynurenine by a Na+-independent transporter of neutral amino acids in astrocytes. The Journal of Neuroscience. 1989;9(6):2066–2072. doi: 10.1523/JNEUROSCI.09-06-02066.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Speciale C., Schwarcz R. Uptake of kynurenine into rat brain slices. Journal of Neurochemistry. 1990;54(1):156–163. doi: 10.1111/j.1471-4159.1990.tb13296.x. [DOI] [PubMed] [Google Scholar]
- 50.Fukui S., Schwarcz R., Rapoport S. I., Takada Y., Smith Q. R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. Journal of Neurochemistry. 1991;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 51.Eastman C. L., Guilarte T. R., Lever J. R. Uptake of 3-hydroxykynurenine measured in rat brain slices and in a neuronal cell line. Brain Research. 1992;584(1-2):110–116. doi: 10.1016/0006-8993(92)90883-B. [DOI] [PubMed] [Google Scholar]
- 52.Stone T. W., Darlington L. G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. British Journal of Pharmacology. 2013;169(6):1211–1227. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito K., Markey S. P., Heyes M. P. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51(1):25–39. doi: 10.1016/0306-4522(92)90467-G. [DOI] [PubMed] [Google Scholar]
- 54.Moroni F., Russi P., Carla V., Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neuroscience Letters. 1988;94(1-2):145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- 55.Moroni F., Russi P., Carla V., De Luca G., Politi V. The regulation of brain kynurenic acid content: focus on indole-3-pyruvic acid. Advances in Experimental Medicine and Biology. 1991;294:299–308. doi: 10.1007/978-1-4684-5952-4_27. [DOI] [PubMed] [Google Scholar]
- 56.Politi V., Lavaggi M. V., Di Stazio G., Margonelli A. Indole-3-pyruvic acid as a direct precursor of kynurenic acid. Advances in Experimental Medicine and Biology. 1991;294:515–518. doi: 10.1007/978-1-4684-5952-4_57. [DOI] [PubMed] [Google Scholar]
- 57.Pocivavsek A., Wu H. Q., Elmer G. I., Bruno J. P., Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. The European Journal of Neuroscience. 2012;35(10):1605–1612. doi: 10.1111/j.1460-9568.2012.08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riedel W. J. Cognitive changes after acute tryptophan depletion: what can they tell us? Psychological Medicine. 2004;34(1):3–8. doi: 10.1017/S0033291703008924. [DOI] [PubMed] [Google Scholar]
- 59.Richard D. M., Dawes M. A., Mathias C. W., Acheson A., Hill-Kapturczak N., Dougherty D. M. L-Tryptophan: basic metabolic functions, behavioral research and therapeutic indications. International Journal of Tryptophan Research. 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widner B., Leblhuber F., Walli J., Tilz G. P., Demel U., Fuchs D. Degradation of tryptophan in neurodegenerative disorders. Advances in Experimental Medicine and Biology. 1999;467:133–138. doi: 10.1007/978-1-4615-4709-9_19. [DOI] [PubMed] [Google Scholar]
- 61.Widner B., Leblhuber F., Walli J., Tilz G. P., Demel U., Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. Journal of Neural Transmission (Vienna) 2000;107(3):343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 62.Keegan M. R., Chittiprol S., Letendre S. L., et al. Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. International Journal of Tryptophan Research. 2016;9:79–88. doi: 10.4137/IJTR.S36464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulaj E., Pawlak K., Bien B., Pawlak D. Kynurenine and its metabolites in Alzheimer’s disease patients. Advances in Medical Sciences. 2010;55(2):204–211. doi: 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- 64.Chouraki V., Preis S. R., Yang Q., et al. Association of amine biomarkers with incident dementia and Alzheimer’s disease in the Framingham Study. Alzheimers Dement. 2017;13(12):1327–1336. doi: 10.1016/j.jalz.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwarz M. J., Guillemin G. J., Teipel S. J., Buerger K., Hampel H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. European Archives of Psychiatry and Clinical Neuroscience. 2013;263(4):345–352. doi: 10.1007/s00406-012-0384-x. [DOI] [PubMed] [Google Scholar]
- 66.Forrest C. M., Mackay G. M., Oxford L., et al. Kynurenine metabolism predicts cognitive function in patients following cardiac bypass and thoracic surgery. Journal of Neurochemistry. 2011;119(1):136–152. doi: 10.1111/j.1471-4159.2011.07414.x. [DOI] [PubMed] [Google Scholar]
- 67.Hayaishi O., Yoshida R. Specific induction of pulmonary indoleamine 2,3-dioxygenase by bacterial lipopolysaccharide. Ciba Foundation Symposium. 1978;65:199–203. doi: 10.1002/9780470715413.ch12. [DOI] [PubMed] [Google Scholar]
- 68.Stone T. W., Darlington L. G. Endogenous kynurenines as targets for drug discovery and development. Nature Reviews Drug Discovery. 2002;1(8):609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 69.Wirleitner B., Neurauter G., Schrocksnadel K., Frick B., Fuchs D. Interferon-γ-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Current Medicinal Chemistry. 2003;10(16):1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 70.Schiepers O. J. G., Wichers M. C., Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Neumeister A., Nugent A. C., Waldeck T., et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Archives of General Psychiatry. 2004;61(8):765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- 72.Yuwiler A., Oldendorf W. H., Geller E., Braun L. Effect of albumin binding and amino acid competition on tryptophan uptake into brain. Journal of Neurochemistry. 1977;28(5):1015–1023. doi: 10.1111/j.1471-4159.1977.tb10664.x. [DOI] [PubMed] [Google Scholar]
- 73.Markus C. R., Firk C., Gerhardt C., Kloek J., Smolders G. J. F. Effect of different tryptophan sources on amino acids availability to the brain and mood in healthy volunteers. Psychopharmacology. 2008;201(1):107–114. doi: 10.1007/s00213-008-1254-0. [DOI] [PubMed] [Google Scholar]
- 74.Suga H., Asakura K., Kobayashi S., Nojima M., Sasaki S., Three-generation Study of Women on Diets and Health Study Group Association between habitual tryptophan intake and depressive symptoms in young and middle-aged women. Journal of Affective Disorders. 2018;231:44–50. doi: 10.1016/j.jad.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 75.Raison C. L., Dantzer R., Kelley K. W., et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: relationship to CNS immune responses and depression. Molecular Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The cognitive and biochemistry data used to support the findings of this study are available with the corresponding author upon request.