Abstract
Aims and Objectives:
The colonization of the respiratory pathogens has been found in the oral cavity. In the high-risk patients for lung infection; the risk factor is the high colonization of the respiratory pathogens in the oral cavity. The present study was performed with an aim to investigate the relationship between periodontal health and respiratory diseases.
Material and Methods:
The present cross-sectional study was conducted in the individuals who were attending the outpatient department of dentistry and those who signed the informed consent to participate in the study. A total of 198 patients were included in the present study. Ninety-nine patients with respiratory diseases were included in the test group and 99 patients with normal pulmonary function were added in the control group. Spirometry was used for the confirmation of the lung diseases. We had assessed the clinical parameters such as plaque index, gingival index, loss of attachment, and community periodontal index with the help of SPSS software 15 and compared between cases and controls.
Results:
The mean age of control patients were 35.12 and for respiratory patients was 43.32. There were 65 males and 34 females in case group with respiratory diseases. The controls consisted of 63 males and 36 females. The high scores for various gingival and periodontal indexes in respiratory patients confirmed more periodontal destruction with respect to that group, compared to the nonrespiratory group.
Conclusion:
It is concluded that a strong association between periodontitis and chronic obstructive pulmonary disease was found. The assessment of the risk factors along with patient education regarding the risk should be done so that suitable intervention strategies can be implemented.
KEYWORDS: Chronic obstructive pulmonary disease, periodontitis, respiratory disease, risk factors
INTRODUCTION
There is a high prevalence of the respiratory diseases in the world. They are responsible for the high in the mortality and morbidity rate of the humans. In 1990s, the lower respiratory tract infections (RTI) are considered to be the cause of mortality and the chronic obstructive pulmonary disease (COPD) was at the sixth place.[1] Periodontitis is also a highly prevalent health problem and manifests as a chronic inflammatory reaction to bacterial infections. Periodontitis is a destructive disease that affects the tooth-supporting tissues and ultimately leads to tooth loss in 15% of adults. Over the past decade, intense studies had been carried out to understand the relationship between periodontitis and respiratory disease. This is due to the anatomical continuity between lungs and oral cavity, making the oral cavity as a site of colonization of respiratory pathogens.[2,3,4,5] The recent research has suggested that periodontitis not only causes gingival bleeding but also is a risk factor for systemic inflammation-related chronic diseases, such as cardiovascular disease, diabetes, and COPD. Furthermore, the recent increase of tobacco usage had increased the severity of periodontal disease and chronic respiratory disease. According to the recent research, the systemic condition like pulmonary diseases was affected, and the prognosis was decreased with the presence of periodontal infection.[6,7,8,9,10] The two-way associations between periodontal health systemic diseases are being widely investigated. Respiratory diseases include COPD, asthma, pleural effusion, and tuberculosis. Studies have shown that there lies a strong link between the periodontal health and pulmonary diseases. When there is the aspiration of the bacteria from oropharynx into the lower RTI, it causes the respiratory infection. Hence, good oral hygiene and good periodontal health play an important role in the prevention and treatment of the respiratory diseases. Hence, the present study was done to investigate the correlation between the respiratory diseases and periodontal health.
MATERIALS AND METHODS
The present cross-sectional study was carried in the individuals who were attending the outpatient department of dentistry and who gave informed consent to participate in this study. The ethical clearance was taken from the Ethical Committee of the Institute. ([Letter no: IIDI/11/2017]) A total of 198 patients who attended at the outpatient department were selected for the study. Of the total sample size, 99 patients were diagnosed with the respiratory problem. Ninety-nine patients with respiratory diseases were included in the test group, and 99 patients with normal pulmonary function were added in the control group. The sample size for this study was selected from 1423 population at 95% confidence level and confidence interval of 122.11. The patients of age group ranging from above 15 years and below 65 years were included in the study. The patients who had <15 teeth, the influence of any other systemic diseases, and who have undergone periodontal therapy in the past 6 months were excluded from the study. Patients who met the eligibility criteria, and were willing to participate, signed a consent form.
METHODOLOGY
The following parameters were evaluated Community periodontal index (WHO. 1982), plaque index (Loe modification - Silness and Loe., 1967), gingival index (Loe modification - Loe and Silness., 1967), loss of attachment (LOA), and pocket depth measurement were performed using the WHO probe. Spirometry was used to check the Lung function. Trained and technicians who were certified was asked to perform the spirometric measurements.
STATISTICAL ANALYSIS
Qualitative data will be expressed as percentages and proportions. Standard deviation and mean were used to express the quantitative data. T-test was used to analyze the difference of the continuous variable between the two groups and Chi-squared test was used to analyze the categorical variables. Statistical Package for the Social Sciences (SPSS 15 software) was used to perform the statistical tests. With P < 0.05, it was considered as statistically significant and P < 0.01 was considered as highly statistically significant. The between-group comparison of compressive strength of samples in Group A and B was done using One-way analysis of variance test. Within-group comparison was done using Mann–Whitney test.
RESULTS
A total of 34 females and 65 males were included in the respiratory disease group. The mean age in the control group patients was found to be 35.12 years and that in the patients included in the respiratory patients were found to be 43.32 years. The controls consisted of 63 males and 36 females. The frequency of brushing did not show much difference among the two groups. In both groups, 44.5% of the individuals brushed once daily. In the case group, 44.9% of the individuals brushed twice; and in the control group, 48.5% did the same. The gingival index scores were used to evaluate the gingival status. There was a comparison of the gingival score indices, and the result was compared to be found as statistically significant. The mean value of the gingival score index in the controls was found to be 1.53 and that for cases were found to be 1.89. With P < 0.05 the difference was found to statically significant. It was found that patients with respiratory diseases were found to have superior gingival index as compared to the normal patients with normal pulmonary function [Table 1].
Table 1.
Gingival index

Mean values of cases and controls for plaque index were found to be 2.11 and 1.59 respectively which was significant statistically (P ≤ 0.05). When the comparison of the plaque score was done between the respiratory patients and nondisease patients, it was found to significantly high [Table 2]. The mean values of the controls and cases were found to be 2.20 and 2.26, respectively. The difference was found to be statistically significant (P < 0.05) [Table 3]. The mean values of cases and controls for the corruption perceptions index-LOA (CPI- LOA) were found to be 1.70 and 0.80, and the difference between them was found to be highly significant.
Table 2.
Plaque index

Table 3.
Corruption perceptions index score

The high CPI-LOA scores in respiratory patients confirmed more periodontal destruction with respect to that group, compared to the nonrespiratory group [Table 4].
Table 4.
Corruption perceptions index-loss of attachment score

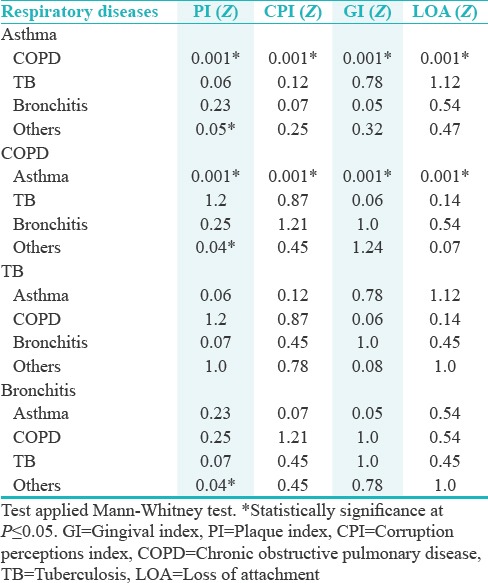
The COPD had significantly highest mean values (P ≤ 0.05) for all the clinical parameters for different respiratory diseases followed by Asthma and various other respiratory diseases [Table 5]. In post hoc analysis performed with Mann–Whitney test for the association between respiratory diseases, it was observed that COPD and Asthma significantly linked to each other while other respiratory diseases had nonsignificant relations [Table 6].
Table 5.
Relationship of respiratory diseases and clinical parameters of periodontal health
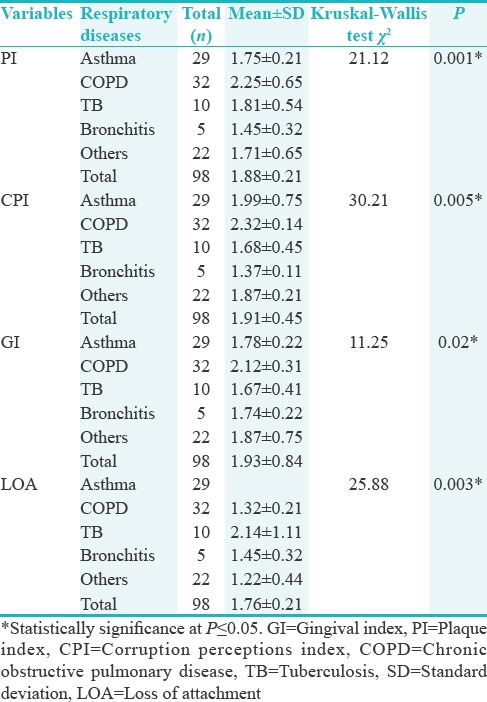
Table 6.
Post hoc analysis of various variables
DISCUSSION
Recent studies have proven that the oral cavity is a sit of colonization of respiratory pathogen. The lack of attention to oral hygiene results in increased mass and complexity of dental plaque which may lead to bacterial interaction, indigenous plaque bacteria, and respiratory pathogen. This result in formation of dental plaque by respiratory pathogen. This plaque may shed into saliva and contaminate the distal portion of respiratory tree on aspiration. Furthermore, the respiratory pathogen in dental plaque is difficult to eradicate.[1] Studies by Oberoi et al. and Chung et al. made the suggestion that a potential association exists between poor oral health and COPD in spite of controlling other confounding factors such as smoking, age, gender, and sex.[3,4]
The aim of the present study was to compare the periodontal status of the respiratory patients and the healthy controls to discover the relationship between the two. When there is the aspiration of the respiratory bacteria from the oropharynx in the lower pulmonary tract, it leads to respiratory problems. The results from the present study showed the significantly higher index in the periodontal index findings, plaque index findings, and the gingival index findings in the test diseases group patients as compared to the control group. This suggested the significant relationship between the pulmonary disease and the periodontal diseases.
In the different longitudinal study done in more than 1100 men, the data showed that there was association of loss of alveolar bone with the increased of COPD. When there was comparison done between the loss of bone, the patients with more severe bone loss were at higher risk of developing the COPDs as compared to the patients with less bone loss.[9,10]
The findings of the present study are in agreement with the Sharma and Shamsuddin study.[11] The study also demonstrates parallel results with that of Wang et al.[12] study which states that poor periodontal health along with oral care were significantly associated with an elevated risk of developing COPD. Similar observations were made by Kowalski et al. in their research.[13]
The present study yielded superior plaque index scores which were in harmony with Mojon et al.[14] study in which they observed that the dentate subjects with a history of RTI had higher plaque score (P = 0.02).[15]
The Third National Health and Nutrition Examination Survey examined the probable role of smoking in the association between these two periodontal health and COPD. The findings recommended that smoking may be a cofactor in the association between periodontal disease and COPD.[16,17,18,19,20,21]
Lot of spotlight was given to evaluate the effects of good periodontal status with systemic status of the patients. Studies have shown that the enhancement of oral health care reduced the hazard of developing aspiration pneumonia and the risk of mortality from aspiration pneumonia directly. A small number of studies also revealed that the sufficient oral health care reduced the amount of virulent respiratory pathogens and suggested a reduction in the risk of aspiration pneumonia by improving the swallowing reflex and cough reflex sensitivity.[22]
Plaque accumulation is clearly an essential initial etiological factor in periodontitis, although the exact mechanisms for the relationship between oral hygiene hypotheses suggest that bacteria in oral cavity may be aspirated along with respiratory pathogens and affect adhesion of the later organisms to the respiratory epithelium, which subsequently cause lung disease. Dental plaque may also provide nutrition to the pathogens in respiratory tract, especially in patients with poor oral hygiene.[23] Periodontal disease may alter environmental conditions to permit mucosal colonization and infection by respiratory pathogens.[24] Aspiration of oral-pharyngeal contents, such as food particles and saliva rich in bacteria, would link periodontitis and respiratory infections.[25] Concentration of bacteria in saliva is very high, and species of bacteria in oral cavity has been found in the lungs of patients with COPD. COPD and Periodontitis are believed to share a similar pathophysiology which ultimately results in the destruction of connective tissue. Such data, if confirmed by future prospective studies with larger sample size, would provide valuable information for the planning of oral health care for patients with COPD. The authors also hope that this study will stimulate and support future function studies to offer new insights into the pathogenic mechanisms underlying the relationship of periodontal health and COPD.
CONCLUSION
It is concluded that a strong association between periodontitis and COPD was found. The assessment of the risk factors along with patient education regarding the risk should be done so that suitable intervention strategies can be implemented. These findings indicate the potential importance of promoting oral hygiene in the prevention and treatment of COPD, although additional studies are needed to clarify the causal relationship between periodontitis and respiratory health and explore the biologic mechanisms underlying the observed association.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Rose LF, Genco RJ, Mealey MF, Cohen DW. Periodontal Medicine. Toronto, Canada: BC Decker Inc; 2000. pp. 83–93. [Google Scholar]
- 2.Moghadam SA, Shirzaiy M, Risbaf S. The associations between periodontitis and respiratory disease. J Nepal Health Res Counc. 2017;15:1–6. doi: 10.3126/jnhrc.v15i1.18023. [DOI] [PubMed] [Google Scholar]
- 3.Oberoi SS, Harish Y, Hiremath S, Puranik M. A cross-sectional survey to study the relationship of periodontal disease with cardiovascular disease, respiratory disease, and diabetes mellitus. J Indian Soc Periodontol. 2016;20:446–52. doi: 10.4103/0972-124X.186946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung JH, Hwang HJ, Kim SH, Kim TH. Associations between periodontitis and chronic obstructive pulmonary disease: The 2010 to 2012 Korean national health and nutrition examination survey. J Periodontol. 2016;87:864–71. doi: 10.1902/jop.2016.150682. [DOI] [PubMed] [Google Scholar]
- 5.Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol. 2017;44:456–62. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- 6.Ryan ME, Carnu O, Kamer A. The influence of diabetes on the periodontal tissues. J Am Dent Assoc. 2003;134(Spec No):34S–40S. doi: 10.14219/jada.archive.2003.0370. [DOI] [PubMed] [Google Scholar]
- 7.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia RI, Nunn ME, Vokonas PS. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol. 2001;6:71–7. doi: 10.1902/annals.2001.6.1.71. [DOI] [PubMed] [Google Scholar]
- 9.Gomes-Filho IS, Soledade-Marques KR, Seixas da Cruz S, de Santana Passos-Soares J, Trindade SC, Souza-Machado A, et al. Does periodontal infection have an effect on severe asthma in adults? J Periodontol. 2014;85:e179–87. doi: 10.1902/jop.2013.130509. [DOI] [PubMed] [Google Scholar]
- 10.Lindhe J, Karring T, Lang NP. Clinical Periodontology and Implant Dentistry. 5th ed. Munksgaard: Oxford Blackwell; 2008. pp. 475–93. [Google Scholar]
- 11.Sharma N, Shamsuddin H. Association between respiratory disease in hospitalized patients and periodontal disease: A cross-sectional study. J Periodontol. 2011;82:1155–60. doi: 10.1902/jop.2011.100582. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Zhou X, Zhang J, Zhang L, Song Y, Hu FB, et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodontol. 2009;36:750–5. doi: 10.1111/j.1600-051X.2009.01448.x. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski M, Kowalska E, Split M, Split W, Wierzbicka-Ferszt A, Pawlicki L, et al. Assessment of periodontal state in patients with chronic obstructive pulmonary disease--Part II. Pol Merkur Lekarski. 2005;19:537–41. [PubMed] [Google Scholar]
- 14.Mojon P, Budtz-Jørgensen E, Michel JP, Limeback H. Oral health and history of respiratory tract infection in frail institutionalised elders. Gerodontology. 1997;14:9–16. doi: 10.1111/j.1741-2358.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: Findings from NHANES III. National health and nutrition examination survey. J Periodontol. 2000;71:743–51. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 16.Tegos TJ, Kalodiki E, Sabetai MM, Nicolaides AN. The genesis of atherosclerosis and risk factors: A review. Angiology. 2001;52:89–98. doi: 10.1177/000331970105200201. [DOI] [PubMed] [Google Scholar]
- 17.Mannino DM. COPD: Epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121:121S–6. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL. Newer risk factors for stroke. Neurology. 2001;57:S31–4. doi: 10.1212/wnl.57.suppl_2.s31. [DOI] [PubMed] [Google Scholar]
- 19.Boden-Albala B, Sacco RL. Lifestyle factors and stroke risk: Exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2:160–6. doi: 10.1007/s11883-000-0111-3. [DOI] [PubMed] [Google Scholar]
- 20.England LJ, Kendrick JS, Gargiullo PM, Zahniser SC, Hannon WH. Measures of maternal tobacco exposure and infant birth weight at term. Am J Epidemiol. 2001;153:954–60. doi: 10.1093/aje/153.10.954. [DOI] [PubMed] [Google Scholar]
- 21.Page RC. The pathobiology of periodontal diseases may affect systemic diseases: Inversion of a paradigm. Ann Periodontol. 1998;3:108–20. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- 22.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: A systematic literature review. Gerodontology. 2013;30:3–9. doi: 10.1111/j.1741-2358.2012.00637.x. [DOI] [PubMed] [Google Scholar]
- 23.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. Bacterial topography of the healthy human lower respiratory tract. MBio. 2017;8:e02287–16. doi: 10.1128/mBio.02287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadiraj S, Nayak R, Choudhary GK, Kudyar N, Spoorthi BR. Periodontal pathogens and respiratory diseases-Evaluating their potential association: A clinical and microbiological study. J Contemp Dent Pract. 2013;14:610–5. doi: 10.5005/jp-journals-10024-1373. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Han J, Liu Z, Song Y, Wang Z, Sun Z, et al. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: A 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41:564–72. doi: 10.1111/jcpe.12247. [DOI] [PubMed] [Google Scholar]


