Abstract
The cyclic GMP‐AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway mediates anti‐microbial innate immunity by inducing the production of type I interferons (IFNs) and inflammatory cytokines upon recognition of microbial DNA. Recent studies reveal that self‐DNA from tumors and by‐products of genomic instability also activates the cGAS–STING pathway and either promotes or inhibits tumor development. This has led to the development of cancer therapeutics using STING agonists alone and in combination with conventional cancer treatment or immune checkpoint targeting. On the other hand, for cancers lacking the cGAS–STING pathway and thus a regular innate immunity response, oncolytic virus therapy has been shown to have therapeutic potential. We here review and discuss the dichotomous roles of the cGAS–STING pathway in cancer development and therapeutic approaches.
Keywords: cancer, cGAS, immune therapy, oncolytic virus, STING
Subject Categories: Cancer, Immunology
Glossary
- 2′3′‐cGAMP
2′3′‐linked cGAMP
- 3′3′‐cGAMP
3′3′‐linked cGAMP
- 5‐FU
fluorouracil
- ALT
alternative lengthening of telomeres
- AOM/DSS
azoxymethane/dextran sodium sulfate
- ATRX
alpha‐thalassemia/mental retardation syndrome X‐linked homolog
- CAC
colitis‐associated cancer
- c‐di‐AMP
cyclic di‐adenylate monophosphate
- c‐di‐GMP
cyclic di‐guanylate monophosphate
- CDN
cyclic dinucleotide
- cGAMP
cyclic adenosine monophosphate‐guanosine monophosphate
- cGAS
cyclic GMP‐AMP synthase
- CIN
chromosomal instability
- CLL
chronic lymphocytic leukemia
- CTLA‐4
cytotoxic T‐lymphocyte‐associated protein 4
- DAMP
danger‐associated molecular pattern
- Daxx
death‐associated protein 6
- DC
dendritic cell
- DMBA
7,12‐dimethylbenz(a)anthracene
- DMXAA
5,6‐dimethylxanthenone‐4 acetic acid
- ECTR
extrachromosomal telomere repeat
- ER
endoplasmic reticulum
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- HR
homologous recombination
- HSV
herpes simplex virus
- IFN
interferon
- IFN‐β
interferon‐beta
- IFN‐γ
interferon‐gamma
- IL‐12
interleukin‐12
- IL‐2
interleukin‐2
- IRF3
interferon regulatory transcription factor 3
- MAGE
melanoma antigen gene
- MCP‐1
monocyte chemotactic protein 1
- MEF
mouse embryonic fibroblast
- ML‐RR‐S2‐CDA
mixed‐linkage Rp, Rp dithio diastereomer c‐di‐AMP
- MUS81
methyl methanesulfonate, UV‐sensitive 81
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated β cells
- NLR
NOD‐like receptor
- NOD
nucleotide‐binding oligomerization domain
- PAMP
pathogen‐associated molecular pattern
- PD‐1
programmed cell death‐1
- PD‐L1
programmed death‐ligand 1
- PRR
pattern recognition receptor
- RIG‐I
retinoid acid‐inducible gene I
- SASP
senescence‐associated secretory phenotype
- STAT1
signal transducer and activator of transcription 1
- STING
stimulator of interferon genes
- TBK1
TANK‐binding kinase 1
- TLR
Toll‐like receptor
- TNF‐α
tumor necrosis factor‐alpha‐like
- TRF2
telomeric repeat‐binding factor 2
- TVEC
talimogene laherparepvec
Introduction
Innate immunity is the first line of host defense against various pathogenic infections 1. It is based on recognition of pathogen‐associated molecular patterns (PAMPs) and danger‐associated molecular patterns (DAMPs) through pattern recognition receptors (PRRs) such as Toll‐like receptors (TLRs), nucleotide‐binding oligomerization domain (NOD)‐like receptors (NLRs), retinoid acid‐inducible gene I (RIG‐I)‐like receptors, or cytosolic DNA sensors 2, 3. The cGAS–STING pathway is an important cytosolic DNA sensing pathway that activates expression of type I IFNs and other inflammatory cytokines to induce innate immunity for anti‐microbial effects in response to viral and bacterial DNA 4, 5. Recent studies suggest that the cGAS–STING pathway is also involved in modulating cancer formation. Tumor‐derived DNA, such as DNA of dead tumor cells, micronuclei, cytoplasmic chromatin fragments, and free telomeric DNA, can activate the cGAS–STING pathway and induce cell senescence, inflammation, and anti‐tumor immunity, which can have divergent effects on tumorigenesis 6, 7, 8, 9, 10, 11, 12. Hence, in this review, we highlight recent findings regarding how the cGAS–STING pathway is activated in response to tumor‐derived DNA and its subsequent effects in terms of suppressing and promoting tumorigenesis. We then describe the approaches for developing anti‐cancer interventions using STING agonists and targeting the vulnerabilities associated with loss of the cGAS–STING pathway.
The cGAS–STING pathway
Studies revealed that STING is an essential signal adaptor that mediates cytosolic DNA‐induced innate immune responses 13, 14, 15, 16 (Fig 1). It was shown that STING directly senses the bacterial cyclic dinucleotides (CDNs) c‐di‐AMP, c‐di‐GMP, and 3′3′‐cGMP‐AMP (3′3′–cGAMP) 17, 18, 19 (Fig 1), which have two 3′‐5′ phosphodiester linkages, to activate host immune responses. Furthermore, an endogenous cGAMP with a 2′‐5′ and a 3′‐5′ phosphodiester linkage 19, 20, 21, 22, 23, designated 2′3′‐cGAMP, is produced by cGAS upon engaging with cytosolic DNA 24, thereby establishing a signaling cascade of cGAS–STING 4, 25. Structural studies revealed that dsDNA binding leads to conformational changes of cGAS and promotes cGAS catalytic activity for cGAMP production 21, 26. Binding of cGAMP to a small pocket of the STING dimer 19, 20, 23, 27 promotes translocation of STING from the endoplasmic reticulum (ER) via the Golgi apparatus to perinuclear microsomes 28, 29. Here, STING recruits and activates TANK‐binding kinase 1 (TBK1) and interferon regulatory transcription factor 3 (IRF3) through serial phosphorylation events 30, 31. NF‐κB is also activated by STING in a TBK1‐dependent manner in response to cytosolic dsDNA and collaborates with IRF3 to mediate dsDNA‐induced gene expression of type I IFN 4, 25, 32. Through the detection of cytosolic DNA and initiating the production of type I IFNs and inflammatory cytokines, the cGAS–STING pathway is essential for host defense against various DNA viruses and retroviruses 28, 33, 34. Likewise, STING is activated by the secondary messengers c‐di‐AMP and c‐di‐GMP released from intracellular bacterial pathogens (such as Listeria monocytogenes and Francisella tularensis) to trigger host production of type I IFNs 17, 35, 36, 37. Apart from pathogen detection, the cGAS–STING pathway also detects self‐DNA and thereby promotes inflammatory autoimmune diseases 38, 39 and is implicated in the development of cancer, as will be discussed next.
Figure 1. CDNs activate the cGAS‐STING pathway and induce production of cytokines.
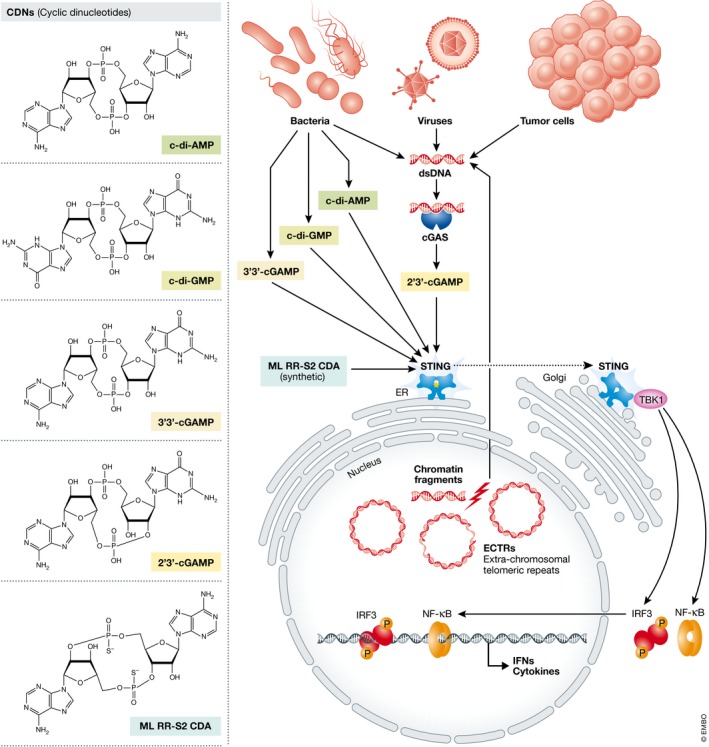
cGAS synthesizes 2′3′‐cGAMP (cyclic [G(2′,5′)pA(3′,5′)p]) upon engaging with cytosolic DNA from bacteria, viruses, tumor cells or chromatin fragments. 2′3′‐cGAMP and bacterial c‐di‐AMP (bis‐(3′–5′) cyclic diadenylic acid), c‐di‐GMP (bis‐(3′‐5′) cyclic diguanylic acid), and 3′3′‐cGAMP (cyclic [G(3′,5′)pA(3′,5′)p]) activate STING and stimulate its translocation from endoplasmic reticulum (ER) via Golgi apparatus to perinuclear microsomes. Active STING coordinates multiple phosphorylation events involving TBK1 kinase to induce nuclear translocation of IRF3 and TBK1, leading to transcriptional induction of cytokines, including IFNs. ML‐RR‐S2‐CDA (Rp, Rp dithio diastereomer cyclic [A(2′,5′)pA(3′,5′)p), is a synthetic CDN that triggers STING activation for cancer therapy.
Involvement of the cGAS–STING pathway in host immuno‐surveillance of cancer
Activation of the host cGAS–STING pathway contributes to anti‐tumor immunity (Fig 2). Cross‐presentation of tumor antigens by dendritic cells (DCs) to CD8+ T cells is critical for anti‐tumor immunity. Priming of CD8+ T cells against immunogenic tumors involves type I IFN production by DCs 40, 41. It has been shown that in the tumor microenvironment, IFN‐β expression in DCs is STING‐dependent and correlates with the uptake of tumor‐derived DNA by DCs 6. CD8+ T‐cell activation and tumor rejection are defective in mice lacking STING. In addition, the anti‐tumorigenic role of STING has been observed in a colitis‐associated cancer mouse model induced by azoxymethane/dextran sodium sulfate (AOM/DSS) 42. AOM triggers DNA damage and induces expression of inflammatory cytokine genes via STING‐dependent signaling. Upon AOM/DSS treatment, STING‐deficient mice are prone to colitis, polyp formation, and tumor development 42. Moreover, cGAS and STING are required for tumor regression by radiation and immune checkpoint inhibitor therapies in mice 43, 44. These findings show that defective innate immune‐sensing of tumor DNA in host bone marrow‐derived DCs that lack cGAS or STING impairs the generation of tumor‐infiltrating CD8+ T cells. Thus, activation of the host cGAS–STING pathway and type I IFN induction in DCs promotes cross‐presentation of tumor antigens to activate T cells for tumor control.
Figure 2. Model of the tumor suppressive roles of the cGAS‐STING pathway and possibilities of utilizing CDNs for tumor treatment.
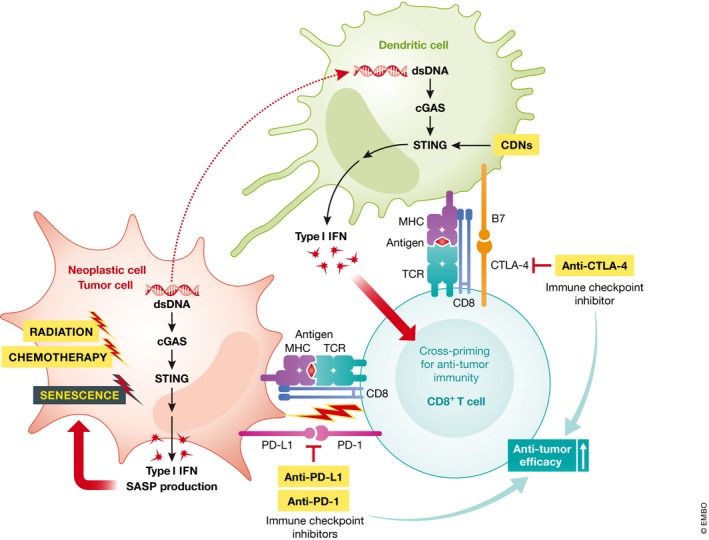
Cytoplasmic chromatin DNA activates the cGAS‐STING pathway and induces type I IFN and senescence‐associated secretory phenotype (SASP) production, which promote cellular senescence. Uptake of tumor‐derived DNA may lead to activation of the cGAS‐STING pathway in DCs and production of type I IFN, which regulates cross‐priming of CD8+ T cells for anti‐tumor immunity. Immune checkpoint pathways of PD‐L1/PD‐1 and B7/CTLA‐4 negatively regulate CD8+ T cell activity killing cancer cells. Combinatory treatments with CDNs augment the anti‐tumor efficacy of immune checkpoint inhibitors (anti‐PD‐1, ‐PD‐L1, ‐CTLA4 antibodies), radiation therapy, and chemotherapy.
Intrinsic activation of the cGAS–STING pathway suppresses cancer development
Recent studies suggest that cytosolic self‐DNA activates the cGAS–STING pathway in cancer cells and affects tumor development (Fig 2). Stalled replication forks resulting from dysregulation of DNA replication in cancer cells are processed for repair by the DNA structure‐specific endonuclease MUS81 45. Cleavage of detrimental DNA structures by MUS81 leads to an accumulation of cytosolic DNA in cancer cells, which promotes type I IFN production through STING 46. Moreover, DNA damage followed by mitosis progression generates micronuclei due to chromosome mis‐segregation in cancer cells 47. Rupture of the micronuclei envelopes exposes chromatin DNA that is recognized by cGAS and activates the cGAS–STING pro‐inflammatory response 7, 8.
Activation of the intrinsic cGAS–STING pathway by cytosolic DNA has been suggested to play a role in cellular senescence, a prominent tumor suppression mechanism. Various stress conditions cause cells to enter senescence, a state of irreversible cell cycle arrest 48, 49. Culturing mouse embryonic fibroblasts (MEFs) at ambient atmospheric O2 levels induces cellular senescence. Intriguingly, acceleration of cell proliferation and attenuation of senescent phenotypes occur in MEFs deficient for cGAS 9, 10 and STING 10. In addition, the cGAS–STING pathway also promotes senescence in response to oxidative stress, genotoxic stress, irradiation, and oncogene expression in MEFs and primary human fibroblasts 9, 10. Activation of the cGAS–STING pathway in senescent cells is associated with loss of the nuclear lamina protein lamin B1 and recognition of aberrant cytosolic chromatin fragments by cGAS 10, 11. Accordingly, the cGAS–STING pathway mediates production of type I IFNs and senescence‐associated secretory phenotype (SASP) factors to promote senescence 9, 10. Notably, the cGAS–STING pathway also facilitates in vivo production of SASP factors 10, 11 and senescence induced by irradiation and oncogenic Ras protein 10. In addition, the cGAS–STING pathway is required for immune‐mediated clearance of senescent hepatocytes induced by oncogenic Ras in mice 10, 11.
Together, these studies indicate that cGAS–STING pathway activation may suppress cancer development by inducing cellular senescence and by promoting immuno‐surveillance. Consequently, disruption of the cGAS–STING pathway may facilitate tumor development. Consistently, cGAS–STING pathway suppression has been observed in colorectal carcinoma, melanoma, and cancer cells lacking telomerase (see below) 12, 50, 51. In addition, there is a high mortality rate among gastric cancer patients with low STING expression 52. Mutation in cGAS or STING is rare in cancer, so the epigenetic and post‐translational mechanisms 5 by which cGAS and STING are suppressed remain to be elucidated.
Sensing of free telomeric DNA from ALT by cGAS–STING
A recent study revealed that activation of the cGAS–STING pathway by free cytosolic DNA derived from telomeres may have a tumor‐suppressive role in the development of cancers using the ALT (alternative lengthening of telomeres) mechanisms for telomere maintenance 12. Telomeres obscure the ends of linear chromosomes from surveillance by the DNA repair machinery, thereby maintaining genome stability 53, 54. In human somatic cells, telomeres gradually shorten over time due to a lack of telomerase activity, and ultimately, critically short telomeres trigger a DNA damage response, resulting in cell senescence 55. However, by regaining telomerase expression, telomeres can be maintained in a majority of tumors 56, 57. In the absence of telomerase, cancer cells utilize the ALT mechanisms that are based on homologous recombination (HR) to maintain telomeres 58, 59. ALT is particularly common in tumors of the central nervous system and soft‐tissue sarcomas 60. Accompanying the ALT mechanism, extrachromosomal telomere repeat (ECTR) DNA is generated by spontaneous telomere trimming and telomeric HR in ALT cancer cell lines and in vitro‐transformed ALT cells 61, 62, 63, 64. It has been shown that ALT cells can contain ~50% of telomeric DNA in the form of ECTR 65.
Apart from serving as a hallmark of ALT, ECTRs in human fibroblast cells can activate cGAS–STING signaling 12. Chen et al artificially generated ECTRs in fibroblast cells through ectopically expressing TRF2ΔB, a dominant‐negative mutant form of the telomeric protein TRF2 63. TRF2 promotes the formation and stabilization of telomere loop (T‐loop) structures, whereas TRF2ΔB triggers homologous recombination and T‐loop excision 63. ECTRs generated by TRF2ΔB induce IFN‐β expression via the cGAS–STING–TBK1–IRF3 signaling axis and impair cell proliferation 12. However, unlike in human fibroblasts, the presence of ECTRs in ALT cancer cell lines does not elicit type I IFN production nor cause cell growth impairment, even when TRF2ΔB is expressed 12. This might be attributed to a universal defect in the cytosolic DNA sensing mechanism in ALT cancer cells due to a lack of STING expression. Analyses of in vitro‐derived ALT cell lines demonstrated that inhibition of STING expression, via transcriptional and post‐transcriptional controls, is associated with ALT‐mediated cell immortalization 12.
Chen et al 12 also showed that ATRX, Daxx, and H3.3 are required for ECTR‐induced IRF3 phosphorylation and IFN‐β expression in BJ fibroblasts. ATRX–Daxx–H3.3 deficiency in various human cancers and cell lines is highly associated with ALT activity 66, 67, 68, 69. ATRX and Daxx form a histone chaperon complex, which deposits histone variant H3.3 at heterochromatic regions including telomeres 70. Loss of ATRX causes ALT phenotypes, such as ECTR production, and replication fork stalling, which can promote telomere recombination 71, 72. Based on its role in ECTR sensing, ATRX–Daxx–H3.3 mutations may attenuate IFN‐β induction in response to ECTR accumulation during initial ALT development. Following ALT establishment, however a further loss of cGAS or STING expression may be required for cancer cells to tolerate abundant ECTRs that normally impede cell proliferation.
The mechanism by which ATRX–Daxx–H3.3 senses ECTRs remains to be determined. It is also unknown whether ATRX–Daxx–H3.3 is involved in activation of the cGAS–STING pathway by micronuclei and other cytoplasmic chromatin fragments. It is clear that ECTR generation is associated with ALT development and high levels of ECTRs are present in ALT cells. Thus, ALT cancer can serve as a model to investigate the role of cGAS–STING signaling in tumor formation and the mechanisms leading to inactivation of the cGAS–STING pathway during tumor progression.
Pro‐tumor role of cGAS–STING
As discussed in previous sections, activation of the cGAS–STING signaling pathway upregulates IFN production and exerts anti‐tumor roles by mediating innate immune responses 6. However, other studies revealed that cGAS–STING pathway activation promotes tumor development. Chronic stimulation of the cGAS–STING pathway may induce inflammation‐driven carcinogenesis. For example, 7,12‐dimethylbenz(a)anthracene (DMBA) is a carcinogen that induces DNA breakage and promotes skin tumorigenesis in mice through STING activation 73. Nuclear DNA leakage activates STING and induces production of inflammatory cytokines and skin inflammation. Importantly, bone marrow transplant experiments suggest that STING in hematopoietic stem cells plays a significant role in DMBA‐induced skin tumorigenesis 73. Similar roles of the host cGAS–STING pathway have been reported in the induction of tumor growth of Lewis lung carcinoma 74.
The cGAS–STING pathway was shown to promote brain metastasis in both cell‐autonomous and non‐autonomous manners. STING activation in astrocytes mediates brain metastasis of breast and lung cancer cells 75. Intriguingly, cGAMP is produced in cancer cells and trafficks through carcinoma–astrocyte gap junctions to activate STING in astrocytes. In response to STING activation, inflammatory cytokines and tumor necrosis factor are produced and in turn activate the STAT1 and NF‐κB pathways in cancer cells. These paracrine effects support cancer cell growth and confer metastatic brain cell chemo‐resistance 75.
cGAS–STING activation also mediates metastasis in a cell‐autonomous manner. Chromosomal instability (CIN) caused by mis‐segregation of chromosomes during cell division is associated with human brain cell metastasis. In metastasis models, CIN promotes micronuclei formation and activates the cGAS–STING pathway, which induces non‐canonical NF‐κB signaling but not type I IFN signaling 76. CIN‐driven metastasis depends on STING and NF‐κB signaling and associates with the induction of epithelial‐to‐mesenchymal transition and inflammation‐related genes 76.
Targeting the cGAS–STING pathway as a cancer therapy
While keeping in mind the complex roles of the cGAS–STING pathway in regulating cancer development, its role in anti‐tumor immunity supports the use of STING agonists as cancer therapeutics (Fig 2). Administration of the STING agonist 5,6‐dimethylxanthenone‐4 acetic acid (DMXAA) to mouse models with solid tumors results in tumor elimination 77. DMXAA disrupts the vascular architecture of tumors by triggering innate immune responses through STING activation 78, 79. However, DMXAA is only effective on mouse STING, despite there being high amino acid sequence homology between mouse and human STING 80, 81. To develop compounds specific for human STING, the structure–function analysis of mouse STING targeting by DMXAA may facilitate future structure‐guided design 81.
Upon treatment with the CDN 3′3′‐cGAMP, STING is activated together with upregulation of type I IFNs in mouse embryonic fibroblasts (MEFs), as well as mouse models of melanoma, hepatoma, and Lewis lung cancer cells 82. Additionally, intraperitoneal injection of 3′3′‐cGAMP resulted in leukemic elimination from Eμ‐TCL1 transgenic mice exhibiting chronic lymphocytic leukemia (CLL) 83. Furthermore, cGAMP was shown to be an anti‐tumor agent against murine colon adenocarcinoma, with a reduced tumor volume and enhanced survival rate being observed in adenocarcinoma‐bearing mice upon cGAMP treatment 83. The anti‐tumor effect of cGAMP was linked to activation of the cGAS–STING pathway and upregulation of pro‐inflammatory cytokine expression, such as of IFN‐β, IFN‐γ, IL‐2, IL‐12, TNF‐α, and MCP‐1, in tumor‐bearing mice 83. Moreover, the competence of cGAMP as an anti‐tumor agent was demonstrated by its inhibition of melanoma tumor growth, and an increased survival rate in tumor‐bearing mice 43.
Irradiation and chemotherapy agents cause genotoxic effects that induce the formation of micronuclei and cytoplasmic chromatin fragments, which activate the cGAS–STING pathway 7, 8, 9, 10, 11, 44. Accordingly, STING agonists may be used as adjuvants in combination with chemotherapy and radiotherapy. Consistently, synergistic radiation therapy and intra‐tumoral administration of cGAMP promote the anti‐tumor effects of radiation through enhanced T‐cell responses in mice in a STING‐dependent manner 44. Moreover, combination treatment of fluorouracil (5‐FU) together with cGAMP not only improves the anti‐tumor activity of 5‐fluorouracil (5‐FU), but also reduces the tissue toxicity of 5‐FU 83. Similarly, combination treatment of cyclic di‐guanylate (c‐di‐GMP) with a Listeria monocytogenes‐based vaccine expressing the tumor‐associated antigen MAGE‐b (Listeria‐Mage‐b) eliminated metastases and retarded tumor growth in a metastatic breast cancer model 84. The anti‐tumor effect of this combination treatment correlated with increased CD8+ T‐lymphocyte activity in response to MAGE‐b in the tumor‐bearing mice.
Activation of immune checkpoint pathways allows tumor cells to evade the host immune response (Fig 2). Cancer cells expressing the checkpoint molecule PD‐L1 suppress T‐cell functions through binding with the PD‐1 receptor 85. CTLA‐4+‐expressing CD8+ T cells also contribute to immunological tolerance toward tumors 86. Drugs of immune checkpoint blockade, such as anti‐PD‐1, anti‐PD‐L1, and anti‐CTLA‐4 antibodies, can unleash anti‐tumor immunity and result in durable tumor regression. However, immune checkpoint blockade is inefficient in “cold” tumors, which are poorly infiltrated by immune cells (Fig 3).
Figure 3. Targeting cancer cells lacking the cGAS–STING pathway.
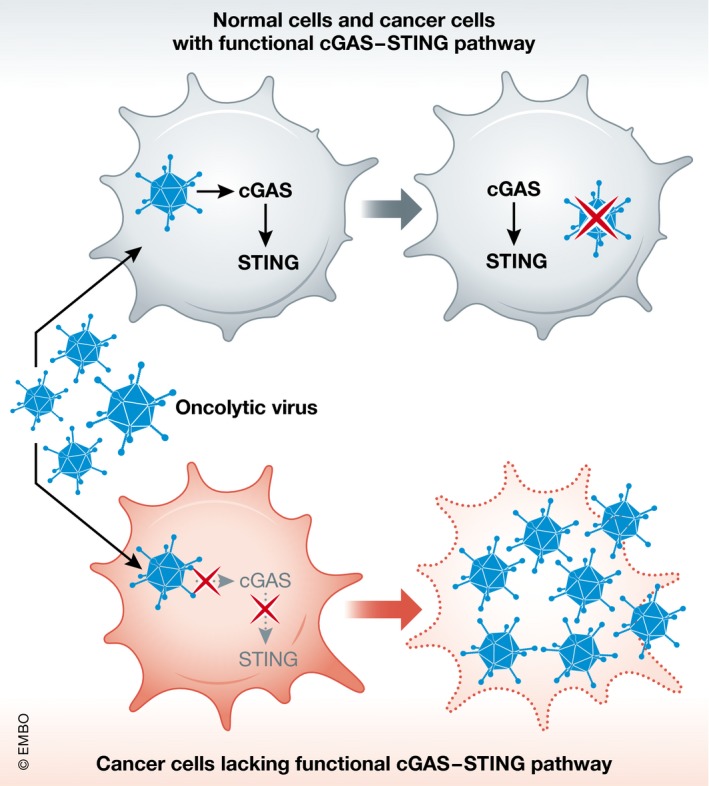
Viral infection is suppressed by the innate immune response following cGAS–STING pathway activation. Cancer cells with a defective cGAS–STING pathway are susceptible to oncolytic virus infection. Virus replication and cell lysis result in tumor regression.
Combination treatment with STING agonists has been shown to enhance the effects of immune checkpoint blockade. The cancer vaccine STINGVAX is formulated based on granulocyte–macrophage colony‐stimulating factor (GM‐CSF) with bacterial or synthetic CDNs 87. Treatment of STINGVAX induced anti‐tumor responses in multiple tumor models 87. STINGVAX formulated with ML‐RR‐S2‐CDA, a rationally designed phosphodiesterase‐resistant c‐di‐AMP (CDA) diastereomer with the phosphate bridge linkage as 2′3′‐cGAMP (Fig 1), has shown enhanced anti‐tumor efficacy compared with canonical c‐di‐AMP. Importantly, ML‐RR‐S2‐CDA broadly activates different human STING variants identified by the 1000 Genomes Project 78, 87, 88. STINGVAX also upregulates PD‐L1 expression in tumors 87; however, co‐treatment of STINGVAX with a PD‐1‐blocking antibody enhances anti‐tumor responses and tumor regression 87. Similarly, 2′3′‐cGAMP enhances anti‐tumor effects of the PD‐L1 antibody 43. Interestingly, STINGVAX can induce tumor infiltration of CD8+ T cells in the tumor microenvironment, suggesting that it can render tumors “hot”. This is consistent with the fact that the cGAS–STING pathway is required for the anti‐tumor effects of immune checkpoint blockade 43.
Oncolytic DNA viruses for cancers lacking an effective cGAS–STING pathway
As mentioned above, ALT cancer cells and some colorectal carcinoma and melanoma cells lack either cGAS or STING expression 12, 50, 51. Accordingly, targeting the cGAS–STING pathway for anti‐tumor therapy might not be feasible for these types of cancers. Given that cGAS–STING signaling is important for host defense mechanisms against viral infections, colorectal carcinoma and melanoma cancer cell lines with a defective cGAS–STING pathway might be more susceptible to infection and the oncolytic activity of DNA viruses, such as herpes simplex virus (HSV) and vaccinia virus 50, 51. Thus, oncolytic viruses represent a potential therapeutic approach for cancers with defective cGAS or STING functions. Oncolytic virus therapy involves employing genetically modified viruses to selectively replicate in and lyse cancer cells, leaving normal cells unaffected 89. Talimogene laherparepvec (TVEC) is an FDA‐approved oncolytic virus therapy for advanced and metastatic melanoma 90. It is based on a recombinant HSV‐1‐based virus expressing human GM‐CSF 91. A clinical study showed that local intra‐lesional injections of TVEC in advanced melanoma patients resulted in improved durable response rates, rates of complete response plus partial response lasting ≥ 6 months, and prolonged overall survival up to 23.3 months 92. Furthermore, a genetically engineered vaccinia virus (JX‐594 or Pexa‐Vec) expressing human GM‐CSF is used for hepatocellular carcinoma (HCC) therapy 93. A phase II clinical evaluation reported that intravenous administration of JX‐594 to HCC patients prolonged dose‐related survival up to 14.1 months, with a 15% response rate 94.
Collectively, preclinical studies of STING agonists have shown that they trigger cGAS–STING pathway activation and immune‐mediated anti‐tumor responses. Moreover, oncolytic virus therapies against cancers with defective cGAS or STING functions are broadening the spectrum of available cancer interventions.
Concluding remarks and future perspectives
Recent studies demonstrate that activation of the cGAS–STING pathway by cellular DNA from tumors influences the development of cancer. Although the cGAS–STING pathway is thought to be involved in host immune‐mediated tumor control, it remains unclear how tumor‐derived DNA is delivered to antigen‐presenting cells, and how DNA leaks from the nucleus into the cytosol to activate the cGAS–STING pathway. A better understanding of how the cGAS–STING pathway mediates anti‐tumor effects will inform the utilization of STING agonists in cancer therapy. However, given that cGAS–STING pathway activation also associates with tumor metastasis and autoimmune disorders, therapeutic windows and side effects must be considered for the potential use of STING agonists in cancer therapy. Furthermore, it has been shown that an intensified STING response occurs in T cells, but not in MEFs, DCs, or macrophages, and triggers apoptosis 95. This cell type‐specific effect of STING activation suggests that a more detailed understanding of STING signaling responses in different cell types is necessary and crucial for using CDNs in cancer therapy. Moreover, for the cancer cell types that exhibit loss of either cGAS or STING functions (such as colorectal, melanoma, and ALT cancer cells), oncolytic viruses may represent an alternative therapeutic approach. Studies have shown that the cGAS–STING signaling pathway can exhibit cell‐ and context‐dependent anti‐tumor and tumorigenic effects. The distinct roles of the cGAS–STING pathway may be determined by differential activation of the downstream IRF3 and NF‐κB pathways. Although the underlying mechanisms causing pathway deregulation in cancer cells remain unknown, such alterations may promote immunosuppression in the tumor microenvironment and thereby result in tumorigenesis and metastasis. Accordingly, the dichotomous roles of the cGAS–STING pathway in different cancers must be clearly and better characterized before targeting this pathway as a therapeutic approach.
Box 1: In need of answers.
We need to decipher the complexity of the cytosolic DNA response in the tumor microenvironment. Type I IFN production through activation of the host cGAS–STING pathway is crucial for tumor control. However, it remains to be determined how tumor DNA activates the cGAS–STING pathway in antigen‐presenting cells and what are the cell types within the tumor microenvironment that produce type I IFN to functionally contribute to the anti‐cancer effects. Answers for these questions may help to dissect the therapeutic effects of STING agonists and design a better treatment regimen.
We need to establish an ALT cancer model. ECTRs generated along with ALT cancer development are comprised of easily traceable telomere repeat DNA. Activation of the cGAS–STING pathway by ECTRs suggests that ALT cancer is an attractive system for investigating how the cGAS–STING pathway regulates tumor progression and shapes tumor evolution. Such work will rely on the development of an ALT cancer model that is currently not available.
We need to understand the effects of STING agonists on different cancer types. Activation of the cGAS–STING pathway can have anti‐tumor or pro‐tumor roles, which impacts on the use of STING agonists for cancer therapy. As discussed in the review, the diverse effects seem to be associated with cancer types and the stage of cancer progression. Therefore, for individual cancer types it is essential to have a comprehensive understanding of the functional consequences on tumorigenesis and cancer metastasis upon activation of the cGAS–STING pathway. Elucidating the molecular mechanisms determining the treatment effects by STING agonists can facilitate the identification of predictive markers for STING agonist‐based cancer therapy.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We are grateful to Y.‐A. Chen for discussions and reading this manuscript. Research in the laboratory of L.‐Y. Chen was supported by Career Development Award CDA‐105‐L01 from Academia Sinica and grants from the Ministry of Science and Technology (105‐2311‐B‐001‐055‐MY3).
EMBO Reports (2018) 19: e46935
See the Glossary for abbreviations used in this article.
References
- 1. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783–801 [DOI] [PubMed] [Google Scholar]
- 2. Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140: 805–820 [DOI] [PubMed] [Google Scholar]
- 3. Barber GN (2014) STING‐dependent cytosolic DNA sensing pathways. Trends Immunol 35: 88–93 [DOI] [PubMed] [Google Scholar]
- 4. Barber GN (2015) STING: infection, inflammation and cancer. Nat Rev Immunol 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Q, Sun L, Chen ZJ (2016) Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat Immunol 17: 1142–1149 [DOI] [PubMed] [Google Scholar]
- 6. Woo S‐R, Fuertes Mercedes B, Corrales L, Spranger S, Furdyna Michael J, Leung Michael YK, Duggan R, Wang Y, Barber Glen N, Fitzgerald Katherine A et al (2014) STING‐dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41: 830–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackenzie KJ, Carroll P, Martin C‐A, Murina O, Fluteau A, Simpson DJ, Olova N, Sutcliffe H, Rainger JK, Leitch A et al (2017) cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548: 461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA (2017) Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548: 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang H, Wang H, Ren J, Chen Q, Chen ZJ (2017) cGAS is essential for cellular senescence. Proc Natl Acad Sci USA 114: E4612–E4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glück S, Guey B, Gulen MF, Wolter K, Kang T‐W, Schmacke Niklas A, Bridgeman A, Rehwinkel J, Zender L, Ablasser A (2017) Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 19: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z et al (2017) Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550: 402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y‐A, Shen Y‐L, Hsia H‐Y, Tiang Y‐P, Sung T‐L, Chen L‐Y (2017) Extrachromosomal telomere repeat DNA is linked to ALT development via cGAS–STING DNA sensing pathway. Nat Struct Mol Biol 24: 1124–1131 [DOI] [PubMed] [Google Scholar]
- 13. Ishikawa H, Barber GN (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455: 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC (2008) MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol 28: 5014–5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhong B, Yang Y, Li S, Wang Y‐Y, Li Y, Diao F, Lei C, He X, Zhang L, Tien P et al (2008) The adaptor protein MITA links virus‐sensing receptors to IRF3 transcription factor activation. Immunity 29: 538–550 [DOI] [PubMed] [Google Scholar]
- 16. Sun W, Li Y, Chen L, Chen H, You F, Zhou X, Zhou Y, Zhai Z, Chen D, Jiang Z (2009) ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA 106: 8653–8658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burdette DL, Monroe KM, Sotelo‐Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE (2011) STING is a direct innate immune sensor of cyclic di‐GMP. Nature 478: 515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud A‐L, Cambier JC, Lenz LL (2011) MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic‐di‐AMP and cyclic‐di‐GMP. J Immunol 187: 2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V (2013) cGAS produces a 2′‐5′‐linked cyclic dinucleotide second messenger that activates STING. Nature 498: 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ (2013) Cyclic GMP‐AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339: 826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao P, Ascano M, Wu Y, Barchet W, Gaffney Barbara L, Zillinger T, Serganov Artem A, Liu Y, Jones Roger A, Hartmann G et al (2013) Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA‐activated cyclic GMP‐AMP synthase. Cell 153: 1094–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diner Elie J, Burdette Dara L, Wilson Stephen C, Monroe Kathryn M, Kellenberger Colleen A, Hyodo M, Hayakawa Y, Hammond Ming C, Vance Russell E (2013) The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3: 1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen Zhijian J (2013) Cyclic GMP‐AMP containing mixed phosphodiester linkages is an endogenous high‐affinity ligand for STING. Mol Cell 51: 226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP‐AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li T, Chen ZJ (2018) The cGAS‐cGAMP‐STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 215: 1287–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, Hopfner K‐P (2013) Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498: 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L et al (2013) Structure‐function analysis of STING activation by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. Cell 154: 748–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishikawa H, Ma Z, Barber GN (2009) STING regulates intracellular DNA‐mediated, type I interferon‐dependent innate immunity. Nature 461: 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T et al (2009) Atg9a controls dsDNA‐driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci USA 106: 20842–20846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka Y, Chen ZJ (2012) STING Specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal 5: ra20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, Du F, Ren J, Wu YT, Grishin NV et al (2015) Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347: aaa2630 [DOI] [PubMed] [Google Scholar]
- 32. Abe T, Barber GN (2014) Cytosolic‐DNA‐mediated, STING‐dependent proinflammatory gene induction necessitates canonical NF‐kappaB activation through TBK1. J Virol 88: 5328–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao D, Wu J, Wu Y‐T, Du F, Aroh C, Yan N, Sun L, Chen ZJ (2013) Cyclic GMP‐AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341: 903–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V et al (2013) Pan‐viral specificity of IFN‐induced genes reveals new roles for cGAS in innate immunity. Nature 505: 691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sauer J‐D, Sotelo‐Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA et al (2011) The N‐Ethyl‐N‐nitrosourea‐induced goldenticket mouse mutant reveals an essential function of sting in the in vivo interferon response to listeria monocytogenes and cyclic dinucleotides. Infect Immun 79: 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burdette DL, Vance RE (2012) STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol 14: 19–26 [DOI] [PubMed] [Google Scholar]
- 37. Storek KM, Gertsvolf NA, Ohlson MB, Monack DM (2015) cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol 194: 3236–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahn J, Gutman D, Saijo S, Barber GN (2012) STING manifests self DNA‐dependent inflammatory disease. Proc Natl Acad Sci USA 109: 19386–19391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao D, Li T, Li X‐D, Chen X, Li Q‐Z, Wight‐Carter M, Chen ZJ (2015) Activation of cyclic GMP‐AMP synthase by self‐DNA causes autoimmune diseases. Proc Natl Acad Sci USA 112: E5699–E5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fuertes MB, Kacha AK, Kline J, Woo S‐R, Kranz DM, Murphy KM, Gajewski TF (2011) Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med 208: 2005–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U et al (2011) Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 208: 1989–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahn J, Konno H, Barber GN (2015) Diverse roles of STING‐dependent signaling on the development of cancer. Oncogene 34: 5302–5308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, Chen ZJ (2017) cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci USA 114: 1637–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li X‐D, Mauceri H, Beckett M, Darga T et al (2014) STING‐dependent cytosolic DNA sensing promotes radiation‐induced type I interferon‐dependent antitumor immunity in immunogenic tumors. Immunity 41: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hanada K, Budzowska M, Davies SL, van Drunen E, Onizawa H, Beverloo HB, Maas A, Essers J, Hickson ID, Kanaar R (2007) The structure‐specific endonuclease Mus81 contributes to replication restart by generating double‐strand DNA breaks. Nat Struct Mol Biol 14: 1096–1104 [DOI] [PubMed] [Google Scholar]
- 46. Ho Samantha SW, Zhang Wendy YL, Tan Nikki Yi J, Khatoo M, Suter Manuel A, Tripathi S, Cheung Florence SG, Lim Weng K, Tan Puay H, Ngeow J et al (2016) The DNA structure‐specific endonuclease MUS81 mediates DNA sensor STING‐dependent host rejection of prostate cancer cells. Immunity 44: 1177–1189 [DOI] [PubMed] [Google Scholar]
- 47. Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D (2012) DNA breaks and chromosome pulverization from errors in mitosis. Nature 482: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campisi J, d'Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740 [DOI] [PubMed] [Google Scholar]
- 49. Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age‐related disease: from mechanisms to therapy. Nat Med 21: 1424–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. w?>Xia T, Konno H, Barber GN (2016) Recurrent loss of STING signaling in melanoma correlates with susceptibility to viral oncolysis. Can Res 76: 6747–6759 [DOI] [PubMed] [Google Scholar]
- 51. Xia T, Konno H, Ahn J, Barber GN (2016) Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep 14: 282–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song S, Peng P, Tang Z, Zhao J, Wu W, Li H, Shao M, Li L, Yang C, Duan F et al (2017) Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep 7: 39858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jain D, Cooper JP (2010) Telomeric strategies: means to an end. Annu Rev Genet 44: 243–269 [DOI] [PubMed] [Google Scholar]
- 54. de Lange T (2004) T‐loops and the origin of telomeres. Nat Rev Mol Cell Biol 5: 323–329 [DOI] [PubMed] [Google Scholar]
- 55. Shay JW, Wright WE (2005) Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 26: 867–874 [DOI] [PubMed] [Google Scholar]
- 56. Shay JW, Wright WE (2011) Role of telomeres and telomerase in cancer. Semin Cancer Biol 21: 349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pfeiffer V, Lingner J (2013) Replication of telomeres and the regulation of telomerase. Cold Spring Harb Perspect Biol 5: a010405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pickett HA, Reddel RR (2015) Molecular mechanisms of activity and derepression of alternative lengthening of telomeres. Nat Struct Mol Biol 22: 875–880 [DOI] [PubMed] [Google Scholar]
- 59. Cesare AJ, Reddel RR (2010) Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 11: 319–330 [DOI] [PubMed] [Google Scholar]
- 60. Durant ST (2012) Telomerase‐independent paths to immortality in predictable cancer subtypes. J Cancer 3: 67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pickett HA, Cesare AJ, Johnston RL, Neumann AA, Reddel RR (2009) Control of telomere length by a trimming mechanism that involves generation of t‐circles. EMBO J 28: 799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Compton SA, Choi J‐H, Cesare AJ, Özgür S, Griffith JD (2007) Xrcc3 and Nbs1 are required for the production of extrachromosomal telomeric circles in human alternative lengthening of telomere cells. Can Res 67: 1513–1519 [DOI] [PubMed] [Google Scholar]
- 63. Wang RC, Smogorzewska A, de Lange T (2004) Homologous recombination generates T‐loop‐sized deletions at human telomeres. Cell 119: 355–368 [DOI] [PubMed] [Google Scholar]
- 64. Henson JD, Cao Y, Huschtscha LI, Chang AC, Au AY, Pickett HA, Reddel RR (2009) DNA C‐circles are specific and quantifiable markers of alternative‐lengthening‐of‐telomeres activity. Nat Biotechnol 27: 1181–1185 [DOI] [PubMed] [Google Scholar]
- 65. Fasching CL, Neumann AA, Muntoni A, Yeager TR, Reddel RR (2007) DNA damage induces alternative lengthening of telomeres (ALT) associated promyelocytic leukemia bodies that preferentially associate with linear telomeric DNA. Can Res 67: 7072–7077 [DOI] [PubMed] [Google Scholar]
- 66. Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482: 226–231 [DOI] [PubMed] [Google Scholar]
- 67. Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S et al (2011) Altered telomeres in tumors with ATRX and DAXX mutations. Science 333: 425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA et al (2011) DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331: 1199–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, De S, Petrini JH, Sung PA, Jasin M et al (2012) Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet 8: e1002772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD (2010) Daxx is an H3.3‐specific histone chaperone and cooperates with ATRX in replication‐independent chromatin assembly at telomeres. Proc Natl Acad Sci USA 107: 14075–14080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Napier CE, Huschtscha LI, Harvey A, Bower K, Noble JR, Hendrickson EA, Reddel RR (2015) ATRX represses alternative lengthening of telomeres. Oncotarget 6: 16543–16558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Clynes D, Jelinska C, Xella B, Ayyub H, Scott C, Mitson M, Taylor S, Higgs DR, Gibbons RJ (2015) Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nat Commun 6: 7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN (2014) Inflammation‐driven carcinogenesis is mediated through STING. Nat Commun 5: 5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, Munn D, Mellor AL (2016) STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Can Res 76: 2076–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez‐Soto A, Jacob L, Patwa R, Shah H, Xu K et al (2016) Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533: 493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bakhoum SF, Ngo B, Laughney AM, Cavallo J‐A, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK et al (2018) Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553: 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhao L, Ching LM, Kestell P, Baguley BC (2002) The antitumour activity of 5,6‐dimethylxanthenone‐4‐acetic acid (DMXAA) in TNF receptor‐1 knockout mice. Br J Cancer 87: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Corrales L, Glickman Laura H, McWhirter Sarah M, Kanne David B, Sivick Kelsey E, Katibah George E, Woo S‐R, Lemmens E, Banda T, Leong Justin J et al (2015) Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 11: 1018–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim S, Li L, Maliga Z, Yin Q, Wu H, Mitchison TJ (2013) Anticancer flavonoids are mouse‐selective STING agonists. ACS Chem Biol 8: 1396–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, Rathinam VAK, Monks B, Jin T, Xiao TS et al (2013) Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6‐Dimethylxanthenone‐4‐acetic acid. J Immunol 190: 5216–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gao P, Zillinger T, Wang W, Ascano M, Dai P, Hartmann G, Tuschl T, Deng L, Barchet W, Patel Dinshaw J (2014) Binding‐pocket and lid‐region substitutions render human STING sensitive to the species‐specific drug DMXAA. Cell Rep 8: 1668–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tang C‐HA, Zundell JA, Ranatunga S, Lin C, Nefedova Y, Del Valle JR, Hu C‐CA (2016) Agonist‐mediated activation of STING induces apoptosis in malignant B cells. Can Res 76: 2137–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li T, Cheng H, Yuan H, Xu Q, Shu C, Zhang Y, Xu P, Tan J, Rui Y, Li P et al (2016) Antitumor activity of cGAMP via stimulation of cGAS‐cGAMP‐STING‐IRF3 mediated innate immune response. Sci Rep 6: 19049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chandra D, Quispe‐Tintaya W, Jahangir A, Asafu‐Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DKR, Gravekamp C (2014) STING ligand c‐di‐GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res 2: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Iwai Y, Hamanishi J, Chamoto K, Honjo T (2017) Cancer immunotherapies targeting the PD‐1 signaling pathway. J Biomed Sci 24: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Callahan MK, Wolchok JD, Allison JP (2010) Anti‐CTLA‐4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol 37: 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W et al (2015) STING agonist formulated cancer vaccines can cure established tumors resistant to PD‐1 blockade. Sci Transl Med 7: 283ra52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yi G, Brendel VP, Shu C, Li P, Palanathan S, Cheng Kao C (2013) Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS ONE 8: e77846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kaufman HL, Kohlhapp FJ, Zloza A (2015) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14: 642–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rehman H, Silk AW, Kane MP, Kaufman HL (2016) Into the clinic: talimogene laherparepvec (T‐VEC), a first‐in‐class intratumoral oncolytic viral therapy. J Immunother Cancer 4: 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, McGrath Y, Thomas SK, Thornton M, Bullock P et al (2003) ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti‐tumour properties. Gene Ther 10: 292–303 [DOI] [PubMed] [Google Scholar]
- 92. Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS et al (2015) Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33: 2780–2788 [DOI] [PubMed] [Google Scholar]
- 93. Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, Roh MS, Je JE, Yoon JH, Thorne SH et al (2006) Systemic armed oncolytic and immunologic therapy for cancer with JX‐594, a targeted poxvirus expressing GM‐CSF. Mol Ther 14: 361–370 [DOI] [PubMed] [Google Scholar]
- 94. Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW et al (2013) Randomized dose‐finding clinical trial of oncolytic immunotherapeutic vaccinia JX‐594 in liver cancer. Nat Med 19: 329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gulen MF, Koch U, Haag SM, Schuler F, Apetoh L, Villunger A, Radtke F, Ablasser A (2017) Signalling strength determines proapoptotic functions of STING. Nat Commun 8: 427 [DOI] [PMC free article] [PubMed] [Google Scholar]


