Abstract
Background
An increasing number of cancer patients die of cardiovascular diseases. The cardiotoxicity of chemotherapy is particularly important in triple-negative breast cancer (TNBC) with limited therapeutic options. Cardiac autophagy is an important mechanism of cardiotoxicity. This research was aimed to investigate the cardiotoxicity of chemotherapy in TNBC, screen the susceptible population, and determine the relationship between cardiotoxicity and autophagy-related polymorphisms.
Methods
From a total of 2450 stage I-III TNBC patients, 147 met the inclusion criteria and finally recruited. Electrocardiography (ECG) was performed before most chemotherapy cycles, and echocardiography (UCG) was performed according to clinical needs. All ECG and UCG records were re-interpreted by cardiologists at the National Center for Cardiovascular Disease, Fuwai Hospital. According to the National Center for Biotechnology Information and the Catalog of Somatic Mutations in Cancer database, we selected 25 single nucleotide polymorphisms (SNPs) related to autophagy and genotyped the 147 TNBC patients. Paired-sample T tests, Chi squared tests, and logistic regression models were employed for the analysis.
Results
Only 46 (31.3%) patients had normal ECG records after every chemotherapy cycle. Among the 16 patients who underwent UCG, 2 (12.5%) had a reversible decrease of left ventricular ejection fraction. The use of anthracyclines and excessive alcohol consumption were risk factors of ECG abnormalities. With the continuation of chemotherapy, heart rate gradually increased. Anthracyclines were associated with QRS duration abnormalities (P = 0.043). After genotyping for 25 autophagy-related SNPs, we found that the G allele of autophagy-related 13 (ATG13) rs10838611 was significantly associated with ECG abnormalities (odds ratio = 2.258, 95% confidence interval = 1.318–3.869; P = 0.003).
Conclusion
ECG abnormalities caused by chemotherapy are common in the real world. Autophagy-related SNPs are associated with chemotherapy-induced cardiotoxicity, thereby providing new evidence for autophagy as a cause of chemotherapy-induced cardiac damage.
Electronic supplementary material
The online version of this article (10.1186/s40880-018-0343-7) contains supplementary material, which is available to authorized users.
Keywords: Triple-negative breast cancer, Chemotherapy, Cardiotoxicity, Autophagy, Single nucleotide polymorphisms
Background
With the application of many new antineoplastic drugs and improved treatment options, the number of cancer survivors is rapidly rising. However, with increasing life expectancy, cancer patients also receive more cycles or more types of treatment, resulting in an increase in the cumulative dose of some effective drugs that are implicated in long-term adverse cardiovascular outcomes [1]. Triple-negative breast cancer (TNBC) accounts for 15% of breast cancers and exhibits special biological and clinicopathological characteristics, as well as high proliferation, poor differentiation, and poor prognosis [2]. In the absence of approved targeted therapy, chemotherapy containing anthracyclines and taxanes remains the mainstay of the limited treatment options for TNBC at present [2, 3].
Anthracyclines have high efficacy on solid tumors and hematological malignancies. However, many studies have shown cardiotoxicity of anthracyclines and some molecular targeted drugs [4–6]. With repeated cycles of chemotherapy, cumulative doses gradually increase in TNBC patients, and so does the risk of cardiac adverse events [7, 8]. At present, the widely accepted consensus is that although cardiac damage may appear as early as the first dose, the risk of adverse events increases with increasing cumulative doses; when the cumulative dose of doxorubicin (ADM) reaches 250 mg/m2 or that of epirubicin (EPI) reaches 600 mg/m2, the risk of adverse events is significantly elevated [7–9]. In addition to anthracyclines, trastuzumab, tyrosine kinase inhibitors (e.g., lapatinib), antimetabolite drugs (e.g., 5-fluorouracil [5-FU], capecitabine), cyclophosphamides, and antimicrotubule agents (e.g., docetaxel, paclitaxel) can cause mild or severe cardiac damage via different pathophysiological mechanisms [7, 8].
Many mechanistic studies have been conducted, and hypotheses have been proposed about the cardiotoxicity of antineoplastic drugs; free iron-based or radical-induced oxidative stress [4] and dysregulation of autophagy [10] have been investigated. Autophagy has dual functions and plays important roles in homeostasis, and thus the regulation of autophagy is very important. In the heart, autophagy may play a role in adaptation to hemodynamic stress and the prevention of age-dependent dysfunction, but it can also cause cardiac hypertrophy [11]. Autophagy induced by drugs, especially chemotherapy agents, is a new area of study. Most studies concluded that anthracyclines up-regulate cardiac autophagy [10], whereas trastuzumab down-regulates autophagy [12]. Cardiac adverse events, especially type I cardiomyopathy caused by some antineoplastic drugs, is often progressive and irreversible [13]; therefore, early detection and prevention are of great importance. Early-onset cardiac damage may result in arrhythmia, a reduction in left ventricular ejection fraction (LVEF; a significant decrease corresponds to > 10% drop) and electrocardiographic changes [7–9]. Therefore, because of the advantages of the techniques, it is necessary to perform electrocardiography (ECG) and echocardiography (UCG) regularly to monitor acute cardiotoxicity; however, there are still many mysteries about the relationship between electrocardiographic or echocardiographic changes and cardiotoxicity, as well as their associations with prognosis [7, 8].
Close attention must be paid to cardiotoxicity of chemotherapy. In addition to performing early diagnosis, we are interested to establish a prognostic model of cardiac events at the molecular level. Although several single nucleotide polymorphisms (SNPs) have been used to evaluate the associations between genetics and cardiac events in cancer patients, relevant studies have usually been performed in patients on anthracyclines [14]. Based on previous studies and databases, we selected 25 candidate SNPs to determine their relationships with abnormalities on ECG or UCG records during chemotherapy. The purpose of this study was to investigate cardiac events caused by neoadjuvant or adjuvant chemotherapy in TNBC patients and to determine the association between abnormalities on ECG or UCG records and certain autophagy-related SNPs.
Materials and methods
Study subjects
We reviewed the electronic records of breast cancer patients treated at the Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China) between January 2008 and December 2015. The patient selection criteria were as follows: (1) volunteered to participate in this study; (2) female patients; (3) pathologically diagnosed with stage I–III TNBC; (4) complete clinicopathological data including age, tumor size, TNM stage, pathological type, adjuvant chemotherapy, past medical history, family history, smoking and drinking history; (5) available reports of ECG and/or UCG; and (6) sufficient blood samples for SNP genotyping. Exclusion criteria were as follows: (1) symptomatic heart failure; (2) acute phase of coronary heart disease; and (3) previous history of fatal arrhythmia. We followed all patients until August 6, 2017. This study was approved by the Institutional Review Boards of the Cancer Hospital, Chinese Academy of Medical Sciences (No. CH-BC-019).
Breast cancer subtype definition
Estrogen and progesterone receptor statuses were evaluated based on immunohistochemistry (IHC) of formalin-fixed, paraffin-embedded breast cancer tissue samples obtained from the patients. IHC was performed with anti-estrogen receptor and anti-progesterone receptor antibodies. A positive estrogen or progesterone receptor status was defined by nuclear staining of more than 1% cells based on the guidelines of American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) released in 2010. To determine the human epidermal growth factor 2 (HER2) status, IHC was performed or gene amplification was determined using fluorescence in situ hybridization (FISH). Tumors are classified as HER2-positive if scored as 3+ for HER2 in ≥ 10% tumor cells as demonstrated by IHC or if gene amplification is demonstrated by FISH. Tumors negative for estrogen and progesterone receptors and HER2 were defined as TNBCs.
Exposure dose of chemotherapeutic drugs
All TNBC patients received adjuvant/neoadjuvant treatment. The chemotherapy regimens were as follows: (1) the EC regimen [EPI 90 mg/m2 or pirarubicin (THP) 40–50 mg/m2 on day 1 and cyclophosphamide (CTX) 600 mg/m2 on day 1, repeated every 21 days for 4–6 cycles]; (2) the EC-T regimen [EPI 90 mg/m2 and CTX 600 mg/m2 on day 1, repeated every 14 or 21 days for 4 cycles, followed by docetaxel (DTX) 75 mg/m2 on day 1, repeated every 21 days for 4 cycles, or paclitaxel (PTX) 175 mg/m2 on day 1, repeated every 14 or 21 days for 4 cycles]; (3) the ET regimen (EPI 75 mg/m2 or THP 40–50 mg/m2 on day 1 and DTX 75 mg/m2 or PTX 175 mg/m2 on day 2, repeated every 21 days for 6 cycles); (4) the TAC regimen (EPI 75 mg/m2 or THP 40–50 mg/m2, CTX 500 mg/m2, and PTX 175 mg/m2 or DTX 75 mg/m2 on day 1, repeated every 21 days for 6 cycles); (5) the CAF regimen [CTX 500 mg/m2 on day 1, EPI 75 mg/m2 or THP 40–50 mg/m2 or ADM 50 mg/m2 on day 1, 5-fluorouracil (5-FU) 500 mg/m2 on days 1 and 8, repeated every 21 days for 6 cycles]; (6) the TC regimen (DTX 75 mg/m2 or PTX 175 mg/m2 and CTX 600 mg/m2 on day 1, repeated every 21 days for 4 cycles); (7) the carboplatin-taxane regimen [DTX 75 mg/m2 or PTX 175 mg/m2 on day 1, and carboplatin (CAPE) area under receiver-operating curve (AUC) = 5 mg/mL on day 2, repeated every 21 days for 6 cycles].
The EC and CAF regimens were classified as anthracycline-based regimens; the TC regimen was a taxane-based regimen; the EC-T, ET, and TAC regimens were classified as anthracycline–taxane-based regimens.
Electrocardiography and echocardiography
Standard 12-lead ECG was performed before chemotherapy and after most chemotherapy cycles. As we mainly study early-onset cardiac events, we chose to analyze ECG records after chemotherapy. Abnormal electrocardiogram refers to the presence of new abnormalities after any chemotherapy cycle compared with baseline electrocardiogram, regardless of whether the abnormality disappeared during the subsequent chemotherapy cycle. For patients with abnormal electrocardiograms, UCG was performed according to clinical needs. All ECG and UCG records were sent to Fuwai Hospital and re-interpreted by specialists at the Department of Cardiology. The following parameters were analyzed: heart rate (HR), PR interval, QRS duration, and QT(c) interval. We classified cardiac events by ST-T segment abnormalities, elevated myocardial enzymes, arrhythmia, and QRS pattern or duration abnormalities. To clarify the relationship between treatment duration and ECG abnormalities, we defined early abnormalities as those appearing before four cycles of chemotherapy.
Candidate SNP selection and genotyping
In total, 25 SNPs related to autophagy were selected according to the National Center for Biotechnology Information (NCBI) SNP database (https://www.ncbi.nlm.nih.gov/snp/) and the Catalog of Somatic Mutations in Cancer (COSMIC) database (http://cancer.sanger.ac.uk/cosmic). The final candidate SNPs were ataxia telangiectasia mutated (ATM) (rs1003623, rs227060, rs228589, rs664143, and rs664677), autophagy-related (ATG)5 (rs473543 and rs3761796), ATG7 (rs2594971, rs111595248, and rs4684789), ATG12 (rs1058600 and rs5870670), ATG13 (rs13448 and rs10838611), microtubule-associated protein 1 light chain (MAP1LC)-3A (rs4911429 and rs6088521), MAP1LC-3B (rs9903, rs35227715, rs7865, and rs16944733), caspase 3 (CASP3) (rs1049216, rs12108497, and rs2720376), crystallin alpha B (CRYAB) (rs14133), and stathmin 1 (STMN1) (rs182455).
Genomic DNA was extracted from a 1- to 2-mL blood sample that was collected from each patient upon recruitment using a blood DNA kit (BioTeKe Corporation, Beijing, China). A MassARRAY MALDI-TOF System (Sequenom Inc., San Diego, CA, USA) was used to genotype candidate SNPs according to the protocol. The PCR primers and probes (forward 5′-ACGTTGGATGAGTTTCCTCGCTCCTGTTTC-3′ and reverse 5′-ACGTTGGATGCTCTCTCTCTGGATCTGCTC for ATG13 rs10838611; see other probes in Additional file 1: Table S1) were designed according to Assay design 3.1 (Sequenom Inc.) and synthesized by the Beijing Genomics Institute (Beijing, China).
Purified primer extension reaction products were dispensed onto a 384-well Spectro CHIP bioarray using a MassARRAY Nanodispenser RS1000 (Sequenom Inc.) and determined using a matrix-assisted laser desorption/ionization time-off light mass spectrometer. Genotype analysis was performed through the MassARRAY Typer software version 4.0 (Sequenom Inc.). Duplicate samples and negative controls (without DNA) were used for quality control of genotyping. Concordance for duplicate samples was 100% for all assays. The group information of each sample was concealed for genotyping analysis.
Statistical analyses
We used paired-sample T tests to compare the differences in HR, PR interval, QRS duration, and QT(c) interval before and after chemotherapy. Chi squared test (Pearson’s χ2 test) or binary logistic regression was employed to determine the relationship among abnormalities on ECG or UCG records, demographic characteristics, and drugs used. Continuous correction of the Chi squared test was used when necessary. The Hardy–Weinberg equilibrium test was performed to validate the genotype distributions of each SNP using the χ2 test. The association between abnormalities on ECG or UCG records and genotype distributions of SNPs was estimated by calculating odds ratios (ORs) and their 95% confidence intervals (95% CIs) with multivariate logistic regression analysis. A P value of less than 0.05 was considered statistically significant, and all statistical tests were two-sided. All analyses were performed with SPSS 19.0 (IBM Inc., Chicago, IL, USA) software.
Results
Patient characteristics
From a total of 2450 stage I–III TNBC patients, 409 signed consent forms to participate in the study. Furthermore, 147 TNBC patients had relatively complete ECG records and were finally enrolled (Fig. 1). All patients were Han Chinese. The median age of the patients was 51 (range 30–75) years. The basic characteristics of patients with or without electrocardiographic changes are presented in Table 1. Among the 147 TNBC patients, only 46 (31.3%) had normal ECG records after each chemotherapy cycle. For the 16 patients who underwent UCG, 2 (12.5%) had a reversible decrease of more than 10% in LVEF with no cardiovascular symptoms. The rate of anthracycline administration was significantly lower in patients with normal ECG than in those with abnormal ECG records (47.8% vs. 66.3%, P = 0.033). The rate of excessive alcohol consumption (corresponding to more than 10 g of ethanol per day for females [15]) in the normal ECG group was also significantly lower than that in the abnormal ECG group (2.2% vs. 15.8%, P = 0.034). The differences in hypertension and diabetes, potential risk factors for abnormal ECG, were not statistically significant because of the relatively small number of patients.
Fig. 1.
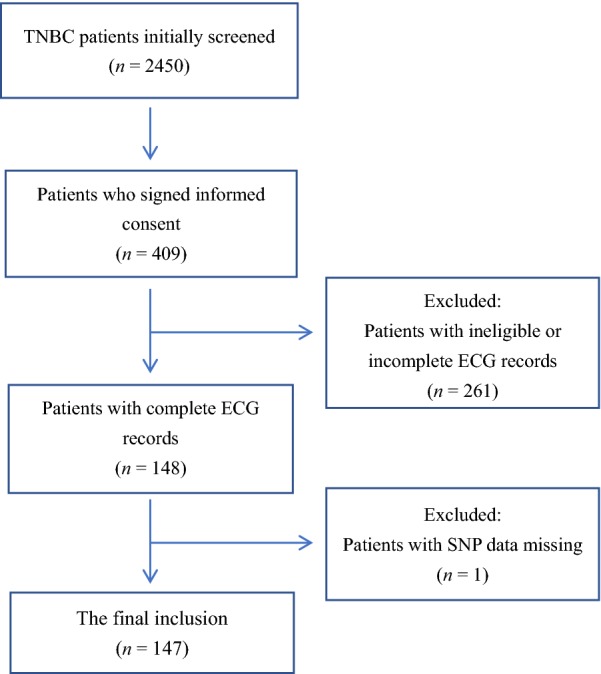
Flowchart of TNBC patient enrollment for the SNP test. TNBC triple-negative breast cancer, ECG electrocardiography, SNPs single nucleotide polymorphisms
Table 1.
Clinicopathological characteristics of 147 triple-negative breast cancer (TNBC) patients with normal or abnormal electrocardiography (ECG)
| Characteristic | Normal ECG group | Abnormal ECG group | P value |
|---|---|---|---|
| Total [cases (%)] | 46 (31.3) | 101 (68.7) | – |
| Age [years, median (range)] | 51 (30–75) | 50 (32–72) | 0.731 |
| Baseline heart rate [bpm, mean ± SD] | 75.1 ± 10.7 | 75.3 ± 11.7 | 0.931 |
| Disease-free survival [months, median (range)] | 64.3 (7.7–183.2) | 58.6 (3.6–181.7) | 0.473 |
| Hypertension [cases (%)] | 5 (10.9) | 17 (16.8) | 0.490 |
| Diabetes [cases (%)] | 1 (2.2) | 7 (6.9) | 0.250 |
| Smoking [cases (%)] | 2 (4.3) | 5 (5.0) | 0.874 |
| Excessive alcohol consumption [cases (%)] | 1 (2.2) | 16 (15.8) | 0.034 |
| Family history of heart disease [cases (%)] | 4 (8.7) | 7 (6.9) | 0.969 |
| Chemotherapy [cases (%)] | 1.000 | ||
| Neoadjuvant | 3 (6.5) | 5 (5.0) | |
| Adjuvant | 43 (93.5) | 96 (95.0) | |
| Drug uses [cases (%)] | |||
| Anthracyclines | 22 (47.8) | 67 (66.3) | 0.033 |
| Cyclophosphamide | 24 (52.2) | 57 (56.4) | 0.630 |
| Paclitaxel | 20 (43.5) | 42 (41.6) | 0.829 |
| Docetaxel | 19 (41.3) | 40 (39.6) | 0.845 |
| Carboplatin | 16 (34.8) | 27 (26.7) | 0.320 |
| 5-Fluorouracil | 1 (2.2) | 4 (4.0) | 0.949 |
| Chemotherapy regimens [cases (%)] | 0.242 | ||
| Anthracycline-based | 6 (13.0) | 16 (15.8) | |
| Taxane-based | 23 (50.0) | 33 (32.7) | |
| Anthracycline–taxane-based | 16 (34.8) | 50 (49.5) | |
| Others | 1 (2.2) | 2 (2.0) | |
SD standard deviation
Parametric analysis of ECG records
For the 147 TNBC patients, we collected 686 ECG records. We did not include the data after the eighth cycle of chemotherapy in our analysis because of limited data and different ECG recording times. As shown in Fig. 2 and Table 2, the heart rate of patients not only increased with almost every chemotherapy cycle compared with baseline but also showed a gradual increasing trend with the continuation of chemotherapy. In patients who were treated with anthracyclines or cyclophosphamide, the heart rate was significantly increased after every cycle, whereas the increases were not significant in patients who were treated with paclitaxel, docetaxel, or carboplatin. However, no meaningful positive association was found regarding PR interval, QRS duration, and QT(c) interval (data not shown).
Fig. 2.
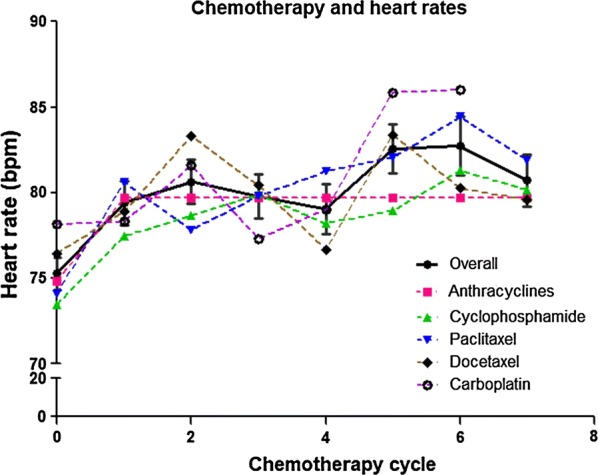
The association between heart rate changes in TNBC patients and chemotherapy drugs. No matter what the chemotherapy drugs are, the heart rate of the patients is increased with nearly every chemotherapy cycle compared with baseline. TNBC triple-negative breast cancer
Table 2.
Heart rates of TNBC patients after cycles of chemotherapy
| Regimen | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 | Cycle 7 | |
| Overall | ||||||||
| Cases (%) | 142 (96.6) | 85 (57.8) | 90 (61.2) | 87 (59.2) | 85 (57.8) | 73 (49.7) | 60 (40.8) | 34 (23.1) |
| Heart rate (bpm, mean ± SD) | 75.3 ± 11.4 | 79.4 ± 11.8 | 80.6 ± 12.3 | 79.8 ± 12.2 | 79.0 ± 13.4 | 82.6 ± 12.2 | 82.7 ± 13.3 | 80.7 ± 12.0 |
| P value | Ref | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.003 |
| Anthracyclines | ||||||||
| Cases (%) | 87 (97.8) | 57 (64.0) | 58 (65.2) | 56 (62.9) | 54 (60.7) | 45 (50.6) | 41 (46.1) | 33 (37.1) |
| Heart rate (bpm, mean ± SD) | 74.8 ± 11.1 | 79.7 ± 11.7 | 80.1 ± 11.5 | 80.5 ± 11.8 | 79.7 ± 14.9 | 81.4 ± 11.3 | 82.7 ± 12.2 | 80.7 ± 12.1 |
| P value | Ref | 0.001 | < 0.001 | < 0.001 | 0.008 | < 0.001 | < 0.001 | 0.004 |
| Cyclophosphamide | ||||||||
| Cases (%) | 80 (98.8) | 50 (61.7) | 49 (60.5) | 49 (60.5) | 44 (54.3) | 36 (44.4) | 39 (48.1) | 30 (37.0) |
| Heart rate (bpm, mean ± SD) | 73.5 ± 11.2 | 77.4 ± 12.0 | 78.7 ± 12.7 | 79.9 ± 13.1 | 78.2 ± 10.1 | 78.9 ± 11.8 | 81.3 ± 13.5 | 80.2 ± 12.1 |
| P value | Ref | 0.004 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.001 |
| Paclitaxel | ||||||||
| Cases (%) | 60 (96.8) | 37 (59.7) | 39 (62.9) | 39 (62.9) | 43 (69.4) | 28 (45.2) | 28 (45.2) | 18 (29.0) |
| Heart rate (bpm, mean ± SD) | 74.1 ± 10.0 | 80.6 ± 12.1 | 77.8 ± 10.9 | 79.8 ± 10.5 | 81.2 ± 9.0 | 82.0 ± 10.5 | 84.4 ± 14.2 | 81.9 ± 12.7 |
| P value | Ref | < 0.001 | < 0.001 | 0.002 | < 0.001 | < 0.001 | 0.002 | 0.086 |
| Docetaxel | ||||||||
| Cases (%) | 56 (94.9) | 35 (59.3) | 39 (66.1) | 36 (61.0) | 34 (57.6) | 36 (61.0) | 24 (40.7) | 14 (23.7) |
| Heart rate (bpm, mean ± SD) | 76.4 ± 13.2 | 78.9 ± 12.3 | 83.3 ± 14.0 | 80.4 ± 14.9 | 79.0 ± 12.2 | 83.4 ± 13.9 | 80.3 ± 13.5 | 79.6 ± 12.2 |
| P value | Ref | 0.137 | 0.001 | 0.037 | 0.036 | 0.004 | 0.231 | 0.004 |
| Carboplatin | ||||||||
| Cases (%) | 40 (93.0) | 20 (46.51) | 25 (58.1) | 23 (53.5) | 25 (58.1) | 26 (60.5) | 14 (32.6) | NA |
| Heart rate (bpm, mean ± SD) | 76.1 ± 11.6 | 78.3 ± 9.2 | 81.6 ± 11.3 | 77.3 ± 9.9 | 79.0 ± 9.1 | 85.8 ± 11.9 | 86.0 ± 14.5 | NA |
| P value | Ref | 0.442 | 0.023 | 0.690 | 0.034 | 0.001 | 0.157 | NA |
All data are compared with baseline values. NA, not applicable (platinum-based chemotherapy was used for only 4–6 cycles)
Cardiac events
Among the 101 TNBC patients with abnormal ECGs, ST-T segment abnormalities were observed on 45 (44.6%) patients, elevated myocardial enzymes on 4 (4.0%), arrhythmia on 52 (51.5%), and QRS pattern or duration abnormalities on 14 (13.9%). ST-T segment abnormalities included ST segment depression and elevation, as well as flat, inverted, and bidirectional T waves. Fourteen (13.9%) patients had two types of cardiac event at the same time. However, among the 45 patients with ST segment abnormalities, only 4 patients displayed clinically significant changes in ST segments, and their electrocardiograms turned normal after postponing chemotherapy and adding secondary prevention drugs for coronary heart disease. Among the 52 patients with arrhythmia, sinus tachycardia was the most common (37, 71.2%), followed by sinus irregularity (8, 15.4%), sinus bradycardia (4, 7.7%), premature ventricular contraction (3, 5.8%), premature atrial contraction (2, 3.8%), complete right bundle branch block (2, 3.8%), atrial flutter and junctional premature beating (1, 1.9%). Among the 16 patients who underwent UCG, 2 (12.5%) had a significant reversible decrease of more than 10% in LVEF. The LVEF of the 2 patients became greater than 45% after treatment for heart failure, and the subsequent treatment was administered as scheduled.
The use of anthracyclines was associated with QRS duration abnormalities (P < 0.05), whereas the use of CBP was associated with reduced frequencies of ST-T segment abnormalities (P < 0.05) (Table 3). Anthracycline-based regimens were associated with ST-T segment abnormalities (P < 0.05), whereas taxane-based regimens were associated with QRS duration abnormalities (P < 0.05) (Table 4). However, in an effort to explore the timing of cardiac events, there was no difference in cardiac events during or after the first four cycles.
Table 3.
Relationships between ECG abnormalities and chemotherapeutic drugs
| Drug | Total (cases) | ST-T segment abnormalities | Elevated myocardial enzymes | Arrhythmia | QRS pattern or duration abnormalities | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of events [cases (%)] | χ2 | P value | No. of events [cases (%)] | χ2 | P value | No. of events [cases (%)] | χ2 | P value | No. of events [cases (%)] | χ2 | P value | ||
| Anthracyclines | 89 | 32 (36.0) | 3.031 | 0.082 | 3 (3.4) | 0.007 | 0.935 | 30 (33.7) | 0.274 | 0.601 | 12 (13.5) | 4.104 | 0.043 |
| Cyclophosphamide | 81 | 27 (33.3) | 0.629 | 0.428 | 2 (2.5) | 0.000 | 1.000 | 28 (34.6) | 0.051 | 0.821 | 10 (12.3) | 1.667 | 0.197 |
| Paclitaxel | 62 | 18 (29.0) | 0.126 | 0.723 | 2 (3.2) | 0.000 | 1.000 | 20 (32.3) | 0.455 | 0.500 | 6 (9.7) | 0.003 | 0.957 |
| Docetaxel | 59 | 13 (22.0) | 3.414 | 0.065 | 2 (3.4) | 0.000 | 1.000 | 25 (42.4) | 2.112 | 0.146 | 4 (6.8) | 0.411 | 0.521 |
| Carboplatin | 43 | 8 (18.6) | 4.126 | 0.042 | 2 (2.3) | 0.135 | 0.713 | 16 (37.2) | 0.090 | 0.765 | 2 (4.6) | 0.971 | 0.324 |
Table 4.
Relationships between ECG abnormalities and chemotherapy regimens
| Chemotherapy regimen | Total (cases) | ST-T segment abnormalities | Elevated myocardial enzymes | Arrhythmia | QRS pattern or duration abnormalities | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of events [cases (%)] | χ2 | P value | No. of events [cases (%)] | χ2 | P value | No. of events [cases (%)] | χ2 | P value | No. of events [cases (%)] | χ2 | P value | ||
| Anthracycline-based | 22 | 12 (54.5) | 6.977 | 0.008 | 0 (0) | 0.020 | 0.889 | 4 (18.2) | 2.519 | 0.112 | 4 (18.2) | 1.224 | 0.269 |
| Taxane-based | 56 | 13 (23.2) | 2.331 | 0.127 | 1 (1.8) | 0.001 | 0.980 | 21 (37.5) | 0.179 | 0.672 | 2 (3.6) | 2.687 | 0.001 |
| Anthracycline–taxane-based | 66 | 19 (28.8) | 0.188 | 0.665 | 3 (4.5) | 0.515 | 0.430 | 25 (37.9) | 0.329 | 0.566 | 8 (12.1) | 0.938 | 0.333 |
| Others | 3 | 1 (33.3) | 0.000 | 1.000 | 0 | 0.000 | 1.000 | 2 (66.7) | 0.287 | 0.592 | 0 | 0.000 | 1.000 |
Autophagy-related SNPs and ECG abnormalities
All selected patients underwent SNP test. According to previous studies, we selected 25 SNPs associated with autophagy.
The proportion of patients with ECG abnormalities was different among the three ATG13 rs10838611 genotype groups (Fig. 3). The G allele of ATG13 rs10838611 was significantly associated with ECG abnormalities (OR = 2.258, 95% CI = 1.318–3.869; P < 0.01), indicating an increased risk of cardiac events. However, the other SNPs did not display significant associations with ECG abnormalities (Table 5).
Fig. 3.
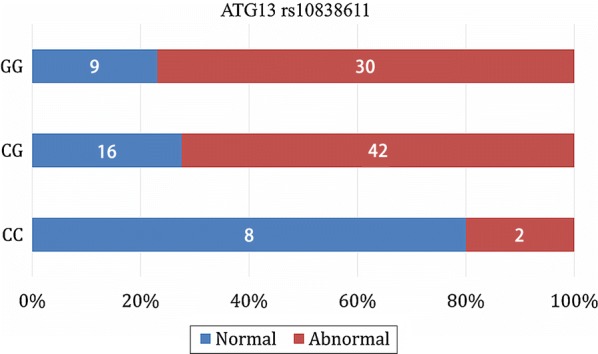
Association between ATG13 rs10838611 genotype and ECG abnormalities in TNBC patients. Patients with GG genotype of ATG13 rs10838611 had significantly higher rate of ECG abnormalities than did those with CC or CG genotype (odds ratio [OR] = 2.258, 95% confidence interval (CI) = 1.318–3.869; P = 0.003)
Table 5.
Associations of 25 SNPs of 10 genes with ECG abnormalities
| Gene | SNP | Nucleotide change | MAF | HWE P value | χ 2 | Association P value |
|---|---|---|---|---|---|---|
| ATM | rs1003623 | C/T | 0.49 | 0.00 | 1.57 | 0.46 |
| rs227060 | C/T | 0.32 | 0.50 | 0.85 | 0.65 | |
| rs228589 | A/T | 0.46 | 0.00 | 0.82 | 0.57 | |
| rs664143 | C/T | 0.37 | 0.93 | 0.20 | 0.91 | |
| rs664677 | A/C/T | 0.35 | 0.23 | 0.95 | 0.62 | |
| ATG5 | rs473543 | C/T | 0.39 | 0.51 | 1.42 | 0.49 |
| rs3761796 | A/G | 0.07 | 0.25 | 2.06 | 0.36 | |
| ATG7 | rs2594971 | C/T | 0.50 | 0.01 | 0.37 | 0.83 |
| rs111595248 | C/T | 0.05 | 0.48 | 0.33 | 0.56 | |
| rs4684789 | G/T | 0.42 | 0.34 | 0.50 | 0.78 | |
| ATG12 | rs1058600 | A/G | 0.42 | 0.96 | 1.77 | 0.41 |
| rs5870670 | Del/T | 0.39 | – | 1.37 | 0.24 | |
| STMN1 | rs182455 | C/T | 0.36 | 0.35 | 2.84 | 0.24 |
| ATG13 | rs13448 | C/T | 0.40 | 0.20 | 3.25 | 0.20 |
| rs10838611 | C/G/T | 0.38 | 0.08 | 20.19 | 0.00 | |
| MAP1LC3A | rs4911429 | A/G | 0.36 | 0.75 | 1.17 | 0.56 |
| rs6088521 | A/C/G/T | 0.35 | 0.43 | 0.67 | 0.72 | |
| MAP1LC3B | rs9903 | C/T | 0.19 | 0.38 | 0.55 | 0.76 |
| rs35227715 | C/G | 0.25 | 0.12 | 0.53 | 0.77 | |
| rs7865 | C/G/T | 0.31 | 0.00 | 0.57 | 0.45 | |
| rs16944733 | A/C/T | 0.20 | 0.00 | 1.58 | 0.45 | |
| CASP3 | rs1049216 | C/T | 0.40 | 0.58 | 1.75 | 0.42 |
| rs12108497 | C/G/T | 0.39 | 0.25 | 0.97 | 0.61 | |
| rs2720376 | C/T | 0.46 | 0.25 | 0.46 | 0.79 | |
| CRYAB | rs14133 | C/G | 0.24 | 0.05 | 6.31 | 0.10 |
HWE Hardy–Weinberg equilibrium, MAF minor allele frequency, ATM ataxia telangiectasia mutated, ATG autophagy-related, MAP1LC microtubule-associated protein 1 light chain, CASP caspase, CRYAB crystallin alpha B, STMN1 stathmin 1
Because of the differences in the mechanisms of cardiotoxicity of various chemotherapy drugs, we performed subgroup analysis to determine their relationships with autophagy-related SNPs. With simple data analysis, SNP genotype differences in MAP1LC3B (rs7865 and rs9903) in the anthracyclines subgroup, ATM (rs1003623) and ATG13 (rs10838611) in the CTX subgroup, MAP1LC3B (rs7865) in the PTX subgroup, and MAP1LC3A (rs6088521) in the DTX subgroup demonstrated potential associations with cardiac toxicity. However, after Hardy–Weinberg equilibrium test and detailed genotypic analysis of each SNP, we considered that these associations were likely false positives. Therefore, no associations between SNPs and chemotherapy drugs were identified.
Discussion
In the present study, we demonstrated that ECG abnormalities were caused by chemotherapy, often asymptomatic, more common than expected. Only 46 (31.3%) patients had normal electrocardiograms after each chemotherapy cycle, consistent with the rate observed by Carver et al. [16]. These data indicate that chemotherapy-induced cardiac adverse events are common and need to be monitored in the real world.
We also found that patient heart rate increased during chemotherapy. High resting heart rate is a known cardiovascular disease risk factor [17] and increases the relative risk of all-cause mortality [18]. The elevated heart rate is another factor illustrating the adverse effects of chemotherapy drugs on the cardiovascular system. Recent studies have demonstrated that a high heart rate would increase the incidence and mortality of cancer [19, 20]. However, the measurement of heart rate is influenced by many factors, and increased heart rate could also be a marker of stress, physical activity, cigarette smoking, blood pressure level, blood viscosity, and plasma level of catecholamines [19, 20]; thus, its clinical predictive value is limited.
All SNPs investigated in the present study have been confirmed to be associated with autophagy. Autophagy has dual functions. Under physiological conditions, autophagy is essential for maintaining normal functions of cells and for removing excess proteins. Under pathological conditions, autophagy can be abnormally activated by stressors of survival and can eventually lead to cell death [10]. Dysregulation of autophagy is one of numerous mechanisms of cardiotoxicity. The present study confirmed the relationship between autophagy-related SNPs and ECG abnormalities and provided new evidence connecting autophagy to cardiac damage caused by chemotherapy.
The only identified SNP associated with ECG abnormalities is located in the ATG13 gene. ATG proteins regulate autophagosome formation. Among the 35 ATG proteins identified in yeast thus far, ATG1–10, 12–14, 16, and 18 comprise the core machinery for membrane formation [11, 21]. In addition to being a core member, ATG13 functions as a regulator of other ATG family members, such as ATG1, and thus plays an important role in autophagy [21]. However, at present, there are no studies on the relationship between chemotherapy-induced cardiac events and the ATG family. The present study demonstrated the association between cardiac adverse events and autophagy-related SNPs, providing not only evidence for the involvement of autophagy in the cardiotoxicity of chemotherapy but also ideas for subsequent research.
In previous studies, many SNPs, such as retinoic acid receptor gamma (RARG) rs2229774 [22], UDP glucuronosyltransferase family 1 member A6 (UGT1A6) rs6759892, spastic paraplegia 7 (SPG7) rs2019604 [14, 23], and solute carrier family 28 member 3 (SLC28A3) rs7853758 [23], have been associated with cardiotoxicity. As increasing SNPs related to the cardiotoxicity of chemotherapy are discovered, along with the novel SNP found in the present study, we may be able to establish a model to predict the susceptibility of cancer patients to cardiac events and help guide clinical treatment.
Hardy–Weinberg equilibrium (HWE), also known as the law of genetic equilibrium, is the most important principle in population genetics. In the present study, we found that the HWE P values of several SNP loci were < 0.05. One possible cause may be that the study participants were breast cancer patients rather than healthy individuals. In addition, the relatively small sample size may also lead to this phenomenon.
Nevertheless, several limitations existed in the present study. First, there might be an inherent selection bias because all patients were from single hospital. However, at the National Cancer Center, patients come from all over China, which may minimize this bias. Second, UCG was performed according to clinical needs rather than a regular requirement. However, most of the recruited patients were at early stages, and the cumulative dose of chemotherapy drugs was low, which may have resulted in a lack of significant cardiotoxicity and thus a low number of UCG records collected [7, 8]. Third, the ECG abnormalities in the present study were mainly non-specific, making the clinical significance of the results less relevant. Last, the relative small sample size might have limited the statistical power of the present study.
Conclusions
Our study not only investigated the occurrence of early cardiac events caused by chemotherapy in TNBC patients but also identified some differences in clinical characteristics and risk factors associated with ECG abnormalities. Upon autophagy-related SNP detection, we found carriers of the G allele of ATG13 rs10838611 to have higher frequencies of cardiac events. Our findings may help clinicians better screen high-risk groups for chemotherapy-induced cardiac events and improve treatment decision for cancer patients.
Additional file
Additional file 1: Table S1. The polymerase chain reaction primers and probes involved.
Authors’ contributions
BL, YZ, FM, and BX conceived of the study and participated in its design. BL and ML collected the blood samples and clinical data. BL, ZY, and CL performed the statistical analyses. TA, YZ, and YW re-interpreted all ECG and UCG records. XG, LL, and XS provided study materials or patients. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and analyzed in the present study are available from the corresponding author upon reasonable request.
Consent for publication
We have obtained consent from the participants to publish and report individual patient data.
Ethics approval and consent to participate
This study was approved by the Institutional Review Boards of the Cancer Hospital, Chinese Academy of Medical Sciences (No. CH-BC-019). All patients voluntarily signed an informed consent form.
Funding
The study was supported by the National Natural Science Foundation of China (No. 81472453).
Abbreviations
- TNBC
triple-negative breast cancer
- ECG
electrocardiography
- UCG
echocardiography
- SNP
single nucleotide polymorphisms
- IHC
immunohistochemistry
- FISH
fluorescence in situ hybridization
- EPI
epirubicin
- PTX
paclitaxel
- DTX
docetaxel
- CTX
cyclophosphamide
- CBP
carboplatin
- 5-FU
5-fluorouracil
- HR
heart rate
- LVEF
left ventricular ejection fraction
Contributor Information
Binliang Liu, Email: liubinliang_onco@163.com.
Tao An, Email: Antrq@126.com.
Meiying Li, Email: axsymay@163.com.
Zongbi Yi, Email: yizongbi@163.com.
Chunxiao Li, Email: campuscx@163.com.
Xiaoying Sun, Email: sunxiaoyingdalian@126.com.
Xiuwen Guan, Email: guanxiuwen7@163.com.
Lixi Li, Email: 13552075722@163.com.
Yanfeng Wang, Email: wangyfh@126.com.
Yuhui Zhang, Email: Yuhuizhangjoy@163.com.
Binghe Xu, Email: xubinghe@medmail.com.cn.
Fei Ma, Phone: 8610-87787652, Email: drmafei@126.com.
Yixin Zeng, Email: Zengyx@sysucc.org.cn.
References
- 1.Truong J, Yan AT, Cramarossa G, Chan KK. Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Can J Cardiol. 2014;30:869–878. doi: 10.1016/j.cjca.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist. 2016;21:1050. doi: 10.1634/theoncologist.2016-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oualla K, Elzawahry HM, Arun B, Reuben JM, Woodward WA, Gamal EH, et al. Novel therapeutic strategies in the treatment of triple-negative breast cancer. Ther Adv Med Oncol. 2017;9:493. doi: 10.1177/1758834017711380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochette Luc, Guenancia Charles, Gudjoncik Aurélie, et al. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. 2015;36:326–348. doi: 10.1016/j.tips.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Jain D, Ahmad T, Cairo M, Aronow W. Cardiotoxicity of cancer chemotherapy: identification, prevention and treatment. Ann Transl Med. 2017;5:348. doi: 10.21037/atm.2017.06.35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Dalen EV, Kremer L. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2005;6:D3917. doi: 10.1002/14651858.CD003917.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines the task force for cancer treatments and cardiovascular toxicity of the European Society of cardiology (ESC) Kardiol Pol. 2016;74:1193. doi: 10.5603/KP.2016.0156. [DOI] [PubMed] [Google Scholar]
- 8.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 9.Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol. 2016;13:172. doi: 10.1038/nrclinonc.2015.171. [DOI] [PubMed] [Google Scholar]
- 10.Dirks-Naylor AJ. The role of autophagy in doxorubicin-induced cardiotoxicity. Life Sci. 2013;93:913–916. doi: 10.1016/j.lfs.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Mohan N, Shen Y, Endo Y, ElZarrad MK, Wu WJ. Trastuzumab, but not pertuzumab, dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol Cancer Ther. 2016;15:1321–1331. doi: 10.1158/1535-7163.MCT-15-0741. [DOI] [PubMed] [Google Scholar]
- 13.Abdulfattah S, Rami A. Management of chemotherapy induced cardiomyopathy. Curr Cardiol Rev. 2011;7:245–249. doi: 10.2174/157340311799960681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magdy T, Burmeister BT, Burridge PW. Validating the pharmacogenomics of chemotherapy-induced cardiotoxicity: what is missing? Pharmacol Therapeut. 2016;168:113–125. doi: 10.1016/j.pharmthera.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castelnuovo AD, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, Gaetano GD. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 16.Carver JR, Szalda D, Ky B. Asymptomatic Cardiac Toxicity in Long-Term Cancer Survivors: defining the population and recommendations for surveillance. Semin Oncol. 2013;40:229–238. doi: 10.1053/j.seminoncol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the chicago heart association detection project in industry. Am J Epidemiol. 1999;149:853–862. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 18.Kristalboneh E, Silber H, Harari G, Froom P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study) Eur Heart J. 2000;21:116. doi: 10.1053/euhj.1999.1741. [DOI] [PubMed] [Google Scholar]
- 19.Tu H, Wen CP, Tsai SP, Chow WH, Wen C, Ye Y, et al. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ. 2018;360:k134. doi: 10.1136/bmj.k134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Kruijsdijk RC, Van DGY, Bemelmans RH, Nathoe HM, Peeters PH, Visseren FL. The relation between resting heart rate and cancer incidence, cancer mortality and all-cause mortality in patients with manifest vascular disease. Cancer Epidemiol. 2014;38:715–721. doi: 10.1016/j.canep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Bio. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 22.Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015;47:1079–1084. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dubé MP, Al-Saloos H, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:1422. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The polymerase chain reaction primers and probes involved.
Data Availability Statement
The datasets used and analyzed in the present study are available from the corresponding author upon reasonable request.


