Abstract
Background
Craniopharyngiomas are neoplasms of the sellar/parasellar region that are classified into adamantinomatous craniopharyngioma (ACP) and papillary craniopharyngioma (PCP) subtypes. Surgical resection of craniopharyngiomas is challenging, and recurrence is common, frequently leading to profound morbidity. BRAF V600E mutations render PCP susceptible to BRAF/MEK inhibitors, but effective targeted therapies are needed for ACP. We explored the feasibility of targeting the programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) immune checkpoint pathway in ACP and PCP.
Methods
We mapped and quantified PD-L1 and PD-1 expression in ACP and PCP resections using immunohistochemistry, immunofluorescence, and RNA in situ hybridization. We used tissue-based cyclic immunofluorescence to map the spatial distribution of immune cells and characterize cell cycle and signaling pathways in ACP tumor cells which intrinsically express PD-1.
Results
All ACP (15 ± 14% of cells, n = 23, average ± SD) and PCP (35 ± 22% of cells, n = 18) resections expressed PD-L1. In ACP, PD-L1 was predominantly expressed by tumor cells comprising the cyst lining. In PCP, PD-L1 was highly expressed by tumor cells surrounding the stromal fibrovascular cores. ACP also exhibited tumor cell–intrinsic PD-1 expression in whorled epithelial cells with nuclear-localized beta-catenin. These cells exhibited evidence of elevated mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) signaling. Profiling of immune populations in ACP and PCP showed a modest density of CD8+ T cells.
Conclusions
ACP exhibit PD-L1 expression in the tumor cyst lining and intrinsic PD-1 expression in cells proposed to comprise an oncogenic stem-like population. In PCP, proliferative tumor cells express PD-L1 in a continuous band at the stromal–epithelial interface. Targeting PD-L1 and/or PD-1 in both subtypes of craniopharyngioma might therefore be an effective therapeutic strategy.
Keywords: craniopharyngioma, brain neoplasms, neuropathology, immunotherapy, programmed cell death 1 receptor
Importance of the study
Craniopharyngiomas are associated with substantial morbidity, which is often exacerbated by treatment, and frequent recurrences. Case reports of dramatic therapeutic responses in patients with BRAF V600E mutant PCP have prompted testing of BRAF/MEK inhibitors in these tumors, currently in a phase II clinical trial; however, no effective targeted therapies are available for ACP. In multiple cancers, elevated expression of PD-L1 correlates with therapeutic responsiveness to PD-1/PD-L1 inhibition, but the feasibility of targeting this pathway in craniopharyngioma has not been explored. We found significant expression of PD-L1 in all ACP and PCP resections analyzed. ACP also exhibit tumor cell–intrinsic PD-1 expression with concomitant elevation of downstream MAPK and mTOR signaling within a putative stem-like population. Tumor cell–intrinsic PD-1 expression has previously been reported only in melanoma, where it promotes tumor cell growth. Our findings suggest multiple mechanisms whereby PD-1/PD-L1 inhibitors might benefit patients with ACP and PCP.
Craniopharyngiomas are epithelial neoplasms of the sellar/parasellar region that are hypothesized to arise from developmental derivatives of the stomodeal ectoderm and Rathke’s pouch.1 They comprise 1.2%–4.6% of all intracranial tumors, with an incidence of 0.5–2.5 new cases per 1 million population per year across all age groups. Craniopharyngiomas are the most common nonglial primary intracranial tumors in children and comprise up to 10% of all pediatric brain tumors.2
Two subtypes of craniopharyngioma—adamantinomatous craniopharyngioma (ACP) and papillary craniopharyngioma (PCP)—are currently recognized based on integrated genetic and histologic analyses.3 Most ACP harbor recurrent activating mutations in CTNNB1, the gene encoding beta-catenin,4–7 while nearly all PCP harbor recurrent BRAF V600E mutations.6,8 ACP and PCP also demonstrate distinct patterns of DNA methylation and gene expression, further suggesting that they are distinct entities.9
Surgical resection is the first-line treatment for craniopharyngioma,10 but the sellar/suprasellar location of these tumors and their proximity to critical neural and endocrine structures make resection challenging.11 Thus, while gross total resection limits the risk of recurrence, subtotal resection with adjuvant radiation is often pursued to reduce morbidity.12 In cases with residual tumor, expansion of cystic components can be problematic and may require frequent aspiration, shunting, or repeat surgical resection.13
Craniopharyngiomas are associated with significant morbidity, including panhypopituitarism, diabetes insipidus, profound obesity, cognitive impairment, personality changes, and retarded growth and sexual maturation in children.14 These problems often require lifelong management.15,16 Novel treatment strategies that address refractory disease, avoid the use of radiation treatment in recurrent or subtotally resected tumors, or ultimately avoid surgery and radiation altogether could reduce the profound morbidity associated with ACP and PCP.
Neoplasms evade immune surveillance by multiple mechanisms.17 One such mechanism involves elevated levels of programmed death ligand 1 (PD-L1), a mediator of peripheral immune tolerance which may be expressed by tumor cells and tumor-associated immune cells. Inhibition of programmed cell death protein 1 (PD-1)/PD-L1 immune checkpoint signaling via competitive antibody inhibition has demonstrated remarkable efficacy in a variety of cancers,18 particularly when tumor-infiltrating lymphocytes are abundant, and such drugs are approved by the Food and Drug Administration (FDA) for multiple indications.19 Craniopharyngiomas frequently exhibit a prominent inflammatory infiltrate, but their immune microenvironment has not been studied in detail and expression of immune checkpoint proteins such as PD-L1 and PD-1 has not been characterized. The importance of the immune microenvironment in ACP is further suggested by markedly elevated levels of numerous immunomodulatory cytokines in ACP cyst fluid.20 Moreover, a PCP treated with BRAF/MEK inhibitors developed a profound inflammatory infiltrate correlating with significant radiologic reduction in tumor volume.21 In this study, we sought to better understand the immune microenvironment in ACP and PCP, characterize expression of PD-L1 and PD-1, and explore the feasibility of targeting the PD-1/PD-L1 pathway in patients.
Methods
Tissue Characterization
Formalin fixed and paraffin embedded (FFPE) tissues from 21 ACP and 14 PCP patients, including 6 recurrent ACPs and 2 paired primary-recurrent ACP resections (23 total specimens) and 3 matched primary-recurrent PCP resections (18 total specimens), were retrieved from the archives of Brigham and Women’s Hospital with institutional review board (IRB) approval as part of discarded/excess tissue protocol. Detailed characteristics of all cases are provided in Supplementary Tables S1 and S2. Cases were classified according to the revised World Health Organization (WHO) 2016 Classification of Tumors of the Central Nervous System (S.C., S.S.). All PCP had BRAF V600E mutation by genotyping and immunohistochemistry (IHC) and lacked CTNNB1 mutations. Twenty-one of 22 ACP had CTNNB1 activation by genotyping and/or IHC and lacked BRAF V600E mutations by genotype and/or IHC.
Biomarker Characterization
Immunohistochemistry, immunofluorescence, image acquisition, and processing were performed using standard methods. Multiplexed tissue-based cyclic immunofluorescence (t-CyCIF) is described in detail elsewhere.22–24 Briefly, t-CyCIF is a method for assembling high-plex immunofluorescence images of FFPE tissue sections via successive rounds of 4-channel imaging followed by fluorophore oxidation. A DNA dye (Hoechst 33342) is included in each cycle to allow images to be aligned at subpixel resolution; subcellular resolution images of dozens of biomarkers can thereby by obtained. Detailed protocols are available in the Supplementary Methods. The percentage of tumor cells with membranous PD-L1 expression was estimated by IHC and quantified by immunofluorescence (Supplementary Tables S1, S2).
PD-L1 and PD-1 RNAscope
PD-L1 and PD-1 mRNA transcripts were assayed as previously described.25 Probes are listed in the Supplementary Methods.
PD-L1 Transcriptome Analysis
Snap-frozen surgical tissues from 27 pediatric ACP and a selected panel of tumors, including 5 choroid plexus papillomas (CPP), 45 medulloblastomas (2 Wnt, 12 sonic hedgehog [SHH], 14 Group 3, 17 Group 4), and 27 normal brain samples obtained from autopsy or epilepsy surgery were reviewed at Children’s Hospital Colorado with IRB approval and patient consent. CPP were selected for comparison to another low-grade epithelial pediatric tumor, while medulloblastoma was selected for comparison by molecular subgroup, including Wnt-pathway tumors which exhibit CTNNB1 mutations, similar to ACP. Transcriptomic profiling of these samples was performed using Human Genome U133plus2 Arrays (Affymetrix) as described previously.26 In this dataset, Geneprobe #227458 was assessed as a measure of PD-L1 (CD274) transcript levels.
Results
PD-L1 and PD-1 Expression in Adamantinomatous Craniopharyngioma
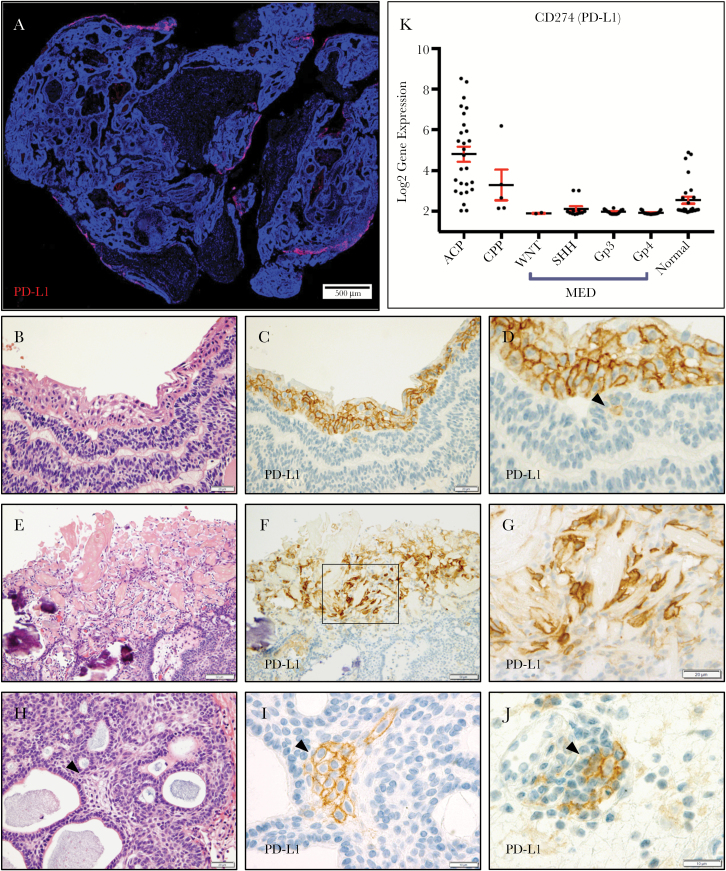
Using IHC, we observed membranous PD-L1 expression in neoplastic epithelial cells (15 ± 14% of cells; range 1%–50%) in all 23 ACP resections examined (Fig. 1A). Expression was predominantly present in regions of well-keratinized squamous tumor epithelium (Fig. 1B–D), which often line the cystic structures of ACP. In addition, strong membranous staining was often present in the cells surrounding “wet keratin” and “ghost cells” (Fig. 1E–G). Generally, PD-L1 was not highly expressed in the basaloid and stellate reticular epithelium, but expression was occasionally observed in rare single cells (16/23 cases, 70%; Fig. 1D) and cell clusters (8/23 cases, 35%; Fig. 1H, I). One case (ACP-15) showed increased PD-L1 expression in the basaloid epithelium and atypical histologic features following multiple resections and radiation. However, PD-L1 expression was not clearly correlated to recurrence status or prior radiation treatment (Supplementary Table S1). Immune cells with membranous expression were present in all cases (Fig. 1J). Analysis of transcriptomic data from a separate cohort of 27 ACP at a second institution similarly showed significantly elevated PD-L1 expression in most ACP (24/27 cases; 89%). PD-L1 mRNA expression was significantly higher in ACP than normal brain, CPP, and medulloblastoma, including Wnt-activated tumors (Fig. 1K).
Fig. 1.
PD-L1 expression in ACP is predominantly localized to well-keratinized cyst-lining epithelium and regions adjacent to wet-keratin and ghost cells (A; PD-L1, red; 4′,6′-diamidino-2-phenylindole [DAPI], blue). Hematoxylin and eosin (H&E) (B) and PD-L1 IHC (C, D) of cyst-lining epithelium. Single cells are occasionally positive in the basaloid epithelium (D, arrowhead). H&E (E) and PD-L1 IHC (F, G) show PD-L1 expression in cells near “wet-keratin/ghost cells.” H&E (H) and PD-L1 IHC (I) show basaloid epithelium with a cluster of PD-L1 expressing cells. Scattered immune cells show PD-L1 expression (J). Transcriptomic analysis of PD-L1 mRNA in a separate cohort of 27 ACPs and 77 other brain tumors and non-neoplastic brain specimens including choroid plexus papillomas (CPP) and medulloblastomas (MED) (K). Values expressed as log2 gene expression. Scale bars 20 μm (B–D, F–L), 50 μm (E).
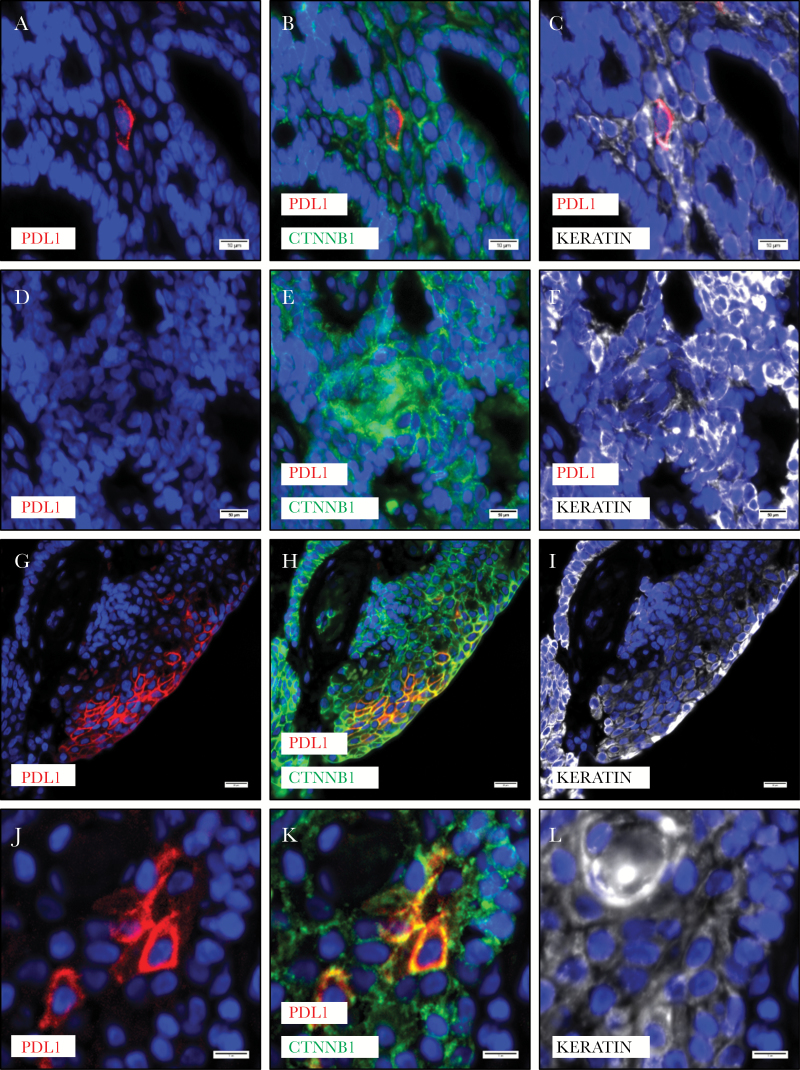
While nearly all ACP harbor activating CTNNB1 mutations, it has been previously determined that nuclear translocation of beta-catenin (necessary for its activity as a transcription factor) is spatially restricted, being most pronounced in small clusters (“whorls”) of epithelial cells. These cells comprise a putative quiescent stem-like niche characterized by coexpression of cluster of differentiation (CD)133, CD44, and C-X-C chemokine receptor type 4, elevated p21Cip/Waf1, and low Ki-67.27 Using co-immunofluorescence, we found that scattered single cells and clusters of cells expressing PD-L1 also expressed keratin, confirming their epithelial nature. These cells had membranous beta-catenin (5/5 cases; Fig. 2A–C). Whorls with nuclear beta-catenin were invariably negative for PD-L1 (5/5 cases; Fig. 2D–F). Well-keratinized cyst-lining epithelium, which nearly always had strong and diffuse expression of PD-L1, had membranous localization of beta-catenin (5/5 cases; Fig. 2G–I). PD-L1 expressing cells surrounding “wet keratin” were typically keratin positive, and these cells had membranous localization of beta-catenin (5/5 cases; Fig. 2J–L). These data suggest that PD-L1 is not expressed in cells with transcriptionally active nuclear beta-catenin.
Fig. 2 .
PD-L1 expressing cells in the basaloid epithelium of ACP (A) showed membranous (inactive) beta-catenin (B) and were typically keratin positive (C). Whorls of epithelium with nuclear beta-catenin do not express PD-L1, but are weakly positive for keratin (D–F). Regions of cyst lining epithelium with PD-L1 expression have membranous beta-catenin (G–I). PD-L1 expressing cells near “wet keratin” were keratin expressing epithelial cells with membranous beta-catenin (J–L). Scale bars 20 µm.
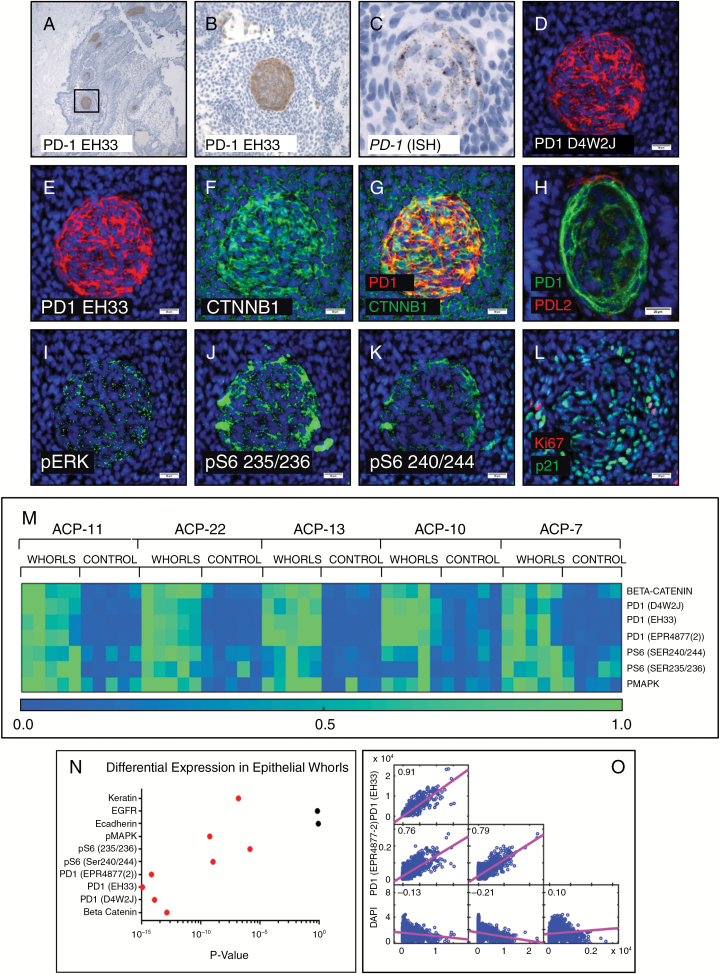
Remarkably, IHC revealed strong membranous expression of PD-1 in neoplastic epithelial cells in all 23 ACP resections. PD-1 expression was only present in whorls of epithelial cells within the basaloid epithelium (Fig. 3A, B). Both the rare basaloid epithelial cells and well-keratinized epithelium with PD-L1 expression were geographically distinct from PD-1 expressing whorls (Supplementary Fig. S1A), suggesting that these cellular populations do not directly interact. In situ hybridization showed elevated expression of PD-1 mRNA in whorls but not other regions of the tumor epithelium (Fig. 3C).
Fig. 3 .
PD-1 IHC in ACP showed tumor cell–intrinsic PD-1 expression in whorls of tumor epithelium (A, B). In situ hybridization showed expression of PD-1 mRNA in epithelial whorls (C). Multiplexed cyclic immunofluorescence (t-CyCIF) showed co-localization of PD-1 (D, E) using multiple antibodies, and nuclear beta-catenin (F, G) in the whorled cells. PD-L2 positive cells were observed closely associated with whorls (H) (different whorl pictured than D–L). Whorls showed elevated phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (I), phospho-S6 (Ser235/236) (J), and (Ser240/244) (K). PD-1 expressing whorls had diffuse nuclear p21Cip1/Waf1 but MIB-1/Ki-67 staining was not typically present (L). Quantitative analysis (heatmaps of 5 ACP resections depicted with 0.0 [low] to 1.0 [high] expression by normalized mean intensity) (M) showed significantly increased expression (P < 0.025, red dots indicate significance) (N) of PD-1, pS6, and pERK in whorls compared with adjacent non-whorled epithelium. Multiple PD-1 antibodies showed high pixel-by-pixel correlation coefficients (O). Scale bar 100 µm (C); scale bars 20 µm (D–L).
To further characterize tumor cells with intrinsic PD-1 expression we used t-CyCIF, a multicycle immunofluorescence methodology that generates multiparametric subcellular resolution images from FFPE tissue sections22–24 (Fig. 3, Supplementary Fig. S1). Three different antibodies raised against distinct PD-1 synthetic peptide or recombinant protein immunogens showed nearly identical staining patterns in ACP (Fig. 3D, E, Supplementary Fig. S1G–I), with a high level of pixel-by-pixel correlation (Fig. 3O). In all instances, PD-1 specifically localized to epithelial whorls comprising cells with nuclear-localized beta-catenin (Fig. 3F, G). PD-L1 expressing cells were not associated with PD-1 expressing whorls in any case examined, consistent with IHC data. However, cells with membranous PD-L2 expression were occasionally observed in close association with whorls (Fig. 3H, Supplementary Fig. S1S–T), but were not found in other regions of the tumor epithelium.
Tumor intrinsic PD-1 expression was recently described in melanoma,28 where it supports tumor growth through activation of downstream mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) signaling. We identified elevated phospho-p44/42 MAPK(Erk1/2) (Thr202/Tyr204) (Fig. 3I), phospho-S6 (Ser235/236) (Fig. 3J), and (Ser240/244) (Fig. 3K) in PD-1 expressing whorls in all cases examined (5/5), suggesting that PD-1 may activate intracellular signaling pathways in ACP cells similar to those activated in melanoma. Quantitative analysis (by normalized mean intensity, see Supplementary Methods for detailed methodology) of whorls and other regions of tumor epithelium showed significant (P < 0.025) enrichment of PD-1, p44/42 (Erk1/2), and pS6 (Ser235/235) in the whorls compared with other regions (Fig. 3M, N, Supplementary Table S3). Rare cells with intense pS6 (Ser235/236) were sometimes associated with whorls; these cells coexpress ionized calcium binding adaptor molecule 1 (IBA1), suggesting that they are of monocytic derivation (Supplementary Fig. S1B, C). The whorled epithelial cells rarely expressed the proliferation marker MIB-1/Ki67 but had strong nuclear expression of p21Cip1/Waf1 (Fig. 3L), suggesting they are quiescent, consistent with prior data.29
PD-L1 and PD-1 Expression in Papillary Craniopharyngioma
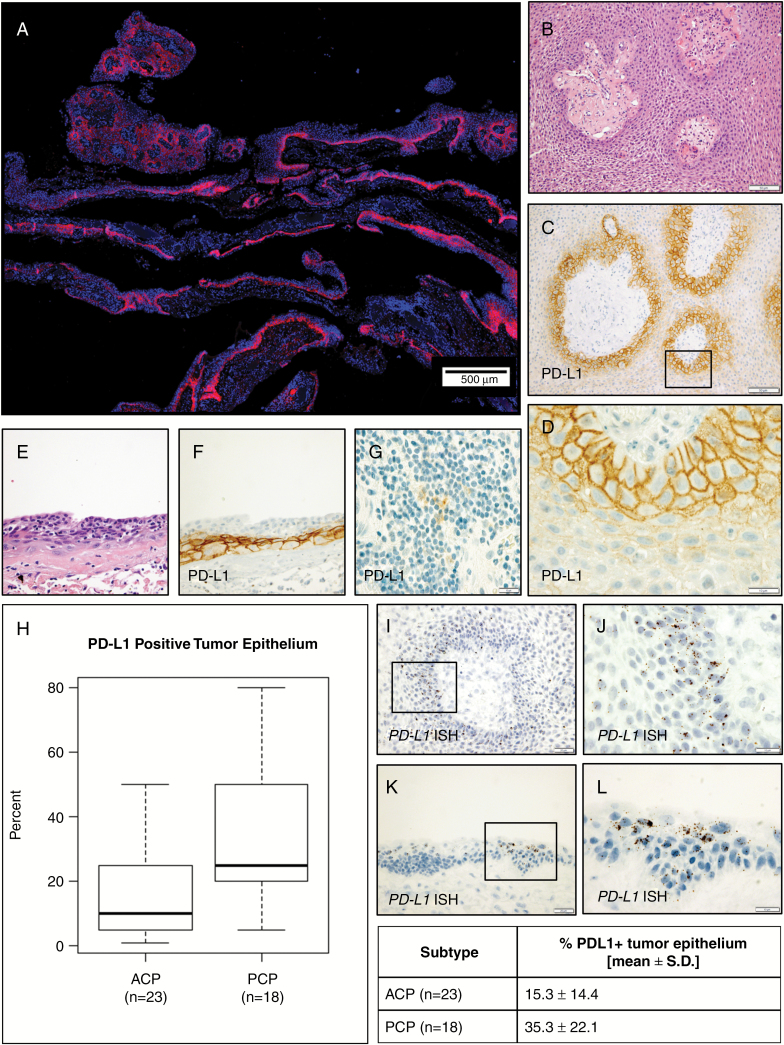
Using IHC, we observed membranous PD-L1 expression in neoplastic epithelial cells (35 ± 22% of cells, range 5%–80%) in all 18 PCP resections examined (Fig. 4A). PD-L1 expression was present in multiple layers of tumor cells circumferentially surrounding the fibrovascular stroma (Fig. 4B–D). Expression was strongest in the basal cells and decreased with increasing distance from the stroma. PD-L1 expression was also present in a continuous band in flat regions of epithelium (Fig. 4E, F). As with ACP, we did not observe a clear correlation between PD-L1 expression and recurrence status or prior radiation. In all cases examined, PD-L1 was expressed in scattered immune cells (Fig. 4G). PD-L1 expression was typically present in a larger percentage of tumor cells in PCP than ACP (Fig. 4H).
Fig. 4.
PD-L1 is diffusely expressed in papillary and flat epithelium in PCP (A). Hematoxylin and eosin (B) and PD-L1 IHC (C, D) in PCP show strong membranous expression in basal cells circumferentially surrounding fibrovascular stroma. Continuous expression was also present in basal cells in regions of flat epithelium (E, F). Scattered immune cells with PD-L1 expression were present in each case (G). ACP and PCP each show significantly increased PD-L1 expression, with higher levels in PCP (H). In situ hybridization shows PD-L1 mRNA expression in PCP (I, J) and ACP (K, L) epithelium in the same distribution as the protein expression observed by IHC. Scale bars 20 μm (D, F, G, I, K, L), 50 μm (B, C, H, J).
Co-immunofluorescence for PD-L1 using clone E1L3N and clone 28-8, an FDA-approved complementary diagnostic for evaluating PD-L1 expression in lung cancer, showed a similar pattern of staining in both ACP (Supplementary Fig. S3) and PCP (Supplementary Fig. S4). The 2 antibodies exhibited a high pixel-by-pixel correlation, suggesting specificity of antigen binding for PD-L1 (Supplementary Fig. S3M). We further confirmed the pattern of PD-L1 expression in both PCP (Fig. 4I, J) and ACP (Fig. 4K, L, Supplementary Fig. S3N–P) using in situ hybridization for PD-L1 mRNA. PCP tumor cells did not express PD-1 (0/18 cases). Nuclear MIB-1/Ki-67 expression, indicating proliferative activity, was predominantly present in basally oriented cells near the stroma, frequently in PD-L1 expressing cells (Supplementary Fig. S2J–L), suggesting that these proliferative tumor cells may suppress or evade immune surveillance.
Immune Cell Profiling in Craniopharyngioma
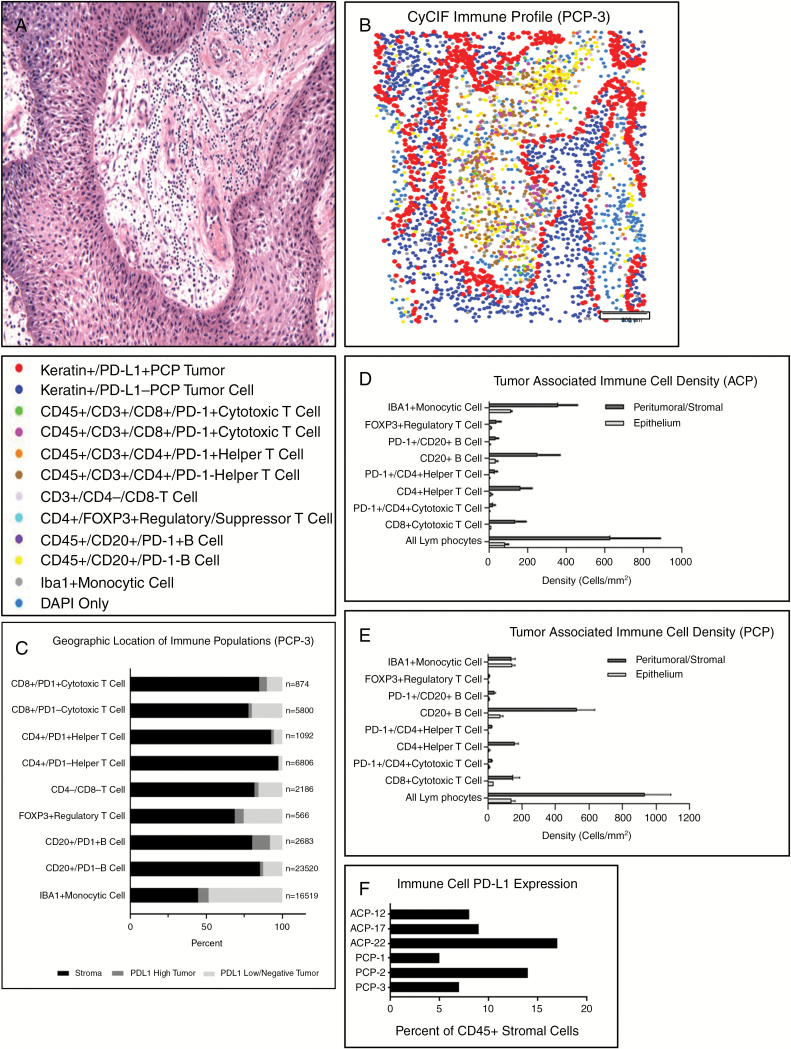
Numerous lymphocytes were present in the stroma and epithelium in all PCP cases (Fig. 5A). To characterize these cells and other components of the immune microenvironment, we profiled immune cell populations in PCP using t-CyCIF. We defined specific populations by surface antigen expression, plotted their spatial distribution (Fig. 5B), and quantified cells in each of 3 compartments: stroma, PD-L1 expressing basal epithelium, and PD-L1 low/negative tumor epithelium further removed from the stroma (Fig. 5C; Supplementary Table S4).
Fig. 5 .
Tissue-based CyCIF was used to profile, quantify, and generate spatial representations of tumor and immune cell populations in whole slide sections (A, hematoxylin and eosin; B, representative “dot-plot”). Each immune population was quantified in the PD-L1 expressing tumor epithelium, PD-L1 negative epithelium, and stroma (C, representative data from PCP-3). The density of immune cells was quantified in ACP (n = 3) (D) and PCP (n = 3) (E) (mean ± SEM). The percent of stromal immune cells (CD45+) with PD-L1 expression was quantified in ACP (n = 3) and PCP (n = 3) (F).
Numerous IBA1-expressing monocytic cells were present in PCP but not enriched in any of the 3 compartments. PD-1 positive and negative B and T cells were more often found in the stromal compartment. Localization of B- and T-cell populations showed no clear correlation with PD-1 expression status. Similarly, in ACP, B and T cells were more often found in the stroma (Supplementary Table S5). Forkhead box protein 3–positive (FoxP3+) regulatory/suppressor T cells were present in all compartments in PCP and ACP, but predominantly localized in the stroma in most cases. Histologically, neutrophils were rare in ACP; however, neutrophils were present at least focally in 12/18 (66%) PCP; these cells did not express PD-L1.
We used t-CyCIF to characterize the expression of PD-L1 in immune cells and the density of immune cell infiltrates in both ACP (Fig. 5D) and PCP (Fig. 5E) within the stromal and epithelial compartments (Supplementary Table S6). Numerous lymphocytes were present in the stroma of ACP (630 ± 450 cells/mm2) and PCP (930 ± 270 cells/mm2). Direct comparison of the density of each subpopulation of immune cell in the stroma and epithelium of ACP and PCP showed no significant differences between subtypes (P > 0.05; Supplementary Table S8, Supplementary Fig. S7). PD-L1 was expressed in a substantial subpopulation of the CD45+ immune cells within the stromal compartment of both ACP (11 ± 5%), and PCP (9 ± 5% of cells) in all cases examined (Fig. 6F, Supplementary Table S7). CD20+ B cells were the most common lymphocyte population in both ACP (250 ± 200 cells/mm2) and PCP (530 ± 190 cells/mm2), of which 17 ± 7% and 11 ± 5% expressed PD-L1, respectively. The overall density of FoxP3+ lymphocytes was low in both ACP (25 ± 30 cells/mm2) and PCP (6 ± 4 cells/mm2). The density of tumor infiltrating CD8+ T cells was low compared with previously characterized human malignancies,30,31 and higher in PCP (35 ± 22 cells/mm2) than ACP (14 ± 12 cells/mm2). Higher densities of CD8+ T cells were present in the peritumoral/stromal compartment in both PCP (150 ± 70 cells/mm2) and ACP (140 ± 90 cells/mm2).
Discussion
While ACP and PCP are uncommon tumors with excellent long-term survival, they frequently lead to profound morbidity under current treatment strategies. Thus, a pressing need exists for new therapeutic options. Our work identifies at least 3 potential ways in which craniopharyngiomas might be vulnerable to PD-1/PD-L1 immune checkpoint blockade. First, ACP and PCP consistently express PD-L1 in a substantial percentage of tumor cells, with a distinctive spatial distribution of expression in each subtype. Secondly, ACP demonstrate tumor cell–intrinsic PD-1 expression in a putative stem-like niche that also exhibits evidence of active MAPK and pS6 signaling. These findings resemble those recently described in melanoma model systems, where tumor cell–intrinsic PD-1 pathway activity promotes tumor growth. Finally, both ACP and PCP contain significant numbers of PD-L1 expressing immune cells, as well as numerous CD8+ T cells.
Our study demonstrates a consistent spatial localization of PD-L1 and PD-1 expression in craniopharyngiomas which is unique to these tumors, highlighting the important insights that may be obtained from studying rare tumors. PD-L1 is predominantly localized to the cyst lining in ACP, and to basally oriented tumor cells circumferentially surrounding the fibrovascular stroma in PCP. PD-L1 expression was typically higher in PCP (35 ± 22%) than ACP (15 ± 14%). In ACP, the proportion of cells with PD-L1 expression generally appeared to reflect the amount of cyst lining epithelium present in the sample. Transcriptomic profiling of a separate ACP cohort confirmed that PD-L1 mRNA expression was elevated in most cases (89%). The few cases with low/undetectable PD-L1 mRNA expression may represent samples with a low amount of cyst-lining epithelium. Cystic regions are a common and clinically recalcitrant problem in recurrent ACP. We found PD-L1 expression in the cyst lining in all 6 recurrent ACPs examined. The presence of numerous inflammatory mediators within the cyst fluid of ACP20 raises the possibility that the strong PD-L1 expression in cyst-lining cells may represent an immune checkpoint barrier that modulates the activity of immune cells within the cyst fluid. Similarly, expression of PD-L1 in basal tumor cells at the stromal–epithelial interface in PCP may modulate the activity of tumor-associated immune cells, which are predominantly found in the stroma.
Our work shows that PD-1 expression in ACP is distributed in a pattern that has not been previously described in human cancer. All ACP resections examined exhibited strong membranous expression of PD-1 in whorled tumor cells with nuclear beta-catenin. The conventional paradigm is that tumor cells express PD-L1 to suppress PD-1 expressing immune cells. PD-1 expression that is intrinsic to tumor cells was recently described in melanoma models, where it promotes tumorigenesis through activation of downstream MAPK and mTOR signaling.28 ACP show consistent spatial localization of PD-1 expression to whorled tumor cells, in contrast to the less well-defined localization of PD-1 expression in melanoma. While tumor cell–intrinsic PD-1 expression in both ACP and melanoma is associated with increased levels of phosphorylated extracellular signal-regulated kinase (ERK) and S6, the whorled cells in ACP rarely stained for MIB-1/Ki-67 and instead strongly expressed p21Cip1/Waf1, suggesting that the cells are not proliferative. Thus, in ACP, the PD-1 pathway does not appear to be directly mitogenic in the whorled cell population. Nevertheless, activating CTNNB1 mutations are present in nearly all ACP and epithelial whorls with nuclear beta-catenin and are thought to represent a population of cells fundamental to ACP tumorigenesis.7 Consistent expression of PD-1 in this tumor cell niche is an entirely unexplored phenomenon, and further investigation in ACP and other tumors may shed light on a wider role for PD-1 in tumor biology.
Of note, PD-L1 expressing cells were not found near the PD-1 expressing whorled tumor cells in any of the ACP resections examined. In contrast, cells with membranous PD-L2 expression were occasionally observed closely associated with the PD-1 expressing whorls. PD-L2 could therefore be an activating ligand for the PD-1 receptor in this population. Consistent strong expression of PD-1 in the proposed stem-like population in ACP suggests that therapeutic targeting of PD-1 signaling in ACP may have intrinsic therapeutic efficacy independent of any interaction with the immune system.
The mechanism by which PD-L1 expression is upregulated in craniopharyngiomas is not known. Expression may be intrinsically upregulated by rearrangements of the 9p24.1 locus; however, these alterations have not been described to date in craniopharyngiomas. PD-L1 expression can be intrinsically upregulated by intracellular signal transduction pathways as in non–small cell lung carcinoma (NSCLC) with epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) translocation.32 ACP frequently exhibit activation of EGFR and SHH pathways via paracrine signaling, which could in principle mediate upregulation of PD-L1.33,34 However, while PD-L1 expression significantly correlates with EGFR activation in NSCLC, clinical trials have shown reduced efficacy of PD-1 inhibitors in patients with EGFR or ALK activation.35 PD-L1 may also be upregulated by inflammatory cytokines, particularly interferon-gamma, and high levels of inflammatory cytokines in the cyst fluid of ACP provide one possible explanation for the localization of PD-L1 expression to the cyst lining.20
Intracystic interferon-alpha has been used to manage recurrent ACP, and this treatment is thought to drive multiple immunomodulatory effects and to have direct antiproliferative effects in tumor cells. While patients in our cohort did not receive this intervention prior to resection, it is an intriguing question whether the effects of this treatment may be in part mediated by modulation of PD-1/PD-L1 pathway activity in tumor cells, or by overcoming suppression of CD8+ cells by the PD-L1+ tumor cyst-lining epithelium. The mechanisms underlying elevated PD-1 expression in ACP are also not yet known. The consistent co-occurrence of nuclear beta-catenin and PD-1 expression may implicate beta-catenin transcriptional activity.
Given that BRAF V600E signals in part through downstream MAPK/ERK, it is possible that BRAF V600E drives PD-L1 expression in PCP. Notably, in one report, inhibition of BRAF/MEK in PCP dramatically increased the number of infiltrating CD8+ lymphocytes and macrophages and led to a dramatic reduction in tumor volume.21 It is possible that the antitumor effects of BRAF/MEK inhibition resulted from inhibition of both oncogenic signaling and disruption of PD-L1 expression.
At baseline, numerous lymphocytes are present in PCP and ACP. We used t-CyCIF to show that the immune population in both subtypes consists of a mixed infiltrate of B and T cells with a significant percentage of immune cells expressing PD-L1 and only rare FoxP3+ regulatory/suppressor T cells. The distribution of B- and T-cell populations is not clearly correlated with PD-1 expression, suggesting that PD-L1 expression in the basal epithelium of PCP may not strictly act as a barrier that blocks passage of PD-1+ lymphocytes across the stromal–epithelial interface. Instead, the expression of PD-L1 may represent an adaptive response to stromal or tumor-infiltrating lymphocytes or as a modulator of immune activity. Given that the basal cells of PCP demonstrate the highest proliferative activity, strong PD-L1 expression in the basal layers may allow the most proliferative cells in the tumor to suppress immune surveillance.
The density of CD8+ lymphocytes in ACP and PCP is low compared with malignancies such as melanoma and NSCLC in which a low density of CD8+ lymphocytes, typically <100–200 cells/mm2, correlates with poor responsiveness to PD-1/PD-L1 blockade.30 The most common lymphocyte population in craniopharyngiomas was CD20+ B cells, many of which expressed PD-L1 (11 ± 5% in ACP and 17 ± 7% in PCP). The significance of tumor-infiltrating lymphocyte B cells (TIL-B) is less well understood than CD8+ T cells with conflicting protumor and antitumor effects in different tissues and model systems. Nevertheless, increased TIL-B correlate with improved survival in multiple human cancers.36 We did not observe tertiary lymphoid structures or germinal centers, or significant numbers of plasma cells, suggesting that immunomodulatory functions of TIL-B may predominate in craniopharyngioma, rather than antibody production.
While neutrophils are rare in ACP, dense neutrophilic inflammation was present in many PCPs (66%). Neutrophils may mediate antitumor immunity via release of cytokines, and activated neutrophils may function as myeloid-derived suppressor cells in human tumors. We did not observe a clear correlation between PD-L1 expression and the density of neutrophils or significant expression of PD-L1 on neutrophils by IHC; however, the significance of these cells in the PCP tumor microenvironment warrants further study.
It is notable that in previously studied tumors, PD-L1 expression varied substantially between patients, and no known morphologic features predicted the pattern of expression. In contrast, PD-L1 expression in ACP and PCP is reproducibly observed in specific histologic regions. Thus, it is not clear that the density of infiltrating immune cells has the same significance in craniopharyngiomas as for more phenotypically heterogeneous malignant tumors. Moreover, the density of immune cells within the cyst fluid of ACP is not yet characterized. Given the localization of PD-L1 expression and presence of immunomodulatory proteins, this compartment may prove biologically important.
Craniopharyngiomas also differ substantially from melanoma and NSCLC in that they have significantly fewer somatic alterations6; thus, they are likely to have fewer neoantigens and may therefore be less intrinsically immunogenic. However, the more limited genetic and phenotypic heterogeneity in craniopharyngioma suggests that these tumors are likely to have a more restricted repertoire of mechanisms for evading the immune system and may be less able to adapt to checkpoint inhibition or generate resistant subclones with different means of immune evasion.
Drugs that block PD-1/PD-L1 are typically well tolerated, with fewer adverse events than with conventional chemotherapy. Targeting the cyst lining epithelium by PD-1/PD-L1 inhibitors in ACP may raise concern for iatrogenic cyst rupture; however, this is not a common complication following intracystic treatment with bleomycin, radionuclides, or interferon. The consistent expression of PD-L1 ligand in both primary and recurrent craniopharyngioma of both histologic subtypes in physiologically relevant patterns and the typically manageable side effect profile of PD-1/PD-L1 targeted therapeutics support the feasibility of exploring this treatment option clinically. Given the risk of autoimmune events, we expect that initial testing of these therapies would be most appropriate in symptomatic patients with refractory disease not easily amenable to radiation or surgery. PD-1/PD-L1 inhibitors could prove to be an attractive new avenue for the systemic treatment of recurrent craniopharyngioma patients, as well as a new possibility for avoiding the long-term risks of radiation and surgery in a wider cohort of patients with these highly morbid and clinically challenging tumors.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was funded in part by P50GM107618 and U54HL127365 (P.K.S.) and the Ludwig Center at Harvard. Dana-Farber/Harvard Cancer Center is supported in part by an NCI Cancer Center Support Grant # NIH 5 P30 CA06516.
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1. Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst. 2005;21(8–9):622–627. [DOI] [PubMed] [Google Scholar]
- 2. Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89(4):547–551. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.. WHO Classification of Tumors of the Central Nervous System. Lyon: IARC; 2016. [DOI] [PubMed] [Google Scholar]
- 4. Sekine S, Shibata T, Kokubu A et al. Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am J Pathol. 2002;161(6):1997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apps JR, Martinez-Barbera JP. Molecular pathology of adamantinomatous craniopharyngioma: review and opportunities for practice. Neurosurg Focus. 2016;41(6):E4. [DOI] [PubMed] [Google Scholar]
- 6. Brastianos PK, Taylor-Weiner A, Manley PE et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46(2):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buslei R, Nolde M, Hofmann B et al. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. 2005;109(6):589–597. [DOI] [PubMed] [Google Scholar]
- 8. Brastianos PK, Santagata S. Endocrine tumors: BRAF V600E mutations in papillary craniopharyngioma. Eur J Endocrinol. 2016;174(4):R139–R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hölsken A, Sill M, Merkle J et al. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commun. 2016;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robinson LC, Santagata S, Hankinson TC. Potential evolution of neurosurgical treatment paradigms for craniopharyngioma based on genomic and transcriptomic characteristics. Neurosurg Focus. 2016;41(6):E3. [DOI] [PubMed] [Google Scholar]
- 11. Cohen M, Bartels U, Branson H, Kulkarni AV, Hamilton J. Trends in treatment and outcomes of pediatric craniopharyngioma, 1975–2011. Neuro Oncol. 2013;15(6):767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann A, Warmth-Metz M, Gebhardt U et al. Childhood craniopharyngioma—changes of treatment strategies in the trials KRANIOPHARYNGEOM 2000/2007. Klin Padiatr. 2014;226(3):161–168. [DOI] [PubMed] [Google Scholar]
- 13. Lamiman K, Wong KK, Tamrazi B et al. A quantitative analysis of craniopharyngioma cyst expansion during and after radiation therapy and surgical implications. Neurosurg Focus. 2016;41(6):E15. [DOI] [PubMed] [Google Scholar]
- 14. Adamson TE, Wiestler OD, Kleihues P, Yaşargil MG. Correlation of clinical and pathological features in surgically treated craniopharyngiomas. J Neurosurg. 1990;73(1):12–17. [DOI] [PubMed] [Google Scholar]
- 15. Clark AJ, Cage TA, Aranda D, Parsa AT, Auguste KI, Gupta N. Treatment-related morbidity and the management of pediatric craniopharyngioma: a systematic review. J Neurosurg Pediatr. 2012;10(4):293–301. [DOI] [PubMed] [Google Scholar]
- 16. Yano S, Kudo M, Hide T et al. Quality of life and clinical features of long-term survivors surgically treated for pediatric craniopharyngioma. World Neurosurg. 2016;85:153–162. [DOI] [PubMed] [Google Scholar]
- 17. Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JY, Lee HT, Shin W et al. Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat Commun. 2016;7:13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donson AM, Apps J, Griesinger AM et al. ; Advancing Treatment for Pediatric Craniopharyngioma Consortium Molecular analyses reveal inflammatory mediators in the solid component and cyst fluid of human adamantinomatous craniopharyngioma. J Neuropathol Exp Neurol. 2017;76(9):779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brastianos PK, Shankar GM, Gill CM et al. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst. 2015;108(2):doi: 10.1093/jnci/djv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin JR, Izar B, Mei S, Wang S, Shah P, Sorger PK. A simple open-source method for highly multiplexed imaging of single cells in tissues and tumours. bioRxiv. 2017;doi:10.1101/151738. [Google Scholar]
- 23. Lin JR, Fallahi-Sichani M, Chen JY, Sorger PK. Cyclic immunofluorescence (CycIF), a highly multiplexed method for single-cell imaging. Curr Protoc Chem Biol. 2016;8(4):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin JR, Fallahi-Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun. 2015;6:8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du Z, Abedalthagafi M, Aizer AA et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffman LM, Donson AM, Nakachi I et al. Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol. 2014;127(5):731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hölsken A, Stache C, Schlaffer SM et al. Adamantinomatous craniopharyngiomas express tumor stem cell markers in cells with activated Wnt signaling: further evidence for the existence of a tumor stem cell niche?Pituitary. 2014;17(6):546–556. [DOI] [PubMed] [Google Scholar]
- 28. Kleffel S, Posch C, Barthel SR et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162(6):1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stache C, Hölsken A, Schlaffer SM et al. Insights into the infiltrative behavior of adamantinomatous craniopharyngioma in a new xenotransplant mouse model. Brain Pathol. 2015;25(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tumeh PC, Harview CL, Yearley JH et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kakavand H, Jackett LA, Menzies AM et al. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol. 2017;30(12):1666–1676. [DOI] [PubMed] [Google Scholar]
- 32. Akbay EA, Koyama S, Carretero J et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coy S, Du Z, Sheu SH et al. Distinct patterns of primary and motile cilia in Rathke’s cleft cysts and craniopharyngioma subtypes. Mod Pathol. 2016;29(12):1446–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hölsken A, Gebhardt M, Buchfelder M, Fahlbusch R, Blümcke I, Buslei R. EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clin Cancer Res. 2011;17(13):4367–4377. [DOI] [PubMed] [Google Scholar]
- 35. Bylicki O, Paleiron N, Margery J et al. Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol. 2017;12(5):563–569. [DOI] [PubMed] [Google Scholar]
- 36. Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–4982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.