Abstract
In several recent guidelines, transcatheter aortic valve implantation (TAVI) has been recommended as a therapeutic option for inoperable or high surgical risk patients with severe aortic stenosis. TAVI has various specific complications that seldom occur in surgical aortic valve replacement. Among them, coronary obstruction (CO) is an infrequent but serious complication. Previous case series have reported symptomatic CO cases diagnosed by hemodynamic instability, electrocardiographic changes, and abnormal findings on aortography. We report a case of silent CO in an 86-year-old female. Monitoring of coronary flow by transesophageal echocardiography led to a diagnosis of CO. Silent CO is probably an underdiagnosed complication of TAVI.
<Learning objective: Coronary obstruction is an infrequent but serious complication of transcatheter aortic valve implantation (TAVI). Previous case series have reported only symptomatic coronary obstruction cases diagnosed by hemodynamic instability, electrocardiographic changes, and abnormal findings on aortography. Transesophageal echocardiography monitoring of coronary ostium flow is useful for detecting coronary obstruction. Silent coronary obstruction is probably an underdiagnosed complication of TAVI.>
Keywords: Transcatheter aortic valve implantation, Coronary obstruction, Transesophageal echocardiography
Introduction
Transcatheter aortic valve implantation (TAVI) has been used as an alternative treatment to surgical aortic valve replacement for inoperable or high surgical risk patients. TAVI has specific complications including aortic root rupture, coronary obstruction (CO), and valve migration. Of these, CO is considered to be a life-threatening but rare complication. In a multicenter registry, the incidence of symptomatic CO was 0.66% and most patients manifested severe hypotension or electrocardiographic changes [1].
We report a patient with post-TAVI silent CO, which was detected by transesophageal echocardiography (TEE). CO is probably an underdiagnosed complication of TAVI and may be a cause of cardiac death after TAVI.
Case report
An 86-year-old female was admitted to our hospital for treatment of severe aortic stenosis (AS). She had experienced recurrent syncope and dyspnea on effort. Transthoracic echocardiography showed severe AS due to degenerative change (aortic valve area 0.42 cm2 by equation of continuity, peak transvalvular pressure gradient 109 mmHg, mean transvalvular pressure gradient 68 mmHg). She had chronic kidney disease, mild obesity (body mass index 29.8), and restrictive ventilatory impairment (% forced vital capacity 54.7%). Estimated operative mortality for isolated surgical aortic valve replacement was 17.4% using the logistic EuroSCORE and 5.9% using the Society of Thoracic Surgeons score.
Our heart team decided to perform TAVI via a trans-femoral approach. On computed tomography, her aortic annulus area was 383 mm2 and calculated diameter was 22.7 mm. The aortic valve was tricuspid and left coronary cusp (LCC) had bulky calcification. The distance from the annulus to left coronary artery (LCA) was 11.0 mm and that to right coronary artery was 11.7 mm. We selected a 23-mm Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA).
Under general anesthesia, we surgically cut down the right common femoral artery and inserted a 16-French long sheath. After bronchial intubation, TEE was started to guide the procedures.
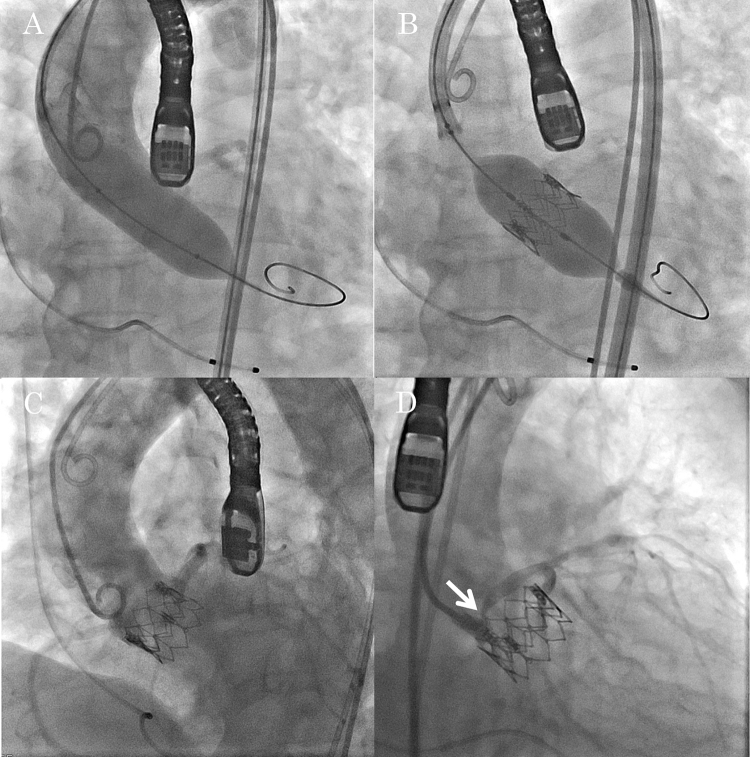
Aortic valvuloplasty using a 20-mm balloon under right ventricular rapid pacing was performed without incident. Simultaneous aortography showed good coronary flow (Fig. 1A). A 23-mm Edwards SAPIEN XT valve was deployed in an appropriate position under rapid pacing (Fig. 1B). After deployment, blood pressure recovered to baseline uneventfully and no ischemic change was found on electrocardiogram. Post-deployment aortography showed no CO (Fig. 1C). On TEE, left ventricular wall motion was normal and para-valvular leakage was trivial.
Fig. 1.
Transcatheter aortic valve implantation. (A) Balloon aortic valvuloplasty with simultaneously performed aortography. (B) Implantation of a 23-mm Edwards SAPIEN XT valve. (C) No apparent coronary obstruction on aortography. (D) Selective left coronary angiography showing significant ostial stenosis (arrow).
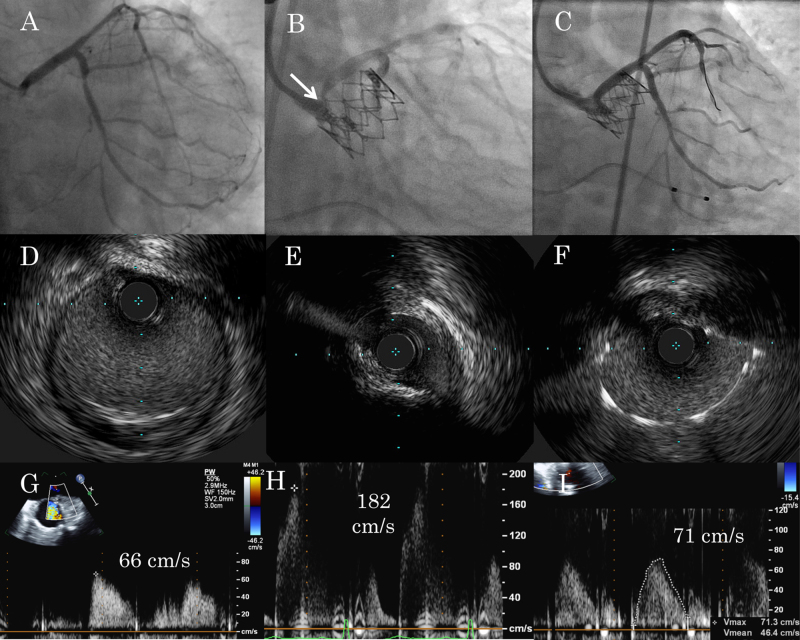
Because she was at risk of CO in terms of coronary height and calcification of LCC, the echocardiographer assessed coronary ostium and noticed acceleration of coronary flow at the LCA ostium from 66 cm/s to 182 cm/s, a few minutes later from valve deployment (Fig. 2G, H). We performed selective left coronary angiography using a guiding catheter, which revealed partial but significant obstruction of the ostial portion (Fig. 1, Fig. 2). On intravascular ultrasound (IVUS), LCA ostium was compressed by an extra-vessel structure and minimum lumen area (MLA) was 3.7 mm2 (Fig. 2E). Taking into account MLA and accelerated coronary flow, we performed bail-out percutaneous coronary intervention (PCI) for the CO. We deployed one drug-eluting stent to the LCA ostium (Fig. 2C). After confirming optimal expansion and apposition by IVUS (Fig. 2F), we finalized the procedure. The final coronary flow of LCA was 71 cm/s (Fig. 2I).
Fig. 2.
(A–C) Left coronary angiography. (D–F) Intravascular ultrasound at the left coronary artery ostium (E, F) and at a distal reference site (D). (G–I) Left coronary flow velocity at the ostium measured by transesophageal echocardiography. (A, G) Pre-procedural. (B, D, E, H) Following valve implantation showing significant ostial stenosis (arrow). (C, F, I) After bail-out percutaneous coronary intervention.
Post-procedural clinical course was good and the patient was discharged on day 10. There were no cardiovascular events including stent thrombosis during follow-up of 1 year.
Discussion
CO is an infrequent but potentially fatal complication of TAVI. Ribeiro et al. [1] reported the frequency, predictive factors, and clinical outcomes of symptomatic CO from a multicenter registry. Of 6685 patients, 44 patients (0.66%) had symptomatic CO following TAVI, with 30-day mortality of 40.9%. Older age, female sex, no previous coronary artery bypass graft, use of a balloon-expandable valve, and previous surgical aortic bioprosthesis were identified as baseline and procedural variables associated with CO. However, their study only collected symptomatic cases manifesting severe hypotension and electrocardiographic changes. Our patient had been hemodynamically stable and presented no abnormal electrocardiographic changes. Although post-deployment aortography is generally useful for the diagnosis of CO, we detected no signs of CO on aortography. In this case, CO would have been undiagnosed during procedure by conventional approach. In fact, most reported cases of CO were diagnosed based on hemodynamic instability, electrocardiographic changes, and abnormal findings on aortography [2]. Some patients who are not diagnosed with CO at procedure may develop delayed coronary obstruction [3], [4], [5], which could lead to sudden cardiac death after TAVI.
To the best of our knowledge, there is no report in which acceleration of coronary ostium flow detected by intraoperative TEE leads to a diagnosis of CO following TAVI. Hozumi et al. [6] reported the utility of transthoracic color Doppler echocardiography in detecting coronary restenosis following successful PCI. They searched for localized color aliasing and measured coronary flow velocity at aliasing. Prestenotic to stenotic mean diastolic velocity ratio less than 0.45 identified as the optimal cut-off value had a sensitivity of 86% and a specificity of 93% for the presence of restenosis in left anterior descending artery lesions. Likewise, the presence of stenotic flow at coronary ostium after TAVI could be an indicator of CO, but the cut-off value of coronary flow velocity is undetermined. If significant elevation of coronary ostium flow velocity compared to pre-procedural value is observed, selective coronary angiography by a guiding catheter is recommended.
In this case, the distance from the annulus to LCA was 11.0 mm and LCC had severe calcification. As lower-lying coronary ostium (<12 mm) was related anatomic factor of CO [1], we should have guidewire protection to LCA before valve deployment. However, TEE monitoring of coronary flow led to timely detection of silent CO without serious consequence.
In addition, there are no definite criteria to intervene for silent CO after TAVI. Because there is a risk of delayed adverse coronary events [3], [4], [5], we should intervene for silent CO taking into consideration several findings which include % diameter stenosis, MLA, and accelerated coronary flow.
We encountered a case of silent CO following TAVI. The initial sign leading to the diagnosis was provided by TEE. Silent CO is probably an underdiagnosed complication of TAVI, and TEE monitoring of coronary ostium flow is useful for its detection.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the other members of the TAVI team: Kenichi Hagiya, MD, Yuko Utanohara, MD, and Harutoshi Tamura, MD.
References
- 1.Ribeiro H.B., Webb J.G., Makkar R.R., Cohen M.G., Kapadia S.R., Kodali S., Tamburino C., Barbanti M., Chakravarty T., Jilaihawi H., Paradis J.M., de Brito F.S., Jr., Cánovas S.J., Cheema A.N., de Jaegere P.P. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552–1562. doi: 10.1016/j.jacc.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro H.B., Nombela-Franco L., Urena M., Mok M., Pasian S., Doyle D., DeLarochellière R., Côté M., Laflamme L., DeLarochellière H., Allende R., Dumont E., Rodés-Cabau J. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv. 2013;6:452–461. doi: 10.1016/j.jcin.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Bagur R., Dumont E., Doyle D., Larose E., Lemieux J., Bergeron S., Bilodeau S., Bertrand O.F., De Larochellière R., Rodés-Cabau J. Coronary ostia stenosis after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:253–255. doi: 10.1016/j.jcin.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Spiro J., Nadeem A., Doshi S.N. Delayed left main stem obstruction following successful TAVI with an Edwards SAPIEN XT valve: successful resuscitation and percutaneous coronary intervention using a non-invasive automated chest compression device (AutoPulse) J Invasive Cardiol. 2012;24:224–228. [PubMed] [Google Scholar]
- 5.Jategaonkar S.R., Dimitriadis Z., Hakim-Meibodi K., Gummert J., Horstkotte D., Scholtz W. Delayed coronary ischemia after transfemoral aortic valve implantation. J Heart Valve Dis. 2013;22:762–766. [PubMed] [Google Scholar]
- 6.Hozumi T., Yoshida K., Akasaka T., Asami Y., Kanzaki Y., Ueda Y., Yamamuro A., Takagi T., Yoshikawa J. Value of acceleration flow and the prestenotic to stenotic coronary flow velocity ratio by transthoracic color Doppler echocardiography in noninvasive diagnosis of restenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 2000;35:164–168. doi: 10.1016/s0735-1097(99)00501-x. [DOI] [PubMed] [Google Scholar]