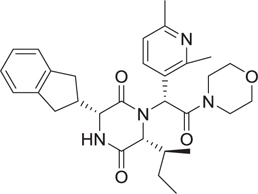
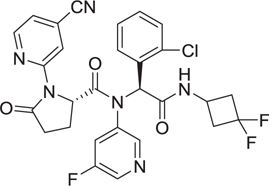
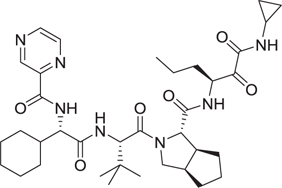
Abstract
Macrocycles are an emerging and largely underexploited part of chemical space where potential drugs for difficult genomic targets can be discovered. Macro-cycles can have advantages over their natural twins such as better control over synthesis, physicochemical properties and target binding. Fast and convergent synthesis pathways are underdeveloped. Multicomponent reaction (MCR) chemistry is very well suited for the synthesis of a diverse range of macrocycles and is also able to generate great levels of molecular diversity and complexity at low synthetic costs.
Introduction
Macrocycles have been defined as ring-systems consisting of 12 or more atoms. In the past decade, interest into macro-cycles strongly increased in medicinal chemistry. More than 100 macrocyclic drugs and clinical candidates are currently marketed or in drug discovery programs [1].
In spite of their higher molecular weight, often polar backbone and increased number of H-bond donors and acceptors, macrocycles are capable to dynamically change their conformation and therefore can become more drug-like than expected based on their physicochemical and pharma-cokinetic properties [2]. Moreover, macrocycles with their high and dynamically adaptable surface area are perfect candidates to mimic some structural features of protein interfaces. Protein–protein interactions (PPIs) are critical in many of physiological and pathological processes including protein biosynthesis, viral diffusion and virus survival in host cell and signaling transduction. Thus, there is great interest in developing approaches for identifying therapeutically useful inhibitors for numerous targets that are not conventionally ‘‘druggable’’, such as protein–protein interfaces [3]. An example of an exciting PPI target is IL17 with approved antibodies for use in rheumatoid arthritis. Recently artificial macrocycles have been described targeting IL17 (Fig. 1A) [4].
Fig. 1.
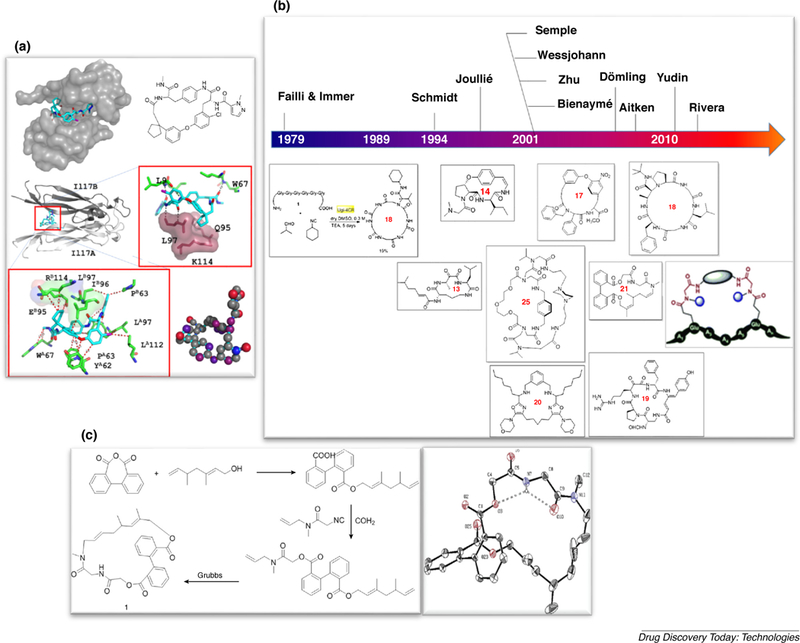
(A) An artificial macrocycle binding to IL17. Above: 2D structure of macrocycle and the overall binding mode into the IL17 dimer interface. Below: close-up view of the macrocycle receptor interaction highlighting hydrophobic and hydrogen bonding interactions; (B) landmarks of macrocycle syntheses by MCR; (C) three step macrocycle synthesis based on a Passerini-MCR and 3D structure of a representative macrocycle (CCDC 200226).
As the role of macrocycles in medicinal chemistry increases, there is an urgent need for new approaches to synthesize these compounds. Classically, macrocycles are synthesized similar to small molecules in sequential fashion. Natural products often can be obtained by fermentation. For example the artificial macrocycle targeting IL17 has been synthesized in a sequence of 5 steps (Fig. 1A) [5]. One of the challenges for exploration of the macrocycles for drug discovery is the difficulty in synthesizing such compounds, especially when there is a need for a series of molecules for structure–activity relationship (SAR) elucidation or to build screening libraries. A number of synthetic routes toward macrocycles have been successfully developed including rapid and efficient methodologies. Acceleration of chemistry is an important aim in contemporary chemistry due to the need of synthesizing, screening and property optimizing large compound libraries in a circular repetitive way [6]. A complementary modern approach to access chemical diversity in a one-pot fashion is by using an MCR. MCRs become increasingly a drug discovery technology for the rapid access of macrocycles due to its advantages over a sequential multistep approach [7]. Here we review the recent developments in this research area, focusing on synthetic strategies to artificial macrocycles which have been developed in our group [8].
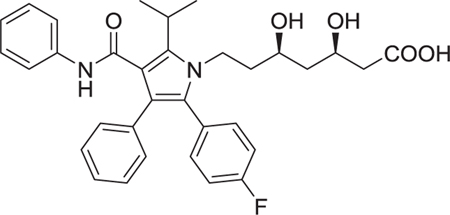
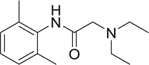
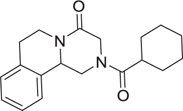
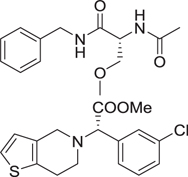
MCR have been defined as reactions of more than 2 different starting materials. Typically the reactants are stirred in a one pot fashion and the majority of atoms of the staring materials can be found in the product molecule [9]. MCR are an old class of synthetically useful reactions and often are name reactions. Examples include the Mannich, Biginelli, Reppe, Bucherer–Bergs, Gewald, Passerini, Stecker, Asinger or Hantzsch reactions. Often the MCR are based on simple and commercially in large numbers available starting materials such as carboxylic acids, oxo components or β-keto esters. Therefore large libraries of products can be easily accessed in great chemical diversity. Notably, MCRs have been described for the convergent synthesis of multiple drugs on the market or in clinical trials (Table 1). This nicely underscores the relevance of MCR chemistry for drug discovery. MCRs should not be mixed up with domino or tandem reactions, which comprise a consecutive series of intramolecular reactions.
Table 1.
Small selection of marketed drugs or clinical candidates which can be synthesized advantageously by MCR technology.
The fist macrocycles were synthesized in 1979 by Failli and Immer using MCR technology [18]. Other notable groups working in the field of macrocyclisation by MCR include Zhu, Yudin, Wessjohann, and Rivera (Fig. 1B) [19–21]. In our laboratory we focus mostly on non peptidic artificial macrocycles. Artificial macrocycles can provide attractive ligands for disease-significant targets and at the same time easy tunability of their drug-like properties. We early on recognized the power of MCR for the synthesis of macrocycles [8,22–24]. One of our first attempts to use the advantages of MCR chemistry is shown in Fig. 1C [25]. A general, convergent and fast strategy was devised for the rapid assembly of structurally diverse artificial macrocycles. The first step comprised of a nucleophilic ring opening reaction of cyclic carboxylic acid anhydrides with terminal ene-ols to yield ω-ene carboxylic acids. Next a Passerini-3CR was performed using isocyano-ω-enes and carbonyl building blocks. The final ring macro closure was accomplished by a metathesis reaction. Notably, some products had natural product-like features including a number of stereocenters, high O content and a non-flat conformation. For example compound 1 exhibits a atropisomeric biphenyl axis, each a secondary and a tertiary amide, two ester groups, two double bonds and a bifurcated intramolecular hydrogen bond (Fig. 1C). A similar strategy using the Ugi MCR was also described. The assembly strategy worked well in the ring opening and the subsequent MCR step with a broad range of building blocks. However, during the process of library expansion, we realized that the subsequent metathesis reaction was generally low yielding and had a very limited substrate scope in terms of ring size, side-chain diversity, and positioning of the orthogonal functional groups. For a useful reaction to be taken up by industry, however, the substrate scope needs to be broad in terms of shape, size and functional group content [26]. Thus, we switched our attention to MCR based macrocyclisations. We devised a powerful synthetic strategy were we assembled a α,ω-difunctionalized linear precursor which was then macroring-closed by a MCR (Fig. 2A and B). In our laboratory focus on short reaction sequences while not jeopardizing functional group compatibility and scaffold diversity is key. Thus we devised the shortest ever artificial macrocycle synthesis known, a 2-step sequence from generally available starting materials (Fig. 2C) [27].
Fig. 2.
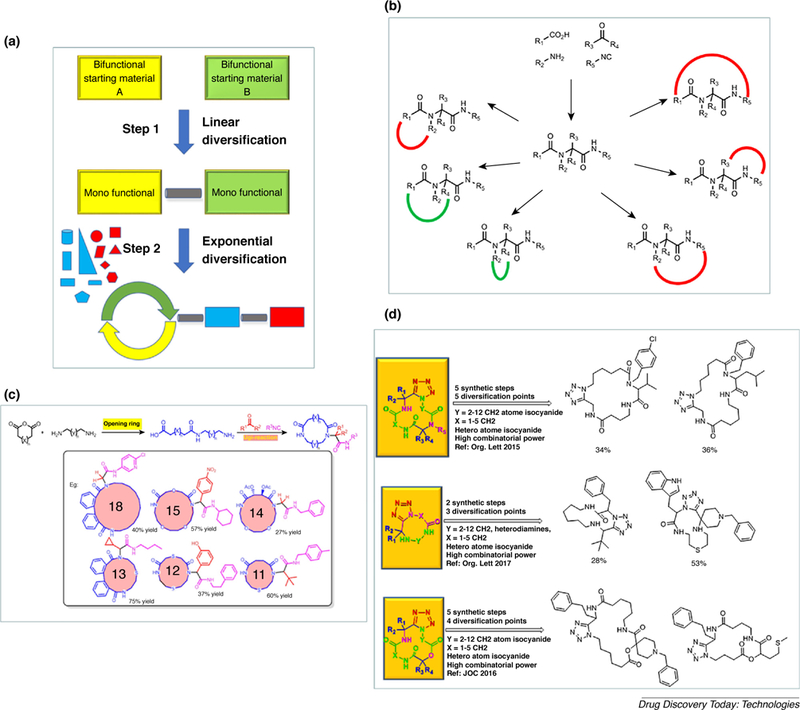
(A) Left: synthetic strategy toward artificial macrocycles. Right: manifolds of topologically possible macroring closures with the Ugi-4-CR; (B) topologically possible macrorinclosures by using the U-4CR; (C) shortest artificial macrocycle synthesis ever; (D) part of the macrocycle synthesis toolbox designed, published and used in the Dömling laboratory.
The 2-step sequence involves a nucleophilic ring opening of cyclic carboxylic acid anhydrides with unprotected primary diamines yielding α-amino-ω-carboxylic acids of varying length followed by a Ugi ring closure upon addition of an isocyanide and a oxo component (Fig. 2C). The procedure can also be performed in one pot without the isolation of the intermediate amino acid. The building blocks used here are generally commercially available in high diversity or easy to access synthetically. Thousands of interesting highly functionalized building blocks are available. For example, due to the combinatorial nature of the Ugi reaction for 100 each of the four building blocks, cyclic anhydride, diamine, oxo component and isocyanide a chemical space not including stereoisomers of 1004 = 100 million macrocycles is ready to be investigated. Other macrocyclisation protocols have been developed in our laboratory (Fig. 2D). Key features of the scaffolds include the use of convergent MCR chemistries, commercially available building blocks, broad functional group compatibility, scalability, inclusion of heterocyclic amide bioisosteres and short economical synthesis routes.
Examples of bioactive macrocycles from our group
The Dömling laboratory has a longstanding tradition in the discovery of p53-MDM2 anatgonists [28–32]. Blocking the protein–protein interaction between the murine double minute (MDM) homologues MDM2/X and the tumor-suppressor protein p53 is a promising strategy in oncology. Inhibiting the binding between wild-type (WT) p53 and its negative regulators MDM2 and/or MDMX has become an important target in oncology to restore the antitumor activity of p53. Recently, the p53-MDM2 complex is the best studied and most targeted protein–protein interaction. Thus, the discovery of new p53– MDM2 inhibitors with diverse structures to improve their properties is of importance [33,34]. Several macrocyclic stapled peptides have been described with great affinity toward MDM2 and MDMX [35]. We designed a series of nonpeptidic artificial macrocyclic compounds that inhibit the p53–MDM2 interaction [27,28] These macrocycles target for the first time the large hydrophobic surface area formed by Tyr67, Gln72, His73, Val93, and Lys94 as shown by 2D NMR thus potentially increasing the affinity to the receptor(Fig. 3A). Moreover, we recently described several peptidic macrocycles which form potent complexes with the immune oncology target PD-L1 (Fig. 3B). Intriguingly, these macrocycles show comparable cell-based activities to the marketed monoclonal antibodies [36].
Fig. 3.
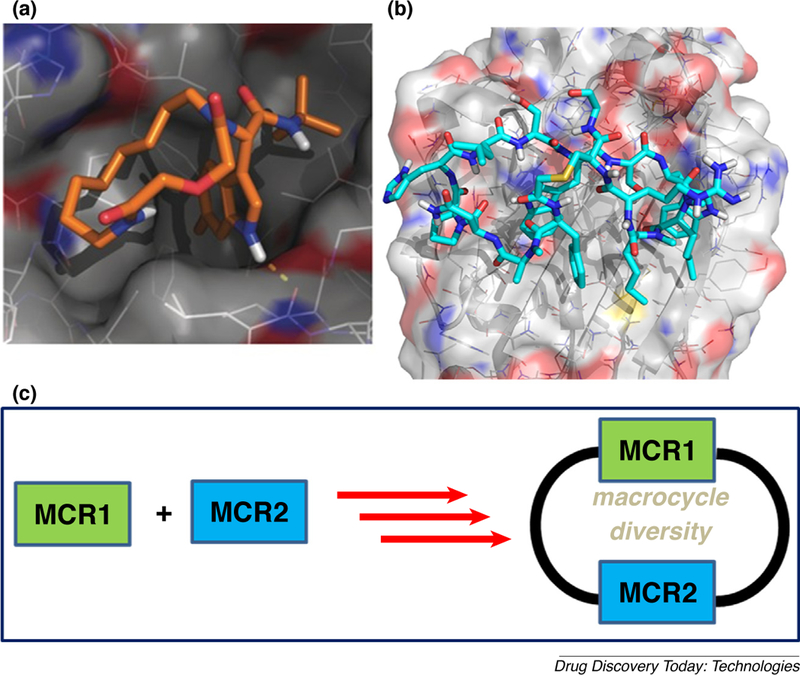
(A) A docking model of an artificial macrocycle which binds to MDM2; (B) the crystal structure of a macrocyclic peptide binding to PD-L1 (PDB ID 504Y); (C) the union and inclusion of several MCRs into the backbone of macrocycles as a synthetic strategy to increase the chemical space and diversity of macrocycles.
Future prospects
Macrocycles are magic and their use will likely expand and take a prominent place in the drug discovery space [8]. However, synthetic accessibility and tunability of properties in a timely and economical fashion is an issue with currently available macrocycles syntheses. MCR can be a solution! Maximal diversity can be reached by the concept of unions of MCR which was recently introduced (Fig. 3C) [9,37]. The convergent, economical and fast nature of MCR chemistry allows to access interesting artificial or peptidic macrocycles with a broad functional group compatibility. Introduction of drug like properties into artificial macro-cycles is key for a successful use of this compound class in pharmaceutical industry. Ideas how to tune drug-like properties, first of all passive membrane permeation, have been recently published [8]. First reports on their biological activities are promising and the future will provide more examples.
Acknowledgments
The work in the PIs laboratory was financially supported from the NIH (NIH 2R01GM097082-05) and by the Innovative Medicines Initiative Joint Undertaking under grant agreement No. 115489, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in-kind. Moreover, funding has also been received from the European Union’s Horizon 2020 research and innovation programme under MSC ITN ‘‘Accelerated Early stage drug dIScovery’’ (AEGIS), grant agreement No. 675555. SS is grateful for a postdoctoral fellowship from KWF (Grant Agreement No. 10504).
References
- [1].Giordanetto F, Kihlberg J. Macrocyclic drugs and clinical candidates: what can medicinal chemists learn from their properties? J Med Chem 2014;57:278–95. [DOI] [PubMed] [Google Scholar]
- [2].Whitty A, Zhong M, Viarengo L, Beglov D, Hall DR, Vajda S. Quantifying the chameleonic properties of macrocycles and other high-molecular-weight drugs. Drug Discov Today 2016;21:712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Am J Health Syst Pharm 2009;66:82–98. [DOI] [PubMed] [Google Scholar]
- [4].Wang W, Groves MR, Do¨mling A. Artificial macrocycles as IL-17A/IL-17RA antagonists. MedChemComm 2018;9:22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu S, Dakin LA, Xing L, Withka JM, Sahasrabudhe PV, Li W, et al. Binding site elucidation and structure guided design of macrocyclic IL-17A antagonists. Sci Rep 2016;6:30859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin S, Dikler S, Blincoe WD, Ferguson RD, Sheridan RP, Peng Z, et al. Mapping the dark space of chemical reactions with extended nanomole synthesis and MALDI-TOF MS. Science 2018. http://dx.doi.org/10.1126/science.aar6236. [DOI] [PubMed]
- [7].Wessjohann LA, Ruijter E, Garcia-Rivera D, Brandt W. What can a chemist learn from nature’s macrocycles? — A brief, conceptual view. Mol Divers 2005;9:171–86. [DOI] [PubMed] [Google Scholar]
- [8].Abdelraheem EMM, Shaabani S, Do¨mling A. Artificial macrocycles. Synlett 2018;29:1136–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dömling A, Ugi I. Multicomponent reactions with isocyanides. Angew Chem Int Ed 2000;39:3168–210. [DOI] [PubMed] [Google Scholar]
- [10].Hulme C, Gore V. Multi-component Reactions: Emerging Chemistry in Drug Discovery ‘From Xylocain to Crixivan’, vol. 10 Bentham Science Publishers; 2003. [DOI] [PubMed] [Google Scholar]
- [11].Haixia L, Samia W, Eberhardt H, Sanaa B, Alexander D. MCR synthesis of praziquantel derivatives. Chem Biol Drug Des 2012;79:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wehlan H, Oehme J, Scha¨fer A, Rossen K. Development of scalable conditions for the Ugi reaction—application to the synthesis of (R)-lacosamide. Org Process Res Dev 2015;19:1980–6. [Google Scholar]
- [13].Kalinski C, Lemoine H, Schmidt J, Burdack C, Kolb J, Umkehrer M, et al. Multicomponent reactions as a powerful tool for generic drug synthesis. Synthesis 2008;2008:4007–11. [Google Scholar]
- [14].Borthwick AD, Liddle J, Davies DE, Exall AM, Hamlett C, Hickey DM, et al. Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: synthesis, pharmacokinetics, and in vivo potency. J Med Chem 2012;55:783–96. [DOI] [PubMed] [Google Scholar]
- [15].Popovici-Muller J, Lemieux RM, Artin E, Saunders JO, Salituro FG, Travins J, et al. Discovery of AG-120 (Ivosidenib): a first-in-class Mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett 2018;9:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Malaquin S, Jida M, Gesquiere J-C, Deprez-Poulain R, Deprez B, Laconde G. Ugi reaction for the synthesis of 4-aminopiperidine-4-carboxylic acid derivatives. Application to the synthesis of carfentanil and remifentanil. Tetrahedron Lett 2010;51:2983–5. [Google Scholar]
- [17].Znabet A, Polak MM, Janssen E, de Kanter FJJ, Turner NJ, Orru RVA, et al. A highly efficient synthesis of telaprevir by strategic use of biocatalysis and multicomponent reactions. Chem Commun 2010;46:7918–20. [DOI] [PubMed] [Google Scholar]
- [18].Failli A, Immer H, Go¨tz M. The synthesis of cyclic peptides by the four component condensation (4 CC). Can J Chem 1979;57:3257–61. [Google Scholar]
- [19].Cristau P, Vors JP, Zhu JP. A rapid access to biaryl ether containing macrocycles by pairwise use of Ugi 4CR and intramolecular SNAr-based cycloetherification. Org Lett 2001;3:4079–82. [DOI] [PubMed] [Google Scholar]
- [20].Rivera DG, Wessjohann LA. Architectural chemistry synthesis of topologically diverse macromulticycles by sequential multiple multicomponent macrocyclizations. J Am Chem Soc 2009;131:3721–32. [DOI] [PubMed] [Google Scholar]
- [21].Hili R, Rai V, Yudin AK. Macrocyclization of linear peptides enabled by amphoteric molecules. J Am Chem Soc 2010;132:2889–91. [DOI] [PubMed] [Google Scholar]
- [22].Rudrakshula M, Abdelraheem EMM, Rossetti A, Twarda-Clapa A, Musielak B, Kurpiewska K, et al. Two-step synthesis of complex artificial macrocyclic compounds. Angew Chem Int Ed 2017;56:Error: FPage (10725) is higher than LPage (–10729)!. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abdelraheem EMM, de Haan MP, Patil P, Kurpiewska K, Kalinowska-Tuscik J, Shaabani S, et al. Concise synthesis of tetrazole macrocycle. Org Lett 2017;19:5078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abdelraheem EMM, Kurpiewska K, Kalinowska-Tuscik J, Do¨mling A. Artificial macrocycles by ugi reaction and passerini ring closure. J Org Chem 2016;81:8789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Beck B, Larbig G, Mejat B, Magnin-Lachaux M, Picard A, Herdtweck E, et al. Short and diverse route toward complex natural product-like macrocycles. Org Lett 2003;5:1047–50. [DOI] [PubMed] [Google Scholar]
- [26].Shevlin M Practical high-throughput experimentation for chemists. ACS Med Chem Lett 2017;8:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Madhavachary R, Abdelraheem EMM, Rossetti A, Twarda-Clapa A, Musielak B, Kurpiewska K, et al. Two-step synthesis of complex artificial macrocyclic compounds. Angew Chem Int Ed 2017;129:10865–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Estrada-Ortiz N, Neochoritis CG, Twarda-Clapa A, Musielak B, Holak TA, Do¨mling A. Artificial macrocycles as potent p53–MDM2 inhibitors. ACS Med Chem Lett 2017;8:1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shaabani S, Neochoritis C, Twarda-Clapa A, Musielak B, Holak T, Do¨mling A. Scaffold hopping via ANCHOR. QUERY: β-lactams as potent p53-MDM2 antagonists. MedChemComm 2017;8:Error: FPage (1046) is higher than LPage (–1052)!. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Popowicz GM, Do¨mling A, Holak TA. The structure-based design of MDM2/MDMX–p53 inhibitors gets serious. Angew Chem Int Ed 2011;50:2680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Neochoritis CG, Wang K, Estrada-Ortiz N, Herdtweck E, Kubica K, Twarda A, et al. 2,3ˊ-bis (1ˊ H-indole) heterocycles: new p53/MDM2/MDMX antagonists. Bioorg Med Chem Lett 2015;25:5661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang Y, Wolf S, Beck B, Ko¨hler L-M, Khoury K, Popowicz GM, et al. Discovery of highly potent p53-MDM2 antagonists and structural basis for anti-acute myeloid leukemia activities. ACS Chem Biol 2014;9:802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao Y, Aguilar A, Bernard D, Wang S. Small-molecule inhibitors of the MDM2–p53 protein–protein interaction (MDM2 inhibitors) in clinical trials for cancer treatment. J Med Chem 2015;58:1038–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov 2014;13:217. [DOI] [PubMed] [Google Scholar]
- [35].Estrada-Ortiz N, Neochoritis CG, Dömling A. How to design a successful p53-MDM2/X interaction inhibitor: a thorough overview based on crystal structures. ChemMedChem 2016;11:757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Magiera-Mularz K, Skalniak L, Musielak B, Rudzinska-Szostak E, Berlicki, et al. Bioactive macrocyclic inhibitors of the PD-1/PD-L1 immune checkpoint. Angew Chem Int Ed 2017;56:Error: FPage (13732) is higher than LPage (–13735)!. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zarganes-Tzitzikas T, Chandgude AL, Dömling A. MUlticomponent reactions, union of MCRs and beyond. Chem Rec 2015;15:Error: FPage (981) is higher than LPage (–996)!. [DOI] [PubMed] [Google Scholar]