Acute myocarditis is an uncommon complication of influenza with a high mortality [1]. Early therapy with neuraminidase inhibitors (NI) is recommended in patients hospitalized for influenza, notably those with myocarditis. Oral oseltamivir is generally used as the first line NI therapy, whereas parenteral zanamivir and peramivir represent alternatives in selected patients who might not respond to oseltamivir, as may occur in conditions of gut failure and defective enteral drug absorption [2, 3]. We present two patients with influenza myocarditis complicated by enteral drug malabsorption, who received early intravenous zanamivir therapy with excellent clinical outcomes.
Patient 1 is a 49-year old woman, admitted with hypotension (71/47 mmHg), high hsTroponin T (up to 1570 ng/l), severe cardiac dysfunction (left ventricular ejection fraction (LVEF) of 20%), and positive naso-pharyngeal swabs for influenza B. Patient 2 is a 30-year old woman admitted with hypotension (82/54 mmHg), severe cardiac dysfunction (LVEF of 15%), elevated hsTroponin T (up to 3643 ng/l), and positive swabs for influenza A. Both patients received oral oseltamivir (300 mg daily) and intravenous catecholamines (Fig. 1). In both, extracorporeal life support (ECLS) was started at day 2 for refractory cardiogenic shock. Given clinical deterioration, enteral drug absorption was assessed at day 4 for patient 1 and day 2 for patient 2 by a paracetamol absorption test [4], which measures sequential plasma paracetamol levels after enteral loading (via nasogastric tube) with 1000 mg paracetamol. A marked impairment of enteral drug absorption was identified in both patients, leading to a switch from oral oseltamivir to parenteral zanamivir (patient 1, 600 mg daily, adapted to renal function, from days 5 to 15; patient 2, 1200 mg daily, from days 2 to 12). In the following days, catecholamines were steadily reduced, LVEF progressively improved, and ECLS was weaned (patient 1, day 12; patient 2, day 10). At ICU discharge, LVEF was 55% and 45%, respectively.
Fig. 1.
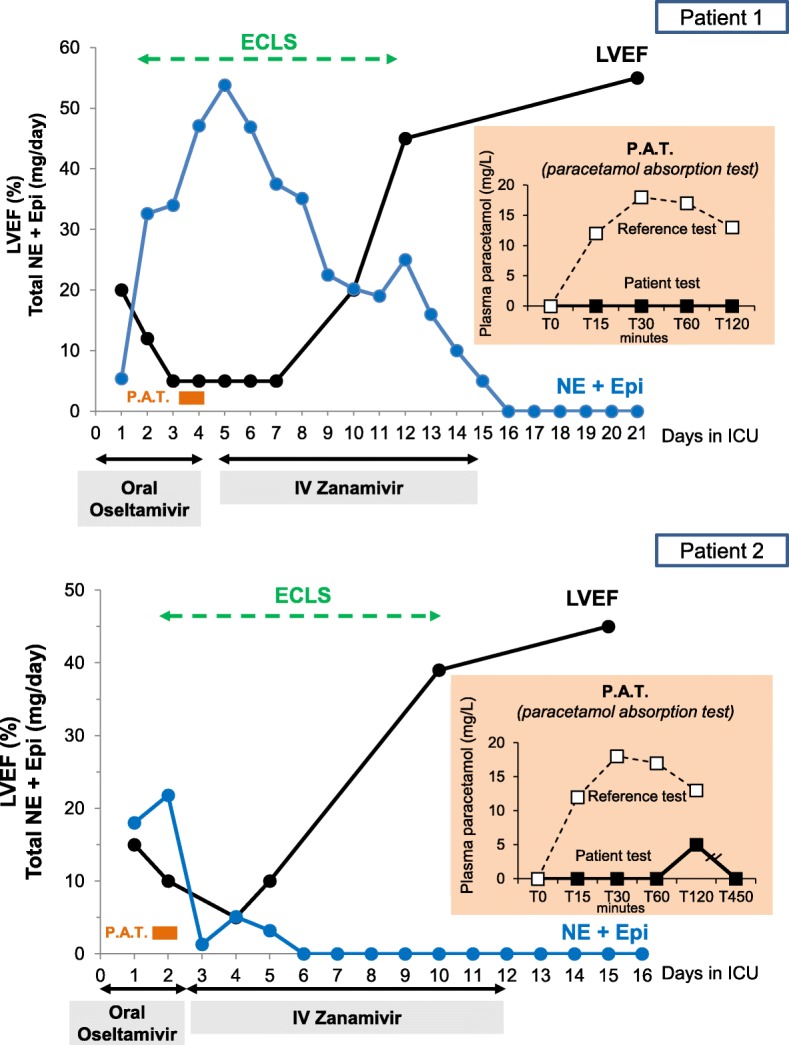
Time-course of LVEF, catecholamines, and ECLS in two patients with fulminant influenza myocarditis. LVEF rapidly increased, allowing weaning of norepinephrine (NE), epinephrine (Epi), and extracorporeal life support (ECLS), after switching from oral oseltamivir to intravenous (IV) zanamivir, following a paracetamol absorption test (P.A.T.) showing enteral drug malabsorption (insert). The reference values for the test were obtained from [4]
In these two patients with influenza myocarditis and refractory cardiogenic shock, the rapid identification of poor drug absorption using a simple paracetamol absorption test allowed the early introduction of parenteral zanamivir instead of oral oseltamivir, with excellent clinical outcomes. Since drug malabsorption is frequent in critically ill patients with circulatory shock [5], we propose that a paracetamol pharmacokinetic study be performed early in patients with influenza myocarditis and cardiogenic shock to avoid any delay in the administration of parenteral therapy if enteral drug malabsorption is demonstrated.
Acknowledgements
None.
Funding
None.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
FPJ, data collection and analysis and writing the manuscript. NBH, data collection and analysis, writing the manuscript, and submission. MK, data collection and analysis and writing the manuscript. AR, data collection and analysis and writing the manuscript. LL, data collection and analysis and writing the manuscript and final revision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Retrospective collection of data. Standard of care.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ukimura A, Satomi H, Ooi Y, Kanzaki Y. Myocarditis associated with influenza A H1N1pdm2009. Influenza Res Treat. 2012;2012:351979. doi: 10.1155/2012/351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marty FM, Vidal-Puigserver J, Clark C, Gupta SK, Merino E, Garot D, et al. Intravenous zanamivir or oral oseltamivir for hospitalised patients with influenza: an international, randomised, double-blind, double-dummy, phase 3 trial. Lancet Respir Med. 2017;5:135–146. doi: 10.1016/S2213-2600(16)30435-0. [DOI] [PubMed] [Google Scholar]
- 3.Marty FM, Man CY, van der Horst C, Francois B, Garot D, Mánez R, et al. Safety and pharmacokinetics of intravenous zanamivir treatment in hospitalized adults with influenza: an open-label, multicenter, single-arm, phase II study. J Infect Dis. 2014;209:542–550. doi: 10.1093/infdis/jit467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger MM, Berger-Gryllaki M, Wiesel PH, Revelly JP, Hurni M, Cayeux C, et al. Intestinal absorption in patients after cardiac surgery. Crit Care Med. 2000;28:2217–2223. doi: 10.1097/00003246-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Roberts DJ, Hall RI. Drug absorption, distribution, metabolism and excretion considerations in critically ill adults. Expert Opin Drug Metab Toxicol. 2013;9:1067–1084. doi: 10.1517/17425255.2013.799137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.


