Abstract
T helper (TH) cells constitute an important arm of the adaptive immune system because they coordinate defence against specific pathogens, and their unique cytokines and effector functions mediate different types of tissue inflammation. The recently discovered TH17 cells, the third subset of effector T helper cells, have been the subject of intense research aimed at understanding their role in immunity and disease. Here we review emerging data suggesting that TH17 cells have an important role in host defence against specific pathogens and are potent inducers of autoimmunity and tissue inflammation. In addition, the differentiation factors responsible for their generation have revealed an interesting reciprocal relationship with regulatory T (Treg) cells, which prevent tissue inflammation and mediate self-tolerance.
The hallmark of adaptive immunity in advanced vertebrates is the existence of lymphocytes, which induce and regulate immune responses. When activated by pathogens in a specific cytokine environment, naive CD4+ T cells differentiate into different subsets with distinct effector functions aimed at orchestrating and mobilizing other cell types to effectively clear invading pathogens. Based on cytokine phenotypes, initially the existence of two distinct effector TH subsets was proposed: TH1 and TH2 (ref. 1). TH1 cells produce interferon-γ (IFN- γ) and mediate protection against intracellular pathogens, whereas TH2 cells produce interleukin-4 (IL-4), IL-13 and IL-25 (also known as IL-17E) and orchestrate the clearance of extracellular pathogens1,2 (Fig. 1). Recently this paradigm has been updated following the discovery of a third subset of TH cells; these cells, known as TH17 cells (ref. 3), produce IL-17 and exhibit distinct effector functions. In the past four years there has been an explosion of information regarding this T-cell subset: the cytokines for their differentiation have been identified, the key transcription factors that are involved in their generation have been recognized and their function in tissue inflammation has been established. This review summarizes this information to develop a comprehensive view of the generation and function of TH17 cells.
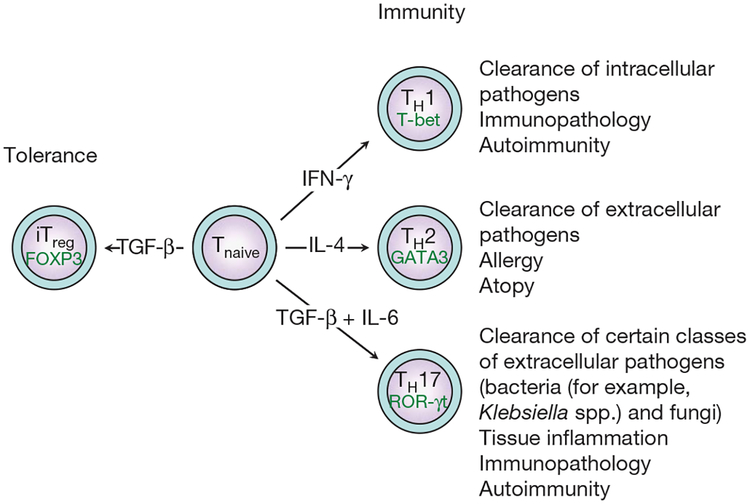
Figure 1 |. Subsets of T helper cells.
Depending on the cytokine milieu present at the time of the initial engagement of their T-cell receptor and costimulatory receptors in the peripheral immune compartment, naive CD41 T cells can differentiate into various subsets of T helper cells (TH1, TH2 and TH17). However, in the presence of TGF-β, naive T cells convert into FOXP3-expressing induced Treg (iTreg) cells. For each T helper cell differentiation programme, specific transcription factors have been identified as master regulators (T-bet, GATA3 and ROR-γt). Terminally differentiated T helper cells are characterized by a specific combination of effector cytokines that orchestrate specific and distinct effector functions of the adaptive immune system.
TH17 cells and TH17-specific effector cytokines
TH17 cells are characterized by the production of IL-17A (also called IL-17), IL-17F and IL-22 (Box 1) and are thought to clear extracellular pathogens not effectively handled by either TH1 or TH2 cells (Fig. 1). Because TH17 cells produce large quantities of IL-17A, most TH17-mediated effects are attributed to this cytokine. IL-17A is the prototypic cytokine of the IL-17 family, which includes six members: IL-17A, B, C, D, E and F4. IL-17 is a phylogenetically old cytokine that is also detected in non-mammalian vertebrates5.
Box 1 |. Effector cytokines produced by TH17 cells
IL-17A
Source. TH17 cells3,55,56, CD81 T cells73, cd T cells74, neutrophils75, eosinophils76 and monocytes77.
Receptor(s). IL-17RA is the cognate receptor for IL-17A. It is expressed at high levels on haematopoietic cells, and at lower levels on osteoblasts, fibroblasts, endothelial cells and epithelial cells78. Human IL-17RC binds human IL-17A with high affinity, but mouse IL-17RC does not bind mouse IL-17A79. Human IL-17RA–IL-17RC can form a heterodimer that binds human IL-17A80.
Effects. IL-17A induces pro-inflammatory cytokines (IL-6, TNF-α and IL-1β78) and chemokines (CXCL1, GCP-2, CXCL8 or IL-8, CINC, MCP-1; ref. 81). It increases the production of prostaglandin E2 (ref. 81), nitric oxide82 and matrix-metalloproteinases55, increases the recruitment of neutrophils14,75 and modulates neutrophil homeostasis83.
IL-17F
Source. TH17 cells3,6,9, monocytes84 and possibly other cell types.
Receptor(s). IL-17RC is the cognate receptor for IL-17F. It is expressed at low levels on haematopoietic cells, and at high levels on nonhaematopoietic cells80. Human IL-17RA–IL-17RC heterodimers can bind human IL-17F80.
Effects. IL-17F induces pro-inflammatory cytokines (IL-6, ref. 85) and chemokines (CXCL1, GCP-2, CXCL8 or IL-8, ref. 85), and increases the recruitment of neutrophils86.
IL-22
Source. TH17 cells6,9, activated T cells and natural killer cells87.
Receptor(s). The receptor for IL-22 is a heterodimer consisting of IL-22R1 and IL-10R2 (ref. 88). IL-10R2 is ubiquitously expressed in haematopoietic and non-haematopoietic cells89. IL-22R1 (CRF2–9) is expressed on a variety of epithelial and parenchymal tissues (skin, liver, kidney, pancreas, intestine and lung)90.
Effects. IL-22 increases acute-phase reactants in hepatocytes91 and protects them from acute liver inflammation35. It induces the expression of b-defensins in epithelial cells6,90 and promotes epidermal hyperplasia9.
IL-21
Source. CD41 T cells stimulated with IL-6, T 17 cells10–12, T follicular helper cells13, natural killer cells and natural killer T cells10,92.
Receptor(s). The receptor for IL-21 is a heterodimer consisting of common cytokine-receptor γ chain (γc) and IL-21R93 γc is expressed in lymphoid, but not in non-lymphoid and non-haematopoietic cells94. IL-21R is restricted to haematopoietic cells with highest levels of expression on B cells, but also on T cells, natural killer cells, and some populations of myeloid cells92.
Effects. IL-21 participates in the differentiation/amplification of TH17 cells10–12. In combination with IL-7 or IL-15, IL-21 stimulates the proliferation and differentiation of CD81 T cells95,96. It promotes B-cell differentiation and antibody class switching (IgG1, IgG3)96, induces the differentiation and cytotoxic programme of natural killer cells92 and natural killer T cells97, and induces CXCL8 in macrophages98.
In addition to IL-17A, TH17 cells co-produce IL-17F3,6. IL-17A and IL-17F have similar functions. They induce the production of proinflammatory cytokines, chemokines and metalloproteinases from various tissues and cell types (Box 1). As a result, they recruit neutrophils to tissues.
Although there is often a coordinated expression of IL-17A and IL-17F in TH17 cells and other cell types, it is now clear that there are TH cells expressing only IL-17A, IL-17F or an IL-17A–IL-17F heterodimer7,8. In addition to IL-17A and IL-17F, TH17 cells produce other effector cytokines, namely IL-21 and IL-22 (refs 6, 9–12). Neither IL-21 nor IL-22 are TH17-exclusive cytokines, but are preferentially expressed in TH17 cells.
Recent work from our group and others showed that IL-21, a member of the IL-2 family of cytokines, is produced in large amounts by T 17 cells and ICOS+CXCR5+CCR7+ T follicular helper cells13. These T follicular helper cells home to the B-cell areas of secondary lymphoid tissue and provide cognate help to B cells. However, it remains to be seen whether T follicular helper cells could represent activated or memory TH17 cells that help B cells in secondary or tertiary lymphoid organs.
IL-22 is a member of the IL-10 family of cytokines, produced by activated T cells and natural killer cells. It mediates its effects through a receptor complex composed of the IL-10R2 and the IL-22R chains. Interestingly, high concentrations of transforming growth factor-β (TGF-β) can inhibit IL-6-induced IL-22 expression9. Furthermore, whereas the combination of TGF-β plus IL-6 induces large quantities of IL-17A and IL-17F by TH17 cells, the secretion of large amounts of IL-22 by TH17 cells requires the addition of IL-23 in vitro6,9. This suggests that IL-22 could represent an end point effector cytokine produced by terminally differentiated TH17 cells.
An important role for TH17 cells in host defence
So far it is unclear which class of pathogens preferentially induces a TH17 response because pathogens as diverse as the Gram-positive Propionibacterium acnes, the Gram-negative Citrobacter rodentium, Klebsiella pneumoniae, Bacteroides spp. and Borrelia spp., the acid-fast Mycobacterium tuberculosis, and fungi such as Candida albicans can all trigger a substantial T 17 response14–19. The fungal-cell-wall-derived product zymosan or other dectin-1 ligands as well as muramyldipeptide (a derivative of bacterial peptidoglycan) are able to promote IL-17 production in T cells20. Therefore, TH17 responses are likely to emerge as an early response to a number of pathogens not handled well by TH1- or TH2-type immunity and which require robust tissue inflammation to be cleared.
Indeed, TH17 cells appear at sites of inflammation with rapid kinetics. Through the potent induction of chemokines, TH17 cells could bridge the gap between innate and adaptive immunity and attract other subsets of T helper cells to sites of infection at later stages of the inflammatory process. This has most convincingly been shown for M. tuberculosis infection, for which an early TH17 response is required to bring TH1 cells into the infected lung tissue to control the infection18.
TH17 cells in autoimmune diseases
It is widely conceived that organ-specific autoimmune diseases are the result of dysregulated autoantigen-specific TH1 responses. In many animal models of human autoimmune diseases, TH1 cells have been shown to be pathogenic21. However, the concept that auto-immune diseases were exclusively mediated by TH1 cells has been challenged, and the idea that TH17 cells are an important part of the autoimmune reaction has emerged in light of the following observations: first, mice deficient for the TH1 effector cytokine IFN-γ develop enhanced experimental autoimmune encephalomyelitis (EAE)22; second, deficiency in the IL-12p35 subunit (specific for IL-12) does not alter the progression of EAE, but deficiency in either p40 or p19, which form IL-23, results in a decreased number of TH17 cells and protection from EAE and collagen-induced arthritis23,24; third, the transfer of myelin-reactive IL-17-producing T cells expanded with IL-23 in vitro induces severe EAE3; and fourth, IL-17 has profound pro-inflammatory effects and induces tissue damage during the course of various autoimmune diseases. Indeed, IL-17 can directly or indirectly promote cartilage and bone destruction25. Conversely, IL-17-deficient mice develop attenuated collagen-induced arthritis26 and EAE27. Increased levels of IL-17 have been observed in patients with rheumatoid arthritis28, multiple sclerosis29, inflammatory bowel disease30 and psoriasis31. Furthermore, IL-22 produced by TH17 cells mediates IL-23-induced acanthosis and dermal inflammation9. In addition, IL-22, similarly to IL-17, can disrupt tight junctions between endothelial cells of the blood–brain barrier32. These data indicate a pathogenic role of TH17-associated cytokines and TH17 cells in inducing autoimmune tissue inflammation both in experimental animals and in humans.
Despite the recent major interest in TH17 cells, these cells may not be the only TH cells that can induce autoimmunity because TH1 cells can readily transfer organ-specific autoimmune disease33. It is therefore possible that there is a sequential involvement and different functions of TH17 and TH1 subsets rather than an exclusive role of these subsets during the development of autoimmune diseases and other tissue inflammation34. In this scenario, TH17 cells might facilitate the migration of other TH cells (such as TH1 cells) into the target tissue, which could further propagate and modulate inflammation and tissue damage.
Taken together, these data suggest that TH17 cells are potent inducers of autoimmunity through the promotion of tissue inflammation and the mobilization of the innate immune system. However, in some tissues, such as the gut and perhaps the liver35, TH17 cells, as potent early players of the adaptive immune system, might also have modulatory and protective roles.
At least two cytokines are needed to differentiate TH17 cells
In contrast to TH1 and TH2 cell differentiation, which depend on their respective effector cytokines (IFN-γ and IL-4) for differentiation, TH17 differentiation does not require IL-17. Instead, IL-6 and TGF-β—two cytokines with opposing effects—together induce the development of TH17 cells15,36,37. IL-6 is a pro-inflammatory cytokine strongly induced in cells of the innate immune system on engagement of specific pattern-recognition receptors such as Toll-like receptors and C-type lectin receptors. Thus, infection or local inflammation induces large amounts of IL-6. In the immune system, TGF-β is regarded as an anti-inflammatory cytokine because the loss of TGF-β is associated with a fatal lymphoproliferative disease38. In mice, TGF-β plus IL-6 have also been shown to be the differentiating factors for TH17 cells in vivo. First, TGF-β transgenic animals immunized with the myelin oligodendrocyte glycoprotein MOG35–55 in complete Freund’s adjuvant, which induces high amounts of IL-6, develop exacerbated EAE owing to enhanced frequencies of TH17 cells37. Second, mice with a defect in TGF-β responsiveness in T cells are protected from EAE owing to the lack of generation of TH17 cells39. Third, when TGF- β is not secreted by T cells as a result of a conditionally disrupted Tgfb gene in CD4 cells, TH17 cells cannot be generated and the mice are relatively protected from developing EAE40. Consistent with the idea that TH17 cells require both TGF-β and IL-6, we showed that IL-6-deficient mice fail to develop a TH17 response and are resistant to the development of EAE10,37.
In humans, IL-17-producing T cells have been detected in the memory population of peripheral blood mononuclear cells (PBMCs); in one report they were characterized by the combined expression of CCR4 and CCR6 (ref. 41), and in another they were characterized by the expression of CCR2 and lack of CCR5 (ref. 42). A heterogeneous population of IL-17 and IFN-γ double-producers resided in the CCR6+CXCR3+ human memory-T-cell compartment41. It has been reported that in naive human T cells, the combination of TGF-β plus IL-6 or TGF-β plus IL-21 failed to induce the differentiation of TH17 cells43,44. Recent findings suggested that CD45RA+ human CD4+ T cells can be more efficiently differentiated into TH17 cells by a combination of IL-1β plus IL-6 (ref. 43) or IL-1β plus IL-23 (ref. 31). However, the presence of TGF-β in fetal calf serum or human serum used in these culture conditions cannot be totally excluded. Interestingly, another study indicated that the combination of TGF-β plus IL-6 is capable of inducing the expression of ROR-γt, a transcription factor important for TH17 cells (see below), but not the expression of IL-17 in human T cells45. More recently, TGF-β in combination with IL-1β, IL-6 or IL-21 was shown to induce the differentiation of human TH17 cells99,100, thus highlighting the role of TGF-β in TH17 differentiation. This also underscores the similarities in the differentiation of mouse and human TH17 cells.
IL-21 and other cytokines as amplifiers of TH17 cells
Although IFN-γ and IL-4 produced by TH cells reinforce, TH1 and TH2 differentiation, respectively46, IL-17 does not act on the differentiation and expansion of TH17 cells. Three independent groups reported simultaneously that IL-21, a member of the IL-2 family of cytokines, is produced in overwhelming amounts by TH17 cells and could, in combination with TGF-β, induce T 17-differentiation10–12. Therefore, IL-21 produced by natural killer cells and natural killer T cells could induce the differentiation of TH17 cells in the absence of IL-6 (ref. 10). When IL-6 is present, however, IL-21-receptor-deficient mice show a reduced but detectable TH17 response in vitro and in vivo10. These findings point to a relevant function of IL-21, produced by newly generated TH17 cells, in amplifying the precursor frequency of differentiating TH17 cells (Fig. 2). In addition to IL-21, other cytokines such as tumour-necrosis factor alpha (TNF-α) and IL-1, which are not specifically produced by TH17 cells, have been proposed to have an additional role in the amplification of TH17 responses36,47.
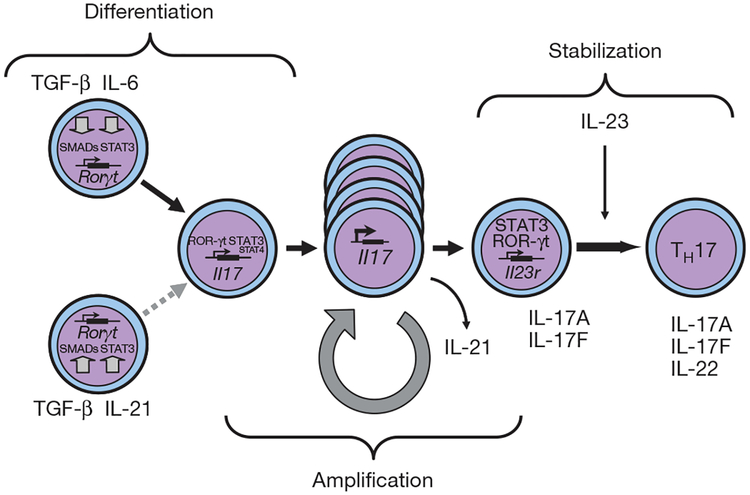
Figure 2 |. Steps in the differentiation of TH17 cells.
Different factors control the initial differentiation of TH17 cells from naive T cells, the amplification of TH17 precursor cells, and finally the stabilization and effector phenotype of TH17 cells. Whereas TGF-β together with IL-6 are the differentiation factors for TH17 cells, IL-21, which is produced by TH17 cells themselves, acts in a positive feedback loop to increase the frequency of TH17 cells. STAT3 is the essential signalling molecule for the differentiation of TH17 cells because the induction of IL-21 is absolutely dependent on STAT3, and STAT3 is also critical in the signal transduction cascades of IL-6, IL-21 and IL-23 receptors. IL-23 expands and stabilizes TH17 cells to produce their effector cytokines IL-17, IL-17F and IL-22.
What is the role of IL-23?
The observation that IL-23p19-deficient animals, which did not develop EAE, lack IL-17-producing T cells24 and the fact that IL-23 could expand a population of IL-17-producing pathogenic cells3 pointed to an important role of IL-23 in the development of pathogenic TH17 cells. It is now clear, however, that IL-23 does not act on naive T cells to induce their differentiation, but instead acts on already differentiated TH17 cells. The maintenance of TH17 cells in vitro for extended periods of time appears to require IL-23, which might also modulate effector functions of TH17 cells both in vitro and in vivo. Recently it has been shown that T cells cultured in the presence of TGF-β plus IL-6 did not induce tissue inflammation unless they are further cultured in the presence of IL-23, which could decrease the secretion of IL-10 by these cells48,49.
Although IL-23 was described eight years ago50, little is known about the role of IL-23 for TH17 cells in vivo. IL-23 signals through a receptor composed of the IL-12Rβ1 chain (which it shares with the IL-12 receptor) and a unique IL-23R subunit51. IL23R mRNA expression has mainly been detected in T cells, natural killer cells and natural killer T cells, but low levels of this receptor can also be found in monocytes, macrophages and dendritic cells52. Both IL-6 and IL-21 are strong inducers of IL-23R in T cells12. Furthermore, IL-23 can enhance the expression of its own receptor through an auto-crine or paracrine feedback loop in mouse3 (M. Oukka, unpublished observation) and human45 T cells. The understanding of the regulation of IL-23R expression on naive T cells as they develop into TH17 cells and on cells of the innate immune system will shed light on the role of this cytokine.
There is new evidence that IL-23 may have a profound impact on the innate immune system as well. Recent work has demonstrated that the development of gut inflammation in T-cell-deficient mice was dependent on IL-23, in that the loss of IL-23 but not IL-12 was associated with a decrease in gut inflammation mediated by anti-CD40-antibody-activated cells of the innate immune system53. IL-23 appears to induce IL-17, IL-1 and IL-6 from cells of the innate immune system47,53. Whether IL-23-mediated gut inflammation is entirely dependent on IL-17 produced by cells of the innate immune system has not been addressed. Consistent with this finding, a genome-wide scan revealed that particular SNPs in the coding sequence (rs11209026, c.1142G > A, p.Arg381Gln) of the IL23R gene conferred strong protection from Crohn’s disease54. It has not been tested whether the different IL-23R variants affect the innate or adaptive immune systems or both.
We propose that the full differentiation of TH17 cells requires three different steps: induction, amplification and stabilization/maintenance (Fig. 2). (1) The differentiation is initiated by the combined actions of IL-6 and TGF-β; (2) the amplification of the TH17 response is driven through the production of IL-21 by TH17 cells; (3) the stabilization/maintenance of the TH17 phenotype is achieved by IL-23. Whereas the first two steps in the development of TH17 cells seem to be distinct, it is possible that the stabilization and the amplification phases overlap or take place simultaneously.
TH17-specific transcription factors
The differentiation of effector TH cells is initiated by proximal signals from the T-cell receptor, co-stimulatory molecules and cytokine receptors. These integrated signals then lead to the induction of lineage-specific transcription factors. TH17 cells have emerged as a truly independent subset because their differentiation was shown to be independent of the TH1- or TH2-promoting transcription factors T-bet, STAT1, STAT4 and STAT6 (refs 55–57). Consistent with the role of IL-6 in the differentiation of TH17 cells, STAT3 appears to be critical for the differentiation of TH17 cells because conditional deletion of STAT3 in T cells prevents the development of TH17 cells58. Activation in the presence of TGF-β1 alone normally induces FOXP3 and generates induced Treg cells (iTreg cells). In Treg cells, the interaction of TGF-β1 with its receptor induces the phosphorylation of SMAD2/3 proteins and the formation of a complex with SMAD4, which then translocates to the nucleus. Whether similar molecules are involved in the differentiation of TH17 cells remains to be determined.
Analogously to TH1 and TH2 subsets, TH17 development relies on the action of a lineage-specific transcription factor, identified as the orphan nuclear receptor ROR-γt. ROR-γt is selectively expressed in TH17 cells differentiated in the presence of TGF-β plus IL-6, and transduction of naive T cells with a retroviral vector containing ROR-ct induces the development of TH17 cells59. Conversely, loss of ROR-ct in T cells prevents the generation of myelin-specific TH17 cells and subsequently the development of EAE in mice immunized with myelin antigens. Furthermore, the analysis of ROR-γt–GFP (green fluorescent protein) reporter mice revealed the existence of a population of IL-171 cells that are constitutively present in the intestinal lamina propria and whose development depends on ROR-γt expression59. These observations argue in favour of a critical role of ROR-γt in the differentiation of the TH17 lineage (Fig. 2). However, the mechanisms by which ROR-γt drives TH17 development have not yet been fully elucidated. A recent report indicates that, similarly to T-bet inducing IL-12Rβ2, ROR-γt would induce IL-23R. More precisely, IL-6-mediated activation of both STAT3 and ROR-γt would promote the production of IL-21 by TH17 cells, and IL-21 would then induce the expression of IL-23R and establish responsiveness of TH17 cells to IL-23. In this model, a sequential involvement of IL-6, IL-21 and IL-23 would lead to the differentiation of TH17 cells12. Another report suggested that the induction of IL-21 required STAT3, but not ROR-γt11. In addition, it has not yet been formally addressed whether ROR-γt directly transactivates the IL17A, IL17F, IL22 and IL21 genes in TH17 cells. A more recent report suggested that TH17 differentiation might be mediated by the combined effect of ROR-γt and ROR-α, both of which are expressed at high levels in differentiated TH17 cells. Loss of only one of these transcription factors resulted in partial loss of TH17 cytokine expression, and loss of both ROR-γt and ROR-α abrogated TH17 differentiation60.
Reciprocal relationship between iTreg and TH17 cells
TGF-β induces the Treg-specific transcription factor FOXP3 and is required for the maintenance of iTreg cells in the peripheral immune compartment. However, addition of IL-6 to TGF-β inhibits the generation of Treg cells and induces TH17 cells. On the basis of these data, we first proposed37 that there is a reciprocal relationship between Treg cells and TH17 cells, and that IL-6 has a pivotal role in dictating the balance between these two cell populations10,37.
This reciprocal relationship between Treg and TH17 cells is further supported by recent data from other laboratories61,62: IL-2, which is a growth factor for Treg cells, has also been shown to inhibit the generation of T 17 cells61. Consistent with these data, mice that lack IL-2 or in which IL-2 signalling is compromised (Stat5–/–), harbour reduced numbers of Treg cells and an increased proportion of TH17 cells in the peripheral repertoire61. Moreover, these mice develop multi-organ inflammatory diseases, which can be prevented by the passive transfer of Treg cells63.
Additional evidence for a reciprocal developmental relationship between FOXP31 Treg cells and TH17 cells came from the discovery that retinoic acid, a vitamin A metabolite, could drive the generation of Treg cells64 by enhancing TGF-β signalling and enhancing FOXP3 promoter activity while abrogating the differentiation of TH17 cells, but not of TH1 cells, through the inhibition of IL-6 signalling62. These findings indicate that retinoic acid can regulate the balance between pro-inflammatory TH17 cells and anti-inflammatory Treg cells (Fig. 3).
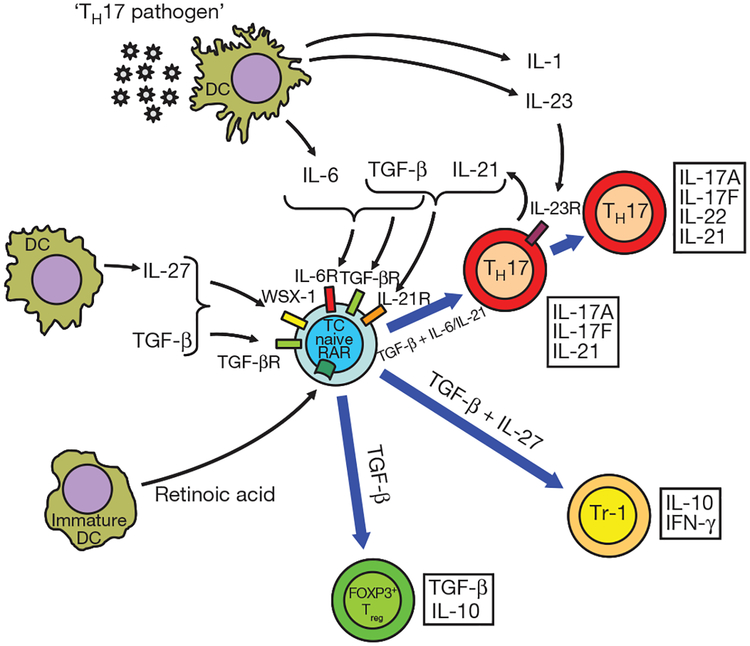
Figure 3 |. The developmental pathways of TH17 cells and FOXP3+ Treg cells require TGF-β signalling and are reciprocally regulated.
TGF-β is ubiquitous although its most relevant source in regulating immune reactions is still unclear. Other factors such as retinoic acid or cytokines such as IL-6, IL-1, IL-23 or IL-27 are provided by cells of the innate immune system (immature or activated dendritic cells (DCs), respectively) and dictate whether a naive T cell (TC) develops into a FOXP3+ Treg cell, a TH17 cell or an IL-10-secreting T cell of the Tr-1 phenotype. IL-6R, IL-6 receptor; IL-21R, IL-21 receptor; IL-23R, IL-23 receptor; RAR, retinoic acid receptor; TGF-βR, TGF-β receptor; WSX-1, IL-27 receptor).
Finally, it has been found that ROR-γt and RORα, the transcription factors for TH17 cells, and FOXP3, the transcription factor for Treg cells, can physically bind to each other and antagonize each other’s functions65,66. In line with this concept, conditional deletion of FOXP3 protein in ‘Treg cells’ in vivo resulted in an increase in ROR-γt, IL-17 and IL-21 expression67,68, further corroborating the reciprocal relationship between TH17 cells and Treg cells.
More than one way to inhibit TH17 cells
So far, several cytokines and pathways have been shown to inhibit the development and expansion of TH17 cells. TH1- and TH2-specific cytokines can antagonize each other. Correspondingly, IL-4, IL-25 (IL-17E) and IFN-γ also inhibit the expansion of T 17 cells55,56,69.
Similarly, IL-27, a member of the IL-12 heterodimeric family of cytokines produced by cells of the innate immune system, can suppress the development of TH17 responses. Consistent with this observation, a lack of IL-27 signalling resulted in an increased TH17 response and enhanced inflammation of the central nervous system in two different disease models70,71. Two recent studies showed that IL-27 together with TGF-β induces the differentiation of IL-10-producing T cells with Tr-1-like properties and that IL-27R-deficient mice (Wsx1–/–) have a defect in generating IL-10-producing Tr-1 cells48,72. Thus, IL-27 might also be necessary to control exaggerated immunopathology indirectly by inducing Tr-1 cells (Fig. 3).
Role of TGF-β in inducing novel TH subsets
Since the discovery of TGF-β and IL-6 as the differentiation factors for TH17 cells, we have proposed that the dual cytokine interaction (TGF-β plus a cytokine X) might be operational in the induction of other novel T-cell subsets as well. Tr-1 differentiation induced by a combination of TGF-β plus IL-27 supports this hypothesis48,72. When TGF-β is involved in the differentiation of novel TH subsets, TH commitment might be accomplished either by TGF-β acting together with another cytokine, where the two cytokines will inhibit each other’s functions and result in the generation of a totally new gene programme (for example, TH17 differentiation induced by TGF-β plus IL-6), or by quantitatively scaling back each other’s functions, thereby resulting in the production of only dominant cytokines in the responding T cells (for example, Tr-1 cells induced by TGF-β plus IL-27; Fig. 4). These observations suggest that T cells would sense multiple cytokine inputs simultaneously from the environment to initiate the differentiation of new T-cell subsets with distinct cytokine phenotypes and effector functions.
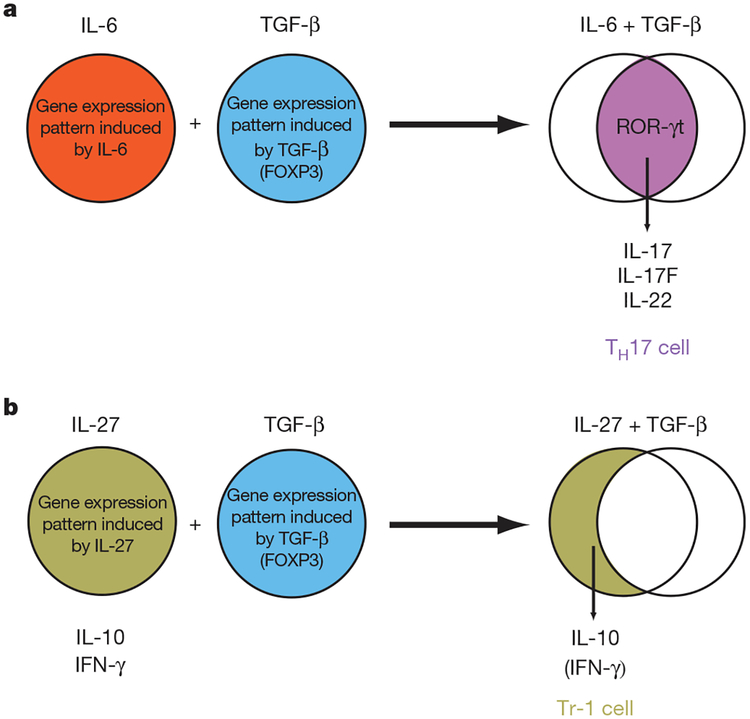
Figure 4 |. Effects of TGF-β in shaping the transcriptional programme of developing T helper cell subsets.
a, IL-6 and TGF-β independently induce specific gene expression programmes in T cells. However, when both cytokines act in concert, an essentially novel and distinct gene expression programme is induced resulting in a qualitatively different outcome such as the TH17 transcriptional programme (IL-17, IL-17F and IL-22). b, In contrast, when TGF-β acts in combination with cytokines such as IL-27, IFN-γ expression is scaled down and IL-10 expression is increased resulting in a Tr-1-like phenotype.
Concluding remarks
It is now established that TH17 cells constitute an independent T-helper-cell subset with major functions in the induction of tissue inflammation and host protection against extracellular pathogens. Since their initial description, substantial progress has been made in the understanding of TH17 differentiation and effector functions. On the basis of recent reports, we propose a three-step model for the differentiation of TH17 cells: induction, amplification and maintenance/stabilization, where TGF-β plus IL-6 induce the differentiation of TH17 cells, IL-21 amplifies the frequency of TH17 cells and IL-23 stabilizes the phenotype of previously differentiated TH17 cells. Loss of any one of the members in this pathway (IL-6, IL-21 or IL-23) limits the TH17 response, and only the combination of these factors leads to a robust and stable TH17 response. The understanding of how different cytokine signalling pathways are integrated to induce the differentiation of novel TH subsets, including TH17 cells, will represent a major step forward in our understanding of T-cell-subset differentiation. Because multiple lines of evidence suggest that there is a reciprocal relationship between Treg cells and TH17 cells, manipulation of this differentiation pathway might result in the generation of pro-inflammatory TH17 cells and induce tissue inflammation or induce protective Treg cells and therefore inhibit autoimmunity and induce tolerance. Targeting nodal points in this pathway will allow one to shift the balance between pro-inflammatory TH17 cells and inhibitory Treg cells and thus provide exciting new targets for the treatment of multiple inflammatory and autoimmune diseases.
Acknowledgements
This work was supported by grants from the National Multiple Sclerosis Society, the National Institutes of Health, the Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard, and the Deutsche Forschungsgemeinschaft. V.K.K. is the recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health.
References
- 1.Mosmann TR & Coffman RL TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol 7, 145–173 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Fort MM et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med 201, 233–240 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolls JK & Linden A Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Gunimaladevi I, Savan R & Sakai M Identification, cloning and characterization of interleukin-17 and its family from zebrafish. Fish Shellfish Immunol. 21, 393–403 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Liang SC et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med 203, 2271–2279 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SH & Dong C A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 17, 435–440 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Liang SC et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol 179, 7791–7799 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Korn T et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurieva R et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Zhou L et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 8, 967–974 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Chtanova T et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol 173, 68–78 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Ye P et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med 194, 519–527 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangan PR et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Chung DR et al. CD41 T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J. Immunol 170, 1958–1963 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Infante-Duarte C, Horton HF, Byrne MC & Kamradt T Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol 165, 6107–6115 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Khader SA et al. IL-23 and IL-17 in the establishment of protective pulmonary CD41 T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature Immunol. 8, 369–377 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Na L, Fidel PL & Schwarzenberger P Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis 190, 624–631 (2004). [DOI] [PubMed] [Google Scholar]
- 20.van Beelen AJ et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27, 660–669 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Charlton B & Lafferty KJ The Th1/Th2 balance in autoimmunity. Curr. Opin. Immunol 7, 793–798 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Ferber IA et al. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol 156, 5–7 (1996). [PubMed] [Google Scholar]
- 23.Becher B, Durell BG & Noelle RJ Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest 110, 493–497 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cua DJ et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Sato K et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med 203, 2673–2682 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakae S, Nambu A, Sudo K & Iwakura Y Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol 171, 6173–6177 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Komiyama Y et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol 177, 566–573 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Chabaud M et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42, 963–970 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Lock C et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature Med. 8, 500–508 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Fujino S et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson NJ et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunol. 8, 950–957 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Kebir H et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nature Med. 13, 1173–1175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Nun A, Wekerle H & Cohen IR The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur. J. Immunol 11, 195–199 (1981). [DOI] [PubMed] [Google Scholar]
- 34.Lohr J, Knoechel B, Wang JJ, Villarino AV & Abbas AK Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J. Exp. Med 203, 2785–2791 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zenewicz LA et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM & Stockinger B TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Bettelli E et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni AB et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA 90, 770–774 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldhoen M, Hocking RJ, Flavell RA & Stockinger B Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nature Immunol. 7, 1151–1156 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Li MO, Wan YY & Flavell RAT Cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26, 579–591 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Acosta-Rodriguez EV et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunol. 8, 639–646 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Sato W, Aranami T & Yamamura T Cutting edge: human Th17 cells are identified as bearing CCR21CCR52 phenotype. J. Immunol 178, 7525–7529 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A & Sallusto F Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nature Immunol 8, 942–949 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Evans HG, Suddason T, Jackson I, Taams LS & Lord GM Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc. Natl Acad. Sci. USA 104, 17034–17039 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z, Tato CM, Muul L, Laurence A & O’Shea JJ Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 56, 2936–2946 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy KM & Reiner SL The lineage decisions of helper T cells. Nature Rev. Immunol 2, 933–944 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Sutton C, Brereton C, Keogh B, Mills KH & Lavelle EC A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med 203, 1685–1691 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stumhofer JS et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature Immunol. 8, 1363–1371 (2007). [DOI] [PubMed] [Google Scholar]
- 49.McGeachy MJ et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nature Immunol. 8, 1390–1397 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Oppmann B et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Kastelein RA, Hunter CA & Cua DJ Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol 25, 221–242 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Parham C et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rb1 and a novel cytokine receptor subunit, IL-23R. J. Immunol 168, 5699–5708 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Uhlig HH et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Duerr RH et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park H et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 6, 1133–1141 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrington LE et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Chen Z et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl Acad. Sci. USA 103, 8137–8142 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang XO et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem 282, 9358–9363 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Ivanov II et al. The orphan nuclear receptor RORct directs the differentiation program of proinflammatory IL-171 T helper cells. Cell 126, 1121–1133 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Yang XO et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORa and RORc. Immunity 28, 29–39 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurence A et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Mucida D et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Antov A, Yang L, Vig M, Baltimore D & Van Parijs L Essential role for STAT5 signaling in CD251CD41 regulatory T cell homeostasis and the maintenance of self-tolerance. J. Immunol 171, 3435–3441 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Coombes JL et al. A functionally specialized population of mucosal CD1031 DCs induces Foxp31 regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med 204, 1757–1764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou L et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORct function. Nature 453, 236–240 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du J, Huang C, Zhou B & Ziegler SF Isoform-specific inhibition of RORα-mediated transcriptional activation by human FOXP3. J. Immunol 180, 4785–4792 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Gavin MA et al. Foxp3-dependent programme of regulatory T-cell differentiation. Natue 445, 771–775 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Williams LM & Rudensky AY Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature Immunol. 8, 277–284 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Kleinschek MA et al. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med 204, 161–170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Batten M et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature Immunol. 7, 929–936 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Stumhofer JS et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature Immunol. 7, 937–945 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Awasthi A et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature Immunol. 8, 1380–1389 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Liu SJ et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-β and interleukin-6. J. Leukoc. Biol 82, 354–360 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Lockhart E, Green AM & Flynn JL IL-17 production is dominated by cd T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol 177, 4662–4669 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Ferretti S, Bonneau O, Dubois GR, Jones CE & Trifilieff A IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol 170, 2106–2112 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Molet S et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol 108, 430–438 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Zhou Q, Desta T, Fenton M, Graves DT & Amar S Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect. Immun 73, 935–943 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao Z et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3, 811–821 (1995). [DOI] [PubMed] [Google Scholar]
- 79.Kuestner RE et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol 179, 5462–5473 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toy D et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol 177, 36–39 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Fossiez F et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med 183, 2593–2603 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martel-Pelletier J, Mineau F, Jovanovic D, Di Battista JA & Pelletier JP Mitogen-activated protein kinase and nuclear factor kB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: possible role of transactivating factor mitogen-activated protein kinase-activated proten kinase (MAPKAPK). Arthritis Rheum. 42, 2399–2409 (1999). [DOI] [PubMed] [Google Scholar]
- 83.Stark MA et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Starnes T et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J. Immunol 167, 4137–4140 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Hymowitz SG et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 20, 5332–5341 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurst SD et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol 169, 443–453 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Wolk K & Sabat R Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 17, 367–380 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Kotenko SV et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rb) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem 276, 2725–2732 (2001). [DOI] [PubMed] [Google Scholar]
- 89.Moore KW, de Waal Malefyt R, Coffman RL & O’Garra A Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol 19, 683–765 (2001). [DOI] [PubMed] [Google Scholar]
- 90.Wolk K et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004). [DOI] [PubMed] [Google Scholar]
- 91.Dumoutier L, Van Roost E, Colau D & Renauld JC Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl Acad. Sci. USA 97, 10144–10149 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parrish-Novak J et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408, 57–63 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Leonard WJ & Spolski R Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nature Rev. Immunol 5, 688–698 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Takeshita T et al. Cloning of the gamma chain of the human IL-2 receptor. Science 257, 379–382 (1992). [DOI] [PubMed] [Google Scholar]
- 95.Zeng R et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med 201, 139–148 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spolski R & Leonard WJ Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol 26, 57–79 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Coquet JM et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol 178, 2827–2834 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Pelletier M, Bouchard A & Girard D In vivo and in vitro roles of IL-21 in inflammation. J. Immunol 173, 7521–7530 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Manel N, Unutmaz D & Littman DR The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgamma. Nat. Immunol advance online publication doi:10.1038/ni.1610 (4 May 2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature advance online publication doi:10.1038/nature07021 (11 May 2008). [DOI] [PMC free article] [PubMed] [Google Scholar]