Abstract
Clopidogrel resistance is associated with stent thrombosis. Prasugrel achieves greater platelet inhibition with less variability among patients than does clopidogrel. Thus, a patient who had stent thrombosis due to clopidogrel resistance may receive prasugrel to prevent repeated episodes of stent thrombosis. This case report describes a case of repetitive stent thrombosis in which resistance not only to clopidogrel, but also to prasugrel, was observed.
<Learning objective: In the face of clopidogrel resistance, prescribing prasugrel may be an acceptable treatment option. However, cross-unresponsiveness may be observed between clopidogrel and prasugrel. Thus, platelet function assay should be performed in patients with stent thrombosis, even when clopidogrel is replaced with prasugrel.>
Keywords: Prasugrel resistance, Clopidogrel resistance, Stent thrombosis, Platelet function test
Introduction
High on-treatment platelet reactivity (HTPR) is associated with adverse cardiovascular events, including stent thrombosis, in patients undergoing percutaneous coronary intervention (PCI). The interpatient variability in the pharmacodynamic response to clopidogrel is well recognized, and patients with less degrees of platelet inhibition in response to clopidogrel have been shown to be at increased risk of cardiovascular events. Prasugrel is a third-generation thienopyridine agent that achieves greater platelet inhibition with less variability among patients than does clopidogrel. Thus, a patient who had stent thrombosis due to HTPR while on clopidogrel may benefit from prasugrel to prevent repeated episodes of stent thrombosis. This case report describes a case of repetitive stent thrombosis in which resistance not only to clopidogrel, but also to prasugrel, was observed.
Case report
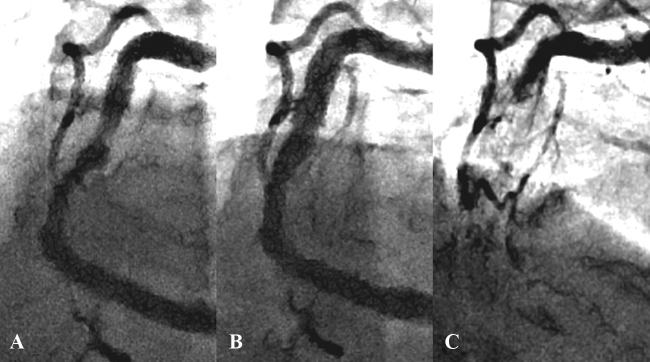
A 60-year-old male on dialysis because of diabetic nephropathy was admitted due to exertional angina. Coronary angiography revealed a 75% stenosis in the proximal left anterior descending artery, a 90% stenosis in the proximal left circumflex artery, and a 75% and a 90% stenosis in the mid- and distal right coronary artery (RCA), respectively (Fig. 1A). The laboratory examination of blood revealed a hemoglobin level of 12.0 g/dL, hemoglobin A1c of 5.7%, and B-type natriuretic peptide of 64.0 pg/ml (Table 1). The patient was referred for coronary angioplasty. He successfully underwent Xience Prime stent (Abbott, Abbott Park, IL, USA) implantation in the proximal left anterior descending artery.
Fig. 1.
(A) Coronary angiography showing a 75% narrowing in the mid-right coronary artery. (B) Angiography shows a good result after drug-eluting stent implantation. (C) Four months later, stent thrombosis is observed.
Table 1.
Laboratory findings.
| Laboratory parameters | Value |
|---|---|
| WBC (103/μl) | 4600 |
| RBC (103/μl) | 4.73 |
| Hgb (g/dL) | 12.0 |
| Ht (%) | 37.4 |
| Plt (103/μl) | 138 |
| PT-INR | 0.99 |
| APTT (s) | 41.5 |
| AST (IU/L) | 5 |
| ALT (IU/L) | 4 |
| UN (mg/dL) | 29 |
| Cre (mg/dL) | 12.71 |
| UA (mg/dL) | 7.9 |
| LDL-Cho (mg/dL) | 92 |
| HDL-Cho (mg/dL) | 28 |
| TG (mg/dL) | 261 |
| HbA1c (%) | 5.3 |
| BNP (pg/ml) | 64.0 |
Abbreviations: WBC, white blood cell count; RBC, red blood cell count; Hgb, hemoglobin; Hct, hematocrit; Plt, platelet count; PT-INR, international normalized ratio of prothrombin time; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; UN, urea nitrogen; Cre, creatinine; UA, uric acid; LDL-Cho, low density lipoprotein cholesterol; HDL-Cho, high density lipoprotein cholesterol; TG, triglyceride; HbA1c, hemoglobin A1c; BNP, B-type natriuretic peptide.
The patient was later readmitted to undergo PCI for the tandem lesions in the RCA. Intravascular ultrasound (IVUS) showed severe calcification in both lesions. Rotational atherectomy using a 1.25 mm burr (Boston Scientific, Natick, MA, USA) was performed. Two Xience Xpedition stents (3.5 mm × 33 mm and 2.5 mm × 12 mm) were deployed at 16 atm and 10 atm for the lesions in the mid- and distal RCA, respectively. IVUS images demonstrated underexpansion of the stent in the mid-RCA. Additional balloon inflation was performed using a 5.0 mm Sapphire NC Plus balloon catheter (OrbusNeich, Hoevelaken, Netherlands) inflated to 20 atm in the mid-RCA. Subsequent angiography showed a good result in both lesions (Fig. 1B). IVUS imaging demonstrated optimal stent expansion in the mid-RCA (Fig. 2A), although IVUS could not be advanced into the distal stent. Following PCI, the patient continued to receive dual antiplatelet therapy with clopidogrel (75 mg daily) and aspirin (100 mg daily).
Fig. 2.
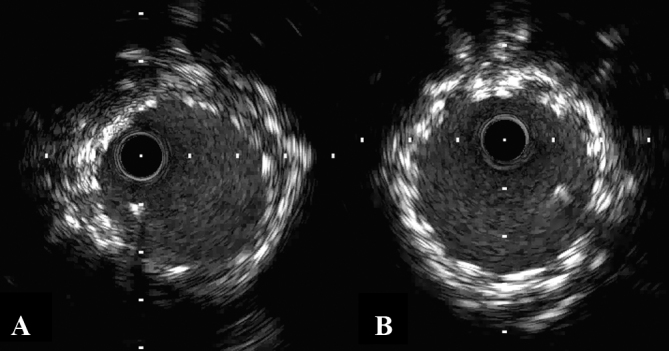
(A) Intravascular ultrasound (IVUS) imaging demonstrates optimal stent expansion in the mid-right coronary artery. (B) IVUS imaging shows no stent underexpansion after drug-eluting stent implantation for stent thrombosis.
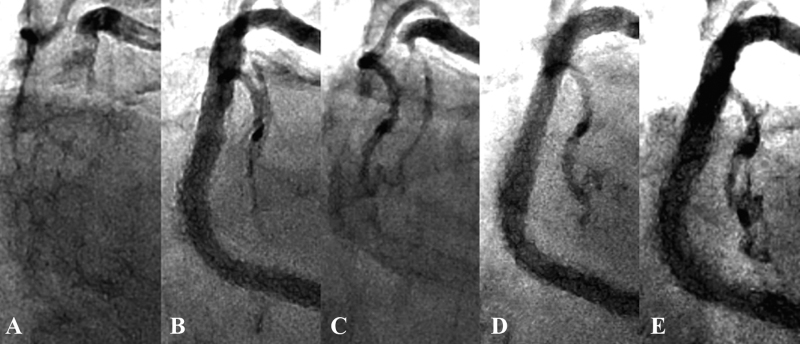
Four months later, the patient was readmitted due to acute chest pain. Coronary angiography revealed stent thrombosis in the mid-RCA (Fig. 1C). After thrombus aspiration and conventional balloon angioplasty, two Nobori stents (3.5 mm × 28 mm) (TERUMO, Tokyo, Japan) were deployed at 14 atm. Five months later, he developed repeat stent thrombosis in the mid-RCA. Two Xience Xpedition stents (3.5 mm × 28 mm and 3.5 mm × 23 mm) were deployed at 18 atm and 12 atm, respectively. Two months later, he developed the third stent thrombosis, presenting with ST-elevation myocardial infarction (STEMI) with a maximum creatine kinase (CK) of 1025 IU/L (Fig. 3A). A Nobori stent (3.5 mm × 18 mm) and a Xience Xpedition stent (3.5 mm × 15 mm) were implanted in the mid-RCA at 14 atm and 18 atm, respectively. Angiography (Fig. 3B) and IVUS (Fig. 2B) showed a good result. Platelet function testing was performed with the VerifyNow system (Accumetrics, San Diego, CA, USA). It indicated 331 P2Y12 reaction units (PRU). Although it is known that PRU evaluated by VerifyNow is affected by low hematocrit levels, his blood test showed only mild anemia, thereby not likely to affect the VerifyNow P2Y12 results. Furthermore, regarding drug compliance, he adhered to the daily antiplatelet drug regimen, despite his blindness, through the help of his wife, who managed his medications. Thus, it appeared that his high residual platelet reactivity was due to clopidogrel resistance. For this reason, clopidogrel was discontinued and prasugrel (3.75 mg daily) was prescribed.
Fig. 3.
(A) The third stent thrombosis develops. (B) Angiography shows a good result. (C) Two months later, the fourth stent thrombosis is observed. (D) Angiography demonstrates a good result. (E) Three months later, follow-up angiography shows no restenosis.
Two months later, the patient developed the fourth stent thrombosis (Fig. 3C). It was treated successfully using three Resolute Integrity stents (4.0 mm × 34 mm, 3.5 mm × 38 mm, and 3.0 mm × 26 mm) (Medtronic, Minneapolis, MN, USA). Angiography and IVUS imaging showed good results (Fig. 3D). The VerifyNow system showed 342 PRU. He was discharged on aspirin (100 mg daily), clopidogrel (75 mg daily), and cilostazol (100 mg twice a day). Three months later, follow-up angiography showed no restenosis (Fig. 3E). Because it is known that cilostazol may lead to inappropriate results of VerifyNow P2Y12 test, we did not perform platelet function testing after administering aspirin, clopidogrel, and cilostazol.
In every PCI procedure for stent thrombosis events, stent implantation was required to achieve full restoration of coronary flow, since a large amount of thrombus could not be removed by aspiration and balloon dilatation. Every episode of stent thrombosis presented as unstable angina without CK elevation, except for the third event with STEMI. Echocardiography after the fourth event revealed asynergy of the left ventricular inferior wall, and a global ejection fraction of about 50%.
Discussion
The mechanisms underlying stent thrombosis are multifactorial, and include patient-related factors, procedural factors, and postprocedural factors (including type and duration of antiplatelet therapy). Among them, insufficient antiplatelet treatment and mechanical problems have been considered two cardinal risk factors, regardless of early, late, or very late stent thrombosis [1]. In this case, mechanical factors, such as stent underexpansion, incomplete apposition, and fracture, were unlikely to cause recurrent stent thrombosis, because every PCI case was performed under IVUS guidance, and no mechanical problems were confirmed at the site of stent thrombosis in the mid-RCA, although IVUS could not be advanced to the distal RCA. The overlapped stents may have been one of the risk factors for stent thrombosis. However, we have not experienced stent thrombosis since the final intervention, even under conditions with most multi-layered stents. On the other hand, results from VerifyNow showed insufficient platelet inhibition on both clopidogrel and prasugrel, suggesting that the absence of P2Y12 inhibitory effect played a crucial role for these thrombotic events.
Clopidogrel resistance is associated with stent thrombosis. Esterases shunt the majority of clopidogrel to a dead-end inactive pathway, with the remaining prodrug requiring a two-step metabolic transformation before binding to the platelet P2Y12 adenosine diphosphate (ADP) receptor. The conversion of clopidogrel to its active metabolite is regulated by the CYP450 system, and the presence of genetic polymorphisms partly determines the extent to which clopidogrel inhibits ADP-induced platelet activation. Clopidogrel resistance has been reported to range between 16% and 50% [2]. HTPR is associated with stent thrombosis and adverse cardiovascular events after stenting. The Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents (ADAPT-DES) registry evaluated the effect of HTPR on clinical outcomes in patients who received aspirin and clopidogrel after drug-eluting stent implantation. HTPR on clopidogrel was strongly related to stent thrombosis and myocardial infarction and was inversely related to bleeding [3].
Prasugrel is a third-generation thienopyridine with more potent, faster, and consistent antiplatelet action compared to clopidogrel. Prasugrel is an inactive prodrug that is transformed first through hydrolyzation by esterases, followed by a single CYP-dependent oxidative step into its active metabolite. Common functional CYP genetic variants do not affect active drug metabolite level, inhibition of platelet aggregation, or clinical cardiovascular event rates in patients treated with prasugrel. However, prasugrel resistance does exist, although it is less frequent compared to clopidogrel. It has been reported to range between 0% and 11.5% [4].
The TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) compared prasugrel to clopidogrel in patients with moderate-to-high-risk acute coronary syndromes who underwent PCI. The primary efficacy end point, defined as death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, occurred in 12.1% of patients receiving clopidogrel and in 9.9% of patients receiving prasugrel (p < 0.001). However, major bleeding was observed in 2.4% of patients receiving prasugrel and in 1.8% of patients receiving clopidogrel (p = 0.03) [5]. Considering the higher average age, lower body weight, and increased bleeding risk with other thrombotic agents in Japanese patients compared to Western patients, meticulous dose-finding was performed. The Japanese Phase II trial (unpublished data) showed that administration of prasugrel (loading dose/maintenance dose: 20/3.75 mg) was associated with a similar incidence of bleeding events compared with a standard clopidogrel regimen (loading dose/maintenance dose: 300/75 mg). This adjusted dose of prasugrel for Japanese patients is approximately one-third of that used in Western patients. In post hoc analysis of the PRASugrel compared with clopidogrel For Japanese patIenTs with Acute Coronary Syndrome undergoing percutaneous coronary intervention (PRASFIT-ACS), PRU >262 was observed in 20.1% of patients receiving prasugrel [6]. In the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial, the prasugrel maintenance dose was 10 mg in patients younger than 75 years who weighed 60 kg or more, and 5 mg for those aged 75 years or older, and younger than 75 years and with a body weight less than 60 kg. In patients younger than 75 years who weighed 60 kg or more, the median PRU values at 30 days were 64 [interquartile range (IQR), 33–128]. In patients younger than 75 years and with a body weight less than 60 kg, the median 30-day PRU values were 139 (IQR, 86–203). In patients 75 years or older, the median PRU values were 164 (IQR, 105–216) [7]. Neubauer et al. demonstrated that doubling of the maintenance dose of 10 mg prasugrel was effective with an adequate platelet inhibitory effect and without bleeding events in all four patients with prasugrel resistance [8]. In the present case, we did not double the maintenance dose of 3.75 mg prasugrel because it is not allowed in Japan. Other mechanisms of prasugrel resistance are poor patient adherence, variations in the absorption of the prodrug and generation and clearance of the active metabolite, differences in receptor expression and post-receptor signaling pathway, and P2Y12 receptor polymorphisms. In the present case, the exact mechanism remained undetermined. Meticulous follow-up is required in patients, even with prasugrel after stenting.
Regarding aspirin resistance, ADAPT-DES registry demonstrated that high platelet reactivity while on aspirin was not significantly associated with stent thrombosis [3]. Furthermore, aspirin resistance has been reported to strongly depend on drug adherence, and true pharmacologic resistance is rare. In our case, self-reported adherence was good, suggesting that aspirin resistance was not related to stent thrombosis.
End-stage renal disease is independently associated with clopidogrel resistance, with high prevalence reaching 60% [9]. Additionally, in hemodialysis patients with clopidogrel resistance, a relatively high rate (19.0%) of prasugrel resistance was also reported [10]. Dysregulated platelet function due to increased platelet turnover, uremia, anemia, and altered drug metabolism may be potential causes for thienopyridine resistance [10]. Some patient-related factors like hemoconcentration after dialysis or severe cardiac dysfunction might promote hemostasis and thrombosis formation. In this case, however, half of the thrombotic events occurred on the non-dialysis day, and infarcted myocardial area was limited to the inferior wall.
Cross-unresponsiveness may be observed between clopidogrel and prasugrel. Thus, platelet function assay should be performed in patients with stent thrombosis, even when clopidogrel is replaced with prasugrel.
Conflict of interest
Yoshio Kobayashi received research grant from Daiichi-Sankyo (Tokyo, Japan), Sanofi (Paris, France), Terumo (Tokyo, Japan), and Abbott Vascular Japan (Tokyo, Japan). The other authors declare that there are no conflicts of interest to disclose.
References
- 1.van Werkum J.W., Heestermans A.A., Zomer A.C., Kelder J.C., Suttorp M.J., Rensing B.J., Koolen J.J., Brueren B.R., Dambrink J.H., Hautvast R.W., Verheugt F.W., ten Berg J.M. Predictors of coronary stent thrombosis. JACC. 2009;53:1399–1409. doi: 10.1016/j.jacc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 2.Mallouk N., Labruyère C., Reny J.-L., Chapelle C., Piot M., Fontana P., Gris J.C., Delavenne X., Mismetti P., Laporte S. Prevalence of poor biological response to clopidogrel: a systematic review. Thromb Haemost. 2012;107:494–506. doi: 10.1160/TH11-03-0202. [DOI] [PubMed] [Google Scholar]
- 3.Stone G.W., Witzenbichler B., Weisz G., Rinaldi M.J., Neumann F.J., Metzger D.C., Henry T.D., Cox D.A., Duffy P.L., Mazzaferri E., Gurbel P.A., Xu K., Parise H., Kirtane A.J., Brodie B.R. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos D., Xanthopoulou I., Davlouros P., Plakomyti T.E., Panagiotou A., Mavronasiou E., Hahalis G. Prasugrel overcomes high on-clopidogrel platelet reactivity in chronic coronary artery disease patients more effectively than high dose (150 mg) clopidogrel. Am Heart J. 2011;162:733–739. doi: 10.1016/j.ahj.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott S.D., Braunwald E., McCabe C.H., Montalescot G., Ruzyllo W., Gottlieb S., Neumann F.J., Ardissino D., De Servi S., Murphy S.A., Riesmeyer J., Weerakkody G., Gibson C.M., Antman E.M., TRITON-TIMI 38 Investigators Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura M., Isshiki T., Kimura T., Ogawa H., Yokoi H., Nanto S., Takayama M., Kitagawa K., Ikeda Y., Saito S. Optimal cutoff value of P2Y12 reaction units to prevent major adverse cardiovascular events in the acute periprocedural period: post-hoc analysis of the randomized PRASFIT-ACS study. Int J Cardiol. 2015;182:541–548. doi: 10.1016/j.ijcard.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Gurbel P.A., Erlinge D., Ohman E.M., Neely B., Neely M., Goodman S.G., Huber K., Chan M.Y., Cornel J.H., Brown E., Zhou C., Jakubowski J.A., White H.D., Fox K.A., Prabhakaran D. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA. 2012;308:1785–1794. doi: 10.1001/jama.2012.17312. [DOI] [PubMed] [Google Scholar]
- 8.Neubauer H., Kaiser A., Busse B., Mügge A. Identification, evaluation and treatment of prasugrel low-response after coronary stent implantation – a preliminary study. Thromb Res. 2010;126:e389–e391. doi: 10.1016/j.thromres.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Muller C., Caillard S., Jesel L., Ghannudi El S., Ohlmann P., Sauleau E., Hannedouche T., Gachet C., Moulin B., Morel O. Association of estimated GFR with platelet inhibition in patients treated with clopidogrel. Am J Kidney Dis. 2012;59:777–785. doi: 10.1053/j.ajkd.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulos D., Panagiotou A., Xanthopoulou I., Komninakis D., Kassimis G., Davlouros P., Fourtounas C., Goumenos D. Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on-treatment platelet reactivity. J Thromb Haemost. 2011;9:2379–2385. doi: 10.1111/j.1538-7836.2011.04531.x. [DOI] [PubMed] [Google Scholar]