Abstract
In pulmonary hypertension (PH), measurement of various echocardiographic parameters that assess right heart function is recommended by current clinical guidelines. Limited data exists on the combined value of clinical and echocardiographic parameters in precapillary PH in the modern era of therapy. We examined the association of clinical and echo-cardiographic parameters with surrogate outcomes (6-minute walk distance) and hard outcomes (hospitalization or death) in patients with precapillary PH. A cohort of patients with an established diagnosis of precapillary PH who underwent transthoracic echocardiography at the Duke Echo Lab were prospectively enrolled from 2010 to 2014. Univariable and multivariable models were constructed to examine the relation of clinical and echo-cardiographic parameters with surrogate and hard outcomes. Of the 98 patients with analyzable echocardiograms with good image quality, 85 were woman, mean age was 59.4 years, and 47% had ≥World Health Organization functional class III symptoms. The mean 6-minute walk distance was 354(±132) m, and 83% were on pulmonary arterial hypertension medications. At 24 months, the cumulative incidence rate for hospitalization or death was 47%. In univariable analyses, the REVEAL (Registry to Evaluate Early and Long-term PAH Disease Management) risk score (HR 1.72 per 1 SD (2.81) increment, 95% CI 1.34, 2.22; p=<0.001), RV global longitudinal strain (RVGLS) (HR 1.54 per 1 SD(5.31) worsening, 95% CI, 2.12; p=0.008) and log-2 NT proBNP (HR 1.43 per 1-fold increase, 95% CI 1.25, 1.63; p=<0.001) were significantly associated with hospitalization or death.
A growing body of evidence supports the prognostic importance of preclinical impairment of right ventricular (RV) function in diseases with precapillary pulmonary hypertension (PH),1 such as pulmonary arterial hypertension (PAH). An assessment of RV function may allow the identification of high-risk patients before they clinically deteriorate.2 To aid in risk assessment, a number of echo-cardiographic and magnetic resonance imaging markers of RV function have been developed,3 ranging from qualitative assessments of RV function to more recently tested quantitative parameters that have been used in patients with congenital heart disease,4,5 pulmonary hypertension,6 and pulmonary embolism.7 Multiple markers of RV function have been evaluated for an association with outcomes in patients with PAH. For example, RV longitudinal strain (RVLS) has been associated with mortality in PAH,6,8 and changes in strain have been associated with a positive therapeutic response.9 With the multitude of echocardiographic parameters reporting on right heart function available at the bedside and recommended by consensus documents,10 it is unclear which parameters are most predictive of outcomes in patients with PAH1 and whether they add value to clinical predictors. The objective of this study was to examine the prognostic value of guideline-recommended echocardiographic parameters, including more novel parameters such as RV strain, combined with clinical parameters in relation to clinical outcomes, ranging from surrogate end points (6-minute walk distance [6MWD]) to hard end points such as hospitalization and death, in patients with precapillary PH.
Methods
This was a single-center, prospective, and observational cohort study. Study participants were recruited from the Center for Pulmonary Vascular Disease at Duke. Inclusion criteria included subjects with an established diagnosis of precapillary PH, defined as mean PA pressure ≥25 mm Hg and PVR ≥3 Woods units, and exclusion criteria including absence of significant left heart disease or lung disease. Ninety-eight patients who underwent transthoracic echocardiography, the majority of whom had PAH (PCWP ≤15 mm Hg), were prospectively enrolled in the study from 2010 to 2014. Patients with significant arrhythmia were excluded from the study.
After providing informed consent, study subjects underwent transthoracic echocardiography using a predefined imaging acquisition protocol (Supplementary Data). Transthoracic echocardiograms were performed using a commercial GE Vivid E9 imaging system (GE Vingmed Ultrasound, Horten, Norway) or Philips IE-33 (Bothell, Washington) ultrasound equipment. Left ventricular (LV) and RV measurements of function and hemodynamics were calculated as per the current American Society of Echocardiography guidelines.2 RV end-diastolic area and RV endsystolic area in square centimeters were measured in the RV focused views. RV fractional area change (RVFAC) was calculated by subtracting RV end-diastolic area from RV end-systolic area. Pulmonary arterial systolic pressure was calculated by the modified Bernoulli equation using the maximum tricuspid regurgitation signal and right atrial (RA) pressure as assessed by inferior vena cava (collapsibility).11 LV ejection fraction was calculated using the Simpson’s biplane method with end-systolic and end-diastolic volumes measured in the apical 4 chamber and apical 2 chamber views. Peak velocities of early (E) and late (A) diastolic filling were derived from mitral inflow velocities. Peak velocities of e’, a’, and s’ were calculated on tissue Doppler images of the lateral tricuspid annulus and the medial and lateral mitral valve annulus.
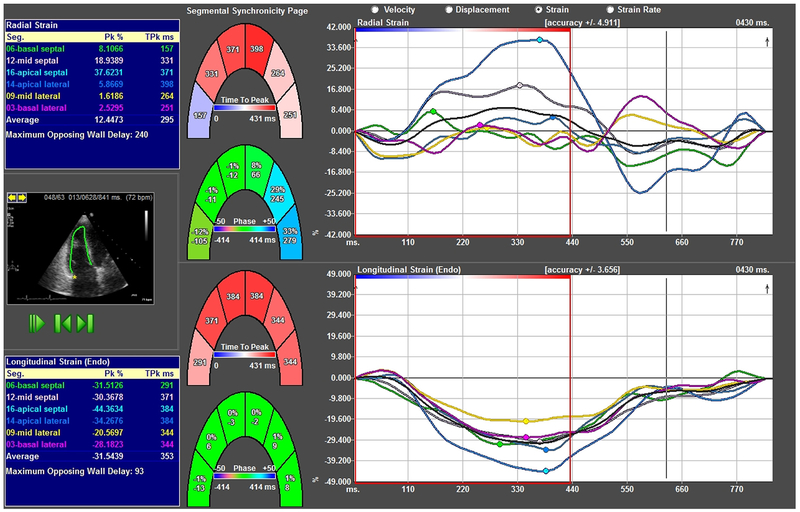
RV longitudinal strain (RVLS) was calculated offline using vendor-independent image analysis software (Tom-Tec 2D Cardiac Performance Analysis, Munich, Germany) on RV-focused views.12 The RV end-diastolic endocardial border was manually traced along the RV free and RV septal walls. RVLS was obtained by averaging basal, mid, and apical strain values of the free wall and septal wall (Figure 1). Because the values of RVLS and RV global longitudinal strain (RVGLS) are highly correlated, we used the RVLS from a 4 chamber view as a surrogate for RVGLS.13 LV GLS was calculated using the apical 4, 3, and 2 chamber views using TomTec Imaging Systems.
Figure 1.
Representative RV global longitudinal strain curves obtained from RV-focused apical 4 chamber view. Individual segments are color-coded to correspond to basal, mid, and apical lateral/septal walls. (Color version of figure is available online.)
Demographics, risk factor data, 6MWD and cardiac catheterization data acquired within 6 months of date of the study echocardiogram were included in the study. Registry to evaluate early and long-term PAH disease management (REVEAL) score was calculated using a composite score of World Health Organization (WHO) Group 1 subgroup, renal function, sex, age, WHO functional class, systolic blood pressure, heart rate, 6MWD, brain natriuretic peptide (BNP), presence of pericardial effusion, pulmonary function test, mean RA pressure, and pulmonary vascular resistance. All patients recruited to this study were clinically followed at the Duke Center for Pulmonary Vascular Disease. Follow-up information regarding hospitalization and death was abstracted by chart review on all patients in December 2014, and a subsequent query of Duke University Health System medical data using the Duke Enterprise Data Unified Content Explorer in March 2018. The main end point studied was the composite of hospitalization or all-cause mortality through 24 months.
Clinical, demographic, and echocardiographic variables were summarized for the 98 patients. Categorical variables were summarized with counts and proportions, and continuous variables were described by their mean (standard deviation, SD) and median (interquartile range [Q1 and Q3]). Cumulative event rates for the composite of rehospitalization or death at 6, 12, 18, and 24 months after baseline echocardiogram were estimated by the Kaplan-Meier method, censoring at the last date known alive. Pearson correlation coefficients were calculated to describe correlation between echocardiographic parameters and 6MWD.
The investigators identified 25 demographic, clinical, and echocardiographic variables from the baseline tables as being of interest based on their relation to RV or LV function. There was missing data in 20 of the 25 variables of interest with rates of missingness ranging from 1% to 16%. Univariable regression analyses was performed on cases with available data. For the multivariable regression analysis, a single imputed dataset was created using a fully conditional specification.14 We decided a single imputed dataset was sufficient due to the low count and rate of missingness in each variable—15 of the 20 covariates with missingness had 5 or fewer missing values (of 98 observations).
Cox proportional hazards regression was used to evaluate the relations between covariates of interest and days from baseline echocardiogram to earliest occurrence of rehospitalization or death, with censoring at the last date known alive. To examine the model assumption of linearity in the log-hazards, a restricted cubic spline was fit to the log-hazard for continuous covariates with the mean being the reference value. Due to non-linear log hazards RVFAC was transformed into a 3-level categorical variable with cutoffs at 28% and 36%, and due to skewness NT-proBNP was log-transformed. All other variables were determined to have sufficiently linear log hazards. Cox regression estimates were calculated by maximizing the partial likelihood and the Efron method was employed to handle tied event times. Ninety-five percent confidence intervals (CIs) and Wald chi-square statistics were calculated to assess the significance of the estimates at a level of 0.05.
Based on the univariable analysis, the two most statistically significant echocardiographic parameters were included in a multivariable model along relevant clinical and demographic covariates. A limit on the number of echocardiographic parameters was set to avoid overfitting. Harrell’s c-index for the multivariable model was computed and compared to c-indices of univariably significant predictors. Potential collinearity between covariates was assessed using scatterplot matrices and the highly correlated variables were reviewed with investigator input to determine which should be considered in the multivariable analysis. The linearity of model variables was reassessed with adjustment for all other covariates in the multivariable model. Transformations were applied as appropriate.
Statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina) and R version 3.1.2. All participants provided informed consent. The study was approved by the Duke Institutional Review Board, IRB # Pro00015241.
Results
Of the 101 patients enrolled, 1 patient withdrew and 2 were excluded from the analysis as they were found to not have precapillary PH. Of the remaining 98 patients enrolled, 85 were woman, 35% were nonwhite, mean age was 59.4 years, and 47% had ≥WHO functional class III symptoms. Baseline demographics are summarized in Table 1. The median (Q1 and Q3) 6MWD was 390.7 (254.0, 460.9) meters, and 83% of patients were on PAH medications. The median (Q1 and Q3) REVEAL risk score, a scoring system for predicting survival in PAH, was 7.0(5.0, 9.0). The hemodynamic and echocardiographic characteristics of the study cohort are also described in Table 1. Of the echocardiographic parameters, the median (Q1 and Q3) RVGLS was −17.9 (−20.7, −14.2), RVFAC was 33.2 (26.1, 38.8), tricuspid annular plane systolic excursion (TAPSE) was 1.8 (1.6, 2.3), and estimated right ventricular systolic pressure (RVSP) was 63.5 (49.0, 78.0). The median time to first event or last follow-up was 721 days (~2 years), with 94% of patients having complete follow-up through 24 months. The Kaplan Meier estimate for the composite of hospitalization or death at 24 months was 47%, with 45 patients having been either hospitalized (n = 37) and/or died (n = 8).
Table 1.
Baseline Characteristics of Study Population of PAH Patients.
| Characteristic | Total (N=98) | Missingness (n (%)) |
|---|---|---|
| Age (mean ± SD) (years) | 59.4 ± 12.77 | 0 (0.0%) |
| Women | 85 (87%) | 0 (0.0%) |
| Race | ||
| White | 62 (65%) | 3 (3.1%) |
| Black | 31 (33%) | 3 (3.1%) |
| 6MWD (mean ± SD) (meters) | 353.6 ± 132.35 | 4(4.1%) |
| WHO Functional Class | ||
| 1 | 1 (1%) | 0 (0%) |
| 2 | 51 (52%) | 0 (0%) |
| 3 | 41 (42%) | 0 (0%) |
| 4 | 5 (5%) | 0 (0%) |
| Medications | ||
| PDE5 Inhibitor | 48 (49%) | 0 (0%) |
| Endothelin Receptor Antagonist | 44 (45%) | 0 (0%) |
| Invasive hemodynamics | ||
| Cardiac Index (median (Q1, Q3)) | 2.4 (2.0, 3.1) | 7 (7.1%) |
| RA Pressure (mmHg) (median (Q1, Q3)) | 9.0 (6.0, 13.0) | 8 (8.2%) |
| PA Mean Pressure (mmHg) (median (Q1,Q3)) | 48.5 (39.8, 54.0) | 0 (0.0%) |
| PA Systolic Pressure (mmHg) (median (Q1,Q3)) | 75.0(61.0, 87.5) | 6(6.1%) |
| PA Diastolic Pressure (mmHg) (median (Q1,Q3)) | 30.5 (23.5, 38.0) | 6(6.1%) |
| PVR (Woods units) (median (Q1, Q3)) | 6.9 (4.1, 11.9) | 0 (0.0%) |
| Echocardiographic Parameters | ||
| LV Ejection Fraction (%) (median (Q1, Q3)) | 59.3 (53.7, 66.3) | 11 (11.2%) |
| RA Area (cm2) (median (Q1, Q3)) | 21.0(17.5, 26.3) | 0 (0.0%) |
| RV Fractional Area Change (%) (median (Q1,Q3)) | 33.2(26.1,38.8) | 2 (2.0%) |
| RV Global Longitudinal Strain (%) (median (Q1,Q3)) | −17.9 (−20.7,−14.2) | 3 (3.1%) |
| TAPSE (cm) (median (Q1, Q3)) | 1.8 (1.6, 2.3) | 1 (1.0%) |
| RVSP (mmHg) (median (Q1, Q3)) | 63.5 (49.0, 78.0) | 6(6.1%) |
| LV Systolic Eccentricity Index (median (Q1,Q3)) | 1.25 (1.09, 1.58) | 4(4.1%) |
6MWD = six minute walk distance, WHO = World Health Organization, RA = right atrium, PA = pulmonary artery, PVR = pulmonary vascular resistance, LV = left ventricle, TAPSE = tricuspid annular plane systolic excursion, RVSP = right ventricular systolic pressure.
The mean (±SD) 6MWD was 353.6 (±132.35) meters. Pearson correlation coefficients revealed that RVGLS (r = −0.36 and p = 0.0005), LVGLS (r = −0.45 and p <0.0001), RVSP (r = −0.35 and p = 0.0007), and TAPSE (r = 0.31 and p = 0.0021) were significantly correlated with 6MWD. RVFAC (r = 0.19 and p = 0.06) and RV myocardial performance index (r = −0.17 and p = 0.11) were not strongly correlated with 6MWD (Table 2).
Table 2.
Correlation of 6-minute walk distance (6MWD) with echo parameters
| Echo variable | Pearson correlation (r) | p value |
|---|---|---|
| Right ventricular longitudinal strain | −0.36 | 0.0005 |
| Left ventricular longitudinal strain | −0.45 | <0.0001 |
| Right ventricular systolic pressure | −0.35 | 0.0007 |
| Tricuspid annular plane systolic excursion | 0.31 | 0.0021 |
| Right ventricular fractional area change | 0.19 | 0.06 |
| RV myocardial performance index | −0.17 | 0.11 |
In the 98 patients, there were 63 with the composite event during follow-up and 45 with an event reported through 24 months. Kaplan-Meir estimates are shown in Table 3. In the univariable analysis, the REVEAL risk score (HR 1.72 per 1 SD (2.81), 95% CI 1.34, 2.22; p =<0.001), NT proBNP (HR 1.43 per 1-fold increase, 95% CI 1.25, 1.63; p=<0.001), RVGLS (HR 1.54 per 1 SD, 95% CI 1.12, 2.12; p = 0.007), TAPSE (HR 0.7 per 1 SD; 95% CI 0.53, 0.92; p=0.01) and RVSP (HR 1.39 per 1 SD; 95% CI 1.10, 1.77; p = 0.007) were associated with hospitalization or death. Notably, visual assessment of RV function (HR 3.33 for Severe vs Mild, 95% CI 1.32, 8.36; overall p=0.07), RVFAC (HR 0.93 for ≥ 36 vs < 28, 95% CI 0.50, 1.70; p=0.92), right atrial (RA) area (HR 1.14 per 1 SD (7.23), 95% CI 0.90, 1.44; p=0.12), and pericardial effusion (HR 1.46 for <1 cm vs None, 95% CI 0.85, 2.49; p=0.17) were not associated with outcomes (Table 5).
Table 3.
Kaplan-Meier estimates for composite end point of rehospitalization or death
| Time postbaseline (months) | KM estimate (95% CI) | Cumulative no. patients with event |
|---|---|---|
| 6 | 20.6% (13.8, 30.1) | 20 |
| 12 | 32.2% (23.9, 42.6) | 31 |
| 18 | 38.7% (29.7, 49.2) | 37 |
| 24 | 47.3% (37.8, 57.8) | 45 |
Table 5.
Univariable Cox Proportional Hazards Model Fits. Hazard ratio estimates for continuous variables are for a 1 SD increment unless indicated otherwise.
| Variable | Hazard Ratio | 95% Confidence Interval (CI) | Chisq Statistic | P value |
|---|---|---|---|---|
| Age (SD = 12.77) | 1.119 | 0.870–1.439 | 0.7676 | 0.3810 |
| Resting HR (SD = 13.74) | 1.245 | 0.968–1.601 | 2.9173 | 0.0876 |
| Gender (Female vs Male) | 0.648 | 0.329–1.278 | 1.5670 | 0.2106 |
| REVEAL Risk Score (SD = 2.81) | 1.723 | 1.336–2.222 | 17.5922 | <.0001 |
| Race (Black vs White) | 1.018 | 0.606–1.710 | 0.0045 | 0.9467 |
| LVEF (SD = 7.37) | 0.911 | 0.698–1.190 | 0.4641 | 0.4957 |
| Peak TR velocity (SD = 61.58) | 1.331 | 1.024–1.728 | 4.5805 | 0.0323 |
| RA area (SD = 7.23) | 1.141 | 0.904–1.440 | 1.2325 | 0.2669 |
| RA volume (SD = 40.90) | 1.100 | 0.873–1.386 | 0.6502 | 0.4200 |
| RV D Base (SD = 0.63) | 1.295 | 1.022–1.641 | 4.5974 | 0.0320 |
| RV D Mid (SD = 0.70) | 1.185 | 0.929–1.513 | 1.8617 | 0.1724 |
| RV DL (SD = 0.92) | 1.136 | 0.879–1.469 | 0.9529 | 0.3290 |
| RVGLS (SD = 5.31) | 1.541 | 1.121–2.119 | 7.1037 | 0.0077 |
| LVGLS (SD = 3.23) | 1.466 | 1.084–1.984 | 6.1530 | 0.0131 |
| TAPSE (SD = 0.56) | 0.701 | 0.534–0.920 | 6.5687 | 0.0104 |
| Est RA pressure (SD = 4.19) | 1.209 | 0.946–1.545 | 2.3025 | 0.1292 |
| EstRVSP (SD= 19.21) | 1.393 | 1.096–1.770 | 7.3516 | 0.0067 |
| RVOT VTI (SD = 4.99) | 0.820 | 0.621–1.084 | 1.9404 | 0.1636 |
| PA accel time (SD = 0.05) | 0.821 | 0.591–1.141 | 1.3744 | 0.2411 |
| Lateral TV annulus (SD = 3.51) | 0.859 | 0.642–1.149 | 1.0511 | 0.3053 |
| LV eccentricity index (diastole) (SD = 0.59) | 1.032 | 0.785–1.357 | 0.0515 | 0.8204 |
| LV eccentricity index (systole) (SD = 0.23) | 1.219 | 0.984–1.511 | 3.2695 | 0.0706 |
| Log2(NT proBNP) (1-fold increase) | 1.431 | 1.254–1.632 | 28.3576 | <.0001 |
| RVMPI equation (SD = 0.13) | 1.097 | 0.869–1.385 | 0.6104 | 0.4346 |
| Tricuspid Valve Regurgitation | ||||
| Mild vs Trivial | 0.884 | 0.464–1.684 | ||
| Moderate vs Trivial | 1.615 | 0.835–3.122 | ||
| Severe vs Trivial | 2.847 | 1.278–6.342 | ||
| Overall Test of Significance | 10.0149 | 0.0184 | ||
| Pericardial Effusion (< 1 cm vs None) | 1.455 | 0.851–2.486 | 1.8810 | 0.1702 |
| Visual RV function | ||||
| Normal vs Mild | 1.471 | 0.802–2.698 | ||
| Moderate vs Mild | 1.628 | 0.822–3.225 | ||
| Severe vs Mild | 3.326 | 1.324–8.358 | ||
| Overall Test of Significance | 6.9606 | 0.0732 | ||
| RV FAC | ||||
| 28 RV FAC < 36 vs RV FAC < 28 | 1.048 | 0.580–1.896 | ||
| RV FAC 36 vs RV FAC < 28 | 0.925 | 0.504–1.700 | ||
| Overall Test of Significance | 0.1580 | 0.9240 |
Finally, a multivariable model was created to include clinically relevant variables noted typically on an initial patient encounter, such as age, gender, heart failure class, and heart rate, in addition to the echocardiographic parameters that were found to be significant predictors of outcome in the univariable analysis, namely RVGLS and RVSP. In this analysis RVGLS was independently predictive of outcome (HR 1.44 per 1 SD (5.28) worsening; 95% CI 1.03, 2.02; p = 0.03; Table 6). Harrell’s c-indices for the REVEAL risk score and RVGLS independently, and for the multivariable model are presented in Table 4. As a sensitivity analysis to the assumption of linearity, this same model was refit, substituting continuous RVGLS with the categorized version, using GLS <−20 as the reference group. In this analysis, the association of RVGLS with outcome was also statistically significant (p = 0.005), and the hazard ratio estimates suggested results consistent with the analysis where we assumed a linear relationship (HR of 1.29, 95% CI 0.69, 2.42 for −20 < GLS < −12.5 vs. GLS <-20; and HR of 3.79, 95% CI 1.61, 8.94 for GLS >-12.5 vs GLS <-20). Consistent with earlier single center studies,6,8 there was a clear relation between RVGLS and outcome.
Table 6.
Multivariable analysis with only clinical and echocardiographic variables (not including REVEAL score). Continuous variables are standardized
| Variable | Hazard ratio | Confidence interval (CI) | Chisq statistic | p value |
|---|---|---|---|---|
| Age (SD = 12.77) | 1.195 | 0.911 – 1.567 | 1.6592 | 0.1977 |
| Gender (female vs male) | 0.597 | 0.302–−1.181 | 2.1960 | 0.1384 |
| RVGLS (SD = 5.28) | 1.444 | 1.029 – 2.024 | 4.5294 | 0.0333 |
| Resting HR (SD = 14.02) | 1.162 | 0.887 – 1.522 | 1.1871 | 0.2759 |
| Estimated RVSP (SD = 18.80) | 1.007 | 0.993 – 1.021 | 0.9746 | 0.3235 |
Table 4.
Harrell’s c-index for three Cox Regression Proportional Hazards models.
| Harrell’s | Confidence | |
|---|---|---|
| Model Predictors | C-index | Interval (CI) |
| REVEAL risk score | 64.5% | 57.4%–71.6% |
| RVGLS | 62.4% | 54.8%–70.1% |
| Age, Gender, RVGLS, RVSP, HR | 63.8% | 56.8%–70.7% |
Discussion
In PAH, the relation between specific echocardiographic parameters and clinical outcomes has been considered in multiple studies6,8,15–18 but it is still relatively unclear as to what specific parameters are best suited for use in clinical practice and add information to clinical risk scores.1,3 The purpose of our study was to compare and evaluate the association of multiple echocardiographic parameters with a commonly used surrogate end point in clinical trials, the 6MWD, and hard clinical outcomes, hospitalization and death. Our study revealed that RVGLS was independently predictive of outcome, similar to the REVEAL risk score but with the benefit of utilizing only noninvasive data. These findings provide a basis for a noninvasive algorithm that does not rely on the often unavailable components of the REVEAL risk score to predict outcomes.
It is frequently difficult to choose between the multiple approaches to assess RV function on an echocardiogram.19 Subjective, or visual, assessment of RV function is performed by almost all echo laboratories. In this study, we found no relation between a visual assessment of RV function and 6MWD and outcomes. In addition, RVFAC and TAPSE have been recommended by current guidelines in assessment of RV function.2 RVFAC has been shown to be an independent predictor of heart failure, stroke, and/or mortality in patients after pulmonary embolism.20 However, RVFAC and other measurements such as the LVEI are not standard and can be cumbersome to measurement, although LVEI does exemplify interventricular interdependence evident in this population.3 In clinical experience, TAPSE is simple, easily measured and reproducible.1 However, it reports on tricuspid annular motion and basal segment motion and may not describe RV global, free wall or other segmental contractile function. TAPSE has previously been reported to be associated with outcome and our study corroborates these findings.15,19 It was predictive of 6MWD and was statistically significant in univariable analysis (p = 0.0104) in predicting outcomes. RV strain, a more global measure of RV function, was also independently predictive of both 6MWD and hard outcomes[KCP1] [SI2]. [KCP1]TAPSE is now statistically significant in univariable analyses. May need to rewrite this last part. [SI2]I have rephrased this statement to reflect the significance of TAPSE.
RV speckle tracking strain from echocardiography has emerged as a method to assess abnormal RV function in a variety of settings5,6 and has been validated as a viable tool for assessment of RV function in pulmonary hypertenstion.21 Given its ability of differentiating active motion from passive motion independent of Doppler angle of incidence, it has the ability to ascertain decreased function even in the setting of normal contractility. RV strain has been previously shown to correlate well with RV ejection fraction by cardiac magnetic resonance imaging24 and elevated PA pressures by right heart catheterization.25 It has also been shown to predict outcomes in patients with known or suspected PAH and has been independently associated with mortality.6,8 Changes in strain on serial echocardiographic studies in PAH have also been shown to correlate with survival.9
This work has implications for standardizing and streamlining echocardiographic protocols for patients with PAH. Qualitative assessment of RV function and certain quantitative assessments appear to be of very limited utility, while RVGLS has now been shown to be predictive of outcomes in multiple disease states associated with RV dysfunction. Moreover, changes in RVGLS are predictive of outcome in patients with PAH,9 suggesting that further studies could test therapy algorithms based on RV function quantified by RVGLS and other reproducible measurements of RV function could be useful.
Our study included patients from a single center. Although the reproducibility of RV strain as measured by 2-D STE is well established9,22,18,23 there may be variability in vendor to vendor software algorithms in calculating strain that has not been explored and requires further study. Although use of a single vendor would result in reproducible 2-D STE, site-to-site variation in the utilization of such software limits the generalizability of the results of the present study. Currently there are no commercially available software packages that allow RV-specific strain measurements. The measurements made were using LV specific algorithms similar to previous studies. The development and testing of such algorithms may be important for future use of RVGLS. Additionally, our modest population size limits its statistical power.
Conclusion
Although risk calculators such as the REVEAL risk score provide prognostic information for patients with PAH, the data required, such as invasive cardiac catheterization measurements, can make routine use of this score difficult in patient follow-up. The present study supports RVGLS as a noninvasive parameter that can be used with clinical parameters to predict patient outcomes of hospitalization or death.
Supplementary Material
Acknowledgments
Funding source: This study was funded by a grant from the American Society of Echocardiography (ASE). ASE was not involved in the study design, the collection, analysis and interpretation of data, the writing of the report, or in the decision to submit the article for publication. Amanda Brucker’s work was funded by NIH grant 5T32HL079896.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
Supplementary Data
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.amjcard.2018.05.019.
References
- 1.Wright LM, Dwyer N, Celermajer D, Kritharides L, Marwick TH. Follow-up of pulmonary hypertension with echocardiography. JACC Cardiovasc Imaging 2016;9:733–746. [DOI] [PubMed] [Google Scholar]
- 2.van de Veerdonk MC, Marcus JT, Westerhof N, de Man FS, Boonstra A, Heymans MW, Bogaard HJ, Vonk Noordegraaf A. Signs of right ventricular deterioration in clinically stable patients with pulmonary arterial hypertension. Chest 2015;147:1063–1071. [DOI] [PubMed] [Google Scholar]
- 3.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. quiz 786–788. [DOI] [PubMed] [Google Scholar]
- 4.Ladouceur M, Kachenoura N, Soulat G, Bollache E, Redheuil A, Azizi M, Delclaux C, Chatellier G, Boutouyrie P, Iserin L, Bonnet D Impaired atrioventricular transport in patients with transposition of the great arteries palliated by atrial switch and preserved systolic right ventricular function: A magnetic resonance imaging study. Congenit Heart Dis 2017;12(no. 4):458–466. [DOI] [PubMed] [Google Scholar]
- 5.Ladouceur M, Redheuil A, Soulat G, Delclaux C, Azizi M, Patel M, Chatellier G, Legendre A, Iserin L, Boudjemline Y, Bonnet D, Mousseaux E. STARS Investigators. Longitudinal strain of systemic right ventricle correlates with exercise capacity in adult with transposition of the great arteries after atrial switch. Int J Cardiol 2016;217:28–34. [DOI] [PubMed] [Google Scholar]
- 6.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013;6:711–721. [DOI] [PubMed] [Google Scholar]
- 7.Dahhan T, Siddiqui I, Tapson VF, Velazquez EJ, Sun S, Davenport CA, Samad Z, Rajagopal S. Clinical and echocardiographic predictors of mortality in acute pulmonary embolism. Cardiovasc Ultrasound 2016;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 2011;139: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 9.Hardegree EL, Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Kushwaha SS, Hsiao JF, McCully RB, Oh JK, Pellikka PA, Kane GC. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am J Cardiol 2013;111: 143–148. [DOI] [PubMed] [Google Scholar]
- 10.Parikh KS, Rajagopal S, Arges K, Ahmad T, Sivak J, Kaul P, Shah SH, Tapson V, Velazquez EJ, Douglas PS, Samad Z. Use of outcome measures in pulmonary hypertension clinical trials. Am Heart J 2015;170(419–429). e3. [DOI] [PubMed] [Google Scholar]
- 11.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S55–S66. [DOI] [PubMed] [Google Scholar]
- 12.Forsha D, Risum N, Kropf PA, Rajagopal S, Smith PB, Kanter RJ, Samad Z, Sogaard P, Barker P, Kisslo J. Right ventricular mechanics using a novel comprehensive three-view echocardiographic strain analysis in a normal population. J Am Soc Echocardiogr 2014;27:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajagopal S, Forsha DE, Risum N, Hornik CP, Poms AD, Fortin TA, Tapson VF, Velazquez EJ, Kisslo J, Samad Z. Comprehensive assessment of right ventricular function in patients with pulmonary hypertension with global longitudinal peak systolic strain derived from multiple right ventricular views. J Am Soc Echocardiogr 2014;27(657–665). [DOI] [PubMed] [Google Scholar]
- 14.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 15.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–1041. [DOI] [PubMed] [Google Scholar]
- 16.Eysmann SB, Palevsky HI, Reichek N, Hackney K, Douglas PS. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation 1989;80:353–360. [DOI] [PubMed] [Google Scholar]
- 17.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jobsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002;39:1214–1219. [DOI] [PubMed] [Google Scholar]
- 18.Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, Vliegen HW, Delgado V. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging 2012;5:628–636. [DOI] [PubMed] [Google Scholar]
- 19.Mazurek JA, Vaidya A, Mathai SC, Roberts JD, Forfia PR. Follow-up tricuspid annular plane systolic excursion predicts survival in pulmonary arterial hypertension. Pulm Circ 2017;7:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nass N, McConnell MV, Goldhaber SZ, Chyu S, Solomon SD. Recovery of regional right ventricular function after thrombolysis for pulmonary embolism. Am J Cardiol 1999;83(804–806). a10. [DOI] [PubMed] [Google Scholar]
- 21.Thenappan T, Glassner C, Gomberg-Maitland M. Validation of the pulmonary hypertension connection equation for survival prediction in pulmonary arterial hypertension. Chest 2012;141:642–650. [DOI] [PubMed] [Google Scholar]
- 22.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012;141:354–362. [DOI] [PubMed] [Google Scholar]
- 23.Meris A, Faletra F, Conca C, Klersy C, Regoli F, Klimusina J, Penco M, Pasotti E, Pedrazzini GB, Moccetti T, Auricchio A. Timing and magnitude of regional right ventricular function: a speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr 2010;23:823–831. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Prakasa K, Bomma C, Tandri H, Dalal D, James C, Tichnell C, Corretti M, Bluemke D, Calkins H, Abraham TP. Comparison of novel echocardiographic parameters of right ventricular function with ejection fraction by cardiac magnetic resonance. Journal of the American Society of Echocardiography 2007;20(no. 9):1058–1064. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda Y, Tanaka H, Sugiyama D, Ryo K, Onishi T, Fukuya H, Nogami M, Ohno Y, Emoto N, Kawai H, Hirata K. Utility of right ventricular free wall speckle-tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. Journal of the American Society of Echocardiography 2011;24(no. 10):1101–1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.