Abstract
Objective:
Although fluorescence imaging is being applied to a wide range of cancers, it remains unclear which disease populations will benefit greatest. Therefore, we review the potential of this technology to improve outcomes in surgical oncology with attention to the various surgical procedures while exploring trial endpoints that may be optimal for each tumor type.
Background:
For many tumors, primary treatment is surgical resection with negative margins, which corresponds to improved survival and a reduction in subsequent adjuvant therapies. Despite unfavorable effect on patient outcomes, margin positivity rate has not changed significantly over the years. Thus, patients often experience high rates of re-excision, radical resections, and overtreatment. However, fluorescence-guided surgery (FGS) has brought forth new light by allowing detection of subclinical disease not readily visible with the naked eye.
Methods:
We performed a systematic review of clinicatrials.gov using search terms ‘‘fluorescence,’’ ‘‘image-guided surgery,’’ and ‘‘near-infrared imaging’’ to identify trials utilizing FGS for those received on or before May 2016. Inclusion criteria: fluorescence surgery for tumor debulking, wide local excision, whole-organ resection, and peritoneal metastases. Exclusion criteria: fluorescence in situ hybridization, fluorescence imaging for lymph node mapping, nonmalignant lesions, nonsurgical purposes, or image guidance without fluorescence.
Results:
Initial search produced 844 entries, which was narrowed down to 68 trials. Review of literature and clinical trials identified 3 primary resection methods for utilizing FGS: (1) debulking, (2) wide local excision, and (3) whole organ excision.
Conclusions:
The use of FGS as a surgical guide enhancement has the potential to improve survival and quality of life outcomes for patients. And, as the number of clinical trials rise each year, it is apparent that FGS has great potential for a broad range of clinical applications.
Keywords: fluorescence contrast-enhanced surgery, fluorescence imaging, fluorescence-guided surgery, surgical oncology
The primary treatment of many solid tumors is surgical resection with negative margins. Currently, the standard of care for achieving these negative margins rests on visual inspection, palpation, intraoperative ultrasound (US), and intraoperative histopathological analysis of frozen tumor margins. Unfortunately, definitive margin assessment and lymph node involvement are not available until several days postsurgery; therefore, not capable of informing intraoperative decision making. Multiple studies have demonstrated that higher rates of negative margins correspond to improved survival and a reduced need for subsequent adjuvant therapies. For example, maximum cytoreduction during surgical debulking of malignant gliomas is beneficial for tumor-free survival.1 In breast-conserving surgery, margin status remains an important prognostic factor for both local recurrence and re-excision rates in patients with breast cancer.2,3 In fact, the direct correlation of positive margins to increased local recurrence results in poorer prognoses for patients with cancers of the head and neck,4 breast,5,6 colorectal,7 lung,8 extremities,9,10 and urogenital11,12 regions. Despite the unfavorable effect on patient outcomes and added healthcare expenditure of reexcision and/or adjuvant therapies to address positive surgical margins, margin positivity rate has not changed significantly over the past several decades,13 with current reports describing margin positivity in 15% to 60% of tumor resections.14–20 Traditional palpation and visualization of malignant versus normal tissue is not reliably sufficient. Intraoperative frozen section analysis is limited to certain tissue types and presents significant challenges by being time-consuming, subjective to sampling error, and associated with discrepancies between frozen and permanent pathology in 5% to 15% of cases.21 In addition, intraoperative difficulties in translating preoperative contrast-enhanced tumor margins into real-time often preclude complete surgical resection. These factors lead to high rates of re-excision, unnecessary radical resections, and in some instances, overtreatment. Together, they result in highly morbid, suboptimal surgical treatments for many tumor types.
In the field of oncologic surgery, the 2 most commonly used intraoperative imaging modalities are US and fluoroscopy.22 Although useful for their respective utilities in mass detection and angiography, they have limited anatomic resolution required for surgical guidance of subclinical tumor tissue. Although intraoperative computed tomography and magnetic resonance imaging (MRI) have been useful in the field of neurosurgery, they are costly, complex, and not widely available for use outside of tertiary medical centers. More importantly, these imaging modalities are neither cancer-specific nor real-time, and difficulties remain in delineating normal from diseased tissue in the operating room (OR).21
The recent advent of optical imaging with fluorescence-guided surgery (FGS) has begun to bridge the gap between preoperative tumor imaging and real-time tumor-specific identification.23 The advantages of real-time fluorescence imaging (FI) to identify cancerous tissue and delineate tumor margins are numerous. Neoplastic tissue detection with FGS has the potential to optimize locoregional control by identifying and excising subclinical disease, improve OR efficiency, and decrease morbidity.13 In fact, cancer-specific FGS with 5-aminolevulinic acid (5-ALA) has been successfully implemented for resection of malignant gliomas in Europe after studies clearly demonstrated clinical benefits with regards to completeness of tumor removal and progression-free survival.1 For patients with oral, esophageal, and anal cancers, the preservation of normal tissue is vitally important in maintaining the very rudimentary functions of daily living.24 In patients with extremity sarcoma, limb salvage decisions are often predicated on marginnegative resections sparing critical neurovascular structures.25 Furthermore, the use of FGS will favorably complement the tactile-limited field of minimally invasive surgery.
Recently, there has been a considerable increase in the number of new clinical trials utilizing FGS; increasing from 9 in 2012 to 22 in 2013, and increasing each year as the clinical applications of FGS continue to broaden (Fig. 1). Although the technique is being applied to a wide range of tumor types, it remains unclear which disease populations will have the greatest benefit from use of this technology. To this end, we review the potential of this technology to improve outcomes in surgical oncology with attention to the differences in surgical procedures and explore clinical trial endpoints that may be optimal for each tumor type (Fig. 2).
FIGURE 1.
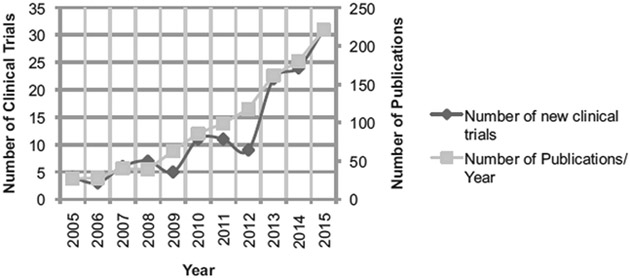
Number of new clinical trials and publications by year. Methods for number of clinical trials: clinicatrials.gov searched for keywords ‘‘fluorescence,’’ ‘‘image-guided surgery,’’ and ‘‘near-infrared imaging’’ in May 2016, which produced 844 entries that were narrowed down to 68 studies by inclusion/exclusion criteria. Methods for publications per year: Searched PubMed Advanced Search Builder on July 7, 2016 for Title/Abstract containing ‘‘fluorescence imaging surgery’’ OR ‘‘fluorescence imaging resection’’ OR ‘‘fluorescence-guided surgery’’ OR ‘‘fluorescence guided resection’’ to produce 1099 results in humans and animals. Results further narrowed by applicable year (ie, 2015: January 1, 2015–Decemer 31, 2015).
FIGURE 2.

Mixed media representation demonstrating images obtained from FGS clinical trials. Debulking: preoperative gadolinium-enhanced MRI (left) and intraoperative 5-ALA-induced tumor fluorescence in GBM (right).26 Circular lines show intraoperative tumor location. Wide local excision: autofluorescence improving tumor margin delineation versus white light.27 Whole-organ resection: intraoperative fluorescence images of the parathyroid gland with (A) bright-field of parathyroid and thyroid from patient undergoing thyroidectomy, (B) NIR fluorescence showing parathyroid in red, and (C) bright-field and fluorescence images superimposed to show parathyroid fluorescence.28
METHODS
We performed a systematic review of clinicatrials.gov using search terms ‘‘fluorescence,’’ ‘‘image-guided surgery,’’ and ‘‘near-infrared imaging’’ to identify ongoing and completed trials using FGS for trials first received on or before May 2016. Each entry was manually reviewed and inclusion criteria consisted of fluorescence surgery for purposes of primary malignant tumor debulking, wide local excision (WLE), and/or whole-organ resection. FGS for peritoneal metastases were additionally included. Exclusion criteria consisted of fluorescence in situ hybridization, FI for lymph node mapping and detection, noncancerous lesions, studies consisting of only spectroscopy without fluorescence, optical coherence tomography, FI for nonsurgical purposes (ie, cancer screening, surveillance, and/or detection, including biopsy and perfusion), stereotactic image guidance, and image-guided surgery without use of FI. In addition, studies withdrawn before enrollment or similar reasons such as termination due to low enrollment were not included for analysis. Each study was systematically organized into a database using Microsoft Excel to evaluate publications associated with the clinical trial number and/or the principal investigator, organ system, tumor type, surgical intervention, imaging probe and targeting mechanism, study phase, and trial dates.
RESULTS
Initial search yielded 844 entries, which was narrowed down to a total of 68 studies using our inclusion and exclusion criteria (Supplementary Table 1, http://links.lww.com/SLA/B167). Organization into the database resulted in 27 debulking, 37 WLE, and 4 whole-organ resection trials. Review of currently published literature and ongoing clinical trials identified 3 primary resection methods for utilizing FGS: (1) debulking, (2) WLE, and (3) whole organ excision. For each category, we review the data and trial outcomes to establish efficacy of FGS as an adjunct to standard surgical methods (Table 1).
TABLE 1.
Summary of Oncologic Procedures Amenable to Fluorescence-guided Surgery, Including Pertinent Tumor Types and Trial Endpoints
| Trial Endpoints |
|||
|---|---|---|---|
| Method of Resection | Tumor Types | Increased | Decreased |
| Debulking | Brain | Progression-free survival | Need for adjuvant therapy |
| Ovarian | Overall survival | Reoperation rates | |
| Testicular | QOL | ||
| Colorectal | Completeness in cytoreduction | ||
| Pancreatic | |||
| Wide local excision | Head and neck cancers | Locoregional control | Local recurrence |
| Bladder | Tissue sparing | Margin positivity | |
| Skin (melanoma, NMSCs) | Morbidity | Radical resections | |
| Hepatocellular and other hepatic lesions | Re-excision and reoperation | ||
| Lung | |||
| Breast | |||
| Sarcoma | |||
| Prostate | |||
| Colorectal | |||
| Pancreatic | |||
| Renal | |||
| Whole-organ resection | Thyroid | Detection/visualization of surrounding, nondiseased tissue | Morbidity |
| Parathyroid | Tissue sparing | Iatrogenic injury (when applicable) | |
| Prostate | |||
NMSC indicates nonmelanoma skin cancer.
DISCUSSION
Debulking
Debulking, or cytoreduction, is the partial surgical resection of an incurable neoplasm that is performed without curative intent (Fig. 3). It is further defined as complete or optimal; where complete may be interpreted as absence of any visible residual tumor and optimal is where amount of residual tumor is 10 mm maximum.29 This is often done to improve quality of life (QOL) or to maximize the efficacy of adjuvant therapy, such as chemoradiation. In patients with glioblastoma multiforme (GBM), complete surgical debulking is the most efficacious treatment available to improve survival.30,31 Similarly, in patients with ovarian cancer, the amount of residual tumor after debulking surgery is the most important prognostic factor for overall survival.29
FIGURE 3.

Illustrative schematic of brain tumor debulking demonstrating (A) GBM in situ, (B) more complete debulking with 5-ALA FGS, and (C) remaining residual disease missed with traditional surgical resection.
The main advantage for use of FGS during cytoreductive surgery is the enhanced visualization of disease extent by offering superior ability to detect subclinical disease as compared to conventional visual and tactile inspection.29 Tumors amenable to debulking with FGS share a number of features that include, but are not limited to, invasion of vital structures, widespread local disease, presence of distant metastasis, and where debulking improves survival. Examples of commonly debulked tumors are brain tumors, ovarian and testicular tumors, intraperitoneal mucinous tumors, and some advanced pancreatic cancers when WLE or whole-organ resection is not possible. In fact, cytoreduction surgery is a key aspect in the primary treatment and staging of any solid tumor with propensity for peritoneal dissemination.32,33
After completion of a phase III clinical trial (NCT00241670), Stummer et al1 revolutionized the field of image-guided surgery by demonstrating that FGS with 5-ALA-induced protoporphyrin IX resulted in more complete resection of malignant gliomas and led to improved progression-free survival. In the present study, contrast-enhancing tumor was completely resected in 65% of patients in the 5-ALA group versus 36% of those in the standard white light group.1 This complete resection translated into a higher 6-month progression-free survival in the study arm as compared to the standard treatment group (41.0% vs 21.1%).1 More recently, a small interim study of 11 patients undergoing surgical resection of newly diagnosed GBM found that tumor contrast enhancement on preoperative MRI correlates to observable intraoperative fluorescence with 5-ALA-induced protoporphyrin IX.34 This correlation was statistically significant and further suggested that pre-op contrast enhancement is important in reliably identifying a patient population that will most benefit from FGS.34 Newer studies have demonstrated 5-ALA FGS combined with intraoperative MRI may be beneficial in maximizing resection of GBM.35,36 This combination presents a potential solution to the problem encountered with 5-ALA in that not all GBM tissue uniformly exhibits 5-ALA fluorescence.36
Although 5-ALA has been the most widely studied agent for FGS in brain tumor debulking, there are additional fluorescent agents currently being studied. The widely available contrast agent, indocyanine green (ICG), is currently being evaluated in 1 study to identify and delineate central nervous system tumors (NCT02710240). In another clinical trial (NCT02740933) out of Massachusetts General Hospital, researchers are studying demeclocycline as an ex vivo agent to detect intraoperative fluorescence of abnormal brain tissue.37 Additional FGS clinical studies (NCT02050243, NCT02462629) have also been directed at the safety and efficacy of FI in the resection of pediatric central nervous system tumors. Although 1 trial studies the safety and efficacy of the widely studied 5-ALA contrast agent, the second uses fluorescent BLZ-100 to delineate normal from diseased tissue. Unlike 5-ALA, however, BLZ-100 is a tumor ligand chlorotoxin conjugated to ICG and thus fluoresces in the near-infrared (NIR) spectrum.38 No human data are currently available to demonstrate the potential benefit of this targeting approach.
In addition, there are 2 phase I clinical trials (NCT02769533, NCT02629549) evaluating the feasibility of the folate analog, OTL38, to detect folate receptor-alpha (FR-alpha)-positive nonfunctioning pituitary adenomas. Although these tumors are frequently benign, they can cause significant morbidity via mass effect. Furthermore, they are particularly prone to residual disease postsurgical resection, which is the primary treatment for all pituitary adenomas except prolactinomas. Thus, optimizing gross total resection will allow both reduced morbidity and need for adjuvant therapy. These studies are currently ongoing.
There are at least 2 (NCT02032485, NCT01982227) studies currently enrolling patients undergoing cytoreductive surgery for peritoneal carcinomatosis of colorectal origin. Recently, published results using ICG in this setting (NCT02032485) have demonstrated moderate tumor-to-background ratio (TBR) for malignant nonmucinous adenocarcinoma nodules when compared to benign ones (1.92 vs 1.02).39 In the small number of patients with mucinous adenocarcinoma, less benefit was seen.39 The reduced TBR correlated with increased incidence of hypofluorescence in the mucinous population (17 vs 0 in the nonmucinous group) and is best explained by lack of vascularization and abundant mucin in these nodules.39 More importantly, however, the study resulted in intraoperative surgical modification for 29% of patients after additional, subclinical peritoneal metastases were identified with NIR ICG fluorescence.39 Surgical modification in these patients led to a more complete surgical debulking procedure.39 Thus researchers concluded that FGS with ICG is best suited for patients with limited peritoneal disease [Peritoneal Cancer Index (PCI) <17] of nonmucinous origin as those patients are most likely to significantly benefit from cytoreductive surgery.39,40 These results indicate that FI not only imparts real-time theranostic implications, but recognizes the importance of identifying the patient populations most likely to benefit from cytoreduction with FGS.
In addition, ICG FGS has been studied in patients with ovarian cancer undergoing cytoreductive surgery.29,41 In the study by Tummers et al,29 researchers found a high false-positive rate using ICG (62%) and concluded NIR fluorescence with ICG provided no added benefits in the small number of patients studied, because it did not identify any additional malignant nodules not previously visualized with the naked eye. In these 3 patients, it was, however, pathologically determined that no occult disease was present.29 Additional studies used tumor-specific fluorophores by targeting FR-alpha, which is overexpressed in 90%to 95% of patients with epithelial ovarian carcinoma.42–44 Van Dam et al45 were able to identify malignant tissue in 3 patients using folate conjugated to fluorescein isothiocyanate (folate-FITC). In a second folate-targeted clinical trial, researchers used OTL38 to identify ovarian cancer in patients undergoing cytoreductive surgery.46 The results of this study are promising and demonstrated improved in situ detection of malignant lesions, which resulted in resection of an additional 29% of lesions not previously identified with inspection and/or palpation.46 In yet another study targeting FR-alpha, researchers used EC17 for intraoperative detection of ovarian carcinoma, which resulted in a 16% increase in the resection of malignant tumors when compared to visual inspection and palpation.42 However, the present study also found a 23% false-positive rate due to tissue autofluorescence in the visible light range and ultimately concluded that while FR-alpha is a suitable tumor-specific target, it can be enhanced by conjugating it to a fluorophore in the NIR spectrum.42 The survival benefit is inferred by increased tumor detection, but this has not been confirmed. Similarly, another clinical trial (NCT02000778) is also studying the feasibility of EC17 to detect occult ovarian carcinoma; however, no data are currently available.
The ability of intraoperative fluorescence to identify subclinical peritoneal nodules in patients undergoing cytoreductive surgery would represent a major advance in achieving optimal results for a population of patients dependent upon correct tumor staging and complete cytoreduction. More research is needed in FGS for cytoreductive surgery to properly establish its effects on progression-free and overall survival.
Optimal Clinical Trial Endpoints for Debulking/Cytoreductive Procedures
Clinical trial endpoints focus on improved overall survival, QOL, efficiency of OR time due to enhanced disease visualization, and fewer missed lesions when compared to standard white light surgery. Early trials report increased detection of lesions using FGS compared with white light, which is helpful to validate fluorescence technology for larger studies but is nevertheless subject to significant surgical bias. The high morbidity and mortality inherent to this patient population should allow measurement of overall- and progression-free survival endpoints in a relatively short time frame. There is little value in measuring the number of positive margins because the primary goal of cytoreductive surgery is elimination of visible disease in noncritical structures.
Wide Local Excision
WLE applies to the vast majority of oncologic tumors and is defined as excision of malignant tissue surrounded by a margin of normal-appearing tissue (Fig. 4), the size of which varies depending on the specific type of cancer. Local recurrence, however, continues to be a common cause of treatment failure in these patients. This is due to presence of clinically invisible and histologically undetectable genetically altered cells,27 or ‘‘satellite’’ tumor cells within a reactive zone surrounding the solid neoplasm.47 Furthermore, frozen margin assessment in the OR is constrained by time and susceptible to sampling error, which has led to false-negative results in up to 23% of lumpectomy cases.48 Together, these factors often lead to unnecessary radical resections and poorer QOL outcomes. The advantages offered by FGS for tumors amenable to WLE include improved margin assessment accuracy, more complete surgical resection through enhanced tumor visualization, and preservation of functional outcomes by increasing the application of organ-sparing oncologic surgery instead of traditional extirpative surgery. These advantages could potentially translate into reduced re-excision rates, more efficient adjuvant therapy, and improved functional outcomes.
FIGURE 4.

Illustrative schematic of WLE demonstrating (A) skin lesion in situ, (B) traditional surgical resection with white light, and (C) improved margin negativity with use of cancer-specific tumor marker fluorescently binding to residual, subclinical disease.
Those tumors undergoing WLE most likely to benefit from FGS include those with high rates of positive margins, those for which tissue sparing favorably affects QOL, and where intraoperative differentiation of normal versus tumor tissue is difficult. Examples of cancer types that would benefit in this manner include breast cancer, skin cancer, some head and neck squamous cell cancers, sarcomas, and a number of urological cancers such as bladder, prostate, and renal cell carcinomas (RCCs).
Bladder Cancer
FGS in patients undergoing transurethral resection of the bladder for urothelial bladder carcinoma has been evaluated in a range of optical imaging studies due to their high propensity for recurrence. In the United States, bladder cancer has a prevalence of more than 520,000 cases and, despite a relatively low mortality rate, requires lifelong surveillance due to a high rate of recurrence.49,50 Nonmuscle invasive bladder cancer, which accounts for approximately 75% of all urothelial carcinomas, has 12-month recurrence rate of approximately 50%.50 Furthermore, residual tumors are detected in 38% to 63% of patients by 2 to 6 weeks, which necessitates repeat transurethral resection of the bladder, and thus contributing to patient anxiety, reduced QOL, and high re-excision rates.51 It is not surprising that this combination of high rate of recurrence and need for repeat tumor resection makes bladder cancer the most expensive cancer to treat, costing approximately $200,000 per patient.52
Early stage lesions can be identified with photodynamic detection using a version of 5-ALA, similar to that used in glioma surgery, but applied topically by intravesicular injection and subsequently removed at the time of cystoscopy. In an international, multicenter trial of 365 patients treated with hexaminolevulinate (HAL, Hexvix), a 5-ALA derivative, 286 patients were found to have Ta or T1 tumors with standard white light cystoscopy, of which HAL fluorescence detected an additional 47 Ta or T1 tumors (16%, P = 0.001).53 In addition, fluorescence cystoscopy revealed carcinoma in situ in 13 of 41 patients (32%), whereas conventional white light failed to demonstrate such findings (P < 0.0001).53 Furthermore, there was no significant difference among false-positive rates in the white light versus HAL group (10% vs 11%), despite the fact that white light biopsies were taken before FI with Hexvix.53 Lastly, there was a 16% relative reduction in the recurrence rate of those treated with HAL and a lower number of tumor recurrences in this group at 3, 6, and 9 months follow-up.53 In fact, during the 9-month follow-up period, tumor recurrence in the HAL group was significantly less than that in the white light group (47% vs 56%, P = 0.026).53
Head and Neck Cancer
Use of autofluorescence in patients with oral cavity cancer has demonstrated improved identification of mucosal margins. In a study by Poh et al,54 20 patients with early (T0-T2) stage oral cancer underwent intraoperative autofluorescence visualization with a handheld device (VELscope), which showed that loss of fluorescence visualization (FVL) significantly correlated with high-grade dysplasia (P < 0.0001). In fact, FVL was identified in 20 out of 20 tumors and, in 19 of 20 tumors, FVL extended beyond the clinically apparent lesion.54 Furthermore, 35 of 36 margin biopsies in the FVL field demonstrated histologic dysplasia/cancer and/or genetic alterations known to have associated molecular risk.54 Only 4 of the 36 (11%) FVL margin biopsies were not dysplastic; however, 3 of those showed loss of heterozygosity with subsequent molecular assessment.54 In the retained fluorescence visualization biopsies, only 1 of 66 margins demonstrated dysplasia (low-grade).54 The subclinical FVL extent ranged from 4 to 25 mm and never extended evenly around any tumor.54 Because the standard of care for resection of oral cancer is to excise 10 mm of normal-appearing mucosa, this suggests that more than 50% of the tumors in the present study would demonstrate malignancy or dysplasia at the surgical margin had the 10 mm standard been followed.54
A number of on-going clinical trials have recently emerged studying tumor-specific FGS by conjugating monoclonal antibodies to fluorescently labeled dyes. This unique application of FGS uses targeted and/or activatable fluorescent probes, which affords improved specificity by further increasing the TBR.55 Rosenthal et al56 hypothesized that fluorescently labeled antiendothelial growth factor receptor (EGFR) antibodies could identify head and neck squamous cell carcinoma based on the presence of previous evidence of demonstrating EGFR overexpression in head and neck tumors.57–59 In a phase I clinical trial (NCT01987375), Rosenthal et al56 studied the safety and tumor specificity of fluorescently labeled cetuximab-IRDye800CW and concluded that targeting EGFR was both safe and effective in providing significant TBR for accurate surgical decision making (mean TBR of 5.2 in the highest dose range). Furthermore, there was a strong association between fluorescence intensity and EGFR expression in the tumor.56 This suggests that widely available antibodies, when conjugated to fluorescent dyes, have the ability to identify subclinical tumors, which translates into improved outcomes in oncologic surgery.56
Hepatic Lesions
Surgical resection of the liver offers survival benefits in patients with either hepatocellular carcinoma (HCC) or liver metastases of colorectal origin.19,60 Small tumors less than 2.0 cm are characterized by indistinct margins and can be missed macroscopically with the naked eye and, as such, necessitate microscopic examination.60 This is particularly true during laparoscopic hepatectomies when tumor delineation is hindered by the loss of real-time, tactile inspection. Histopathological evaluation of the entire resected liver is, however, virtually impossible due to the onerous and costly task associated with such a large, dense organ. Thanks to the serendipitous experiences of Ishizawa et al,60 it was determined that preoperative IV injection of ICG is retained in liver tumors due to concomitant biliary dysfunction.60 In their study of 49 patients [37 with HCC and 12 with metastasis of colorectal carcinoma (CRC)], intraoperative FGS with ICG correctly identified all microscopically confirmed HCCs (n = 63) and CRC metastases (n = 28).60 In addition, 8 HCCs (13%) identified with ICG fluorescence were macroscopically invisible.60 In HCCs alone, the sensitivity of intraoperative ICG fluorescence was 100% and the positive predictive value (PPV) 93% (63/68 fluorescing lesions).60 In the CRC group, sensitivity and PPV were both 100%.60 Ultimately, the results of the present study demonstrate a high sensitivity and satisfactory depth penetration (<8mm) in detection of small, often grossly invisible liver cancers with use of intraoperative ICG fluorescence.60 At present, only 1 clinical trial (NCT01738217) studying ICG fluorescence for hepatic tumor detection is currently listed on clinicaltrials.gov. No results have, however, been published since trial completion. In addition to real-time FI in this population, there is a unique role for near real-time fluorescence with benchtop imaging devices.24 As previously mentioned, microscopic examination of entire large hepatic resections is both time consuming and arduous. Thus, benchtop fluorescent devices represent a useful device for pathologists in evaluating tumor margins and preidentifying fluorescent regions before microscopic evaluation, which would permit improved attention to highly suspicious areas.24
Skin Cancers
In skin cancers, particularly nonmelanoma skin cancers such as squamous cell carcinoma and basal cell carcinoma, there have been 2 clinical trials (NCT02666833, NCT00449358) studying the utility of presurgical FI before WLE. A study by Alkalay et al61 (NCT00449358) used methyl-5-ALA cream and Wood’s light fluorescence in comparison to Mohs micrographic surgery (MMS) for accurate delineation of tumor borders in basal cell carcinoma. Although tumor border was detectable under Wood’s light fluorescence, they concluded that FI underestimated the genuine tumor size as determined by MMS (median tumor size under FI was 72.75 vs 93.05 mm2 with MMS).61 The second trial (NCT02666833), which is currently ongoing, uses an autofluorescent device for presurgical marking of nonmelanoma skin cancer based on their hypothesis that collagen distortion lies within the premarked area.
Lung Cancer
Pleural malignancies have high rates of recurrence, which suggests incomplete detection and subsequent of pulmonary nodules at time of primary surgical resection (NCT01778920). Currently, video-assisted thoracoscopic surgery is the most commonly used minimally invasive technique for treatment of these nodules, which are detected as small as 5 mm in diameter (NCT02570958). These early-stage localized non–small cell lung cancers have 5-year survival rates of less than 60%,62 and thus highlighting the importance of swift detection and subsequent removal. We have identified several clinical trials aimed at improving the detection and resection of lung cancer. Although the majority of these studies look at sputum cytology or bronchoscopy for cancer detection purposes, at least 3 studies (NCT01778920, NCT02653612, NCT02602119) are utilizing contrast agents targeted against FR-alpha, which is known to be expressed by approximately 85% of lung adenocarcinomas.63,64 An early feasibility study by Okusanya et al65 demonstrated fluorescence in 92% (46/50) of pulmonary adenocarcinomas with FR-alpha-targeted molecular imaging using fluorescent folate-FITC. Although 39 of 43 (91%) nodules demonstrated fluorescence following excision, only 7 of 50 (14%) demonstrated in situ fluorescence.65 This study was limited by lack of depth penetration and further concluded FITC was not an ideal fluorophore for tumors deep within the lung parenchyma.65 As such, they have since initiated 2 additional clinical trials (NCT02602119, NCT02769156) utilizing OTL38, which is purportedly associated with improved depth penetration and less background autofluorescence, for the detection and resection of pulmonary nodules. Moreover, this same group out of the University of Pennsylvania has 2 trials (NCT01778920, NCT02653612) targeting FR-alpha with EC17 for the detection of pulmonary adenocarcinoma. All studies are currently on going.
Use of ICG, when injected 24hours before imaging, can detect smaller lung nodules may offer a more sensitive, minimally invasive alternative to the widely used microcoil-guided video-assisted thoracoscopic surgery. In fact, there are several currently ongoing studies using ICG for pulmonary nodule detection and subsequent removal by the University of Pennsylvania group (NCT02280954, NCT02621268, NCT01335893). Published data from 1 trial (NCT01335893) has demonstrated the ability of ICG NIR imaging to properly identify 16 of 18 primary nodules as well as 5 additional malignant nodules up to a depth of 1.3 cm.66 In addition, a separate study (NCT02570815) evaluated the utility of ICG fluorescence in patients with non–small cell lung cancer undergoing robotic pulmonary lobectomy. Researchers use intraoperative computed tomography to attempt pulmonary segmentectomy by identifying the involved pulmonary segment and cutting off its blood supply before venous ICG injection (NCT02570815). By doing so, the involved pulmonary segment will not fluoresce under NIR light and can be removed by the surgeon. Although this example does not use FI to directly exploit the differences between malignant and normal tissue, it demonstrates one of the many applications of FGS for tissue sparing and reducing unnecessary radical procedures. In addition, one of the major applications of FGS for lung cancer may be minimally invasive surgery. Although robotic or video-assisted surgical procedures confer many advantages, they are mainly limited by their inability to provide tactile feedback, which is a significant problem faced by many minimally invasive approaches. As such, FGS has great potential to supplement these minimally invasive techniques for detection and resection of early lung nodules.
Other Tumor Types
Others among this group include 1 trial (NCT02736578) using cetuximab-IRDye800CW for the detection of pancreatic adenocarcinoma. Interestingly, the utility of the tumor-specific probe bevacizumab-IRDye800CW is additionally being used to identify vascular endothelial growth factor-expressing pancreatic adenocarcinomas (NCT02743975). In yet another clinical trial (NCT02048150), researchers are using the fluorescent monoclonal antibody anti–prostate-specific membrane antigen during robot-assisted laparoscopic prostatectomy for patients with prostate cancer. Interestingly, 1 group is additionally studying the utility of EC17 for identifying renal tumor margins during partial nephrectomy in a phase 0 study (NCT01778933). Results from their pilot study demonstrated the ability of EC17 to identify 2 of 4 RCCs with TBRs of 3.7 and 4.6, respectively.67 No false positives were noted.67 Similarly, another study (NCT02645409) is currently evaluating the utility of OTL38 to delineate RCC margins during partial nephrectomy, but no published data are available. A third trial (NCT02497599) uses dual-modality imaging using Indium-111-DOTA-Girentuximab-IRDye800CW, which targets over-expressed carbonic anhydrase IX present in more than 95% of RCC.68–70 Although combination dual modality imaging using radiotracers and optical tracers has been used in several clinical trials for lymph node mapping, this is the first trial we have identified using this combination for the primary purpose of tumor resection.
Although the majority of breast cancer studies use FI for detection of regional metastasis, we have identified multiple clinical trials using FGS for resection of breast tumors. Another tumor-specific probe, bevacizumab-IRDye800CW, is being used in a phase II clinical trial (NCT02583568) to delineate cancerous from normal tissue in patients with breast cancer. In addition, a phase I trial (NCT02496065) is currently using the chlorotoxin BLZ-100 to delineate solid breast cancer tumors. Because certain breast cancers, particularly triple negative breast cancers, overexpress folate receptors,71 one study (NCT01994369) is using EC17 to identify and improve tumor margin delineation in breast-conserving surgery for breast cancer. The remaining 3 completed clinical studies (NCT02027818, NCT02473159, NCT01796041) use ICG to delineate tumor margins in breast cancer; however, no published data have been identified.
Another study (NCT01626066) used a PEGylated, protease-activated fluorescent probe (LUM015, Lumicell, Inc, Wellesley, MA), to demonstrate tumor-specific labeling in both human breast cancer and soft tissue sarcomas.55 In addition, they found LUM015 to be safely administered and tolerated for detection of tumor-specific fluorescence in humans with a significantly higher fluorescent signal in tumor tissue compared with normal (mean TBR 4.1).55 A more recent feasibility study (NCT02438358) will use the same probe for intraoperative imaging in patients with breast cancer.
Optimal Clinical Trial Endpoints for Wide Local Excision
Clinical trial endpoints for fluorescence-guided WLE include decreased positive margin rate due to increased tumor detection, decreased incidence of re-excision and/or reoperation, and ideally, a reduced local recurrence rate (Table 1). These relatively short-term endpoints would demonstrate benefit to patients, which may circumvent the longer, more expensive phase III studies necessary for determining outcomes such as progression-free survival, tumor-free survival, overall survival, and QOL. Improved overall survival therefore may be better suited as secondary outcomes due to the extended duration of observation required in ascertaining such parameters given the survival rates in this population. Patient benefit may be indirectly measured by improved detection of positive margins because it has been previously established that this metric proportionally correlates with overall survival.
Whole-organ Resection
Whole-organ resection is the complete removal of an anatomic structure that encompasses the tumor (Fig. 5). It is further defined as surgery that does not assess margins and does not involve tissue sparing with WLE. Tumors that grow within parts of the body that cannot be partially resected are treated by surgical removal of the entire organ; for example, total laryngectomy for laryngeal tumor, radical nephrectomy for renal tumors, amputation for large extremity sarcomas, or resection of the orbital contents for a tumor extending into the intraorbital fat. The clinical applications of FGS with regards to whole-organ resection are limited to anatomic delineation of structures.
FIGURE 5.

Illustrative schematic of whole-organ resection during thyroidectomy demonstrating improved parathyroid gland detection with use of fluorescence imaging.
To date, FGS in whole-organ resection has been studied with 5-ALA for the detection of positive surgical margins during radical prostatectomy in patients with prostate cancer (trial registration: EudraCT: 2005-004406-93).72 The present study included both laparoscopic and open procedures, demonstrating a PPV of 69% and an overall sensitivity and specificity of FGS with 5-ALA in detecting positive surgical margins was 56% and 92%, respectively.72 Interestingly, sensitivity was higher with endoscopic compared with open procedures (75% vs 38%), whereas specificity was higher with open compared with endoscopic procedures (88.2% vs 100%).72 The results of the present study further concluded that FGS with 5-ALA in radical prostatectomy may be more favorable during endoscopic compared with open surgery.72
In cases in which identification of vascular anatomy is critical, the use of FGS with ICG has been used to facilitate more precise surgical navigation and dissection, which may result in fewer vascular injuries. For example, ICG fluorescence has been applied to laparoscopic73 and robotic74 adrenalectomy procedures to ensure complete resection of adrenal tumors with enhancement of vascular anatomy. Use of FGS is of particular interest in these cases due to the tactile limitations of robotic and laparoscopic surgeries as compared to open surgery, but the significant benefits that often necessitate minimally invasive adrenalectomy.73 Moreover, preservation of the vascular supply, specifically the adrenal vein, during adrenalectomy is further complicated by differences in anatomical laterality, the resultant hemodynamic stress with tumor manipulation during resection of pheochromocytoma, and the low case volume of such procedures, which poses a significant learning curve for less experienced surgeons.73 Superiority of FGS as compared to conventional imaging was dependent upon specific tumor type, with greater benefits observed in adrenocortical tumors demonstrating ICG hyperfluorescenece.74 ICG was less useful in medullary adrenal tumors such as pheochromocytomas, because they appeared largely hypofluorescent with ICG.74 Although depth of imaging may be a limiting factor, endocrine surgery is a prime setting for FGS with ICG due to the abundant blood supply of the associated organs.74
For instance, where organ delineation may be challenging, certain studies have additionally used FGS for the facile detection of certain tissues. For example, both ICG75,76 and methylene blue have been used to reliably identify parathyroid glands during thyroidectomy or parathyroidectomy. ICG has additionally been used to assess vascularization of normal parathyroid glands during total thyroidectomy.77 Although no published results are currently available from these studies (NCT01598727, NCT02089542), preclinical data from this group demonstrated accurate identification of both parathyroid and thyroid glands.78
Anatomic Delineation of Surrounding Structures
Although the majority of aforementioned contrast agents are being used to differentiate neoplastic from normal tissue, significant work is being applied to identify surrounding structures at risk of iatrogenic injury during oncologic surgery. For example, use of FGS for ureteral identification during laparoscopic and open urologic or gynecologic procedures. In addition, use of FGS for nerve identification and preservation may be applied to highly morbid procedures such as radical prostatectomy. For whole-organ resection, use of FGS is most likely to be the beneficial in reducing iatrogenic injury during complicated surgical procedures or enhance both detection and extent of adjacent nodal involvement.
Intraoperative visualization of vital dye-enhanced structures, such as nerves, has great potential to positively affect QOL postoperatively. Current nerve monitoring techniques include visual inspection and electromyography. Although electromyography has good sensitivity and has become the standard of care in some surgical procedure, it is limited by its relatively low specificity and dependence on intact neuronal axons.79,80 In patients undergoing prostatectomy for prostate cancer, for example, the cavernosal nerves in the prostate are not visualized by the naked eye, and thus leading to loss of nerve function with negative effects on patient QOL.81 In addition, in those undergoing parotidectomy for parotid tumors, permanent facial dysfunction due to facial nerve injury can still range from 2% to 8%.82–85 In 1 preclinical study, facial nerve imaging in murine models with the fluorescently labeled peptide NP41 demonstrated increased nerve to surrounding tissue contrast by a factor of 2.86 (P = 0.005).86 FI significantly improved detection of additional nerve branches, particularly those underlying surrounding tissue (7.50 ± 1.60 small nerve branches vs 3.25 ± 0.89 with white light).86 Although nerve imaging has not yet translated into clinical trials, these data suggest that concurrent nerve imaging, when combined with current imaging and monitoring techniques, can improve the functional outcomes and QOL in patients by improving nerve preservation. With emerging preclinical studies, there is, however, evidence to suggest that future intraoperative imaging will expand to include visualization of extratumoral structures and tissues. This strategy can be used for real-time differentiation of nerves and surrounding structures from tumor tissue through multiwavelength, tissue-specific targeted contrast agents.
Optimal Clinical Trial Endpoints for Whole-Organ Resection
Trial endpoints should be directed at demonstrating improved visualization rather than assessing reduction in the frequency of iatrogenic complications among trial arms (ie, with and without fluorescence guidance) since the rate of injury is generally very low, but nonetheless devastating (Table 1). The ability to better delineate structures can be inferred as measurement for improvement of outcomes. For each trial, complications specific to the procedure should be considered, for example, inadvertent removal of parathyroid tissue with thyroidectomy. It should be emphasized that use of fluorescence in whole-organ resection will be used primarily to identify nondiseased tissue in efforts of sparing the uninvolved surrounding tissue.
COMMON CHALLENGES, PITFALLS, AND SETBACKS
Like any new field, FGS faces numerous regulatory barriers that hinder its swift translation into clinical practice, which is not surprising with new technology. This is particularly true as newer target probes are being developed to enhance tumor specificity.87 In fact, a number of studies have continued to experience suboptimal development in preclinical models and poor funding for probe synthesis, toxicity and safety studies, and subsequent translation to phase I clinical trials.55 Furthermore, the regulatory approval of both imaging agents and devices is cumbersome, complex, and can significantly slow the process of developing multicenter phase III clinical trials. There is, however, a need to conduct adequate preclinical studies and lengthy clinical trials to assess safety and reduce these risks.
Another major hurdle to the implementation of FGS into realtime clinical practice is the adoption of standardized manufacturing and quality protocols. This is increasingly important as newer, more specific targeting agents are being developed. This means that minor chemical modifications such as conjugation of a probe to a fluorescent dye requires regulatory approval before clinical use because it is considered a new formulation.88 This regulatory approval falls under good manufacturing practice (GMP), which is costly, but required for all clinical administration of fluorescence probes.88 To minimize any regulatory issues when transitioning from preclinical to clinical trials, Snoeks et al88 suggest that GMP begin at the preclinical stage. Early implementation of GMP manufacturing reduces chemical impurities, production changes, and difficulties when certain steps in preclinical probe development do not properly translate to the clinical trial stage under required GMP guidelines.88 Apart from GMP adherence, there is a need for the large-scale production of fluorescent probes so as to facilitate multicenter studies and minimize the effects of synthesis variation.88 In addition to adequate funding, this requires strict quality control to optimize production stability, probe quality, and purity.88 The exclusive use of 1, or few, production facilities maximizes the efficacy and outcomes of multicenter clinical trials. Moreover, there are substantial differences in the GMP guidelines between the European Union and the United States for use of an imaging agent during first human trials. Therefore, it is recommended that multicenter trials comply with both European Union and US GMP production guidelines.
A separate, albeit-related issue stems from the difficulty in standardization of FGS devices. Because fluorescence depends on a number of factors and is particularly dependent upon tissue optical properties, standardization of specific devices will be challenging to establish. Snoeks et al88 suggest the use of phantoms to combat this issue, which are commonly employed in nuclear imaging.
Interestingly, there has been a recent increase in the use of phase 0 clinical trials in efforts to fast track the implementation of novel FI techniques into phase 1 or 2 clinical trials. In these trials, small groups of volunteers are given a microdose of less than 1% of the therapeutic dose of a drug to evaluate its processing within the human body.89 The benefits of phase 0 studies, which are also called investigational new drug studies, are that they can determine the effectiveness of a new drug in human subjects and quickly eliminate any ineffective drugs.89 Furthermore, they require fewer preclinical studies to support their transition into full clinical trials, which is important if the novel drug is ultimately found to be ineffective. Thus, the Food and Drug Administration’s relatively recent implementation of phase 0 trials may provide a fast track method for testing the utility of novel fluorescent probes and/or devices. For example, this has recently been done in a study evaluating the effects of EC17 for WLE of patients with suspected renal nodules (NCT01778933). Still, this presents confounding results when seeking approval due to the device/probe pairing issue, which is often different for each trial and likely to be a barrier to widespread approval.
Lastly, there is a need for funding and awareness. In contrast to mainstream drug therapies that are typically prescribed on a recurring basis, imaging agents are not intended for long-term use.24 Because imaging agents are, however, regulated by the Food and Drug Administration in the same manner as drugs, they are subjected to rigorous and costly developmental protocols, which can often be more than $100 M US dollars, irrespective of market potential.24 Thus, as we transition from preclinical to phase III multicenter trials, there is a need for large investments from researchers, probe, and/or device manufacturers, but also from the public. Public funding will be particularly essential as support is likely to further attract funding from the private sector, that is, from the imaging and technology companies.88 In fact, it is shocking to consider how little has been financially invested in the development of imaging agents when compared to traditional cancer research and drug therapies, especially when surgery represents one of the few curative treatments available for many solid tumors.24 Still, the focus should be on development of low-cost imaging systems where possible so as to implement them into clinical practice swiftly. There, however, must also be a balance between performance and cost so as not to interfere with trial outcomes nor steer things in a direction that would be cost prohibitive.
CONCLUSIONS
Despite advances in diagnostic imaging, adjuvant therapies, and minimally invasive surgical techniques, the global burden of cancer-related disease remains exceedingly high and the surgical outcomes unnecessarily morbid.90 Still, the primary goal of oncologic surgery continues to be resection with negative margins for the best chance at tumor-free survival.91 Persistent positive margins due to the presence of invisible, subclinical disease have, however, continued to stonewall efforts of improving outcomes in surgical oncology. Real-time imaging in the operative setting presents a major advantage over current non–real-time, preclinical imaging (Fig. 6). The use of FGS as a surgical guide enhancement has the potential to improve survival and QOL outcomes for patients.53 FGS has reduced the visual limitations of human eyesight and has demonstrated strong evidence for improving patient outcomes in surgical oncology. Because the number of clinical trials rise each year, it is overwhelmingly apparent that FGS has great potential for a broad range of clinical applications. So although the challenging search for cancer-curing wonder drugs continues, FI represents a promising field that might just be the best solution to fill the current gap between modern technology and what we know about cancer.
FIGURE 6.

Mixed media schematic illustrating the emerging bridge in preoperative tumor imaging and real-time tumor-specific identification.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (T32CA091078). The authors report no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. [DOI] [PubMed] [Google Scholar]
- 2.Rizzo M, Iyengar R, Gabram SG, et al. The effects of additional tumor cavity sampling at the time of breast-conserving surgery on final margin status, volume of resection, and pathologist workload. Ann Surg Oncol. 2010;17:228–234. [DOI] [PubMed] [Google Scholar]
- 3.Tartter PI, Kaplan J, Bleiweiss I, et al. Lumpectomy margins, reexcision, and local recurrence of breast cancer. Am J Surg. 2000;179:81–85. [DOI] [PubMed] [Google Scholar]
- 4.Haque R, Contreras R, McNicoll MP, et al. Surgical margins and survival after head and neck cancer surgery. BMC Ear Nose Throat Disord. 2006;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. [DOI] [PubMed] [Google Scholar]
- 6.Meric F, Mirza NQ, Vlastos G, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer. 2003;97:926–933. [DOI] [PubMed] [Google Scholar]
- 7.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–312. [DOI] [PubMed] [Google Scholar]
- 8.Snijder RJ, Brutel de la Riviere A, Elbers HJ, et al. Survival in resected stage I lung cancer with residual tumor at the bronchial resection margin. Ann Thorac Surg. 1998;65:212–216. [DOI] [PubMed] [Google Scholar]
- 9.Stojadinovic A, Leung DH, Hoos A, et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertrand TE, Cruz A, Binitie O, et al. Do surgical margins affect local recurrence and survival in extremity, nonmetastatic, high-grade osteosarcoma? Clin Orthop Relat Res. 2016;474:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dotan ZA, Kavanagh K, Yossepowitch O, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol. 2007;178:2308–2312; discussion 2313. [DOI] [PubMed] [Google Scholar]
- 12.Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299–315. [PubMed] [Google Scholar]
- 13.Rosenthal EL, Warram JM, Bland KI, et al. The status of contemporary image-guided modalities in oncologic surgery. Ann Surg. 2015;261:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolgar JA, Triantafyllou A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005;41:1034–1043. [DOI] [PubMed] [Google Scholar]
- 15.McMahon J, O’Brien CJ, Pathak I, et al. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br J Oral Maxillofac Surg. 2003;41:224–231. [DOI] [PubMed] [Google Scholar]
- 16.Ravasz LA, Slootweg PJ, Hordijk GJ, et al. The status of the resection margin as a prognostic factor in the treatment of head and neck carcinoma. J Craniomaxillofac Surg. 1991;19:314–318. [DOI] [PubMed] [Google Scholar]
- 17.Atkins J, Al Mushawah F, Appleton CM, et al. Positive margin rates following breast-conserving surgery for stage I-III breast cancer: palpable versus non-palpable tumors. J Surg Res. 2012;177:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iczkowski KA, Lucia MS. Frequency of positive surgical margin at prostatectomy and its effect on patient outcome. Prostate Cancer. 2011;2011:673021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chagpar AB. Trial I. Cavity shave margins in breast cancer. New Engl J Med. 2015;373:2187–2188. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal EL, Warram JM, de Boer E, et al. Successful translation of fluorescence navigation during oncologic surgery: a consensus report. J Nucl Med. 2016;57:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vahrmeijer AL, Hutteman M, Van der Vorst JR, et al. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parrish-Novak J, Holland EC, Olson JM. Image-guided tumor resection. Cancer J. 2015;21:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Sullivan B, Wylie J, Catton C, et al. The local management of soft tissue sarcoma. Semin Radiat Oncol. 1999;9:328–348. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Ikeda N, Nonoguchi N, et al. Enhanced expression of coproporphyrinogen oxidase in malignant brain tumors: CPOX expression and 5- ALA-induced fluorescence. Neuro Oncol. 2011;13:1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Poh CF, Anderson DW, Durham JS, et al. Fluorescence visualization-guided surgery for early-stage oral cancer. JAMA Otolaryngol Head Neck Surg. 2016;142:209–216. [DOI] [PubMed] [Google Scholar]
- 28.McWade MA, Paras C, White LM, et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab. 2014;99:4574–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tummers QR, Hoogstins CE, Peters AA, et al. The value of intraoperative near-infrared fluorescence imaging based on enhanced permeability and retention of indocyanine green: feasibility and false-positives in ovarian cancer. PLoS One. 2015;10:e0129766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. [DOI] [PubMed] [Google Scholar]
- 31.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764; discussion 264–756. [DOI] [PubMed] [Google Scholar]
- 32.Vergote I, Van Gorp T, Amant F, et al. Timing of debulking surgery in advanced ovarian cancer. Int J Gynecol Cancer. 2008;18(suppl 1):11–19. [DOI] [PubMed] [Google Scholar]
- 33.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. [DOI] [PubMed] [Google Scholar]
- 34.Roberts DW, Valdes PA, Harris BT, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg. 2011;114:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quick-Weller J, Lescher S, Forster MT, et al. Combination of 5-ALA and iMRI in re-resection of recurrent glioblastoma. Br J Neurosurg. 2016;30:313–317. [DOI] [PubMed] [Google Scholar]
- 36.Hauser SB, Kockro RA, Actor B, et al. Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: a histology-based evaluation. Neurosurgery. 2016;78:475–483. [DOI] [PubMed] [Google Scholar]
- 37.Wirth D, Smith TW, Moser R, et al. Demeclocycline as a contrast agent for detecting brain neoplasms using confocal microscopy. Phys Med Biol. 2015;60:3003–3011. [DOI] [PubMed] [Google Scholar]
- 38.Butte PV, Mamelak A, Parrish-Novak J, et al. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus. 2014;36:E1. [DOI] [PubMed] [Google Scholar]
- 39.Liberale G, Vankerckhove S, Caldon MG, et al. Fluorescence imaging after indocyanine green injection for detection of peritoneal metastases in patients undergoing cytoreductive surgery for peritoneal carcinomatosis from colorectal cancer: a pilot study. Ann Surg. 2016;246:1110–1115. [DOI] [PubMed] [Google Scholar]
- 40.Goere D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22:2958–2964. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz N. Laparoscopy in the near infrared with ICG detects microscopic tumor in women with ovarian cancer: 0078. Int J Gynecol Cancer. 2006;16:616–623. [Google Scholar]
- 42.Tummers QR, Hoogstins CE, Gaarenstroom KN, et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget. 2016;7:32144–32155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markert S, Lassmann S, Gabriel B, et al. Alpha-folate receptor expression in epithelial ovarian carcinoma and non-neoplastic ovarian tissue. Anticancer Res. 2008;28:3567–3572. [PubMed] [Google Scholar]
- 45.Van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. [DOI] [PubMed] [Google Scholar]
- 46.Hoogstins CE, Tummers QR, Gaarenstroom KN, et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res. 2016;22:2929–2938. [DOI] [PubMed] [Google Scholar]
- 47.White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1439–1445. [DOI] [PubMed] [Google Scholar]
- 48.Cendan JC, Coco D, Copeland EM 3rd. Accuracy of intraoperative frozen-section analysis of breast cancer lumpectomy-bed margins. J Am Coll Surg. 2005;201:194–198. [DOI] [PubMed] [Google Scholar]
- 49.Grossman HB, Stenzl A, Moyad MA, et al. Bladder cancer: chemoprevention, complementary approaches and budgetary considerations. Scand J Urol Nephrol Suppl. 2008;213–233. [DOI] [PubMed] [Google Scholar]
- 50.Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in a high-risk population? a cost per life-year saved analysis. Cancer. 2006;107:982–990. [DOI] [PubMed] [Google Scholar]
- 51.Jakse G, Algaba F, Malmstrom PU, et al. A second-look TUR in T1 transitional cell carcinoma: why? Eur Urol. 2004;45:539–546; discussion 546. [DOI] [PubMed] [Google Scholar]
- 52.Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. [DOI] [PubMed] [Google Scholar]
- 53.Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010;184:1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poh CF, Zhang L, Anderson DW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12:6716–6722. [DOI] [PubMed] [Google Scholar]
- 55.Whitley MJ, Cardona DM, Lazarides AL, et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci Transl Med. 2016;8:320ra324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenthal EL, Warram JM, De Boer E, et al. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res. 2015;21:3658–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 58.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. [DOI] [PubMed] [Google Scholar]
- 59.Suh Y, Amelio I, Guerrero Urbano T, et al. Clinical update on cancer: molecular oncology of head and neck cancer. Cell Death Dis. 2014;5:e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–2504. [DOI] [PubMed] [Google Scholar]
- 61.Alkalay R, Alcalay J, Maly A, et al. Fluorescence imaging for the demarcation of basal cell carcinoma tumor borders. J Drugs Dermatol. 2008;7:1033–1037. [PubMed] [Google Scholar]
- 62.Howlader N, Noone AM, Krapcho M et al. (eds). SEER cancer statistics review, 1975–2013.Bethesda, MD: National Cancer Institute; Available at: http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. Accessed July 1, 2016. [Google Scholar]
- 63.Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- 64.O’Shannessy DJ, Yu G, Smale R, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okusanya OT, DeJesus EM, Jiang JX, et al. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg. 2015;150:28.e21–35.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg. 2014;98:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guzzo TJ, Jiang J, Keating J, et al. Intraoperative molecular diagnostic imaging can identify renal cell carcinoma. J Urol. 2016;195:748–755. [DOI] [PubMed] [Google Scholar]
- 68.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 69.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. [DOI] [PubMed] [Google Scholar]
- 70.Leibovich BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25:4757–4764. [DOI] [PubMed] [Google Scholar]
- 71.O’Shannessy DJ, Somers EB, Maltzman J, et al. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. Springerplus. 2012;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adam C, Salomon G, Walther S, et al. Photodynamic diagnosis using 5- aminolevulinic acid for the detection of positive surgical margins during radical prostatectomy in patients with carcinoma of the prostate: a multicentre, prospective, phase 2 trial of a diagnostic procedure. Eur Urol. 2009;55:1281–1288. [DOI] [PubMed] [Google Scholar]
- 73.DeLong JC, Chakedis JM, Hosseini A, et al. Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol. 2015;112:650–653. [DOI] [PubMed] [Google Scholar]
- 74.Colvin J, Zaidi N, Berber E. The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J Surg Oncol. 2016;114:153–156. [DOI] [PubMed] [Google Scholar]
- 75.Chakedis JM, Maser C, Brumund KT, et al. Indocyanine green fluorescence-guided redo parathyroidectomy. BMJ Case Rep. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaidi N, Bucak E, Okoh A, et al. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol. 2016;113:771–774. [DOI] [PubMed] [Google Scholar]
- 77.Vidal Fortuny J, Belfontali V, Sadowski SM, et al. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg. 2016;103:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antakia R, Gayet P, Guillermet S, et al. Near infrared fluorescence imaging of rabbit thyroid and parathyroid glands. J Surg Res. 2014;192:480–486. [DOI] [PubMed] [Google Scholar]
- 79.Meier JD, Wenig BL, Manders EC, et al. Continuous intraoperative facial nerve monitoring in predicting postoperative injury during parotidectomy. Laryngoscope. 2006;116:1569–1572. [DOI] [PubMed] [Google Scholar]
- 80.Minahan RE, Mandir AS. Neurophysiologic intraoperative monitoring of trigeminal and facial nerves. J Clin Neurophysiol. 2011;28:551–565. [DOI] [PubMed] [Google Scholar]
- 81.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–360. [DOI] [PubMed] [Google Scholar]
- 82.Marshall AH, Quraishi SM, Bradley PJ. Patients’ perspectives on the short-and long-term outcomes following surgery for benign parotid neoplasms. J Laryngol Otol. 2003;117:624–629. [DOI] [PubMed] [Google Scholar]
- 83.Guntinas-Lichius O, Kick C, Klussmann JP, et al. Pleomorphic adenoma of the parotid gland: a 13-year experience of consequent management by lateral or total parotidectomy. Eur Arch Otorhinolaryngol. 2004;261:143–146. [DOI] [PubMed] [Google Scholar]
- 84.McGurk M, Thomas BL, Renehan AG. Extracapsular dissection for clinically benign parotid lumps: reduced morbidity without oncological compromise. Br J Cancer. 2003;89:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehle ME, Kraus DH, Wood BG, et al. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope. 1993;103(4 pt 1):386–388. [DOI] [PubMed] [Google Scholar]
- 86.Hussain T, Nguyen LT, Whitney M, et al. Improved facial nerve identification during parotidectomy with fluorescently labeled peptide. Laryngoscope. 2016;126:2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garland M, Yim JJ, Bogyo M. A bright future for precision medicine: advances in fluorescent chemical probe design and their clinical application. Cell Chem Biol. 2016;23:122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Snoeks TJ, Van Driel PB, Keereweer S, et al. Towards a successful clinical implementation of fluorescence-guided surgery. Mol Imaging Biol. 2014;16:147–151. [DOI] [PubMed] [Google Scholar]
- 89.Fromer MJ. FDA introduces new phase 0 for clinical trials: some enthusiastic, some skeptical. Oncol Times. 2006;28:18–19. [Google Scholar]
- 90.GLOBOCAN 2012 v1.0. Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr. Accessed July 1, 2016. [Google Scholar]
- 91.Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nat Rev Cancer. 2013;13:653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


