Abstract
The development of resistance to glucocorticoids (GCs) in therapeutic regimens poses a major threat. Generally, GC resistance is congenital or acquired over time as a result of disease progression, prolonged GC treatment or, in some cases, both. Essentially, disruptions in the function and/or pool of the glucocorticoid receptor α (GRα) underlie this resistance. Many studies have detailed how alterations in GRα function lead to diminished GC sensitivity; however, the current review highlights the wealth of data concerning reductions in the GRα pool, mediated by disease-associated and treatment-associated effects, which contribute to a significant decrease in GC sensitivity. Additionally, the current understanding of the molecular mechanisms involved in driving reductions in the GRα pool is discussed. After highlighting the importance of maintaining the level of the GRα pool to combat GC resistance, we present current strategies and argue that future strategies to prevent GC resistance should involve biased ligands with a predisposition for reduced GR dimerization, a strategy originally proposed as the SEMOGRAM–SEDIGRAM concept to reduce the side-effect profile of GCs.
Keywords: glucocorticoid receptor, glucocorticoid resistance, acquired resistance, biased ligands, GRα downregulation
Introduction
Due to the interrelatedness of the stress and inflammatory responses, chronic persistent inflammation may be considered both a cause and a consequence of a prolonged disruption of the central HPA axis, a systemic signalling pathway of the stress response (1). This in turn, has many peripheral effects, such as an increase in circulating glucocorticoids (GCs) (2, 3).
Chronic stress or prolonged exogenous GC treatment also disrupts the central homeostatic nature of GC signalling, often resulting in various peripheral effects, one of which is the tissue-specific reductions in the glucocorticoid receptor α (GRα) functional pool. This reduction in the GRα functional pool may ultimately drive the development of acquired GC resistance and result in the progression of many psychological and pathological conditions.
Endogenous GCs, which are regulated by the HPA axis, are physiological mediators secreted in an ultradian or circadian manner (3) or in response to internal or external signals (2, 3, 4, 5, 6), such as infection, pain or stress, and function within the body to regulate inflammation and maintain internal homeostasis (2, 3, 6, 7). Exogenous GCs, designed to mimic the biological anti-inflammatory action of endogenous GCs, remain the mainstay therapeutic choice (7) for the treatment of chronic inflammation in various psychological and pathological conditions. GCs are currently one of the most widely prescribed drugs in the world with an estimated 1.2% of the population of the United States, using them (8). Although effective anti-inflammatory agents, it is believed that approximately 30% of all patients receiving treatment, experience a degree of GC insensitivity (9). Specifically, 4–10% of asthma patients (10), 30% of rheumatoid arthritis patients (10), almost all chronic obstructive pulmonary disease (COPD) (10) and sepsis patients (5) and 10–30% of untreated acute lymphoblastic leukaemia (ALL) patients (11) experience varying degrees of GC insensitivity.
This stochastic response to GCs within disease groups (10), is compounded by inter-individual variation in patient sensitivity, as well as tissue-specific intra-individual differences in GC responsiveness (1). Thus, research is now focussed on developing diagnostic tools for determining GC sensitivity prior to treatment, for the use in personalized therapeutic regimens (12), which will likely assist in limiting adverse side effects and restrict the development of further GC insensitivity.
This review begins by briefly describing the types of GC resistance and then discusses reductions in the GRα pool in various pathological and psychological conditions, in terms of acquired GC resistance. Primary focus is given to disease- or treatment-associated reductions in the GRα pool, which drive the development of GC insensitivity, followed by the molecular mechanisms involved in mediating these reductions. Furthermore, current methods to restore GRα protein expression and improve GC sensitivity are briefly detailed. Lastly, a potential role for the conformation of GRα in receptor turnover is proposed, and a strategy using conformationally biased ligands is advocated to combat acquired GC resistance.
GC resistance
Following GC secretion into the bloodstream, GCs are transported to various tissues and cells and diffuse across the cell membrane where they bind and mediate their biological effects via their cognate receptor, the ligand-activated transcription factor, GRα (13). Upon ligand binding, the GRα undergoes a conformational change which allows for subsequent translocation to the nucleus (13). Here, the GC-bound GRα mediates the transrepression or transactivation of various GC-responsive genes (13, 14, 15).
Central to the ability of GCs to combat inflammation is the requirement for a significant amount of functional GRα through which they may mediate their effects (16, 17). There are a multitude of factors which can regulate the functional pool of GRα, either at the level of the functionality of the receptor and/or at the level of the GRα pool, thus ultimately contributing to GC resistance. In short, disruptions in GRα function (1, 7, 18) are known to modulate, not necessarily independently of one another, the subcellular localization, ligand binding and transactivation ability of the receptor, and are regulated by, among others, increases in additional GR isoforms (GRβ and GRγ) due to alternative splicing events, inactivating GRα mutations, the inflammatory cytokine profile of the cellular microenvironment and mutations/polymorphisms in the ERK pathway. However, rather than altered GRα function, the focal point of this review is reviewing the importance of the GRα pool, with regards to acquired GC resistance.
GC resistance is multi-faceted and has been extensively identified and studied in healthy and diseased states (9). Broadly speaking, GC resistance may be divided into two major groups: generalized (systemic/primary) or acquired (localized/secondary) GC resistance (1, 9). The generalized form of GC resistance falls beyond the scope of the current review, but for the interested reader is reviewed in several papers (1, 9, 15, 19). Essentially, these two groups of GC resistance are distinctively different in terms of the site of occurrence within a biological system, with acquired GC resistance often affecting specific tissues and/or cells while generalized GC resistance affects almost all tissues (1, 9). However, central to both types of GC resistance is perturbation of the GRα functional pool.
Acquired GC resistance is significantly more common in the general population and has been linked to a number of psychological and pathological conditions/diseases. An apt description for this form of GC resistance is a ‘consequence of a pathophysiological process’ (5) affecting specific tissues/cell types (9). Furthermore, the clinical use of GCs, although effective initially, may lead to the development of acquired GC resistance thus posing a significant challenge for the long-term treatment of these conditions (9).
GC-resistant patients often require higher GC doses for prolonged periods of time in order to efficiently combat chronic inflammation, which likely leads to adverse side effects and may aggravate GC insensitivity (16). Thus, it is of importance for practitioners to be able to evaluate the GC responsiveness, of individual patients, to permit personalized GC treatment to obtain an optimal therapeutic outcome (12). Acquired resistance is more difficult to diagnose than generalized resistance, which generally displays a ‘clinical picture’ of GC resistance (1). In terms of generalized GC resistance, no single, standardized method for determining patient sensitivity to GC treatment exists (12), however, a range of endocrine (1) (e.g. cortisol awakening rise/response (CAR) or the 24-h urinary-free cortisol (UFC)) and biochemical methods (9) (dexamethasone suppression test (DST) or the more recent Dex/CRH suppression test) are employed to determine generalized GC resistance. In contrast, patients with or developing acquired GC resistance are mostly asymptomatic, thus, a range of in depth biochemical diagnostic approaches (12, 20, 21) (e.g. BrdU incorporation lymphocyte steroid sensitivity assay (BLISS) and measuring the GC-responsive gene expression) are required to determine the GC responsiveness of specific tissues and/or cells. Although GC response can be determined, an increasing demand for more sensitive and specific tests remain, to avoid the unnecessary chronic GC use in treatment regimens (22).
Reductions in the GRα pool and implications for acquired GC resistance
In many, but certainly not all, stress-related, psychological and pathological conditions, reductions in the GRα pool have been noted (9) (Table 1). These disease-associated reductions in the GRα pool often produce GC-resistant forms within disease groups, which are exceptionally challenging to manage clinically (9). In addition to the disease-associated reductions in the GRα pool, generally mediated via increased circulating endogenous GCs, GC treatment-associated reductions in the GRα pool are well documented (Table 2). It is often difficult to distinguish between disease- and treatment-associated GRα turnover because withholding GC treatment from patients would not be ethical. Moreover, the treatment-associated effects on the GRα pool often exacerbate those that are disease-associated (23), further contributing to the development of acquired GC resistance.
Table 1.
Disease-associated reductions in the GRα pool.
| Type of condition (general) | Broad category of disease condition | Species | Specific stress/condition/disease | Tissue/cells | GRα mRNA expression | GRα protein expression | Implications for GC sensitivity | References |
|---|---|---|---|---|---|---|---|---|
| Stress | Pre/post-natal stress | Humans | Pre-natal stressChildhood adversity/abuse leading to adult suicide | PBMCsaHippocampus | Reduced | N.Cb | N.Dc | (24, 25, 26) |
| Rodents | Early Life Stress (ELS) (i.e. maternal separation (MS) and preconception paternal stress (PPS)) | Hippocampus, amygdala, limbic regions of brain dentate gyrus | Reduced | Reduced | Cognitive dysfunction, altered behavioural affects, increase in anxiety-like behaviour, anhedonia | (27, 28, 29, 30, 31, 32, 33, 34) | ||
| Physical or psychological stress | Rodents | Restraint stress, psychological stress, forced swim stress (FSS), repeated social defeat (RSD), repetitive restraint stress (RSS), water-immersion and restraint stress (WIRS) | Hippocampus, amygdala, hypothalamus, cerebellum, splenic macrophages, splenocytes, peripheral leucocytes, oligodendrocytes of corpus callosum, prefrontal cortex, lung tissues | Reduced | Reduced | More susceptible to psychological disorders, asthma exacerbations, diminished GC sensitivity | (29, 37, 38, 39, 40, 41, 42, 43, 44, 45) | |
| Psychological condition | Psychological conditions | Humans | Major depression (MD), schizophrenia, bipolar disorderPost-traumatic stress disorder (PTSD), general anxiety disorder (GAD) | Hippocampus, prefrontal-, temporal- and entorhinal cortex, PBMCs, lymphocytes | Reduced | N.D | Diminished GC sensitivityTreatment-resistant depression | (52, 53, 54, 55, 56, 57, 58, 59) |
| Pathological conditions | Autoimmune or inflammatory-linked conditions | Human | Atopic dermatitis (AD) | PBMCs | Reduced | N.D | GC resistant to topical treatment and systemic administration of potent corticosteroid | (61) |
| Systemic lupus erythematosus (SLE) | PBMCs | Reduced | N.D | Diminished GC sensitivity | (62, 63, 64) | |||
| Inflammatory bowel disease (IBD) | PBMCs | Reduced | N.C | Impaired GC response | (76) | |||
| Adult immune thrombocytopenia (ITP) | PBMCs | Reduced | Reduced | GC-resistant ITP | (65) | |||
| Asthma | PBMCs, cells from skin biopsies of patients | N.D | Reduced | GC-resistant asthma | (66, 67) | |||
| Chronic obstructive pulmonary disease (COPD) | PBMCs, lymphocytes, lung tissue | Reduced | Reduced | GC-resistant COPD | (68, 69, 70, 71) | |||
| Arthritis | Chondrocytes and lymphocytes | Reduced | Reduced | Steroid-resistant arthritis | (72, 73, 74) | |||
| Rodents | Experimental encephalomyelitis (EAE) | T cells | Reduced | Reduced | GC-resistant apoptosis | (77) | ||
| Cancer | Human | Acute lymphoblastic leukaemia (ALL)Multiple myeloma (MM)Small-cell lung cancer (SCLC), non-small-cell lung cancer (NSCLC), breast cancer | B-lineage leukaemia, T-ALL resistant, lymphoblasts, T-leukaemic, multiple myeloma, human carcinoma, lung adenocarcinoma cells, breast tissue | Reduced | Reduced | GC-resistant ALLGC-resistant MM and diminished GC sensitivity (transactivation and GC-mediated apoptosis)GC-resistant SCLC | (11, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93) | |
| Rodents | Liver cancer | HTC cells | Reduced | Reduced | Reduced sensitivity to Dex | (94) | ||
| Infection and other conditions | Human | Sepsis | Neutrophils and T-cells | Reduced | Reduced | Diminished GC sensitivity | (95, 96) | |
| Idiopathic nephrotic syndrome (NS) | PBMCs | NC | Reduced | Steroid-resistant Nephrotic syndrome (SRNS) | (97) | |||
| Keloid disease | Keloid tissue | Reduced | Reduced | Diminished GC sensitivity | (98) | |||
| Rodents | Stroke | mouse brain capillary endothelial cells (cEND) | N.C | Reduced | Diminished GC sensitivity | (99) |
aPeripheral blood mononuclear cells (PBMCs), bNo change in GRα expression (mRNA or protein) (N.C), cNot detected (N.D).
Table 2.
GC Treatment-associated reductions in the GRα pool.
| Exogenous GC | In vitro/ex vivo/in vivo | Treatment conditions | Cells/tissues | GRα mRNA expression | GRα protein expression | Implications for GC sensitivity | References | |
|---|---|---|---|---|---|---|---|---|
| Concentration | Time | |||||||
| Dex | In vitroa | Various Dex doses (10−10 to 10−6 M) | Generally up to 72 h with one study continuing treatment for up to 4 weeks and one for up to 2 years | Human IM-9 lymphocytes and rat pancreatic acinar (AR42J) cells Hepatoma tissue culture (HTC), HeLa, COS-1, cells NIH 3T3 cells, Chinese Hamster ovary-derived (CHO) cells, BWTG3 cells Mouse brain capillary endothelial (cEND) cells, U2-0S and A459, human respiratory epithelial cells (BEAS-2B) Normal human liver (HL7702) cells L6 muscle cells, fibroblast-like synoviocytes (FLS), RAW264.7 cells Peripheral blood mononuclear cells (PBMCs) |
Reduced | Reduced | Most of the papers demonstrated diminished GC sensitivity | (99, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 116, 119) |
| Ex vivob or in vivoc | 5 μM, 20 μg or 1–5 mg/kg body weight | Up to 48 h, 3–28 days | Variety of mice and rat tissues (liver, kidney, lung and heart), culture mouse podocytes Rat hippocampal neurons Mice frontal cortex and hippocampus tissue Human lymphocytes |
Reduced | Reduced | Most of the papers demonstrated diminished GC sensitivity | (60, 102, 107, 108, 113, 114, 115, 116) | |
| Triamcinolone acetonide (TA) | In vitro | 1 μM | Up to 96 h | L929 cells (a fibroblast-like cell line) | Reduced | Reduced | N.Dd | (117) |
| Hydrocortisone | In vivo | Intraperitoneally 5 mg/100 g body weight | 6 h | Liver tissue | N.D | Reduced | Altered GC sensitivity | (118) |
| Various prednisolone-based steroids | In vitro | 10−5 M | 0 to 24 h | HeLa | Reduced | N.D | N.D | (119) |
| In vivo | 120 mg/kgLow-dose and 1 × mega dosee; 0.01–0.3 mg/kg orally or 10–15 mg/kg i.v. pulse therapyf; 1 mg/kg body weight | 10 daysDaily (oral) or 3 dosese; 4–6 weeks (i.v) | Liver tissueHuman blood monocytesLymphocyte subpopulationsPBMCs | Reduced | Reduced | Diminished GC sensitivity GC resistance based on clinical predictive factors for GC resistance (i.e. fundus depigmentation and chronic disease in VKHg) |
(23, 100, 101, 120, 122) | |
aIn vitro: GC treatment of transiently, stably transfected or endogenous GRα in tissue culture cells. bEx vivo: GC treatment of endogenous GRα in cells/tissues derived directly from animals in a tissue culture assay. cIn vivo: Subjects (rodents or patients) treated with GCs with cells/tissues retrieved and assayed (i.e. GC treatment does not occur in tissue culture). dNot detected (N.D). eSee Berki et al. (122) for details. fIntravenous therapy (i.v). gVogt–Koyanagi–Harada (VKH) disease (102).
Disease-associated reductions in the GRα pool
There is a wealth of evidence associating stress, psychological and pathological conditions, with the development of an acquired GC resistance, through reductions in the GRα pool (Table 1).
Specifically, in terms of stress, the modulation of the GRα pool is fundamentally dependent on the duration of the stressor, the environment in which the stress occurs, and the individual’s sensitivity to stress (24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43). Various stressors ranging from pre- or post-natal to physical and psychological stress, in a number of human and rodent studies, encompassing various different tissues and cells, result in significant reductions in the GRα mRNA and/or protein pool (Table 1). These reductions are generally, but not always (26), correlated with stress-induced increases in circulating endogenous GCs (24, 25). Whilst GC-mediated receptor turnover is thought to be an adaptive mechanism employed by the cell to protect against the damaging effects of unrelenting stress, this reduction in the GRα pool has implications in GC sensitivity, often leading to a blunted GC response (42). Jung et al. (38), supported by Quan et al. (43), noted reductions in the GRα mRNA, and protein pool following repeated social defeat in rodent models, and importantly correlated these reductions to a consequent diminished GC sensitivity. In addition to encouraging the development of GC resistance, certain chronic physical, psychological and/or pre- or post-natal stressors can also increase susceptibility to severe psychological or pathological conditions (44, 45). An example is a recent study by Han et al. (44) where stress-induced hypercortisolemia mediated a decrease in the GRα protein pool in the hypothalamus of mice, which subsequently increased their susceptibility to psychological disorders (e.g. depression).
In many psychological disorders, including depression and schizophrenia, a large cohort of patients, but not all (46, 47), display consistent biological findings (48, 49), namely an increase in inflammation and hyperactivity of the HPA, which drives hypercortisolemia, with consequences for the GRα pool in peripheral tissues (50). Whilst it must be noted that vast heterogeneity in GRα expression exists in patients with psychological conditions (48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59), the current review focuses on conditions/disorders which have been explicitly linked to reductions in the GRα pool (Table 1). Specifically, a number of studies have demonstrated a reduction in the GRα mRNA pool in patients suffering from major depression (MD) (52, 53, 58), schizophrenia (58), bipolar disorder (58) and post-traumatic stress disorder (54, 56, 57, 59) in various tissues of the brain (e.g. the hippocampus and prefrontal cortex) as well as in peripheral blood mononuclear cells (52, 53, 58). Furthermore, in patients suffering from generalized anxiety disorder, a negative correlation was made between circulating GC concentrations and the GRα mRNA pool, which was subsequently shown to result in diminished GC sensitivity (55).
In terms of pathological conditions, it is difficult to tease apart whether modulations in the GRα pool are a pathological consequence of the disease, as in the case of many psychological disorders, or as a result of prolonged GC treatment, which many of these patients require (60). Nevertheless, this review highlights cases in which reductions in the GRα pool are noted in autoimmune or inflammatory-linked conditions, cancers and infection or other conditions, attempting to limit it to cases in which patients were not receiving treatment (Table 1).
In autoimmune and inflammatory-linked conditions, a significant correlation between disease-associated reductions in the GRα pool and GC resistance has been demonstrated for atopic dermatitis (AD) (61), systemic lupus erythematosus (SLE) (62, 63, 64), adult immune thrombocytopenia (65) (ITP), steroid-resistant Type II asthma (66, 67), chronic obstructive pulmonary disease (68, 69, 70, 71) and osteoarthritis in humans (72, 73, 74). However, it has been suggested that the level of the GRα pool is not the primary determinant for GC sensitivity in all inflammatory-linked conditions as in the resistant form of irritable bowel disease (75, 76) and rheumatoid arthritis (73), for example, a reduction in the GRα pool does not always correlate with GC resistance, nevertheless a partial role for the GRα pool likely exists. Furthermore, in a rodent model, T-cells obtained from mice with experimental autoimmune encephalomyelitis, have a reduced GRα mRNA pool, which was linked to diminished GC sensitivity, in terms of GC-resistant apoptosis (77).
GCs are a primary therapeutic choice in cancer for either their pro-apoptotic effects or their use as an adjuvant therapy, in combination with chemotherapeutic agents, to reduce symptoms such as inflammation, allergic reactions, pain and nausea, which may also be caused by the tumour itself (78). However, both the type of cancer cell as well as the level of the GRα pool of certain cancer cells are thought to play a significant role in mediating the response to GC treatment (78, 79, 80, 81, 82). It is fairly well documented that high GRα expression is associated with a good response to GC treatment in lung cancer; however, drastic reductions in the GRα pool, thought, in part, to be a pathological consequence of the tumorigenic process may lead to GC insensitivity. Specifically, a number of authors have detailed that a reduction in the GRα pool is negatively correlated to GC response (78, 79, 80, 81, 82). For example, in acute lymphoblastic leukaemia (ALL) (83, 84, 85, 86), multiple myeloma (MM) (87, 88, 89, 90, 91), lung cancer (i.e. small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC)) (78, 79, 80, 81, 82) and breast cancer (92, 93), reductions in the GRα pool, have been associated with treatment-resistant forms of these cancers and/or diminished GC sensitivity. Furthermore, Vanderbilt et al. (94) established that the GC response in a rat hepatoma cell line was modulated in accordance to the level of the GRα pool.
Apart from autoimmune and inflammatory-linked diseases and certain cancers, disease-associated reductions in the GRα pool have been documented in conditions such as sepsis (95, 96), nephrotic syndrome (NS) (97), keloid disease (98) and stroke (99). Although these reductions in receptor expression were generally negatively correlated to GC sensitivity, in sepsis, the association between the GRα pool and the GC response is, however, highly variable (5). In children with NS, the level of the GRα protein pool was assessed before exogenous GC treatment in two patient groups, namely the steroid-sensitive (SSNS) and the steroid-resistant (SRNS) groups (97). Patients from the SRNS group were reported to have reductions in the cellular GRα protein pool, which Hammad et al. (97) postulated may be one of the pathophysiological mechanisms of acquired GC resistance in these children. As with NS (97), patients with keloid disease may be separated into two groups, namely non-responders (nRPs) or responders (RPs) (98). Before receiving GC therapy, tissue isolated from keloid scars from nRPs displayed reductions in the GRα pool, both mRNA and protein, which was associated with decreased GC sensitivity following treatment (98). Lastly, in an in vitro model of hypoxia (used to mimic stroke events), endothelial cells isolated from mice brains, following O2/glucose deprivation had significant reductions in their GRα protein pool, relative to normoxic cells, which was proposed to be the cause of a decrease in subsequent GC sensitivity (99).
It is clear that chronic stress and certain psychological and pathological conditions drive disease-associated reductions in the GRα pool, often independently of exogenous GC treatment. More importantly, in many cases, these reductions in the GRα pool have been directly correlated to an increase in GC insensitivity and resistant forms of these diseases.
GC treatment-associated reductions in the GRα pool
It is often difficult to discriminate between disease- and treatment-associated reductions in the GRα pool (60). However, some clinical studies have demonstrated treatment-associated reductions in the GRα pool independent of disease-associated reductions (100, 101). Using various in vitro, in vivo and ex vivo human and/or rodent models, a number of studies have demonstrated that exogenous GC treatment, e.g. with dexamethasone (Dex), results in significant dose- and time-dependent reductions in the GRα pool with implications for GC sensitivity (Table 2).
Specifically, in vitro Dex treatment led to time-dependent reductions in the GRα mRNA and/or protein pool, of between 50 and 90% (60, 99, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116). Interestingly, Dex treatment of HeLa cells conducted for 2 years, led to reductions in the GRα mRNA and protein pool to below detectable levels (103). Moreover, in most of these studies, where both the GR mRNA and protein pool was assessed, it would appear that the Dex-mediated reductions in the GRα protein pool were generally greater than that observed for the GRα mRNA pool. In a study by Bellingham et al. (112), the rapid Dex-mediated reduction in GRα protein expression was maintained even after 4 weeks, while GRα mRNA expression displayed a ‘biphasic pattern’, with an initial decrease followed by rise in receptor mRNA expression and a subsequent decline, which was attributed to ligand-induced transcriptional, post-transcriptional and translational regulation in mediating receptor mRNA expression, which was not reflected at the protein level (112). A number of studies using ex vivo and in vivo models mirror results of Dex-mediated reductions in the GRα mRNA and/or protein pool obtained in cell lines. In a variety of mouse tissues and rat liver tissue, prolonged treatment with Dex led to significant reductions in the GRα pool (60, 102, 107, 113, 114, 115, 116), which in some cases was associated with diminished GC sensitivity (102, 116).
Importantly, several in vitro, ex vivo and in vivo studies have demonstrated that GC sensitivity is compromised following prolonged Dex treatment, as a result of a significant reduction in the GRα pool (60, 99, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116), highlighting how long-term GC therapy contributes to the development of acquired GC resistance. In addition to Dex, Table 2 also summarizes the reductions in the GRα mRNA and/or protein pool mediated by other exogenous GCs (23, 101, 117, 118, 119, 120, 121, 122), such as hydrocortisone (118).
Taken together, both disease and/or exogenous GC treatment drive reductions in the GRα pool and development of acquired GC resistance, a major clinical challenge. With the burden of resistance to GC treatment mounting, it is of utmost importance to understand the molecular mechanisms involved in ligand-induced GRα turnover.
Molecular mechanisms of GC-mediated reductions in GRα pool
To date, a number of GC-mediated molecular mechanisms employed by the cell have been identified to tightly regulate the GRα pool (Table 3).
Table 3.
GC-mediated molecular mechanisms involved in reducing GRα expression.
| Level of regulation | Molecular mechanism | Species | GRα mRNA expression | GRα protein expression | References |
|---|---|---|---|---|---|
| Epigenetic | DNA methylation of GRα gene • Rodents: exon 17 • Humans: exon 1F, exon 1C, exon 1B, exon 1H, exon 1D |
Rodent Human |
Reduced Reduced |
Reduced Reduced |
(30, 32, 33, 42)(24, 25, 81, 92) |
| Transcriptional | GRα gene regulation via nGREa • Present in exon 6 |
Human | Reduced | N.Db | (107) |
| Post-transcriptional | miRNA • Rodents: miR-96, miR-101a, miR-142-3p, miR-433, miR-29b, miR-340-5p, miR-18 and miR-124a • Humans: miR-124, miR-130b and miR-142-3p |
Rodent Human |
Reduced Reduced |
Reduced Reduced |
(38, 40, 42, 127, 128, 129)(84, 89, 95, 130) |
| Post-translational | Phosphorylation • Rodents: ◦ Multiple mouse mutations(Ser212, Ser220 and Ser234) ◦ Hyper-phosphorylation at Ser412 • Humans: hyper-phosphorylation at Ser211, Ser226 and Ser404 |
Mouse Human |
N.Ac N.A |
Decreased Decreased |
(136, 137)(135, 136) |
| Ubiquitination • Rodents: K426 • Humans: K419 Proteasome degradation (i.e. use of proteasome inhibitors) • Rodents: MG132 or bortezomib (BZ) • Humans: MG132 or BZ |
Mouse Human Mouse Human |
N.A N.A N.A N.A |
Decreased Decreased Decreased Decreased |
(99, 104, 105, 139, 142)(105, 141, 143, 144, 145, 146)(99, 104, 105, 139, 142)(105, 141, 143, 144, 145, 146) | |
| Sumoylation • Specific site unknown |
Human | N.A | Decreased | (152) |
aNegative glucocorticoid response element (nGRE), bNot detected (N.D), cNot applicable (N.A) as effects exerted on GRα protein.
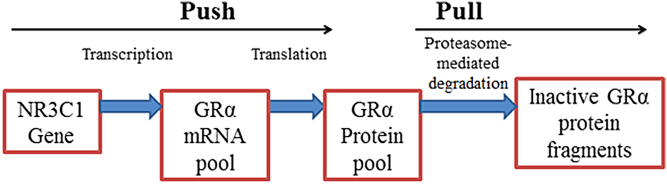
The regulation of the GRα pool may be described using a simple ‘push’ vs ‘pull’ mechanism where, when in a dynamic state of equilibrium and unperturbed, the synthesis of GRα is roughly equivalent to receptor turnover and the level of the GRα pool remains constant (Fig. 1). The ‘push’ is governed by two processes namely transcription and translation while the ‘pull’ is defined by proteasomal degradation, specifically via the ubiquitin-proteasome pathway (UPS). One can assume that perturbations in the equilibrium state of GRα regulation will most likely result in alterations in the GRα pool. One of the ways in which the equilibrium of this dynamic state may be perturbed is via an increase in circulating GCs, either endogenous (i.e. disease-associated increases; Table 1) or exogenous (due to prolonged treatment; Table 2), which subsequently induces GC-mediated GRα turnover.
Figure 1.
Regulation of the GRα protein pool described by a simple ‘push’ vs ‘pull’ mechanism.
GC-mediated regulation of the GRα pool is complex and involves multiple layers of epigenetic, transcriptional, post-transcriptional and post-translational regulation (9, 15). At each level of regulation, the molecular mechanisms function in a highly specific manner to stabilize or destabilize the GRα, which contributes to the complexity of the finely tuned GC/GRα signalling pathway, with receptor destabilization potentially advancing acquired GC resistance. This review focuses specifically on the molecular mechanisms, which function to reduce the GRα mRNA and protein pool in a ligand-dependent manner, however, ligand-independent regulation has been described (1, 9, 15).
GRα mRNA regulation
Epigenetic regulation
DNA methylation of the GRα (NCR31) promoter (123) has been identified as one of the major mechanisms involved in disease-associated acquired GC resistance across species (24, 25, 30, 32, 33, 42, 55, 81, 92) and has been positively correlated with an increase in circulating GCs (42). GC-mediated increases in DNA methylation of the GRα promoter generally, but not always (47), lead to a reduction in the GRα mRNA pool and possibly a corresponding reduction in the GRα protein pool (Table 3).
A specific exonic sequence in the rat GRα gene has been identified as a region that undergoes substantial DNA methylation following stressful events (30, 32, 42). Specifically, increased DNA methylation at the exon 17 promoter, within the GRα promoter, was shown to mediate a reduction in the GRα mRNA pool (30, 32, 42), with Mifsud et al. (42), demonstrating up to a 75% reduction in the GRα mRNA pool in dentate gyrus neurons of male Wistar rats. In mice, methylation of the same exon 17 promoter led to a significant reduction in the GRα protein pool (33). Additionally, human studies have demonstrated that DNA methylation of the GRα gene, specifically at exon1F, exon 1D, exon 1B, exon 1H and exon 1C, resulted in reductions in the GRα mRNA pool (24, 25, 55, 81, 92). DNA methylation of the exon 1F promoter led to reductions in the GRα mRNA pool in tissues/cells from victims with a history of abuse (25) and patients with generalized anxiety disorder (55), with the latter being correlated to diminished GC sensitivity (55). Similarly, for exon 1B, exon 1C and exon 1H, an increase in the methylation status at these sites was associated with a decrease in the GRα mRNA pool, in breast cancer tissue (92) and the hippocampi of suicide completers (24). Furthermore, Kay et al. (81) showed that a 6% increase in GRα methylation resulted in a reduction in the receptor protein pool by up to 50%, in human small-cell lung cancer cells. Collectively, these studies highlight a role for DNA methylation in GC-mediated reductions in the GRα pool and demonstrate that this epigenetic mechanism is likely to contribute to the development of acquired GC resistance.
Transcriptional regulation
The GRα promoter has a negative glucocorticoid response element (nGRE) (107, 124). GC-mediated inhibition of transcription initiation of the GRα gene was shown to be the primary mechanism for up to a 90% reduction in the nascent GRα mRNA pool (107). Specifically occurring through a long-range interaction between the GC-bound GRα, at a nGRE present in exon 6, and a NCOR1 repression complex, which is assembled at the transcription start site of the gene (107). The ability of the GC-bound GRα to regulate its own transcription was neither species nor tissue specific (107). Whilst Ramamoorthy et al. (107) convincingly demonstrated that the GC-mediated auto-regulatory loop to repress the GRα gene occurs via an nGRE in the GRα gene promoter; it appears to be the only study to do so.
Post-transcriptional regulation
Unlike transcriptional regulation of the GRα gene that modulates nascent receptor mRNA expression, post-transcriptional regulation involves the destabilization of mature receptor mRNA via the presence of adenylate uridylate (AU)-rich elements present in the 3′-untranslated region (UTR) of the GRα mRNA transcript, which may ultimately affect receptor protein expression, presenting another level of regulation for fine-tuning GRα expression (125). One of the ways in which this can occur is through the regulatory role of miRNAs, which bind to 3′-UTR of GR mRNA (22). These miRNAs are a family of small non-coding RNAs, which primarily prevent efficient translation of mRNA transcripts but can also induce degradation of these transcripts (126).
The ability of miRNAs to regulate the GRα mRNA pool has been shown to be GC mediated and has been implicated in acquired GC resistance (Table 3). Vandevyver et al. (15) reviews most, but not all (38), of the miRNA target sites in the GRα mRNA transcript; however, the current review will focus only on miRNAs which reduce the GRα pool. Four miRNAs, namely miR-96, miR-101a, miR-142-3p and miR-433, drive reductions in the GRα mRNA pool by up to 40% in mice (127). Additionally, social stress in mice (38) and acute stress in rats (42), resulted in an increase in miR-29b and miR-340-5p and miR‐124a expression, respectively, which was associated with a significant reduction in the GRα mRNA pool. Reductions of the GRα protein pool in rats not necessarily reflected at the mRNA level have also been noted as a result of an increase in miR-18 (128, 129) and miR-124a (40). In humans, a reduction in the GRα pool (both mRNA and protein) was noted following a GC-mediated increase in miR-124, in ALL cells (130) and in T-cells of sepsis patients (95). Moreover, Tessel et al. (89) demonstrated that overexpression of miR-130b mediated a reduction in the GRα protein pool in human MM cell lines; however, knockdown of this miR-130b did not alter GRα protein levels and whilst experiments were conducted in the presence of Dex, it is not clear whether GC’s directly mediated the expression of miR-130b (89). Moreover, an increase in miR-142-3p expression and consequent decrease in the GRα protein pool has been noted in GC-resistant ALL patients (84). Unfortunately, in many of these studies, it is unclear whether up to 80% increase in miRNA expression (38) is directly mediated via an increase in circulating GCs; however, from other studies, one could postulate that a positive correlation between the two exists.
GRα protein regulation
Post-translational regulation
Additionally, the GRα protein is also subjected to GC-mediated regulation in the form of post-translational modifications (PTMs). The nature and degree of these PTMs modulates both GRα function and pool, impacting GC responsiveness in selective tissues, and in some cases, contributes to an acquired GC resistance (15). In this review, we focus on GC-mediated PTMs, which drive reductions in the GRα pool via the proteasome. The effects of PTMs on GRα function are reviewed in several papers (7, 13, 15, 131, 132, 133, 134).
For GRα, the most widely studied and first PTM identified was phosphorylation (15). Since the initial discovery, additional GRα phosphorylation sites have been identified (Fig. 2). Basal GRα phosphorylation may occur in a ligand-independent manner (135, 136), however, hyper-phosphorylation at several of these sites is GC-mediated (135, 136) and modulates GRα function as well as the receptor pool (15, 135, 136). Moreover, various kinases (e.g. p38, ERK, JNK, CDKs and GSK3β (136)) responsible for the phosphorylation of these sites have been described (15).
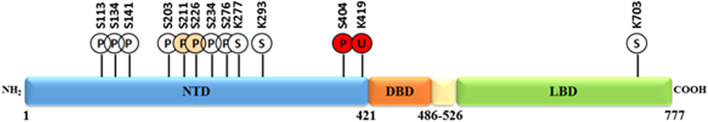
Figure 2.
Post-translational modification sites of human GRα with focus on phosphorylation, ubiquitination and sumoylation. The human GRα protein consists of 777 amino acids and undergoes PTMS at numerous sites. Moreover, many of these PTM sites are contained within the N-terminal domain (NTD) (amino acids 1 to 421) of the receptor, with two present in close proximity to the DNA-binding domain (DBD) (amino acids 421 to 486). Specifically, phosphorylation (P) occurs at serine (e.g. S211, S226 and S404) residues, whilst ubiquitination (U) and sumoylation (S) occurs at lysine residues (i.e. K419 and K277, K293 and K703, respectively). Unlike the others, the K703 sumoylation site occurs within the ligand-binding domain (LBD) of the receptor (amino acids 526 to 777). Moreover, PTMs at these sites are known to modulate GRα function (white) or protein expression (red) and in some cases affect both receptor function and protein expression (pink).
Webster et al. (137) demonstrated that multiple point mutations (i.e. at S212, S220 and S234) in the mouse GRα, which correlate to S203, S211 and S226 of the human GRα (15), respectively, restricted GC-mediated GRα protein turnover. GC-mediated hyper-phosphorylation of the human GRα at S211, S226 (135) and S404 (136) (or Ser412 in mice (136)) led to reductions in the GRα protein pool. Moreover, inhibiting the GC-mediated hyper-phosphorylation at S404, through the use of a mutant or a kinase inhibitor, resulted in a significant increase in GRα protein stability (136). To our knowledge, these are the only sites (135, 136, 137), which directly demonstrate the ability of GC-mediated phosphorylation of the human GRα (135, 136) and the mouse GRα (137) to affect the GRα pool.
It was postulated, but not demonstrated experimentally, that, apart from the inability to be phosphorylated, the phospho-deficient GRα mutants (137, 138), could not be ubiquitinated. Protein ubiquitination is preceded by phosphorylation and is a fundamental requirement for protein degradation via the proteasome; however, GRα ubiquitination is not well documented with only a handful of papers specifically demonstrating GC-mediated GRα ubiquitination (99, 104, 105, 139, 140, 141, 142, 143, 144, 145, 146). Moreover, the idea that ubiquitination of GRα increases following GC treatment seems to be controversial, with one paper demonstrating a Dex-mediated increase in GRα ubiquitination (140) while others noted a Dex-induced reduction in GRα ubiquitination in the presence of a proteasome inhibitor (105, 142). It seems necessary for further research to be conducted in this specific area of GC/GRα signalling. To date, only a single ubiquitination site for GRα that occurs within the PEST degradation motif at Lys426 in mice and Lys419 in humans has been identified, with mutations at these sites restoring the GRα protein pool, by restricting GRα turnover via the proteasome (104, 105). Nevertheless, several studies have through the use of proteasome inhibitors, definitively implicated the ubiquitin-proteasome system (UPS) in the control GRα degradation rates, ultimately contributing to the stringent regulation of the GRα protein pool (99, 102, 104, 105, 139, 142, 145, 147).
Similarly to ubiquitination, sumoylation is a dynamic, reversible process, which involves a multi-step, enzyme-catalysed reaction to mediate the covalent attachment of the SUMO protein (e.g. SUMO-1, SUMO-2/3) to the protein of interest (148). Sumoylation of the GRα is known to modulate GRα function (131, 149, 150, 151, 152) and, less frequently, promote reductions in the GRα pool (152). Specifically, Le Drean et al. (152) demonstrated that overexpression of SUMO-1 aids Dex-mediated receptor downregulation; however, this paper is the only paper to describe the potential of sumoylation to regulate GRα protein expression.
Enzymes of the UPS that mediate GRα protein turnover
Proteasomal degradation of a substrate (i.e. GRα) requires rounds of ubiquitination, mediated by various enzymes of the UPS (Fig. 3) to form a poly-ubiquitin chain, which the proteasome recognizes, resulting in degradation. There are number of UPS enzymes and additional co-regulators (153, 154, 155, 156, 157, 158, 159), which interact with the GRα protein (Fig. 3), in a GC-dependent or independent manner, as regulators of the GRα pool and function. The co-regulator/GRα interactions, which mediate reductions in the GRα pool via the ubiquitin-dependent proteasomal degradation pathway (104, 105) have implications in GC sensitivity and is the primary focus of this section.
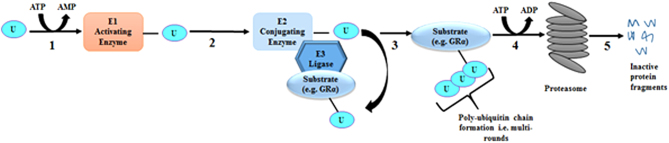
Figure 3.
The ubiquitination of a substrate requires multiple rounds of a multi-step enzymatic process before being targeted to the proteasome. 1. Ubiquitin (U) is activated by an activating enzyme (E1) in an energy (ATP)-dependent manner. 2. The activated U molecule is then transferred to E2, a conjugating enzyme. 3. E3 binds the substrate and the E2 and the transfer of the activated U molecule from E2 to the substrate occurs. 4. This is repeated, until a poly-ubiquitinated chain is formed and the ubiquitinated substrate is then actively (i.e. ATP-dependent) delivered to the proteasome. 5. The catalytically active proteasome recognizes and degrades the substrate to produce inactive protein fragments.
The binding of two enzymes associated with the UPS, namely the inactive E2 conjugating enzyme, tumour susceptibility gene 101 (TSG101) (160) or the E3 ligase, carboxy-terminus of heat shock protein 70-interacting protein (CHIP) (161), to the GRα protein does not require prior ligand binding (Table 4). Moreover, whilst binding of CHIP to GRα is unaffected by GC treatment (139), the formation of the TSG101/GRα complex only occurs in the absence of ligand binding (162). Specifically, TSG101, which like the unliganded GRα is located in the cytoplasm, binds to the N-terminal region of the hypo-phosphorylated unliganded receptor and prevents protein turnover of the unliganded GRα by acting as a dominant negative regulator of ubiquitination due to its catalytically inactive characteristic (162, 163). Knockdown experiments in which TSG101 was targeted demonstrated a decrease in the stability of the hypo-phosphorylated form of GRα, thus suggesting a role for TSG101 in protecting the unliganded GRα from receptor turnover (162). A mutant GRα receptor (S203A/S211A), incapable of undergoing even basal phosphorylation showed enhanced interaction with TSG101 (162), indicating that the association of GRα with TSG101 is dependent on the GRα phosphorylation status. Unlike TSG101, CHIP interactions with GRα seems to be phosphorylation and ligand independent, however, it appears to be a major regulator of unliganded receptor turnover (164) and its presence in the cell is vital for basal GRα protein turnover (139). Overexpression of CHIP in HT22 cells, where steady-state receptor levels were unaffected by prolonged hormone treatment, is able to restore GC-mediated GRα protein turnover, confirming a role for this E3 ligase in reducing the GRα pool (139).
Table 4.
Enzymes of the UPS that mediate GRα protein turnover.
| Enzyme | Type of UPS enzyme | Interactions with GRα depend on | Role in GRα turnover | References | |||
|---|---|---|---|---|---|---|---|
| Ligand-binding status | Phosphorylation status | ||||||
| Unliganded | Liganded | Hypo | Hyper | ||||
| TSG101 | inactive E2 conjugating enzyme | Yes | No | Yes | No | Protects unliganded GRα from turnover | (162) |
| UbcH7 | E2 conjugating enzyme | No | Yes | No | Yes | GC-mediated turnover | (169) |
| CHIP | E3 ligase | Yes | Yes | Yes | Yes | GC-mediated and basal turnover | (139) |
| FBXW7α | E3 ligase | No | Yes | No | Yes; at S404 | GC-mediated turnover | (136, 167) |
| Mdm2/Hdm2 | E3 ligase | Yes, but requires p53 | Yes, but requires p53 | Yes | Yes | GC-mediated turnover | (140,144, 155) |
Binding of F-box/WD repeat-containing protein 7 (FBXW7α), an E3 ligase, to its substrate, requires substrate phosphorylation at a CDC4 phosphodegron motif (165) to mediate phosphorylation-dependent ubiquitination and subsequent proteasomal degradation (166). Specifically, FBXW7α binding to GRα is primarily dependent on GSK3β-mediated phosphorylation at S404 (136), which then targets it for proteasomal degradation (167). Malyukova et al. (167) demonstrated that a GRα phosphorylation mutant (S404A) was incapable of GC-mediated ubiquitination, which partially restricted its degradation via the proteasome. In addition, inactivation of FBXW7α, via mutations, restricted GRα protein turnover (167). From this evidence, it is clear that FBXW7α activity and expression has implications for GC sensitivity by regulating GC-mediated reductions in the GRα pool.
Ubiquitin-conjugating enzyme (UbcH7), an E2-conjugating enzyme, is a known co-regulator of steroid hormone receptors (168), including the GRα. It has been shown to modulate the function and level of the GRα pool, by targeting the receptor for degradation in response to GCs (169). Immunofluorescence studies have elucidated that UbcH7 is predominantly co-localized with GC-bound GRα in the cell’s nucleus, however, cytoplasmic UbcH7 was also observed (169). Overexpression of a dominant negative form of UbcH7 preserved the GRα pool through increasing the stability of the receptor and restricting GC-mediated GRα turnover, thus confirming UbcH7 as a key regulator of the GRα pool and supporting a role for UbcH7 in mediating GC sensitivity (169).
Lastly, another UPS enzyme involved in the regulation of the GRα pool is the E3 ligase, murine double minute 2 (i.e. Mdm2 (144) or Hdm2, the human homologue (170)). Unlike the other enzymes, Mdm2 relies on the presence of p53 to form a trimeric complex with GRα to mediate receptor proteasomal turnover, both in the presence and absence of GCs (155). Dex treatment of human umbilical endothelial cells enhanced GC-mediated ubiquitination of GRα in the presence of all three proteins (i.e. GRα, p53 and Hdm2) (140). Furthermore, disruption of the interaction of p53 with Hdm2 prevented Dex-induced ubiquitination of GRα (140). Interestingly, both the presence of Mdm2 and p53 where required for oestrogen-mediated GRα protein turnover, via the proteasomal degradation pathway (144).
Strategies to restore the GRα pool for improved GC sensitivity
It is clear that reductions in the GRα pool, whether disease-associated (Table 1), treatment-associated (Table 2), or both, contribute to the development of acquired GC resistance. With the increasing incidence of severe stress, psychological and pathological conditions, in combination with the looming threat of acquired GC resistance, a dire need exists for the development of novel GC therapeutics to combat chronic inflammation, without eliciting GC resistance.
Current strategies
In recent years, as discussed, a number of molecular mechanisms involved in GRα turnover have been uncovered and these have been explored and in some cases utilized in a clinical setting (40, 99, 102, 145).
For example, proteasome inhibitors, such as MG132 (104, 105), used in tissue culture cells, and bortezomib (BZ), used clinically (145) may prevent GC-induced GRα downregulation. Moreover, the repurposing of BZ, a Food and Drug Administration (FDA)-approved therapeutic (146), has been shown to restore GC sensitivity by preventing receptor turnover (99, 145). Specifically, in a model of hypoxic blood–brain barrier damage, O2/glucose deprivation led to an approximate 80% reduction in the GRα protein pool, with BZ treatment restoring the receptor pool to 90% (in the absence of Dex) or 50% (in the presence of Dex) (99). Importantly, this restoration in the GRα pool was associated with increased GC sensitivity (99). Additionally, Lesovaya et al. (145) demonstrated the ability of BZ to increase the anticancer activities of GCs, by maintaining the GRα pool through proteasomal inhibition. Although proteasomal inhibition (99, 104, 105, 145) seems promising for restoring GC sensitivity, chronic inhibition of such a vital system for finely tuning the levels of numerous proteins (171) could be risky.
Other compounds, such as Yokukansan (YKS) (a Japanese herbal medicine for the treatment of psychiatric and psychological symptoms (172, 173)) and Ginsenoside Rh1 (102) (a major active compound in Ginseng (174)) have also been shown to exert a protective effect against GC-mediated GRα turnover. Specifically, YKS counteracted by approximately 20% a stress-induced reduction in the GRα protein pool in mice (40) through a molecular mechanism that reduced (by almost 50%) the expression of miR-124, which targets GRα mRNA. Combinatorial treatment of Ginsenoside Rh1, with Dex, restricted reductions in the GRα pool, thus potentiating Dex’s anti-inflammatory potential, specifically in prolonged treatments (102). Whilst the ability of Ginsenoside Rh1 was found to require mRNA transcription and new protein synthesis (102), suggesting its ability to transcriptionally and post-transcriptionally regulate the GRα pool, the exact mechanism, remains to be elucidated.
Future strategies
To date, current strategies to restore GRα levels for improved GC sensitivity have been based on combinatorial treatments and have not focussed on GRα ligands biased towards preventing a decrease in the receptor pool. Biased ligands, defined by Luttrell et al. (175) as ‘novel pharmacologic entities that possess the unique ability to qualitatively change receptor signalling’, may display an increased efficacy and/or a defined functional selectivity (14, 134), which could be harnessed to improve the therapeutic index of GCs. Additionally, Luttrell et al. (175) makes a strong case that the biological responses that arise from the interaction of a ligand with its cognate receptor are all encoded at that single point of contact with a distinct conformational change in the receptor being the initial consequence of ligand binding. Thus, conformationally biased ligands drive the conformational equilibrium towards a particular state, resulting in differential biological responses downstream.
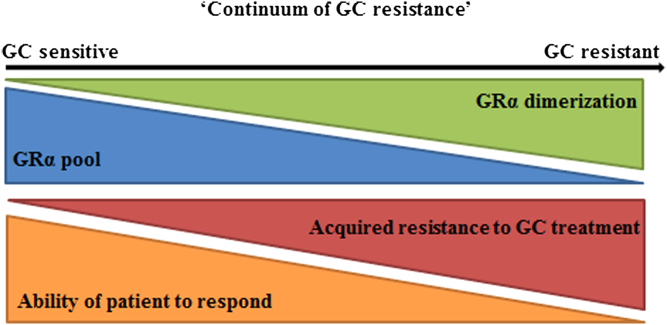
Recently, De Bosscher et al. (176, 177) developed the SEMOGRAM–SEDIGRAM strategy, which is essentially based on conformationally biased ligands that induce either monomers (SEMOGRAMs) or dimers (SEDIGRAMs) of the GR for use as selective therapeutics in chronic or acute inflammation, respectively. Whilst De Bosscher et al. (176, 177) address selectively modulating the dimerization state of GRα in terms of the anti-inflammatory effects of GC signalling vs their adverse side effects, the ligand-selective effects of GRα conformation on receptor turnover, with implications in acquired GC resistance, are not addressed. We now suggest that co-opting the SEMOGRAM–SEDIGRAM strategy for acquired GC resistance could be fruitful and propose the idea of a ‘continuum of resistance’ (Fig. 4), where encouraging GRα dimerization though the use of SEDIGRAMs, may not only have negative implications in terms of the generation of adverse side effects (178, 179), but may also drive reductions in the GRα pool, which encourages a decrease in GC sensitivity. In contrast, the use of SEMOGRAMs, which abrogate GR dimerization, may result in reduced side effects and prevent acquired resistance while maintaining an adequate anti-inflammatory potential, a therapeutic regimen more suited to chronic use.
Figure 4.
A ‘continuum of GC resistance’. As GRα dimerization increases, so increased ligand-induced receptor turnover of the GRα pool, both at the mRNA and protein level, occurs. These significant reductions in receptor turnover, in many cases, drive the development of an acquired resistance to treatment and so the ability of a patient to respond to treatment diminishes.
In support of this, a wealth of pharmacological evidence supports the biased ligand behaviour of the SEMOGRAM, Compound A (CpdA) (180). The biased ligand behaviour of CpdA arises from its ability to abrogate GRα dimerization (181, 182), which favours transrepression of pro-inflammatory genes, which contributes to its potent immunosuppressive effects, over transactivation, generally associated with negative side effects and has proved effective in combatting inflammation in a number of in vivo models (116, 176, 183, 184, 185, 186, 187) without resulting in adverse side effects (116, 184, 186, 188, 189). Furthermore, CpdA does not result in ligand-induced GRα turnover (106, 116, 135), an ability that may be related to its ability to abrogate GR dimerization, and as such may be considered a biased ligand able to prevent acquired resistance. In fact, recent work from our own laboratory demonstrates that dimerization impairment, either through the use of CpdA or the dimerization deficient GR mutant (GRdim), restricts GRα turnover via the proteasome through a molecular mechanism involving a substantial reduction in hyper-phosphorylation at Ser404 and the interaction of GR with the E3 ligase, FBXW7α (190).
Throughout this review reductions in the GRα pool mediated by classical GCs, such as Dex, of anywhere between 10 and 90% have been detailed, which promote GC resistance (Tables 1 and 2). Importantly, these GCs are known to induce GRα dimerization of the GRα (181, 182, 191), prior to eliciting a biological response and subsequently driving receptor turnover, and may thus be termed dimerization promoting GCs or SEDIGRAMs. On the other hand, CpdA, which displays dimerization abrogating potential and is thus a SEMOGRAM, does not induce GRα turnover (106, 116, 135, 190) while maintaining its immunosuppressive capabilities even during prolonged treatment regimens (106, 116). We believe, this begs the question of whether the dimerization state of the GRα is likely to influence development of an acquired resistance to treatment, in prolonged GC regimens.
Caution should, however, be exercised in overenthusiastically embracing GR ligands conformationally biased towards loss of dimerization for prevention of acquired GC resistance as our understanding of the implications of GR dimerization in GC signalling is currently limited. Accordingly, a more prudent approach may be the development of biased ligands positioned along the continuum of GC resistance (Fig. 4) rather than at the extremes of the monomer/dimer dichotomy. Nonetheless, in addition to the current strategy of combinatorial use of compounds (99, 102, 145) that may restrict receptor turnover, we believe that disrupting dimerization through biased ligands, in a tissue-specific manner, may be a fruitful future strategy for developing tailored treatments to counteract the development of acquired GC resistance in a number of disease states. Moreover, an in-depth characterization of the dimerization capabilities (192) of GRα mutants (51), associated with generalized GC resistance, may provide more insight into generalized GC resistance and assist in the treatment of these rare, pathological conditions.
Conclusions
To conclude, acquired GC resistance, due to reductions in the GRα pool, is an ever-increasing therapeutic challenge for patients requiring chronic treatment and occurs ubiquitously throughout a number of psychological and pathological conditions. In recent years, a number of the molecular mechanisms which underpin these GC-mediated reductions in the GRα pool have been elucidated, with attempts to counteract GC-mediated receptor turnover being made through combinatorial treatment of GCs with other compounds, which disrupt transcriptional, post-transcriptional and post-translational GRα regulation. Whilst in some cases, these strategies have proved fruitful, they are not without limitations. Thus, we believe the strategy of using conformationally biased ligands, specifically the SEMOGRAM–SEDIGRAM strategy, which underscores the importance of GRα conformation, with particular reference to the receptor’s dimerization state, requires investigation and offers a novel perspective from which to approach the rational design of drugs that limit GC resistance.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work was supported by the National Research Foundation, South Africa (grant CPRR14072479679 to A L and PhD bursary to L W). Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF do not accept any liability in regard thereto.
Author contribution statement
A L, N J D V and L W participated in drafting the manuscript, revising its intellectual content and approved the final version of the submitted manuscript.
References
- 1.Nicolaides NC, Lamprokostopoulou A, Sertedaki A, Charmandari E. Recent advances in the molecular mechanisms causing primary generalized glucocorticoid resistance. Hormones 2016. 15 23–34. ( 10.1007/BF03401400) [DOI] [PubMed] [Google Scholar]
- 2.Strahler J, Skoluda N, Rohleder N, Nater UM. Dysregulated stress signal sensitivity and inflammatory disinhibition as a pathophysiological mechanism of stress-related chronic fatigue. Neuroscience and Biobehavioral Reviews 2016. 68 298–318. ( 10.1016/j.neubiorev.2016.05.008) [DOI] [PubMed] [Google Scholar]
- 3.Dumbell R, Matveeva O, Oster H. Circadian clocks, stress, and immunity. Frontiers in Endocrinology 2016. 7 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Practice and Research. Clinical Endocrinology and Metabolism 2015. 29 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dendoncker K, Libert C. Cytokine & growth factor reviews glucocorticoid resistance as a major drive in sepsis pathology. Cytokine and Growth Factor Reviews 2017. 35 85–96. ( 10.1016/j.cytogfr.2017.04.002) [DOI] [PubMed] [Google Scholar]
- 6.Howell BR, Sanchez MM. Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Development and Psychopathology 2011. 23 1001–1016. ( 10.1017/S0954579411000460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. Journal of Allergy and Clinical Immunology 2013. 132 1033–1044. ( 10.1016/j.jaci.2013.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care and Research 2013. 65 294–298. ( 10.1002/acr.21796) [DOI] [PubMed] [Google Scholar]
- 9.Quax RA, Manenschijn L, Koper JW, Hazes JM, Lamberts SWJ, van Rossum EFC, Feelders R. Glucocorticoid sensitivity in health and disease. Nature Reviews. Endocrinology 2013. 9 670–686. ( 10.1038/nrendo.2013.183) [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ, Adcock IM. Review glucocorticoid resistance in inflammatory diseases. Lancet 2009. 373 1905–1917. ( 10.1016/S0140-6736(09)60326-3) [DOI] [PubMed] [Google Scholar]
- 11.Haarman EG, Kaspers GJL, Veerman AJP. Glucocorticoid resistance in childhood leukaemia: mechanisms and modulation. British Journal of Haematology 2003. 120 919–929. ( 10.1046/j.1365-2141.2003.04189.x) [DOI] [PubMed] [Google Scholar]
- 12.Williams EL, Stimpson ML, Collins PL, Enki DG, Sinha A, Lee RW, Dhanda AD. Development and validation of a novel bioassay to determine glucocorticoid sensitivity. Biomarker Research 2016. 4 26 ( 10.1186/s40364-016-0079-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weikum ER, Knuesel MT, Ortlund EA, Yamamoto KR. Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nature Reviews Molecular Cell Biology 2017. 18 159–174. ( 10.1038/nrm.2016.152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desmet SJ, De Bosscher K. Glucocorticoid receptors : finding the middle ground. Journal of Clinical Investigation 2017. 127 1136–1145. ( 10.1172/JCI88886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandevyver S, Dejager L, Libert C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocrine Reviews 2014. 35 671–693. ( 10.1210/er.2014-1010) [DOI] [PubMed] [Google Scholar]
- 16.Schaaf MJ., Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. Journal of Steroid Biochemistry and Molecular Biology 2002. 83 37–48. ( 10.1016/S0960-0760(02)00263-7) [DOI] [PubMed] [Google Scholar]
- 17.Cornejo S, Tantisira K, Raby BA, Weiss ST, Kaplan F. Nuclear bioavailability of the glucocorticoid receptor in a pediatric asthma cohort with variable corticosteroid responsiveness. Pediatric Research 2015. 78 505–512. ( 10.1038/pr.2015.148) [DOI] [PubMed] [Google Scholar]
- 18.Merkulov VM, Merkulova TI, Bondar NP. Mechanisms of brain glucocorticoid resistance in stress-induced psychopathologies. Biochemistry 2017. 82 351–365. [DOI] [PubMed] [Google Scholar]
- 19.Beck IM, De Bosscher K, Haegeman G. Glucocorticoid receptor mutants: man-made tools for functional research. Trends in Endocrinology and Metabolism 2011. 22 295–310. ( 10.1016/j.tem.2011.03.009) [DOI] [PubMed] [Google Scholar]
- 20.Kushwah R, Cao H, Hu J, Alerts E. Characterization of pulmonary T cell response to helper-dependent adenoviral vectors following intranasal delivery. Journal of Immunology 2008. 180 4098–4108. ( 10.4049/jimmunol.180.6.4098) [DOI] [PubMed] [Google Scholar]
- 21.Cardinal J, Pretorius CJ, Ungerer JPJ. Biological and diurnal variation in glucocorticoid sensitivity detected with a sensitive in vitro dexamethasone suppression of cytokine production assay. Journal of Clinical Endocrinology and Metabolism 2010. 95 3657–3663. ( 10.1210/jc.2009-2720) [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Gou X, Jiang T, Ouyang J. The effects of microRNAs on glucocorticoid responsiveness. Journal of Cancer Research and Clinical Oncology 2017. 143 1005–1011. ( 10.1007/s00432-017-2388-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreae J, Tripmacher R, Weltrich R, Rohde W, Keitzer R, Wahn U, Paul K, Buttgereit F. Effect of glucocorticoid therapy on glucocorticoid receptors in children with autoimmune diseases. Pediatric Research 2001. 49 130–135. ( 10.1203/00006450-200101000-00025) [DOI] [PubMed] [Google Scholar]
- 24.Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biological Psychiatry 2012. 72 41–48. ( 10.1016/j.biopsych.2012.01.034) [DOI] [PubMed] [Google Scholar]
- 25.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience 2009. 12 342–348. ( 10.1038/nn.2270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perroud N, Rutembesa E, Paoloni-Giacobino A, Mutabaruka J, Mutesa L, Stenz L, Malafosse A, Karege F. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World Journal of Biological Psychiatry 2014. 15 334–345. ( 10.3109/15622975.2013.866693) [DOI] [PubMed] [Google Scholar]
- 27.Bingham BC, Sheela Rani CS, Frazer A, Strong R, Morilak DA. Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior. Psychoneuroendocrinology 2013. 38 2746–2757. ( 10.1016/j.psyneuen.2013.07.003) [DOI] [PubMed] [Google Scholar]
- 28.Navailles S, Zimnisky R, Schmauss C. Expression of glucocorticoid receptor and early growth response gene 1 during postnatal development of two inbred strains of mice exposed to early life stress. Developmental Neuroscience 2010. 32 139–148. ( 10.1159/000293989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SW, Lee JG, Mi K, Ngoc N, Chan H, Hye YC, Hein LT, Jeong A, Kim G, Young HK. Epigenetic modification of glucocorticoid receptor promoter I 7 in maternally separated and restraint-stressed rats. Neuroscience Letters 2017. 650 38–44. ( 10.1016/j.neulet.2017.04.024) [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Wang Y, Yao R, Hao T, Cao J, Huang H, Wang L, Wu Y. Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period. Journal of Neuroinflammation 2017. 14 6 ( 10.1186/s12974-016-0782-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnett MG, Pan MS, Doak W, Cyr PEP, Muglia LM, Muglia LJ. The role of glucocorticoid receptor-dependent activity in the amygdala central nucleus and reversibility of early-life stress programmed behavior. Translational Psychiatry 2015. 5 e542 ( 10.1038/tp.2015.35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Roozbahany NA, Abbaszadeh HA. Hippocampal NR3C1 DNA methylation can mediate part of preconception paternal stress effects in rat offspring. Behavioural Brain Research 2017. 324 71–76. ( 10.1016/j.bbr.2017.02.014) [DOI] [PubMed] [Google Scholar]
- 33.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality following stress early in pregnancy. Journal of Neuroscience 2009. 28 9055–9065. ( 10.1523/JNEUROSCI.1424-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan N, Chiu MPY, Ellis L, Weinberg J. Prenatal alcohol exposure and prenatal stress differentially alter glucocorticoid signaling in the placenta and fetal brain. Neuroscience 2017. 342 167–179. ( 10.1016/j.neuroscience.2015.08.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smart C, Strathdee G, Watson S, Murgatroyd C, McAllister-Williams RH. Early life trauma, depression and the glucocorticoid receptor gene – an epigenetic perspective. Psychological Medicine 2015. 44 1–18. [DOI] [PubMed] [Google Scholar]
- 36.Zannas AS, Chrousos GP. Epigenetic programming by stress and glucocorticoids along the human lifespan. Molecular Psychiatry 2017. 22 640–646. ( 10.1038/mp.2017.35) [DOI] [PubMed] [Google Scholar]
- 37.Witzmann SR, Turner JD, Meriaux SB, Meijer OC, Muller CP. Epigenetic regulation of the glucocorticoid receptor promoter 1 7 in adult rats. Epigenetics 2012. 7 1290–1301. ( 10.4161/epi.22363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung SH, Wang Y, Kim T, Tarr A, Reader B, Powell N, Sheridan JF. Molecular mechanisms of repeated social defeat-induced glucocorticoid resistance: role of microRNA. Brain, Behavior, and Immunity 2015. 44 195–206. ( 10.1016/j.bbi.2014.09.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makhathini KB, Abboussi O, Stein DJ, Mabandla MV, Daniels WMU. Repetitive stress leads to impaired cognitive function that is associated with DNA hypomethylation, reduced BDNF and a dysregulated HPA axis. International Journal of Developmental Neuroscience 2017. 60 63–69. ( 10.1016/j.ijdevneu.2017.04.004) [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S, Tanaka T, Tohyama M, Miyata S. Yokukansan normalizes glucocorticoid receptor protein expression in oligodendrocytes of the corpus callosum by regulating microRNA-124a expression after stress exposure. Brain Research Bulletin 2015. 114 49–55. ( 10.1016/j.brainresbull.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 41.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2012. 39 112–119. ( 10.1016/j.pnpbp.2012.05.018) [DOI] [PubMed] [Google Scholar]
- 42.Mifsud KR, Saunderson EA, Spiers H, Carter SD, Trollope AF, Mill J, Reul JMHM. Rapid down-regulation of glucocorticoid receptor gene expression in the dentate gyrus after acute stress in vivo: role of DNA methylation and microRNA activity. Neuroendocrinology 2016. 104 157–169. ( 10.1159/000445875) [DOI] [PubMed] [Google Scholar]
- 43.Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. Journal of Neuroimmunology 2003. 137 51–58. ( 10.1016/S0165-5728(03)00042-0) [DOI] [PubMed] [Google Scholar]
- 44.Han Q, Yang L, Huang H, Wang Y, Yu R, Wang J. Differential GR expression and translocation in the hippocampus mediates susceptibility vs . resilience to chronic social defeat stress. Frontiers in Neuroscience 2017. 11 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li B, Duan XH, Wu JF, Liu BJ, Luo QL, Jin HL, Du YJ, Zhang HY, Cao YX, Dong JC. Impact of psychosocial stress on airway inflammation and its mechanism in a murine model of allergic asthma. Chinese Medical Journal 2013. 126 325–334. [PubMed] [Google Scholar]
- 46.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS ONE 2012. 7 e30148 ( 10.1371/journal.pone.0030148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyrka AR, Parade SH, Welch ES, Ridout KK, Price LH, Marsit C, Philip NS, Carpenter LL. Methylation of the leukocyte glucocorticoid receptor gene promoter in adults : associations with early adversity and depressive , anxiety and substance-use disorders. 2016. 6 e848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Foundation Symposium 2007. 172 296–316. [DOI] [PubMed] [Google Scholar]
- 49.Lopez JF, Chalmers DT, Little KY, Watson SJ. Regulation of serotonin (1A), glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biological Psychiatry 1998. 43 547–573. ( 10.1016/S0006-3223(97)00484-8) [DOI] [PubMed] [Google Scholar]
- 50.Pariante CM. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. European Neuropsychopharmacology 2017. 27 554–559. ( 10.1016/j.euroneuro.2017.04.001) [DOI] [PubMed] [Google Scholar]
- 51.Spencer R, Williams EL, Stimpson ML, Collins PL, Enki DG, Sinha A, Lee RW, Dhanda AD, Spijker AT, Cardinal J, et al. Synthesis of novel steroidal agonists, partial agonists, and antagonists for the glucocorticoid receptor. Bioorganic and Medicinal Chemistry Letters 2017. 27 347–353. ( 10.1016/j.bmcl.2016.11.007) [DOI] [PubMed] [Google Scholar]
- 52.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology 2013. 38 377–385. ( 10.1038/npp.2012.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikkheslat N, Zunszain PA, Horowitz MA, Barbosa IG, Parker JA, Myint A, Schwarz MJ, Tylee AT, Carvalho LA, Pariante CM. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behavior and Immunity 2015. 48 8–18. ( 10.1016/j.bbi.2015.02.002) [DOI] [PubMed] [Google Scholar]
- 54.Matsubara T, Funato H, Kobayashi A, Nobumoto M, Watanabe Y. Reduced glucocorticoid receptor alpha expression in mood disorder patients and first-degree relatives. Biological Psychiatry 2006. 59 689–695. ( 10.1016/j.biopsych.2005.09.026) [DOI] [PubMed] [Google Scholar]
- 55.Wang W, Feng J, Ji C, Mu X, Ma Q, Fan Y, Chen C, Gao C, Ma X, Zhu F. Increased methylation of glucocorticoid receptor gene promoter 1 F in peripheral blood of patients with generalized anxiety disorder. Journal of Psychiatric Research 2017. 91 18–25. ( 10.1016/j.jpsychires.2017.01.019) [DOI] [PubMed] [Google Scholar]
- 56.Matić G, Milutinović DV, Nestorov J, Elaković I, Jovanović SM, Perišić T, Dunderski J, Damjanović S, Knežević G, Špirić Ž, et al. Lymphocyte glucocorticoid receptor expression level and hormone-binding properties differ between war trauma-exposed men with and without PTSD. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2013. 43 238–245. [DOI] [PubMed] [Google Scholar]
- 57.Gola H, Engler A, Morath J, Adenauer H, Elbert T, Kolassa IT, Engler H. Reduced peripheral expression of the glucocorticoid receptor α isoform in individuals with posttraumatic stress disorder: a cumulative effect of trauma burden. PLoS ONE 2014. 9 e86333 ( 10.1371/journal.pone.0086333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster M, Knable M, O’ Grady J, Orthmann J, Weickert C. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Molecular Psychiatry 2002. 7 985–994. ( 10.1038/sj.mp.4001139) [DOI] [PubMed] [Google Scholar]
- 59.Gotovac K, Sabioncello A, Rabati S, Berki T, Dekaris D. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: Lower quantity of GCR in patients with post-traumatic stress disorder (PTSD). Clinical and Experimental Immunology 2003. 131 335–339. ( 10.1046/j.1365-2249.2003.02075.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bloomfield D, Smith A. Glucocorticoids and lymphocytes. Blood 2017. 61 1086–1090. [PubMed] [Google Scholar]
- 61.Inui S, Sumikawa Y, Asada H, Itami S. Glucocorticoid resistance in atopic dermatitis associated with decreased expression of glucocorticoid receptor-alpha in peripheral blood mononuclear cells. Journal of Dermatology 2010. 37 496–499. ( 10.1111/j.1346-8138.2010.00866.x) [DOI] [PubMed] [Google Scholar]
- 62.Chen H, Fan J, Shou Q, Zhang L, Ma H, Fan Y. Hypermethylation of glucocorticoid receptor gene promoter results in glucocorticoid receptor gene low expression in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. Rheumatology International 2015. 35 1335–1342. ( 10.1007/s00296-015-3266-5) [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Pang C, Ma X, An Y, Feng X. Role of glucocorticoid receptor in the pathogenesis of systemic lupus erythematosus. Journal of Peking University. Health Sciences 2012. 44 229–232. [PubMed] [Google Scholar]
- 64.Guan Y, Zhang Y, Fang M, Guan J, Sun X, Zhang J. The relationship between mRNA level of glucocorticoid receptor α, heat shock protein 90, protein level of macrophage migration inhibitory factor and glucocorticoid resistance in systemic lupus erythematosus. Zhonghua nei ke za zhi 2015. 54 922–926. [PubMed] [Google Scholar]
- 65.Ma L, Fang M, Liang Y, Xiang Y, Jia Z, Sun X, Wang Y, Qin J. Low expression of glucocorticoid receptor alpha isoform in adult immune thrombocytopenia correlates with glucocorticoid resistance. Annals of Hematology 2013. 92 953–960. ( 10.1007/s00277-013-1705-5) [DOI] [PubMed] [Google Scholar]
- 66.Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor β-isoform. Journal of Allergy and Clinical Immunology 2000. 105 943–950. ( 10.1067/mai.2000.106486) [DOI] [PubMed] [Google Scholar]
- 67.Sher ER, Leung DYM, Surs W, Kam JC, Zieg G, Kamada AK, Szefler SJ. Steroid-resistant asthma. Cellular mechanisms contributing to inadequate response to glucocorticoid therapy. Journal of Clinical Investigation 1994. 93 33–39. ( 10.1172/JCI116963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pujols L, Xaubet A, Ramírez J, Mullol J, Roca-Ferrer J, Torrego A, Cidlowski JA, Picado C. Expression of glucocorticoid receptors alpha and beta in steroid sensitive and steroid insensitive interstitial lung diseases. Thorax 2004. 59 687–693. ( 10.1136/thx.2003.013268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marwick JA, Caramori G, Stevenson CS, Casolari P, Jazrawi E, Barnes PJ, Ito K, Adcock IM, Kirkham PA, Papi A. Inhibition of PI3Kδ restores glucocorticoid function in smoking-induced airway inflammation in mice. American Journal of Respiratory and Critical Care Medicine 2009. 179 542–548. ( 10.1164/rccm.200810-1570OC) [DOI] [PubMed] [Google Scholar]
- 70.Hodge G, Jersmann H, Tran HB, Holmes M, Reynolds PN, Hodge S. Lymphocyte senescence in COPD is associated with loss of glucocorticoid receptor expression by pro-inflammatory/cytotoxic lymphocytes. Respiratory Research 2015. 16 2 ( 10.1186/s12931-014-0161-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hodge G, Roscioli E, Jersmann H, Tran HB, Holmes M, Reynolds PN, Hodge S. Steroid resistance in COPD is associated with impaired molecular chaperone Hsp90 expression by pro-inflammatory lymphocytes. Respiratory Research 2016. 17 135 ( 10.1186/s12931-016-0450-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozaci DL, Chernajovsky Y, Chikanza IC. The differential expression of corticosteroid receptor isoforms in corticosteroid-resistant and -sensitive patients with rheumatoid arthritis. Rheumatology 2007. 46 579–585. ( 10.1093/rheumatology/kel276) [DOI] [PubMed] [Google Scholar]
- 73.Schlaghecke R, Beuscher D, Kornely E, Specker C. Effects of glucocorticoids in rheumatoid arthritis. Diminished glucocorticoid receptors do not result in glucocorticoid resistance. Arthritis and Rheumatism 1994. 37 1127–1131. [DOI] [PubMed] [Google Scholar]
- 74.Dibattista JA, Martel-Pelletier J, Antakly T, Tardif G, Cloutier JM, Pelletier JP. Reduced expression of glucocorticoid receptor levels in human osteoarthritic chondrocytes. Role in the suppression of metalloprotease synthesis. Journal of Clinical Endocrinology and Metabolism 1993. 76 1128–1134. [DOI] [PubMed] [Google Scholar]
- 75.Creed TJ, Probert CSJ. Review article: steroid resistance in inflammatory bowel disease-mechanisms and therapeutic strategies. Alimentary Pharmacology and Therapeutics 2007. 25 111–122. [DOI] [PubMed] [Google Scholar]
- 76.Raddatz D, Middel P, Bockemuhl M, Benohr P, Wissmann C, Schworer H, Ramadori G, Bockemühl M, Benöhr P, Schwörer H. Glucocorticoid receptor expression in inflammatory bowel disease: evidence for a mucosal down-regulation in steroid-unresponsive ulcerative colitis. Alimentary Pharmacology and Therapeutics 2004. 19 47–61. ( 10.1046/j.1365-2036.2003.01802.x) [DOI] [PubMed] [Google Scholar]
- 77.Gold SM, Sasidhar MV, Lagishetty V, Spence RD, Umeda E, Ziehn MO, Krieger T, Schulz KH, Heesen C, Hewison M, et al. Dynamic development of glucocorticoid resistance during autoimmune neuroinflammation. Journal of Clinical Endocrinology and Metabolism 2012. 97 1402–1410. ( 10.1210/jc.2012-1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor KM, Ray DW, Sommer P. Glucocorticoid receptors in lung cancer: new perspectives. Journal of Endocrinology 2016. 229 R17–R28. ( 10.1530/JOE-15-0496) [DOI] [PubMed] [Google Scholar]
- 79.Patki M, Gadgeel S, Huang Y, McFall T, Shields AF, Matherly LH, Bepler G, Ratnam M. Glucocorticoid receptor status is a principal determinant of variability in the sensitivity of non-small-cell lung cancer cells to pemetrexed. Journal of Thoracic Oncology 2014. 9 519–526. ( 10.1097/JTO.0000000000000111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sommer P, Cowen RL, Berry A, Cookson A, Telfer BA, Williams KJ, Stratford IJ, Kay P, White A, Ray DW. Glucocorticoid receptor over-expression promotes human small cell lung cancer apoptosis in vivo and thereby slows tumor growth. Endocrine-Related Cancer 2010. 17 203–213. ( 10.1677/ERC-09-0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kay P, Schlossmacher G, Matthews L, Sommer P, Singh D, White A, Ray D. Loss of glucocorticoid receptor expression by DNA methylation prevents glucocorticoid induced apoptosis in human small cell lung cancer cells. PLoS ONE 2011. 6 e24839 ( 10.1371/journal.pone.0024839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlossmacher G, Platt E, Davies A, Meredith S, White A. Glucocorticoid receptor-mediated apoptosis in small-cell lung cancer requires interaction with BCL2. Endocrine-Related Cancer 2013. 20 785–795. ( 10.1530/ERC-13-0402) [DOI] [PubMed] [Google Scholar]
- 83.Schmidt S, Irving JE, Minto L, Matheson E, Nicholson L, Ploner A, Parson W, Kofler A, Amort M, Erdel M, et al Glucocorticoid resistance in two key models of acute lymphoblastic leukemia occurs at the level of the glucocorticoid receptor. FASEB Journal 2006. 20 2600–2602. ( 10.1096/fj.06-6214fje) [DOI] [PubMed] [Google Scholar]
- 84.Lv M, Zhang X, Jia H, Li D, Zhang B, Zhang H, Hong M, Jiang T, Jiang Q, Lu J, et al. An oncogenic role of miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by targeting glucocorticoid receptor-α and cAMP/PKA pathways. Leukemia 2012. 26 769–777. ( 10.1038/leu.2011.273) [DOI] [PubMed] [Google Scholar]
- 85.Jones CL, Gearheart CM, Fosmire S, Delgado-Martin C, Evensen NA, Bride K, Waanders AJ, Pais F, Wang J, Bhatla T, et al MAPK signaling cascades mediate distinct glucocorticoid resistance mechanisms in pediatric leukemia. Blood 2015. 126 2202–2212. ( 10.1182/blood-2015-04-639138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tissing WJE, Meijerink JPP, den Boer ML, Pieters R. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia 2003. 17 17–25. ( 10.1038/sj.leu.2402733) [DOI] [PubMed] [Google Scholar]
- 87.Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T, Anderson KC, Rosen ST. Characterization of the MM.1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Experimental Hematology 2003. 31 271–282. ( 10.1016/S0301-472X(03)00023-7) [DOI] [PubMed] [Google Scholar]
- 88.Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, Gupta D, Richardson P, Schlossman RL, Krett N, et al Identification of genes regulated by Dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene 2002. 6 1346–1358. ( 10.1038/sj.onc.1205205) [DOI] [PubMed] [Google Scholar]
- 89.Tessel MA, Benham AL, Krett NL, Rosen ST, Gunaratne PH. Role for microRNAs in regulating glucocorticoid response and resistance in multiple myeloma. Hormonal Cancer 2013. 18 1199–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sánchez-Vega B, Gandhi V. Glucocorticoid resistance in a multiple myeloma cell line is regulated by a transcription elongation block in the glucocorticoid receptor gene (NR3C1). British Journal of Haematology 2009. 144 856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heuck CJ, Szymonifka J, Hansen E, Shaughnessy JD, Usmani SZ, Van Rhee F, Anaissie E, Nair B, Waheed S, Alsayed Y, et al. Thalidomide in total therapy 2 overcomes inferior prognosis of myeloma with low expression of the glucocorticoid receptor gene NR3C1. Clinical Cancer Research 2012. 18 5499–5506. ( 10.1158/1078-0432.CCR-12-0019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nesset KA, Perri AM, Mueller CR. Frequent promoter hypermethylation and expression reduction of the glucocorticoid receptor gene in breast tumors. Epigenetics 2014. 9 851–859. ( 10.4161/epi.28484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lien HC, Lu YS, Cheng AL, Chang WC, Jeng YM, Kuo YH, Huang CS, Chang KJ, Yao YT. Differential expression of glucocorticoid receptor in human breast tissues and related neoplasms. Journal of Pathology 2006. 209 317–327. ( 10.1002/path.1982) [DOI] [PubMed] [Google Scholar]
- 94.Vanderbilt JN, Miesfeld R, Maler BA, Yamamoto KR. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Molecular Endocrinology 1987. 1 68–74. ( 10.1210/mend-1-1-68) [DOI] [PubMed] [Google Scholar]
- 95.Ledderose C, Möhnle P, Limbeck E, Schütz S, Weis F, Rink J, Briegel J, Kreth S. Corticosteroid resistance in sepsis is influenced by microRNA-124--induced downregulation of glucocorticoid receptor-α. Critical Care Medicine 2012. 40 2745–2753. ( 10.1097/CCM.0b013e31825b8ebc) [DOI] [PubMed] [Google Scholar]
- 96.Van Den Akker ELT, Koper JW, Joosten K, De Jong FH, Hazelzet JA, Lamberts SWJ, Hokken-Koelega ACS. Glucocorticoid receptor mRNA levels are selectively decreased in neutrophils of children with sepsis. Intensive Care Medicine 2009. 35 1247–1254. ( 10.1007/s00134-009-1468-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hammad A, Yahia S, Gouida MS, Bakr A, El-Farahaty RM. Low expression of glucocorticoid receptors in children with steroid-resistant nephrotic syndrome. Pediatric Nephrology 2013. 28 759–763. ( 10.1007/s00467-012-2385-4) [DOI] [PubMed] [Google Scholar]
- 98.Rutkowski D, Syed F, Matthews LC, Ray DW, McGrouther DA, Watson REB, Bayat A. An abnormality in glucocorticoid receptor expression differentiates steroid responders from non-responders in keloid disease. British Journal of Dermatology 2015. 173 690–700. ( 10.1111/bjd.13752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kleinschnitz C, Blecharz K, Kahles T, Schwarz T, Kraft P, Göbel K, Meuth SG, Burek M, Thum T, Stoll G, et al. Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke 2011. 42 1081–1089. ( 10.1161/STROKEAHA.110.592238) [DOI] [PubMed] [Google Scholar]
- 100.Griese M, Kusenbach G, Lüsebring K, Köster W, Roth B, Reinhardt D. Glucocorticoid receptors in mononuclear blood cells and their correlation to endogenous and exogenous corticoids in healthy and asthmatic children. European Journal of Pediatrics 1988. 147 490–495. ( 10.1007/BF00441973) [DOI] [PubMed] [Google Scholar]
- 101.Urzua CA, Guerrero J, Gatica H, Velasquez V, Goecke A. Evaluation of the glucocorticoid receptor as a biomarker of treatment response in Vogt-Koyanagi-Harada disease. Investigative Opthalmology and Visual Science 2017. 58 974 ( 10.1167/iovs.16-20783) [DOI] [PubMed] [Google Scholar]
- 102.Li J, Du J, Liu D, Cheng B, Fang F, Weng L, Wang C, Ling C. Ginsenoside Rh1 potentiates dexamethasone’s anti-inflammatory effects for chronic inflammatory disease by reversing dexamethasone-induced resistance. Arthritis Research and Therapy 2014. 16 R106 ( 10.1186/ar4556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silva CM, Powell-Oliver FE, Jewell CM, Sar M, Allgood VE, Cidlowski JA. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids 1994. 59 436–442. ( 10.1016/0039-128X(94)90013-2) [DOI] [PubMed] [Google Scholar]
- 104.Wallace AD, Cidlowski JA. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. Journal of Biological Chemistry 2001. 276 42714–42721. ( 10.1074/jbc.M106033200) [DOI] [PubMed] [Google Scholar]
- 105.Wallace AD, Cao Y, Chandramouleeswaran S, Cidlowski JA. Lysine 419 targets human glucocorticoid receptor for proteasomal degradation. Steroids 2010. 75 1016–1023. ( 10.1016/j.steroids.2010.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Visser K, Smith C, Louw A. Interplay of the inflammatory and stress systems in a hepatic cell line: interactions between glucocorticoid receptor agonists and interleukin-6. Endocrinology 2010. 151 5279–5293. ( 10.1210/en.2010-0368) [DOI] [PubMed] [Google Scholar]
- 107.Ramamoorthy S, Cidlowski JA. Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Molecular and Cellular Biology 2013. 33 1711–1722. ( 10.1128/MCB.01151-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pujols L, Mullol J, Perez M, Roca-Ferrer J, Juan M, Xaubet A, Cidlowski JA, Picado C. Expression of the human glucocorticoid receptor alpha and beta isoforms in human respiratory epithelial cells and their regulation by dexamethasone. American Journal of Respiratory Cell and Molecular Biology 2001. 24 49–57. ( 10.1165/ajrcmb.24.1.4024) [DOI] [PubMed] [Google Scholar]
- 109.Ling C, Li Y, Zhu X, Zhang C, Li M. Ginsenosides may reverse the dexamethasone-induced down-regulation of glucocorticoid receptor. General and Comparative Endocrinology 2005. 140 203–209. ( 10.1016/j.ygcen.2004.11.003) [DOI] [PubMed] [Google Scholar]
- 110.Dekelbab BH, Witchel SF, & DeFranco DB. TNF-alpha and glucocorticoid receptor interaction in L6 muscle cells: a cooperative downregulation of myosin heavy chain. Steroids 2007. 72 705–712. ( 10.1016/j.steroids.2007.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun Y, Wan X, Ouyang J, Xie R, Wang X, Chen P. Prenatal dexamethasone exposure increases the susceptibility to autoimmunity in offspring rats by epigenetic programing of glucocorticoid receptor. BioMed Research International 2016. 2016 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bellingham DL, Sar M, Cidlowski JA. Ligand-dependent down-regulation of stably transfected human glucocorticoid receptors is associated with the loss of functional glucocorticoid responsiveness. Molecular Endocrinology 1992. 6 2090–2102. [DOI] [PubMed] [Google Scholar]
- 113.Zhang B, Zhang Y, Xu T, Yin Y, Huang R, Wang Y, Zhang J, Huang D, Li W. Chronic dexamethasone treatment results in hippocampal neurons injury due to activate NLRP1 inflammasome in vitro. International Immunopharmacology 2017. 49 222–230. ( 10.1016/j.intimp.2017.05.039) [DOI] [PubMed] [Google Scholar]
- 114.Guess A, Agrawal S, Wei CC, Ransom RF, Benndorf R, Smoyer WE. Dose- and time-dependent glucocorticoid receptor signaling in podocytes. American Journal of Physiology. Renal Physiology 2010. 299 F845–F853. ( 10.1152/ajprenal.00161.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu W, Zhang Y, Wu W, Yin Y, Huang D, Wang Y, Li W, Li W. Chronic glucocorticoids exposure enhances neurodegeneration in the frontal cortex and hippocampus via NLRP-1 inflammasome activation in male mice. Brain, Behavior, and Immunity 2016. 52 58–70. ( 10.1016/j.bbi.2015.09.019) [DOI] [PubMed] [Google Scholar]
- 116.Gossye V, Elewaut D, Van Beneden K, Dewint P, Haegeman G, De Bosscher K. A plant-derived glucocorticoid receptor modulator attenuates inflammation without provoking ligand-induced resistance. Annals of the Rheumatic Diseases 2010. 69 291–296. ( 10.1136/ard.2008.102871) [DOI] [PubMed] [Google Scholar]
- 117.Barrett TJ, Vig E, Vedeckis WV. Coordinate regulation of glucocorticoid receptor and c- jun gene expression is cell type-specific and exhibits differential hormonal sensitivity for down- and up-regulation. Biochemistry 1996. 35 9746–9753. ( 10.1021/bi960058j) [DOI] [PubMed] [Google Scholar]
- 118.Djordjevic-Markovic R, Radic O, Jelic V, Radojcic M, Rapic-Otrin V, Ruzdijic S, Krstic-Demonacos M, Kanazir S, Kanazir D. Glucocorticoid receptors in ageing rats. Experimental Gerontology 1999. 34 971–982. ( 10.1016/S0531-5565(99)00067-4) [DOI] [PubMed] [Google Scholar]
- 119.Shimojo M. Differences in down-regulation of glucocorticoid receptor mRNA by cortisol, prednisolone and dexamethasone in HeLa cells. Journal of the Medical Society of Toho University 1995. 42 331–338. [DOI] [PubMed] [Google Scholar]
- 120.Peeters RP, Hagendorf A, Vanhorebeek I, Visser TJ, Klootwijk W, Mesotten D, Wouters PJ, Koper JW, De Jong FH, Feelders RA, et al. Tissue mRNA expression of the glucocorticoid receptor and its splice variants in fatal critical illness. Clinical Endocrinology 2009. 71 145–153. ( 10.1111/j.1365-2265.2008.03443.x) [DOI] [PubMed] [Google Scholar]
- 121.Vachier I, Roux S, Chanez P, Loubatière J, Térouanne B, Nicolas JC, Godard P. Glucocorticoids induced down-regulation of glucocorticoid receptor mRNA expression in asthma. Clinical and Experimental Immunology 1996. 103 311–315. ( 10.1046/j.1365-2249.1996.d01-628.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berki T, Tavakoli A, Nagy KK, Nagy G, Nemeth P. Alterations of glucocorticoid receptor expression during glucocorticoid hormone therapy in renal transplant patients. Transplant International 2002. 15 132–138. ( 10.1111/j.1432-2277.2002.tb00140.x) [DOI] [PubMed] [Google Scholar]
- 123.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985. 318 635–641. ( 10.1038/318635a0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, Li M, Chambon P. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 2011. 145 224–241. ( 10.1016/j.cell.2011.03.027) [DOI] [PubMed] [Google Scholar]
- 125.Schaaf MJ, Cidlowski JA. AUUUA motifs in the 3’UTR of human glucocorticoid receptor alpha and beta mRNA destabilize mRNA and decrease receptor protein expression. Steroids 2002. 67 627–636. ( 10.1016/S0039-128X(02)00015-6) [DOI] [PubMed] [Google Scholar]
- 126.Lam JKW, Chow MYT, Zhang Y, Leung SWS. siRNA versus miRNA as therapeutics for Gene Silencing. Molecular Therapy—Nucleic Acids 2015. 4 e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Riester A, Issler O, Spyroglou A, Rodrig SH, Chen A, Beuschlein F. ACTH-dependent regulation of microRNA as endogenous modulators of glucocorticoid receptor expression in the adrenal gland. Endocrinology 2012. 153 212–222. ( 10.1210/en.2011-1285) [DOI] [PubMed] [Google Scholar]
- 128.Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, Watanuki T, Wakabayashi Y, Otsuki K, McEwen BS, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: Possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. European Journal of Neuroscience 2008. 27 2250–2261. ( 10.1111/j.1460-9568.2008.06218.x) [DOI] [PubMed] [Google Scholar]
- 129.Vreugdenhil E, Verissimo CSL, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, Champagne DL, Schouten T, Meijer OC, De Kloet ER, et al. Glucocorticoid Receptor : Implications for Glucocorticoid Responsiveness in the Brain. Endocrinology 2009. 150 2220–2228. ( 10.1210/en.2008-1335) [DOI] [PubMed] [Google Scholar]
- 130.Liang YN, Tang YL, Ke ZY, Chen YQ, Luo XQ, Zhang H, Huang LB. MiR-124 contributes to glucocorticoid resistance in acute lymphoblastic leukemia by promoting proliferation, inhibiting apoptosis and targeting the glucocorticoid receptor. Journal of Steroid Biochemistry and Molecular Biology 2017. 172 62–68. ( 10.1016/j.jsbmb.2017.05.014) [DOI] [PubMed] [Google Scholar]
- 131.Anbalagan M, Huderson B, Murphy L, Rowan BG. Post-translational modifications of nuclear receptors and human disease. Nuclear Receptor Signaling 2012. 10 1–13. ( 10.1621/nrs.10001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences 2013. 34 518–530. ( 10.1016/j.tips.2013.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids 2010. 75 1–12. ( 10.1016/j.steroids.2009.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramamoorthy S, Cidlowski JA. Corticosteroids-mechanisms of action in health and disease. Rheumatic Diseases Clinics of North America 2016. 8 583–592. ( 10.1016/j.rdc.2015.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Avenant C, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Role of ligand-dependent GR phosphorylation and half-life in determination of ligand-specific transcriptional activity. Molecular and Cellular Endocrinology 2010. 327 72–88. ( 10.1016/j.mce.2010.06.007) [DOI] [PubMed] [Google Scholar]
- 136.Galliher-Beckley AJ, Williams JG, Collins JB, Cidlowski JA. Glycogen synthase kinase 3β-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Molecular and Cellular Biology 2008. 28 7309–7322. ( 10.1128/MCB.00808-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Webster JC, Jewell CM, Bodwell JE, Munck A, Sar M, Cidlowski JA. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. Journal of Biological Chemistry 1997. 272 9287–9293. ( 10.1074/jbc.272.14.9287) [DOI] [PubMed] [Google Scholar]
- 138.Webster JC, Cidlowski JA. Downregulation of the glucocorticoid receptor. A mechanism for physiological adaptation to hormones. Annals of the New York Academy of Sciences 1994. 746 216–220. ( 10.1111/j.1749-6632.1994.tb39238.x) [DOI] [PubMed] [Google Scholar]
- 139.Wang X, DeFranco DB. Alternative effects of the ubiquitin-proteasome pathway on glucocorticoid receptor down-regulation and transactivation are mediated by CHIP, an E3 ligase. Molecular Endocrinology 2005. 19 1474–1482. ( 10.1210/me.2004-0383) [DOI] [PubMed] [Google Scholar]
- 140.Sengupta S, Wasylyk B. Ligand-dependent interaction of the glucocorticoid receptor with p53 enhances their degradation by Hdm2. Genes and Development 2001. 15 2367–2380. ( 10.1101/gad.202201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sultana R, Theodoraki MA, Caplan AJ. Specificity in the actions of the UBR1 ubiquitin ligase in the degradation of nuclear receptors. FEBS Open Bio 2013. 3 394–397. ( 10.1016/j.fob.2013.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang X, Pongrac JL, DeFranco DB. Glucocorticoid receptors in hippocampal neurons that do not engage proteasomes escape from hormone-dependent down-regulation but maintain transactivation activity. Molecular Endocrinology 2002. 16 1987–1998. ( 10.1210/me.2001-0287) [DOI] [PubMed] [Google Scholar]
- 143.Deroo BJ, Rentsch C, Sampath S, Young J, DeFranco DB, Archer TK. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Molecular and Cellular Biology 2002. 22 4113–4123. ( 10.1128/MCB.22.12.4113-4123.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kinyamu HK, Archer TK. Estrogen receptor-dependent proteasomal degradation of the glucocorticoid receptor is coupled to an increase in mdm2 protein expression. Molecular and Cellular Biology 2003. 23 5867–5881. ( 10.1128/MCB.23.16.5867-5881.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lesovaya E, Yemelyanov A, Kirsanov K, Popa A, Belitsky G, Yakubovskaya M, Gordon LI, Rosen ST, Budunova I. Combination of a selective activator of the glucocorticoid receptor Compound A with a proteasome inhibitor as a novel strategy for chemotherapy of hematologic malignancies. Cell Cycle 2013. 12 133–144. ( 10.4161/cc.23048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yemelyanov A, Bhalla P, Yang X, Ugolkov A, Iwadate K, Karseladze A, Budunova I. Differential targeting of androgen and glucocorticoid receptors induces ER stress and apoptosis in prostate cancer cells: a novel therapeutic modality. Cell cycle 2012. 11 395–406. ( 10.4161/cc.11.2.18945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yemelyanov A, Czwornog J, Gera L, Joshi S, Chatterton RT, Budunova I. Novel steroid receptor phyto-modulator compound a inhibits growth and survival of prostate cancer cells. Cancer Research 2008. 68 4763–4773. ( 10.1158/0008-5472.CAN-07-6104) [DOI] [PubMed] [Google Scholar]
- 148.Müller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nature Reviews. Molecular Cell Biology 2001. 2 202–210. [DOI] [PubMed] [Google Scholar]
- 149.Paakinaho V, Kaikkonen S, Makkonen H, Benes V, Palvimo JJ. SUMOylation regulates the chromatin occupancy and anti-proliferative gene programs of glucocorticoid receptor. Nucleic Acids Research 2014. 42 1575–1592. ( 10.1093/nar/gkt1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Druker J, Liberman AC, Antunica-Noguerol M, Gerez J, Paez-Pereda M, Rein T, Iniguez-Lluhi JA, Holsboer F, Arzt E. RSUME enhances glucocorticoid receptor SUMOylation and transcriptional activity. Molecular and Cellular Biology 2013. 33 2116–2127. ( 10.1128/MCB.01470-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hua G, Paulen L, Chambon P. GR SUMOylation and formation of an SUMO-SMRT/NCoR1-HDAC3 repressing complex is mandatory for GC-induced IR nGRE-mediated transrepression. PNAS 2015. 113 E626–E634. ( 10.1073/pnas.1522821113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drean Y Le, Mincheneau N, Goff P Le, Michel D. Potentiation of glucocorticoid receptor transcriptional activity by sumoylation. Endocrinology 2002. 143 3482–3489. ( 10.1210/en.2002-220135) [DOI] [PubMed] [Google Scholar]
- 153.Vandevyver S, Dejager L, Libert C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic 2012. 13 364–374. ( 10.1111/j.1600-0854.2011.01288.x) [DOI] [PubMed] [Google Scholar]
- 154.Trebble PJ, Woolven JM, Saunders KA, Simpson KD, Farrow SN, Matthews LC, Ray DW. A ligand-specific kinetic switch regulates glucocorticoid receptor trafficking and function. Journal of Cell Science 2013. 126 3159–3169. ( 10.1242/jcs.124784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Davies L, Paraskevopoulou E, Sadeq M, Symeou C, Pantelidou C, Demonacos C, Krstic-Demonacos M. Regulation of glucocorticoid receptor activity by a stress responsive transcriptional cofactor. Molecular Endocrinology 2011. 25 58–71. ( 10.1210/me.2010-0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014 157 1685–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Galigniana NM, Ballmer LT, Toneatto J, Erlejman AG, Lagadari M, Galigniana MD. Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51. Journal of Neurochemistry 2012. 122 4–18. ( 10.1111/j.1471-4159.2012.07775.x) [DOI] [PubMed] [Google Scholar]
- 158.Oakley R, Cidlowski J. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. Journal of Biological Chemistry 2011. 286 3177–3184. ( 10.1074/jbc.R110.179325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ratajczak T, Cluning C, Ward BK. Immunophilin chaperones in steroid signalling steroid receptor-associated immunophilins: a gateway to steroid signalling. Clinical Biochemist Reviews 2015. 36 31–52. [PMC free article] [PubMed] [Google Scholar]
- 160.Hittelman AB, Burakov D, Iñiguez-Lluhí JA, Freedman LP, Garabedian MJ. Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins. EMBO Journal 1999. 18 5380–5388. ( 10.1093/emboj/18.19.5380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Molecular and Cellular Biology 1999. 19 4535–4545. ( 10.1128/MCB.19.6.4535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ismaili N, Blind R, Garabedian MJ. Stabilization of the unliganded glucocorticoid receptor by TSG101. Journal of Biological Chemistry 2005. 280 11120–11126. ( 10.1074/jbc.M500059200) [DOI] [PubMed] [Google Scholar]
- 163.Ponting CP, Cai YD, Bork P. The breast cancer gene product TSG101: a regulator of ubiquitination? Journal of Molecular Medicine 1997. 75 467–469. [PubMed] [Google Scholar]
- 164.Helzer KT, Hooper C, Miyamoto S, Alarid ET. Ubiquitylation of nuclear receptors: New linkages and therapeutic implications. Journal of Molecular Endocrinology 2015. 54 R151–R167. ( 10.1530/JME-14-0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 2001. 413 316–322. ( 10.1038/35095076) [DOI] [PubMed] [Google Scholar]
- 166.Koepp D, Schaefer L, Ye X, Keyomarsi K. Phosphorylation-dependent ubiquitination of Cyclin E by the SCF-Fbw7 ubiquitin ligase. Science 2001. 294 173–177. ( 10.1126/science.1065203) [DOI] [PubMed] [Google Scholar]
- 167.Malyukova A, Brown S, Papa R, O’Brien R, Giles J, Trahair TN, Dalla Pozza L, Sutton R, Liu T, Haber M, et al. FBXW7 regulates glucocorticoid response in T-cell acute lymphoblastic leukaemia by targeting the glucocorticoid receptor for degradation. Leukemia 2013. 27 1053–1062. ( 10.1038/leu.2012.361) [DOI] [PubMed] [Google Scholar]
- 168.Verma S, Ismail A, Gao X, Fu G, Li X, Malley BWO, Nawaz Z. The ubiquitin-conjugating enzyme UBCH7 acts as a coactivator for steroid hormone receptors the ubiquitin-conjugating enzyme UBCH7 acts as a coactivator for steroid hormone receptors. Molecular and Cellular Biology 2004. 24 8716–8726. ( 10.1128/MCB.24.19.8716-8726.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Garside H, Waters C, Berry A, Rice L, Ardley HC, White A, Robinson P, Ray D. UbcH7 interacts with the glucocorticoid receptor and mediates receptor autoregulation. Journal of Endocrinology 2006. 190 621–629. ( 10.1677/joe.1.06799) [DOI] [PubMed] [Google Scholar]
- 170.Sengupta S, Vonesch JL, Waltzinger C, Zheng H, Wasylyk B. Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells. EMBO Journal 2000. 19 6051–6064. ( 10.1093/emboj/19.22.6051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Braten O, Livneh I, Ziv T, Admon A, Kehat I, Caspi LH, Gonen H, Bercovich B, Godzik A, Jahandideh S, Jaroszewski L, Sommer T, Kwon YT, Guharoy M, Tompa P, Ciechanover A. Numerous proteins with unique characteristics are degraded by the 26S proteasome following monoubiquitination. PNAS 2016. 113 E4639–E4647. ( 10.1073/pnas.1608644113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Horiguchi J. Clinical usage of Yi-gan san-schizophrenia, borderline personality disorder, dyskinesia etc. Psychiatria et neurologia Japonica 2012. 114 708–718. [PubMed] [Google Scholar]
- 173.Ikarashi Y, Iizuka S, Imamura S, Yamaguchi T, Sekiguchi K, Kanno H, Kawakami Z, Yuzurihara M, Kase Y, Takeda S. Effects of yokukansan, a traditional Japanese medicine, on memory disturbance and behavioral and psychological symptoms of dementia in thiamine-deficient rats. Biological and Pharmaceutical Bulletin 2009. 32 1701–1709. [DOI] [PubMed] [Google Scholar]
- 174.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochemical Pharmacology 1999. 58 1685–1693. ( 10.1016/S0006-2952(99)00212-9) [DOI] [PubMed] [Google Scholar]
- 175.Luttrell LM. Minireview: More than just a hammer: ligand ‘bias’ and pharmaceutical discovery. Molecular Endocrinology 2014. 28 281–294. ( 10.1210/me.2013-1314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sundahl N, Bridelance J, Libert C, De Bosscher K, Beck IM. Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacology and Therapeutics 2015. 152 28–41. ( 10.1016/j.pharmthera.2015.05.001) [DOI] [PubMed] [Google Scholar]
- 177.De Bosscher K, Beck IM, Ratman D, Vanden Berghe W, Libert C. Activation of the glucocorticoid receptor in acute inflammation: the SEDIGRAM concept. Trends in Pharmacological Sciences 2016. 37 4–16. ( 10.1016/j.tips.2015.09.002) [DOI] [PubMed] [Google Scholar]
- 178.Patel R, Williams-Dautovich J, Cummins CL. Minireview: New molecular mediators of glucocorticoid receptor activity in metabolic tissues. Molecular Endocrinology 2014. 28 999–1011. ( 10.1210/me.2014-1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Reichardt SD, Foller M, Rexhepaj R, Pathare G, Minnich K, Tuckermann JP, Lang F, Reichardt HM. Glucocorticoids enhance intestinal glucose uptake via the dimerized glucocorticoid receptor in enterocytes. Endocrinology 2012. 153 1783–1794. ( 10.1210/en.2011-1747) [DOI] [PubMed] [Google Scholar]
- 180.Louw A, Swart P, De Kock SS. Mechanism for the stabilization in vivo of the aziridine precursor 2-(4-acetoxyphenyl)-2-chloro-n-methyl-ethylammonium chloride by serum proteins. Science 1997. 53 189–197. [DOI] [PubMed] [Google Scholar]
- 181.Robertson S, Allie-Reid F, Vanden Berghe W, Visser K, Binder A, Africander D, Vismer M, Bosscher K De, Hapgood J, Haegeman G, Louw A. Abrogation of glucocorticoid receptor dimerization correlates with dissociated glucocorticoid behavior of Compound A. Journal of Biological Chemistry 2010. 285 8061–8075. ( 10.1074/jbc.M109.087866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dewint P, Gossye V, De Bosscher K, Vanden Berghe W, Van Beneden K, Deforce D, Van Calenbergh S, Muller-Ladner U, Vander Cruyssen B, Verbruggen G, et al. A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. Journal of Immunology 2008. 180 2608–2615. ( 10.4049/jimmunol.180.4.2608) [DOI] [PubMed] [Google Scholar]
- 183.Wüst S, Tischner D, John M, Tuckermann JP, Menzfeld C, Hanisch UK, van den Brandt J, Lühder F, Reichardt HM. Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis. PLoS ONE 2009. 4 e8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.De Bosscher K, Vanden Berghe W, Beck IM, Van Molle W, Hennuyer N, Hapgood J, Libert C, Staels B, Louw A, Haegeman G. A fully dissociated compound of plant origin for inflammatory gene repression. PNAS 2005. 102 15827–15832. ( 10.1073/pnas.0505554102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rauner M, Thiele S, Sinningen K, Winzer M, Salbach-Hirsch J, Gloe I, Peschke K, Haegeman G, Tuckermann JP, Hofbauer LC. Effects of the selective glucocorticoid receptor modulator compound a on bone metabolism and inflammation in male mice with collagen-induced arthritis. Endocrinology 2013. 154 3719–3728. ( 10.1210/en.2012-2221) [DOI] [PubMed] [Google Scholar]
- 186.Zhang Z, Zhang ZY, Schluesener HJ. Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. Journal of Immunology 2009. 183 3081–3091. ( 10.4049/jimmunol.0901088) [DOI] [PubMed] [Google Scholar]
- 187.Huynh T, Uaesoontrachoon K, Quinn JL, Tatem KS, Heier CR, Van Der Meulen JH, Yu Q, Harris M,, Nolan CJ, Haegeman G, et al. Selective modulation through the glucocorticoid receptor ameliorates muscle pathology in mdx mice. Journal of Pathology 2013. 231 223–235. ( 10.1002/path.4231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.van Loo G, Sze M, Bougarne N, Praet J, Mc Guire C, Ullrich A, Haegeman G, Prinz M, Beyaert R, De Bosscher K. Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis. Molecular Endocrinology 2010. 24 310–322. ( 10.1210/me.2009-0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saksida T, Vujicic M, Nikolic I, Stojanovic I, Haegeman G, Stosic-Grujicic S. Compound A, a selective glucocorticoid receptor agonist, inhibits immunoinflammatory diabetes, induced by multiple low doses of streptozotocin in mice. British Journal of Pharmacology 2014. 171 5898–5909. ( 10.1111/bph.12892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wilkinson L, Verhoog N, Louw A. Novel role for receptor dimerization in post-translational processing and turnover of the GRα. Scientific Reports 2018. 8 14266 ( 10.1038/s41598-018-32440-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Robertson S, Hapgood JP, Louw A. Glucocorticoid receptor concentration and the ability to dimerize influence nuclear translocation and distribution. Steroids 2013. 78 182–194. ( 10.1016/j.steroids.2012.10.016) [DOI] [PubMed] [Google Scholar]
- 192.Tiwari M, Oasa S, Yamamoto J, Mikuni S, Kinjo M. A quantitative study of internal and external interactions of homodimeric glucocorticoid receptor using fluorescence cross-correlation spectroscopy in a live cell. Scientific Reports 2017. 7 4336 ( 10.1038/s41598-017-04499-7) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a