Abstract
Introduction
We examined the association between cerebrospinal fluid (CSF) biomarkers of Alzheimer's disease, neural novelty responses, and brain volume in predementia old age.
Methods
We conducted a cross-sectional analysis of the observational, multicentric DZNE-Longitudinal Cognitive Impairment and Dementia Study (DELCODE) study. Seventy-six participants completed task functional magnetic resonance imaging and provided CSF (40 cognitively unimpaired, 21 experiencing subjective cognitive decline, and 15 with mild cognitive impairment). We assessed the correlation between CSF biomarkers and whole-brain functional magnetic resonance imaging novelty responses to scene images.
Results
Total tau levels were specifically and negatively associated with novelty responses in the right amygdala and right hippocampus. Mediation analyses showed no evidence that these associations were dependent on the volume of hippocampus/amygdala. No relationship was found between phosphorylated-tau or Aβ42 levels and novelty responses.
Discussion
Our data show that CSF levels of total tau are associated with anatomically specific reductions in novelty processing, which cannot be fully explained by atrophy.
Keywords: Alzheimer's disease (AD), Subjective cognitive decline (SCD), Mild cognitive impairment (MCI), Longitudinal, Cerebrospinal fluid (CSF), Aβ42, Tau, Apolipoprotein E (APOE), Magnetic resonance imaging (MRI), Positron emission tomography (PET)
1. Background
The hallmarks of Alzheimer's disease (AD) pathology are extracellular amyloid aggregates and intracellular accumulation of hyperphosphorylated tau [1], [2]. In the cerebrospinal fluid (CSF), increasing levels of total tau and phospho tau (p-tau) and decreasing levels of Aβ42 provide indirect measures of the magnitude of these pathologies [3], [4], [5], [6], [7], [8]. Although it is well established that amyloid pathology can be associated with synaptic dysfunction [2], tau pathology is widely considered to reflect neurodegeneration [9], [10], [11], [12]. According to this view, CSF Aβ42 levels should be associated with synaptic dysfunction [13] potentially irrespective of atrophy, whereas any relationship between CSF levels of total tau and synaptic function should depend on neuronal damage, potentially indexed by the volume of the underlying brain region. In the recent NIA-AA research framework [12], models hypothesized to explain cognitive dysfunction as a result of biomarker-related pathology all postulate neurodegeneration as a mediator. However, more recent discussions consider the possibility that tau can also be associated with synaptic dysfunction before neurodegeneration has occurred [14].
We first investigated in which brain regions the magnitude of the functional magnetic resonance imaging (fMRI) novelty response for scenes [15] was independently associated with CSF levels of tau and amyloid. We used an unbiased approach considering whole-brain novelty responses, unlike recent studies that focused on the hippocampus and the medial temporal lobe [16]. We then determined whether any association was dependent on the local volume of the underlying region, which we considered a surrogate for structural integrity, hence neurodegeneration.
2. Methods
2.1. Overall study design
The DZNE-Longitudinal Cognitive Impairment and Dementia Study (DELCODE) is an observational longitudinal memory clinic-based multicenter (10 sites) study of the German Center for Neurodegenerative Diseases (DZNE) in Germany. The detailed study design of DELCODE is reported in the study by Jessen et al. [17]. The participants to be enrolled are 400 subjects with subjective cognitive decline (SCD), 200 patients with mild cognitive impairment (MCI), 100 patients with Alzheimer's dementia, 200 control subjects without subjective or objective cognitive decline, and 100 first-degree relatives of patients with a documented diagnosis of Alzheimer's dementia. All patient groups (SCD, MCI, AD) are referrals, including self-referrals while the control group and the relatives of Alzheimer's dementia patients are recruited by standardized public advertisement. The current analysis is based on the first 400 participants of the still ongoing baseline recruitment.
All local institutional review boards and ethical committees approved the study protocol. All participants gave written informed consent before inclusion in the study. DELCODE is retrospectively registered at the German Clinical Trials Register (DRKS00007966), (04/05/2015). Data handling and quality control are reported in the study by Jessen et al. [17].
2.2. Participants
SCD was defined by the presence of subjectively reported decline in cognitive functioning and a test performance above −1.5 standard deviations below the age-, sex-, and education-adjusted normal performance on all subtests of the CERAD (Consortium to Establish a Registry of Alzheimer's Disease) test battery [18]. MCI (amnestic) was defined by an age-, sex- and education-adjusted performance below −1.5 standard deviations on the delayed recall trial of the CERAD word-list episodic memory tests. Both patient groups (SCD and MCI) fulfilled the current research criteria for SCD [18] or MCI [9], respectively. Mild Alzheimer's dementia [19] included a lower cutoff on the Mini–Mental State Examination of ≥18 points. Additional inclusion criteria for all groups were aged ≥60 years, fluent German language skills, capacity to provide informed consent, and presence of a study partner. For exclusion criteria, see the study by Jessen et al. [17]. Other neuropsychological assessments included ADAS-cog 13, FCSRT-IR, WMS-R Logical Memory Story A, WMS-R Digit Span, semantic fluency (animals), the oral form of the Symbol-Digit-Modalities Test, Trail Making Test A and B, Clock Drawing, and Clock Copying [17].
2.3. CSF AD biomarker assessment
CSF AD biomarkers were determined centrally at the Bonn site using commercially available kits according to vendor specifications (V-PLEX Aβ Peptide Panel 1 (6E10) Kit, K15200E and V-PLEX Human Total tau Kit, K151LAE; Meso Scale Diagnostics LLC, Rockville, USA; and Innotest Phospho-Tau(181P), 81581; Fujirebio Germany GmbH, Hannover, Germany) [17].
2.4. MRI acquisition
MRI data were acquired at nine scanning sites, all with Siemens scanners (3 TIM Trio systems, 4 Verio systems, one Skyra, and one Prisma system). For the current report, T1- (3D GRAPPA PAT 2, 1 mm3 isotropic, 256 × 256 px, 192 slices, sagittal, ∼5 min, TR 2500 ms, TE 4.33 ms, TI 110 ms, FA 7°) and T2-weighted (optimized for medial temporal lobe volumetry, 0.5 × 0.5 × 1.5 mm3, 384 × 384 px, 64 slices, orthogonal to the hippocampal long axis, ∼12 min, TR 3500 ms, TE 353 ms) images and a task-fMRI protocol (2D EPI, GRAPPA PAT 2, 3.5 mm3 isotropic, 64 × 64 px, 47 slices, oblique axial/AC-PC aligned, ∼9 min, TR 2580 ms, TE 30 ms, FA 80°, 206 volumes) were used.
For task fMRI, all sites used the same 30″ MR-compatible LCD screen (Medres Optostim) matched for distance, luminance, color and contrast constant across sites, and the same response buttons (CurrentDesign). All participants underwent vision correction with MR-compatible goggles (MediGlasses, Cambridge Research Systems) according to the same standard operating procedures. Standard operating procedures and quality assurance and assessment (QA) were provided and supervised by the DZNE imaging network (iNET, Magdeburg) as described in the study by Jessen et al. [17].
2.5. Task fMRI
During the fMRI session, subjects performed a modified version of a previously published scene novelty and encoding task (“FADE”) [15]. Here, 44 were novel indoor scenes, 44 were novel outdoor scenes, and 44 were repetitions of the prefamiliarized images (one indoor and one outdoor, 22 times each; all 8-bit gray scale, scaled to 1250 × 750 pixel resolution and matched for luminance, viewing horizontal half-angle was 10.05°) were presented (Neurobehavioral Systems Inc.), and subjects had to classify them as either “indoor” or “outdoor” by button press. Stimuli were shown for 2500 ms each, with an optimized jitter for statistical efficiency [20]. 206 functional volumes were recorded with a TR of 2.58 s. The whole task took around 11 minutes. Following a delay of 70 min, recognition memory for the novel 88 images was tested together with 44 entirely new images (22 indoor, 22 outdoor) outside of the MRI scanner (viewing horizontal half-angle was 10.05°). Recognition memory responses were given on a 5-step scale (1: “I am sure that this picture is new”/2: “I think that this picture is new”/3: “I cannot decide if this picture is new or old”/4: “I think I saw this picture before”/5: “I am sure that I did see this image before”).
2.6. MRI analysis
2.6.1. Single subject analyses
After preprocessing (slice time correction, unwarping, realignment, and spatial smoothing [isotropic gaussian kernel of FWHM 6 × 6 × 6 mm]; SPM, version 12; Wellcome Trust Centre for Neuroimaging, London, UK), first-level general linear models were calculated in native space (including 6 motion regressors from the realignment process) using a hemodynamic response function with a 128-second high-pass filter, no global scaling, and no serial correlations were modeled. Prefamiliarized and novel stimuli were used to calculate a “novelty” contrast (“novel vs. old images”).
2.6.2. Group-level analyses
A study-specific group template was calculated using the RF-bias-corrected (N4-ITK [21]) MPRAGE images using ANTs v2.1 [22]. Four rigid-then-affine iterations were followed by six full runs of a nonlinear multi-resolution routine to ensure stable convergence (three resolutions, maximum of 90 iterations, template update step size of 0.1 mm). The resulting SPM contrast images for the “novelty” contrast were warped to the study-specific group template. Co-registration parameters (Mean EPI to MPRAGE) and spatial normalization parameters resulting from the template creation were applied to individual contrast images using ANTs. The normalized contrast images were entered into planned multiple regression analyses as outlined below.
2.6.3. Cortical volume analysis
FreeSurfer 6.0 (http://surfer.nmr.mgh.harvard.edu/) was used to calculate measures for cortical volume and hippocampal segmentations by combining T1- and T2-weighted images using a multispectral analysis algorithm [23].
2.7. Statistical analyses
Behavioral data were analyzed using SPSS 23 (IBM, Armonk, USA). Regression analyses with the fMRI novelty contrast (blood oxygenation level dependent activity difference between novel and repeated scenes) followed a whole-brain approach and were done using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) applying family-wise error rate cluster-level correction for multiple comparisons. To assess the specific effects of CSF total tau, phospho tau, or amyloid on whole-brain novelty responses, three main models were set up.
-
1.
CSF total tau as a regressor using the MRI site, age, gender, and Aβ42 levels as covariates.
-
2.
CSF p-tau as a regressor using the MRI site, age, gender, and Aβ42 levels as covariates.
-
3.
CSF Aβ42 as a regressor using the MRI site, age, gender, and total tau levels as covariates.
2.7.1. Mediation analyses
The influence of local brain volume on correlations between CSF biomarker levels and local novelty responses were assessed with a mediation analyses (SPSS 24 using the PROCESS module [24]).
2.8. Data availability
Data, study protocol, and biomaterials can be shared with partners based on individual data and biomaterial transfer agreements.
3. Results
3.1. Participants and demographics
Of the first 400 participants of the DELCODE study, 76 who were classified as healthy controls (HCs), SCD, or MCI also completed task fMRI and contributed CSF (Table 1). Among these 76 individuals, the Mini–Mental State Examination scores were similar in HCs and SCD, and lower in MCI than HCs (T = 3.6, P = .001). CSF total tau and p-tau values were not significantly different between HCs and SCD but higher in MCI than in HCs (total tau: T = −3.3, P = .002; p-tau: T = −2.5, P = .013). CSF Aβ42 levels were not significantly different between these groups (P > .08).
Table 1.
Characteristics of participants who completed task-fMRI
| Age | MMSE | ADAS delayed recall | Dprime | Aβ42 | Total tau | p-tau | |
|---|---|---|---|---|---|---|---|
| Healthy controls, N = 40 | |||||||
| Mean | 67.7 | 29.4 | 7.7 | 1.14 | 834.1 | 325.3 | 46.3 |
| SD | 5.15 | 0.9 | 1.7 | 0.37 | 295.8 | 113.0 | 15.1 |
| SCD, N = 21 | |||||||
| Mean | 70.0 | 29.0 | 7.7 | 1.09 | 892.0 | 367.1 | 53.6 |
| SD | 5.96 | 1.1 | 1.9 | 0.48 | 325.5 | 102.5 | 16.8 |
| MCI, N = 15 | |||||||
| Mean | 72.4 | 28.1 | 4.5 | 0.70 | 674.3 | 450.0 | 59.2 |
| SD | 4.88 | 1.6 | 2.4 | 0.39 | 305.0 | 151.7 | 19.6 |
Abbreviations: MMSE, Mini–Mental Status Examination; MCI, mild cognitive impairment; SCD, subjective cognitive decline; fMRI, functional magnetic resonance imaging.
NOTE. Dprime is a measure of memory discrimination of repeated scenes (presented during task-fMRI) and new distractors (not presented during task-fMRI). A value of 0 would correspond to chance-level performance. Mean values are given in bold.
3.2. Behavioral results from the task-fMRI paradigm
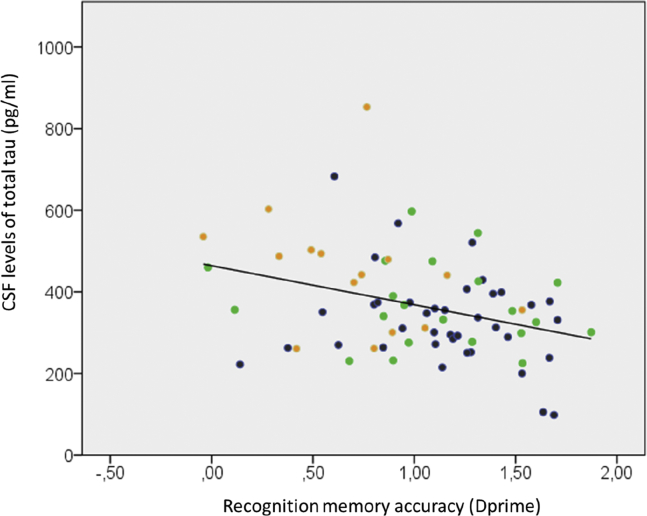
Recognition memory accuracy (dprime) for novel scene images (Table 1) was equivalent between HCs and SCD participants. MCI participants performed significantly lower than HCs (T = 4.3; P = .000) and SCD participants (T = 3.3; P = .001) (Table 1). Partial correlations with age as a covariate showed a significant negative correlation between CSF total tau and dprime (R = −0.313; P = .007) (Fig. 1). Correlations between p-tau and dprime were marginally significant (R = −0.226; P = .053). There were no correlations between dprime and Aβ42 levels (P > .5).
Fig. 1.
Correlation between CSF levels of total tau (pg/mL) and recognition memory accuracy (dprime) for the novel scenes presented during task-fMRI. Color codes indicate healthy controls (blue), subjective complainers (subjective cognitive decline, green), and individuals with mild cognitive impairment (mild cognitive impairment, orange).
3.3. Functional MRI results
3.3.1. Group-level novelty effect
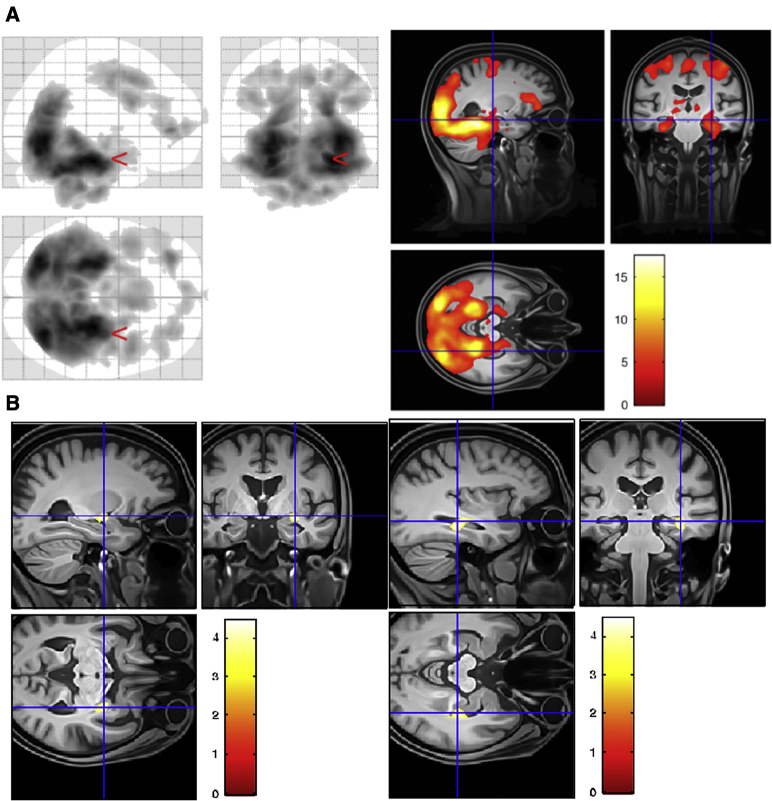
Table 2 lists the brain regions that in a first-level contrast showed increased blood oxygenation level dependent responses to novel as compared to familiar scenes in the entire sample of 76 participants (family-wise error rate cluster < 0.05). As also depicted in Fig. 2, novel images were associated with activation of two large clusters, which comprised the expected network of hippocampus, medial temporal lobes, visual areas, and precuneus, as well as medial frontal and insula.
Table 2.
Group-level results of novelty contrast thresholded at p cluster level < .05 (N = 76)
| Cluster size | Cluster pFWE | Peak T | Template x,y,z (mm) | Brain structures contained in main cluster |
|---|---|---|---|---|
| 47,206 | 0.000 | 17.38 | 28, −42, −14 | L & R each: amygdala, calcarine, sup. cuneus, cerebellum, vermis, G. fusiformis, hippocampus, lingualis, occipital, parahippocampal, parietal, postcentral, precuneus, temporal, thalamus R: Putamen |
| 17.04 | 34, −82, 18 | (submaximum of first cluster) | ||
| 16.58 | −30, −90, 12 | (submaximum of first cluster) | ||
| 10,086 | 0.000 | 8.93 | −34, 32, −10 | L & R each: Med. cingulate, Frontal (Tri., Orb., Oper.), Orb. frontal, insula, postcentral, precentral, rectus, suppl. motor |
| 7.78 | 46, 4, 28 | (submaximum of second cluster) | ||
| 7.69 | 8, 10, 48 | (submaximum of second cluster) |
NOTE. Structure labels in activated clusters were identified using the AAL Toolbox in SPM12. Coordinates in Table 2 are MNI space.
Fig. 2.
Novelty activations and regression analyses. (A) Group-level contrast depicting increased BOLD responses to novel as compared to familiar scenes in the entire sample of 76 participants (depicted at P < .001 uncorrected, k = 400 voxels). Left panel shows entire activation map as a glass brain. Right panel depicts activation on group template (left is left). (B) Whole-brain regression analysis with CSF total tau levels. CSF total tau levels correlate selectively with novelty responses in the amygdala (left panel) and the hippocampus (right panel). Covariates were MRI sites (6 sites), age, gender, and Aβ42 levels. Abbreviations: CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
3.3.2. CSF total tau and p-tau as regressors
Only clusters in the hippocampus and the amygdala (on the right) showed a significant negative correlation between novelty and total tau (FWEcluster < 0.05) (Fig. 2). Thus, higher CSF total tau was associated with lower novelty responses. There was no significant correlation with p-tau.
3.3.3. CSF Aβ42 levels as a regressor
No significant correlations were found.
3.3.4. Mediation analyses with regional volumes
As shown in Fig. 3, the regression of CSF total tau on reduced novelty activation, ignoring the combined right hippocampal and amygdala volumes, was significant [b = −0.016, P < .001]. The regression of CSF total tau on combined right hippocampal and amygdala volume was also significant [b = −1.323, P < .05]. The regression of combined right hippocampal and amygdala volume, controlling for tau pathology, on novelty activation was not significant [b = 0.0003, P = .65]. When controlling for combined right hippocampal and amygdala volumes, CSF total tau still predicted reduced novelty [b = −0.015, P < .001]. The bootstrapped unstandardized indirect effect was 0.0004 (not significantly different from zero, as revealed by a 95% bootstrap confidence interval that did contain zero [confidence interval: −0.0019 to 0.0011]). Thus, the combined right hippocampal and amygdala volume did not mediate the relationship between increased CSF levels of total tau and reduced novelty responses in the hippocampus and amygdala.
Fig. 3.
Mediation analyses. Total tau levels have a significant association with hippocampus + amygdala volume. The volume of hippocampus + amygdala, however, does not have a significant association with novelty. As expected (given the selection of the novelty cluster), there is also a significant influence on novelty responses in the hippocampus/amygdala clusters. More importantly, the direct effect of total tau levels on novelty responses remains significant after controlling for hippocampus/amygdala volume.
3.3.5. Relationship between novelty responses and recognition memory performance
To assess whether the novelty response in the hippocampus/amygdala correlated with recognition memory performance for the novel scenes, we conducted partial correlations with the right hippocampus/amygdala novelty responses and recognition memory dprime using age and right hippocampus volume as covariates. This revealed a significant correlation (R = .244; P = .043). The corresponding analysis for the precuneus was not significant on either side (P > .2).
4. Discussion
The perirhinal and entorhinal cortices, hippocampus, amygdala, precuneus, medial prefrontal, and parietal cortex are core components of an episodic memory network that enables detecting of and orienting toward novel events and their subsequent encoding [15], [25]. Indeed, fMRI studies have consistently shown, as we have observed in this study (Fig. 2A), that these regions are more active in response to novel as compared to repeated/familiarized images of scenes [15], [26], [27]. Given this widespread novelty network, the regional specificity of the correlations between CSF total tau levels and neural novelty responses in the hippocampus/amygdala is striking (Fig. 2B). With increasing levels of total tau, hippocampal/amygdala novelty responses and recognition memory accuracy decreased. This finding suggests that there is a linearly increasing impact of tau pathology on hippocampal and amygdala function across HCs and individuals with MCI.
Neural activation to novel stimuli is a hallmark process of forming new episodic memories [15], [28]. Although a number of previous studies have investigated the relationship between whole-brain resting-state activity and CSF biomarkers (i.e., [29]), very few studies have considered task-related whole-brain activity. Our results reveal a converging relationship between a functional reduction in novelty responses and a reduction in the ability to behaviorally recognize those novel images after a long delay of ca 70 minutes; both measures were related to the same biomarker and were also correlated with each other independent of hippocampal volume. This convergence indicates that the reductions of novelty responses were indeed behaviorally relevant for forming a memory for the novel scenes.
In the recent NIA-AA research framework [12], models hypothesized to explain cognitive dysfunction because of biomarker-related pathology all postulate neurodegeneration as a mediator. Although total tau levels also showed an association with hippocampal/amygdala volumes in our study, the relationship with novelty remained independent of volume (Fig. 3). Hippocampal volume is considered to be an important biomarker for AD and is correlated with a number of medial temporal lobe dependent cognitive functions [30]. CSF tau levels have been associated with neurodegeneration because the release of full-length tau into the CSF has been postulated to be a result of neuronal death [12]. However, a recent study showed that CSF levels of tau also include truncated tau, which can be released into the CSF from intact neurons [31]. Our data suggest an atrophy-independent functional impact of tau pathology on hippocampal memory encoding, which may be mediated by such tau species from intact neurons and which may even be reversible within the preclinical spectrum of AD. This has implications for future tau-based therapies.
The structure-independent functional impact of tau pathology that we observed here is consistent with animal studies showing that misfolded and hyperphosphorylated tau can impair neuronal function [14], [32]. Early local accumulations of pathological tau in axons lead to presynaptic dysfunction, neuronal hypoactivity, and strong behavioral deficits in mice, and this effect can be rectified by restoring neuronal energy balance [33]. Mislocation of tau to dendritic spines can cause synaptic dysfunction [34], and there is evidence that pathological tau reduces network activity [35]. Such a reduction is well compatible with the reduction of a novelty response in the hippocampus and amygdala as observed in our study.
Although CSF total tau and CSF p-tau are known to be highly correlated, they are considered to reflect different pathological processes because they behave differently in other diseases. For instance, unlike total tau, p-tau is not elevated in Creutzfeldt-Jakob disease despite the presence of massive neurodegeneration [36]. Hence, in the current research framework, p-tau is not considered to be a marker of neurodegeneration [12]. In our study, p-tau did not correlate with the hippocampal novelty response. Furthermore, a correlation with recognition memory performance was only marginal.
It is also well established that certain Aβ oligomer species are neurotoxic and cause synaptic dysfunction [1], [13], [37]. A recent study indicated that the earliest accumulation of Aβ oligomers (evident in decreased Aβ42 levels in CSF but no discernible amyloid plaque deposition with PET) reduces the resting-state connectivity of the precuneus [29]. We did not observe a relationship between Aβ42 levels and novelty responses in any brain region. Although it is possible that resting-state connectivity is more sensitive to Aβ42 pathology than the novelty responses, our data indicate that the previously reported [29] connectivity decrease is not likely to be accompanied by decreased memory encoding for novel events. We also observed that the impact CSF total tau on hippocampal/amygdala novelty responses was independent of Aβ42 levels. Indeed, histological studies have noted that early tau pathology has little overlap with Aβ aggregation [38], [39]. Likewise, PET studies showed that amyloid pathology is more ubiquitous in the cortex and shows a preponderance in parietal cortical regions and midline posterior regions including the precuneus [29], [40] rather than medial temporal lobe regions.
This study has a number of limitations. Our sample size was not large enough to conduct analyses within each subgroup or within early and late stages of the Alzheimer's spectrum separately. Furthermore, this was a cross-sectional analysis, and longitudinal data would be helpful to be able to relate synaptic dysfunction to the rate of neurodegeneration as derived from atrophy over time. These extensions would also help to further clarify the dissociation between total tau and p-tau. Finally, although we spend considerable effort to achieve high imaging quality (harmonized standard operating procedures across sites, continuous QA of data quality and participant positioning, estimation of volumes using combined analyses of T1 and bespoke T2 images), it is possible that total tau levels were associated with subtle atrophy of the neuropil that we have not been able to capture at 3T. It would be valuable to follow-up on our results using ultrahigh-resolution imaging at 7T.
To summarize, our results indicate that tau pathology can reduce novelty processing in the core brain regions where it originates irrespective of Aβ42 and irrespective of local gross structural integrity. The independence of synaptic dysfunction potentially caused by tau pathology indicate that regional novelty responses may be used as functional indices of target engagement in proof-of-concept studies targeting tau-related neurotoxicity. Treatment failures with disease-modifying drugs in clinically manifest AD have highlighted that a need to initiate interventions before irreversible neurodegeneration, or atrophy, has occurred [41]. This requires the characterization of the earliest abnormalities of synaptic function rather than atrophy in the preclinical phase of AD [42]. Our data need to be confirmed in a larger sample, but they raise the possibility that tau may cause synaptic dysfunction irrespective of local atrophy and therefore suggest that in the early stages of the AD spectrum dysfunction caused by tau might be partly reversible.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Cerebrospinal fluid (CSF) total tau levels are viewed to reflect neurodegeneration. However, there is also recent evidence that intact neurons may contribute to these CSF levels and that tau may be associated with synaptic dysfunction. These relevant studies are appropriately cited.
-
2.
Interpretation: Our findings indicate a relationship between CSF total tau levels and brain dysfunction. This hypothesis is discussed in relation to the prevailing view that they are primarily related to neurodegeneration.
-
3.
Future directions: The manuscript proposes that the assessment of the brain function with task–functional magnetic resonance imaging, in conjunction with measures of brain volume and CSF biomarkers, may yield valuable insights into our understanding of how biomarkers are related to behavioral impairment. Larger samples are needed to further advance this topic.
Acknowledgments
The study was funded by the German Center for Neurodegenerative Diseases (Deutsches Zentrum für Neurodegenerative Erkrankungen [DZNE]), reference number BN012.
Authors' contributions: Emrah Düzel contributed to study concept and design, acquisition of data, study supervision, and writing of manuscript. David Berron, Hartmut Schütze, Arturo Cardenas-Blanco, Matthew Betts, Gabriel Ziegler, Yi Chen, and Martin Reuter contributed to analysis of data. Laura Dobisch and Oliver Speck contributed to acquisition of data and study supervision. Coraline Metzger, Daniel Bittner, Wenzel Glanz, Annika Spottke, Janna Rudolph, Frederic Brosseron, Katharina Buerger, Daniel Janowitz, Klaus Fliessbach, Michael Heneka, Christoph Laske, Martina Buchmann, Peter Nestor, Oliver Peters, Dominik Diesing, Siyao Li, Josef Priller, Eike Jakob Spruth, Slawek Altenstein, Alfredo Ramirez, Anja Schneider, Barbara Kofler, Stefan Teipel, Ingo Kilimann, Martin Dyrba, Jens Wiltfang, Claudia Bartels, Steffen Wolfsgruber, and Michael Wagner contributed to acquisition of data and critical revision of manuscript for intellectual content. Frank Jessen contributed to study concept and design, acquisition of data, analysis of data, study supervision, and writing of manuscript.
Footnotes
Emrah Düzel and David Berron are co-founders of neotiv GmbH. Emrah Düzel conducts paid consultancy work for Heptares (unrelated to this study).
References
- 1.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman D.M., Goate A., Kelly J., Sperling R. Mapping the road forward in Alzheimer's disease. Sci Transl Med. 2011;3:114ps48. doi: 10.1126/scitranslmed.3003529. [DOI] [PubMed] [Google Scholar]
- 3.Motter n., Pelfrey V.C., Kholodenko D., Barbour R., Wood J.K., Galasko D. Reduction of amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 5.Forlenza O.V., Radanovic M., Talib L.L., Aprahamian I., Diniz B.S., Zetterberg H. Cerebrospinal fluid biomarkers in Alzheimer's disease: Diagnostic accuracy and prediction of dementia. Alzheimer's Demen (Amsterdam, Netherlands) 2015;1:455–463. doi: 10.1016/j.dadm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stomrud E., Minthon L., Zetterberg H., Blennow K., Hansson O. Longitudinal cerebrospinal fluid biomarker measurements in preclinical sporadic Alzheimer's disease: A prospective 9-year study. Alzheimer's Demen (Amsterdam, Netherlands) 2015;1:403–411. doi: 10.1016/j.dadm.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattsson N., Lönneborg A., Boccardi M., Blennow K., Hansson O. for the of Biomarkers G. Clinical validity of cerebrospinal fluid Aβ42, tau, and phospho-tau as biomarkers for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:196–213. doi: 10.1016/j.neurobiolaging.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Vos S.J.B., Gordon B.A., Su Y., Visser P.J., Holtzman D.M., Morris J.C. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. doi: 10.1016/j.neurobiolaging.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos S.J., Verhey F., Frölich L., Kornhuber J., Wiltfang J., Maier W. Prevalence and prognosis of Alzheimer's disease at the mild cognitive impairment stage. Brain. 2015;138:1327–1338. doi: 10.1093/brain/awv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mucke L., Selkoe D.J. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L., McInnes J., Wierda K., Holt M., Herrmann A.G., Jackson R.J. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun. 2017;8:15295. doi: 10.1038/ncomms15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duzel E., Schutze H., Yonelinas A.P., Heinze H.J. Functional phenotyping of successful aging in long-term memory: Preserved performance in the absence of neural compensation. Hippocampus. 2011;21:803–814. doi: 10.1002/hipo.20834. [DOI] [PubMed] [Google Scholar]
- 16.Marks S.M., Lockhart S.N., Baker S.L., Jagust W.J. Tau and β-Amyloid Are Associated with Medial Temporal Lobe Structure, Function, and Memory Encoding in Normal Aging. The J Neurosci. 2017;37:3192–3201. doi: 10.1523/JNEUROSCI.3769-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessen F., Spottke A., Boecker H., Brosseron F., Buerger K., Catak C. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer's disease (DELCODE) Alzheimers Res Ther. 2018;10:15. doi: 10.1186/s13195-017-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinrichs H., Scholz M., Tempelmann C., Woldorff M.G., Dale A.M., Heinze H.J. Deconvolution of event-related fMRI responses in fast-rate experimental designs: tracking amplitude variations. J Cogn Neurosci. 2000;12:76–89. doi: 10.1162/089892900564082. [DOI] [PubMed] [Google Scholar]
- 21.Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes A.F. Guilford Publications; 2017. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. [Google Scholar]
- 25.Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 26.Maass A., Schutze H., Speck O., Yonelinas A., Tempelmann C., Heinze H.J. Laminar activity in the hippocampus and entorhinal cortex related to novelty and episodic encoding. Nat Commun. 2014;5:5547. doi: 10.1038/ncomms6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berron D., Schutze H., Maass A., Cardenas-Blanco A., Kuijf H.J., Kumaran D. Strong Evidence for Pattern Separation in Human Dentate Gyrus. J Neurosci. 2016;36:7569–7579. doi: 10.1523/JNEUROSCI.0518-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duzel E., Bunzeck N., Guitart-Masip M., Duzel S. NOvelty-related motivation of anticipation and exploration by dopamine (NOMAD): implications for healthy aging. Neurosci Biobehav Rev. 2010;34:660–669. doi: 10.1016/j.neubiorev.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Palmqvist S., Scholl M., Strandberg O., Mattsson N., Stomrud E., Zetterberg H. Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun. 2017;8:1214. doi: 10.1038/s41467-017-01150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan P.J., Lim Y.Y., Abbott R., Galluzzi S., Marizzoni M., Babiloni C. Association between CSF biomarkers, hippocampal volume and cognitive function in patients with amnestic mild cognitive impairment (MCI) Neurobiol Aging. 2017;53:1–10. doi: 10.1016/j.neurobiolaging.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Sato C., Barthelemy N.R., Mawuenyega K.G., Patterson B.W., Gordon B.A., Jockel-Balsarotti J. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018;97:1284–1298.e7. doi: 10.1016/j.neuron.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polydoro M., Dzhala V.I., Pooler A.M., Nicholls S.B., McKinney A.P., Sanchez L. Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer's disease model. Acta Neuropathologica. 2014;127:257–270. doi: 10.1007/s00401-013-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennissen F.J., Anglada-Huguet M., Sydow A., Mandelkow E., Mandelkow E.M. Adenosine A1 receptor antagonist rolofylline alleviates axonopathy caused by human Tau DeltaK280. Proc Natl Acad Sci U S A. 2016;113:11597–11602. doi: 10.1073/pnas.1603119113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoover B.R., Reed M.N., Su J., Penrod R.D., Kotilinek L.A., Grant M.K. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menkes-Caspi N., Yamin H.G., Kellner V., Spires-Jones T.L., Cohen D., Stern E.A. Pathological tau disrupts ongoing network activity. Neuron. 2015;85:959–966. doi: 10.1016/j.neuron.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Skillback T., Rosen C., Asztely F., Mattsson N., Blennow K., Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol. 2014;71:476–483. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- 37.Selkoe D.J. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delacourte A., Sergeant N., Champain D., Wattez A., Maurage C.A., Lebert F. Nonoverlapping but synergetic tau and APP pathologies in sporadic Alzheimer's disease. Neurology. 2002;59:398–407. doi: 10.1212/wnl.59.3.398. [DOI] [PubMed] [Google Scholar]
- 39.Price J.L., Morris J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Schöll M., Lockhart S.N., Schonhaut D.R., O'Neil J.P., Janabi M., Ossenkoppele R. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 42.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data, study protocol, and biomaterials can be shared with partners based on individual data and biomaterial transfer agreements.