Abstract
The data presented in this article are related to the research article entitled “An unconventional helical push-pull system for solar cells” (Dova et al., 2019). This article provides: a) the cyclic voltammogram plots in solution of helical push-pull sensitizers and the corresponding precursors; b) the visualization of the leading natural transition orbital (NTO) pairs obtained by theoretical calculation of frontiers orbitals; c) J/V curves of dye-sensitized solar cells (DSSC) sensitized by the dyes, without 3a,7a-dihydroxy-5b-cholic acid (CDCA) as co-adsorbent agent; d) 1H and 13C NMR spectra of dyes.
Specifications table
| Subject area | Chemistry |
| More specific subject area | Organic dyes, solar cells, helicenes |
| Type of data | Synthetic scheme, figures, tables, and graphs |
| How data was acquired | Cyclic voltammograms (AUTOLAB PGSTAT potentiostat, EcoChemie, The Netherlands). |
| Computational data (Gaussian 09 Rev. D.01 program package). | |
| Photovoltaic measurements (Keithley digital source meter). | |
| NMR spectra (Bruker AC-300, Bruker Avance III 400 MHz, Bruker | |
| AMX-500 MHz and Bruker Avance 600 MHz). | |
| Data format | Analyzed |
| Experimental factors | CV scan of dyes and their precursors in solution. |
| Computational data. | |
| Current density-voltage (J/V) scans of dye-sensitized solar cell stained with sensitizers without co-adsorbent agent. | |
| 1H and 13C NMR spectra of dyes. | |
| Experimental features | Electrochemical studies in solution, computational data, and J/V scan of dye-sensitized solar cell |
| Data source location | University of Milano and University of Milano-Bicocca Milano, Italy. |
| Heidelberg University, Heidelberg, Germany | |
| Data accessibility | Data is with this article |
| Related research article | Dova D, Cauteruccio S, Manfredi N, Prager S, Dreuw A, Arnaboldi S, et al. An unconventional helical push-pull system for solar cells. Dyes Pigm. 2019, 161, 382–388. [1] |
Value of the data
-
•
The CV patterns of helical push-pull sensitizers can be used by other researches to compare the redox properties of this helical-based dyes with other push-pull systems.
-
•
Photovoltaic performances of DSSCs sensitized with the two dyes without CDCA could give important information about the efficacy of the helical structure in suppressing the aggregation compared to the data reported in Ref. [1].
-
•
NMR spectra can be used to evaluate the variation of the chemical shift of the helical structure with different substituents.
1. Data
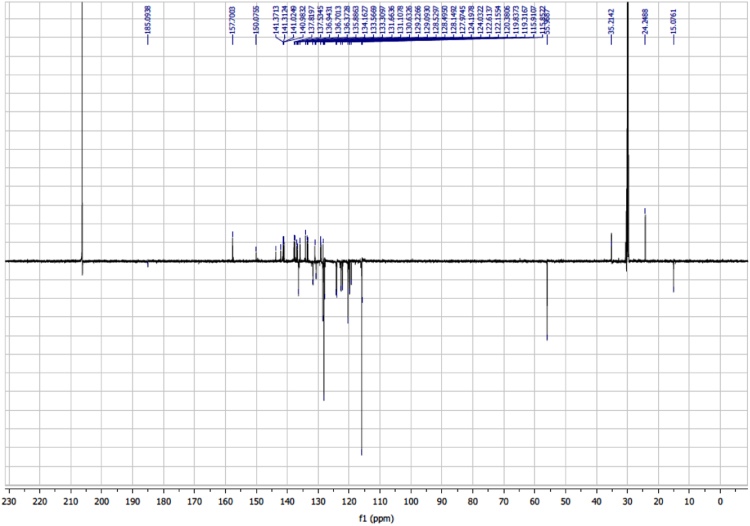
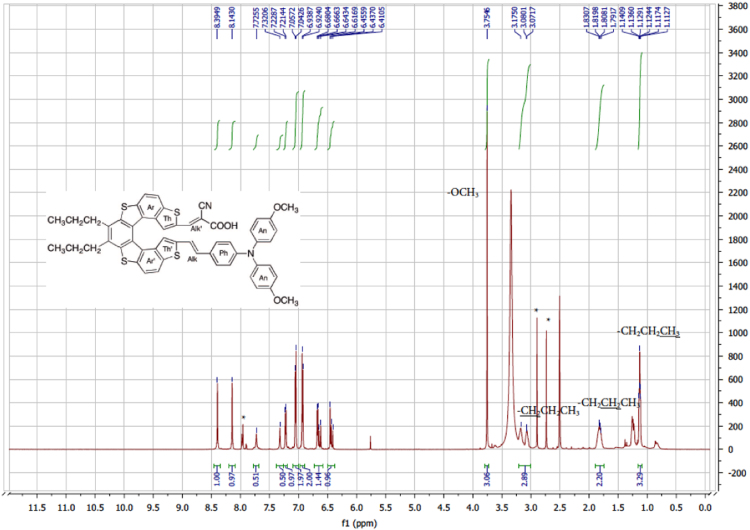
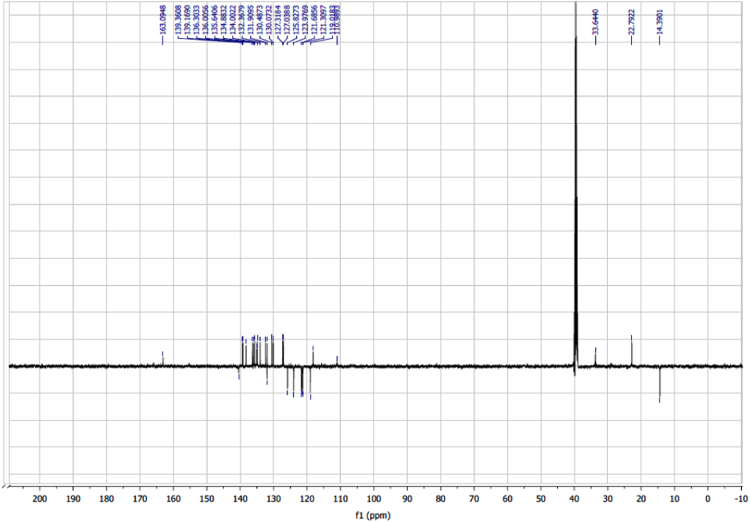
The dataset of this article affords information on the redox properties, photovoltaic performances and NMR spectra of two helical-based push-pull sensitizers. Figs. 1 and 2 show CV patterns of dyes and their corresponding precursors, respectively. Table 1 displays the visualization of the leading NTO pairs. Fig. 3 shows photovoltaic performances of DSSCs sensitized obtained with the two dyes without CDCA. Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13 are related to the 1H-NMR and 13C-NMR spectra of the dyes.
Fig. 1.
CV patterns of dye 5 (on the left) and dye 1 (on the right) recorded at 0.2 V s−1 potential scan rate, on GC electrode in ACN+TBAP 0.1 M.
Fig. 2.
CV patterns of bis(paramethoxyphenyl)phenylamine (on the left) and tetrathiahelicene 7 (on the right) recorded at 0.2 V s−1 potential scan rate, on GC electrode in ACN+TBAP 0.1 M.
Table 1.
Visualization of the leading NTO pairs with contributions of at least 80% of the total excitation. NTO of the hole (left) describing the origin of the excitation and of the electron (right) describing the target. The NTOs are labeled as HONTO and LUNTO for highest occupied natural transition orbital and lowest unoccupied natural transition orbital, respectively. These labels are chosen in analogy to the molecular orbital labelling scheme although it is not fully correct in the case of NTOs.
 |
 |
Fig. 3.
J/V curves of DSSC sensitized by dyes 1 and 5, with different solar intensities.
Fig. 4.
1H NMR (400 MHz, (CD3)2SO) of the precursor of dye 1.
Fig. 5.
1H NMR (400 MHz, (CD3)2SO) of the precursor of dye 1 (aromatic part).
Fig. 6.
13C NMR (150 MHz, (CD3)2CO) of the precursor of dye 1.
Fig. 7.
1H NMR (600 MHz, (CD3)2SO) of dye 1 recorded with some traces of DMF (*) to improve the solubility.
Fig. 8.
1H NMR (600 MHz, (CD3)2SO) of dye 1 (aromatic part).
Fig. 9.
13C NMR (75 MHz, (CD3)2CO) of dye 1.
Fig. 10.
13C NMR (75 MHz, (CD3)2CO) of dye 1 (aromatic part).
Fig. 11.
1H NMR (500 MHz, (CD3)2SO) of dye 5.
Fig. 12.
13C NMR (125 MHz, (CD3)2SO) of dye 5.
Fig. 13.
13C NMR (125 MHz, (CD3)2SO) of dye 5 (aromatic part).
2. Experimental design, materials and methods
All information about the data obtained in electrochemical measurements, computational studies, photovoltaic measurements, and NMR spectra are reported in reference [1].
Acknowledgements
D. D. and S. C. thank the Università degli Studi di Milano for the PhD and post-doctoral fellowship, respectively.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.11.074.
Contributor Information
Silvia Cauteruccio, Email: silvia.cauteruccio@unimi.it.
Norberto Manfredi, Email: norberto.manfredi@unimib.it.
Transparency document. Supplementary material
Supplementary material
Reference
- 1.Dova D., Cauteruccio S., Manfredi N., Prager S., Dreuw A., Arnaboldi S., Mussini P.R., Licandro E., Abbotto A. An unconventional helical push-pull system for solar cells. Dyes Pigm. 2019;161:382–388. doi: 10.1016/j.dib.2018.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material