Abstract
Papillary fibroelastomas are benign cardiac tumors with high embolic potential. The majority of cases of complete obstruction of the left main trunk (LMT) of the coronary artery are diagnosed via autopsy following sudden death; survival is rare in this setting. We present the case of a 60-year-old woman who underwent stent placement in the LMT three years prior to developing chest pain and cold sweats. On coronary arteriography, the catheter could not be advanced into the LMT due to resistance in the ostium. Insertion of the catheter was achieved after the resolution of resistance via catheterization of the LMT by means of an intra-aortic balloon pump drive system. The LMT was normal, and the patient's circulatory failure improved. The cause of the LMT embolism was a cardiac papillary fibroelastoma. Primary surgical excision is the recommended therapy for symptomatic cardiac papillary fibroelastoma. If the patient is hemodynamically stable, it may be possible to delay surgery. However, the patient in question developed cardiogenic shock secondary to two-vessel obstruction by the tumor. Therefore, even if the tumor had been removed using an intra-aortic balloon pump, the patient may not have survived until surgery.
<Learning objective: Primary surgical excision is the recommended therapy for symptomatic cardiac papillary fibroelastoma. If the patient is hemodynamically stable, it may be possible to delay surgery. However, hemodynamically unstable patients may not survive until surgery. Therefore, emergent therapy is a useful stop-gap measure until surgery is feasible.>
Keywords: Papillary fibroelastoma, Acute myocardial infarction, Cardiogenic shock, Intra-aortic balloon pump, Aortic valve
Introduction
Although the majority of patients with cardiac papillary fibroelastomas (CPFs) are asymptomatic, the clinical presentation of this entity may include severe thromboembolic complications, myocardial ischemia, infarction, stroke, and sudden cardiac death [1]. Acute myocardial infarction (AMI) secondary to CPF has historically been attributed to either adherent thrombi on the surfaces of or tumor fragments within the coronary arteries, as well as occlusion of the left coronary artery (LCA) ostium. The majority of cases of LCA occlusion are diagnosed via autopsy following sudden death [2]; survival is rare in this setting [3]. Previous reports [4], [5] have attributed MI to either intermittent obstruction of the LCA ostium or to the obstruction of a single vessel by the tumor; the patients described in these instances were hemodynamically stable. We describe a patient who presented with cardiogenic shock secondary to AMI. The patient's diagnosis was two-vessel obstruction secondary to a left main trunk (LMT) embolus from a CPF.
Case report
A 60-year-old female with a history of hyperlipidemia and prior angina pectoris underwent stenting of the LMT three years prior to hospitalization for chest pain and cold sweats. On examination, the patient's blood pressure was 94/68 mmHg, her pulse was 100 beats per minute, and her body temperature was 36.4 °C. Electrocardiography (ECG) revealed sinus rhythm, ST-segment elevation in leads I, aVL, and V1–V4, and ST-segment depression in leads II, III, aVF, and V5–V6. Chest radiography demonstrated an area of hemostasis at the pulmonary hilum. Transthoracic echocardiography (TTE) demonstrated severe anterior and lateral septal akinesis. Evidence of either aortic stenosis or regurgitation was not observed. At the time of the coronary arteriographic (CAG) examination, the right coronary artery was normal; a 5-Fr catheter could not be advanced into the LCA due to resistance in the ostium. Aortography demonstrated that the LMT was completely obstructed (Fig. 1A); the obstruction was attributed to a thrombus. The patient's blood pressure decreased to 56/34 mmHg, and circulatory failure subsequently developed. Both oxygen and catecholamines were subsequently administered for hypoxemia and hypotension, respectively, and an intra-aortic balloon pump (IABP) was inserted. The catheterization of the LCA under the IABP drive system was initially characterized by resistance in the catheter tip, but the successful insertion of the catheter was ultimately achieved following the resolution of the resistance. On CAG, the LCA was normal (Fig. 1B), and the patient's chest pain resolved. Her peak cardiac enzyme values were as follows: creatine kinase (CK), 7140 U/l; CK-MB, 635 U/l; and troponin I, 406.8 ng/ml. The patient underwent a repeat CAG on the 18th day of her admission due to improvement in her cardiac failure. Slight resistance was encountered at the same location as noted previously. The patient subsequently developed chest pain and dyspnea. Her ECG demonstrated ST-segment elevation, and her blood pressure decreased to 68/42 mmHg. Therefore, an IABP was promptly utilized, and the patient's symptoms eventually resolved. A filling defect in the proximal portion of the LMT inlet was visualized via aortography with the assistance of the IABP (Fig. 1C and D). Therefore, the patient underwent emergency surgery. Preoperative transesophageal echocardiography demonstrated a pendulous mass filling the left coronary cusp (Fig. 2A). During the operation, a tumor attached to the rim of the left coronary cusp of the aortic valve was identified (Fig. 2B). Surgical removal of the tumor without valve replacement was performed without complications. The tumor measured 19 mm × 13 mm × 5 mm (Fig. 2C), and the results of the histopathological examination were consistent with a benign papillary fibroelastoma (Fig. 2D). The patient remained free of symptoms following surgery.
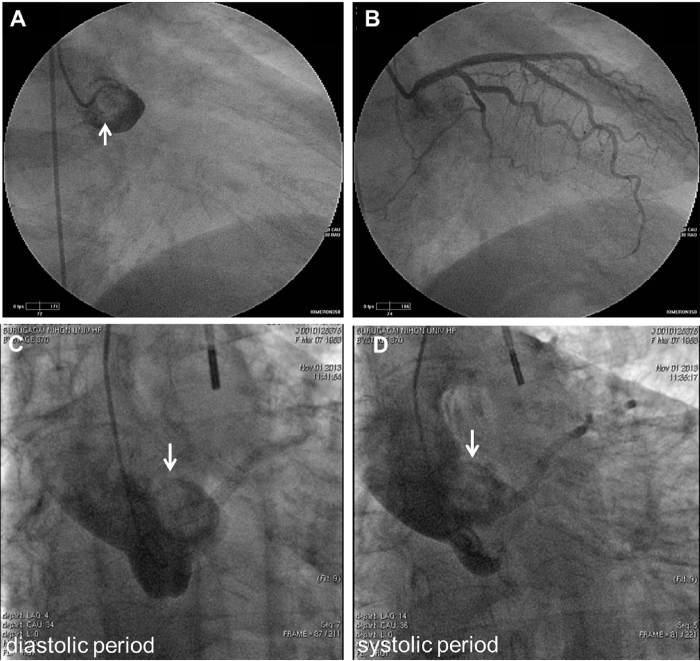
Fig. 1.
(A) A coronary angiogram of the left main coronary artery demonstrating a filling defect (arrow). (B) Under intra-aortic balloon pump drive (IABP), a catheter was inserted into the left coronary artery after encountering resistance. The left coronary artery was normal. (C and D) The tumor (arrow) migrated toward the cardiac chamber in a diastolic period and toward the aortic arch in a systolic period after IABP insertion.
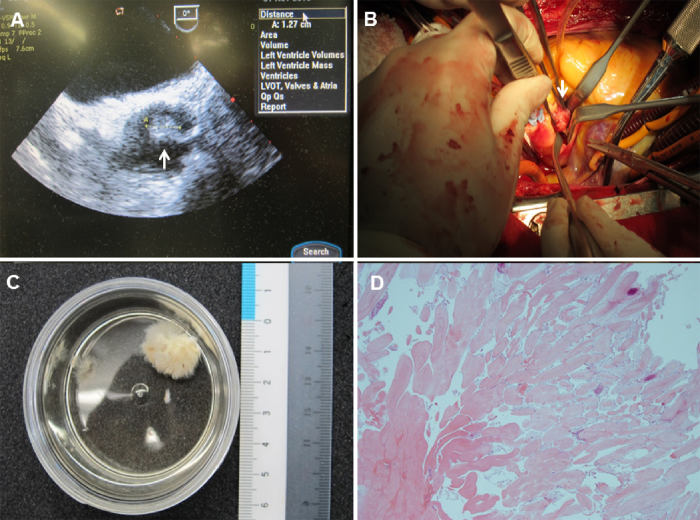
Fig. 2.
(A) A preoperative transesophageal short-axis view demonstrating a pendulous mass filling the left coronary cusp (arrow). (B) An intraoperative photograph demonstrating a papillomatous lesion on the rim of the left coronary cusp of the aortic valve (arrow). (C) This cardiac papillary fibroelastoma image bears a characteristic resemblance to a sea anemone, particularly when placed in saline. (D) Hematoxylin and eosin demonstrating papillary fibroelastoma, with its elongated and branching papillary fronds, central avascular collagen and elastic tissue (Low power view, 20× magnification).
Discussion
Although most cases of MI are caused by atherothrombosis, MI occasionally occurs in patients with normal appearing coronary arteries on angiography. MI with normal coronary arteries can result from multiple conditions, including coronary embolism, coronary spasm, coronary artery anomalies, coronary dissection, hyper-coagulable states, and imbalances between blood flow and blood supply. A rare cause of coronary embolism is CPF, the second most common benign primary cardiac tumor after myxomas which represents 10% of all primary cardiac tumors and exhibits a frequency of approximately 0.02% [2], [6].
CPFs are benign cardiac neoplasms. CPFs are always found incidentally during autopsy, during cardiac surgery, or via echocardiography. Sun et al. reported that the sensitivity and specificity of TTE were 88.9% and 87.8%, respectively, for the detection of CPFs ≥0.2 cm. When CPFs ≤0.2 cm were included in the analysis, the overall sensitivity of TTE was 61.9% [7]. The clinical presentation of this entity may vary from no symptoms to severe thromboembolic complications, myocardial ischemia, infarction, or stroke [1]. In particular, tumors involving the left side of the heart have been frequently associated with serious cardiovascular and neurologic events [2]. Currently, symptomatic CPFs should be surgically removed, and surgical excision should be considered for asymptomatic lesions that are left-sided, mobile, or larger than 1 cm [7], [8]. By contrast, there have been no reports outlining treatment strategies in cases involving a tumor that completely occludes a coronary artery, resulting in circulatory failure.
Our case warranted a surgical approach because the tumor was larger than 1 cm and was attached to the rim of the left coronary cusp, and because the patient developed an MI. However, the tumor was not discovered during the first TTE, which delayed the diagnosis. This delay was caused by the occlusion of the LMT ostium by the tumor. The patient subsequently developed cardiogenic shock due to the obstruction. Therefore, even if the tumor had been removed, the patient may not have survived until surgery.
The treatment strategy for cardiogenic shock entails the recovery of appropriate circulatory dynamics via the administration of catecholamines or the use of either IABP or cardiopulmonary bypass. Our patient's circulatory system was stabilized via the administration of catecholamines and the use of an IABP. Because the presence of a thrombus in the LMT was assumed, catheterization was also utilized. However, the AMI was actually caused by a tumor embolism, and the IABP was effective in not only maintaining circulatory dynamics but also in removing the tumor embolus. When a cardiac valve tumor is discovered, catheter examination is not recommended because the catheter may dislodge a fragment of the tumor or an adherent thrombus, resulting in an embolism [2]. The catheter may actually have forced the tumor farther into the coronary artery initially, but it is likely that the IABP successfully removed the embolus because both the valve and the tumor migrated toward the cardiac chamber by a mechanism of diastolic augmentation, creating a space through which the catheter was inserted, which left us with the impression that the occlusion had been successfully removed. Additionally, because the aortic pressure was decreased compared with the internal pressure of the coronary artery based on the systolic unloading of the IABP, the tumor was easily removed from the coronary artery. Therefore, emergent treatment of hemodynamically unstable patients may be an effective stop-gap measure until surgery is feasible.
In summary, the patient developed cardiogenic shock secondary to two-vessel obstruction by the tumor. Therefore, even if the tumor had been removed, patient may not have survived until surgery. The use of an IABP and a catheter may be attempted to remove the tumor as a bridge to surgery.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jha N.K., Khouri M., Murphy D.M., Salustri A., Khan J.A., Saleh M.A., Canal F.V., Augustin N. Papillary fibroelastoma of the aortic valve – a case report and literature review. J Cardiothorac Surg. 2010;5:84–88. doi: 10.1186/1749-8090-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gowda R.M., Khan I.A., Nair C.K., Metha N.J., Vasavada B.C., Sacchi T.J. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404–410. doi: 10.1016/S0002-8703(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 3.Her A.Y., Kim Y.H., Heo J.W., Yie K., Lee S. Papillary fibroelastoma of the aortic valve with atypical chest pain: late presentation with acute myocardial infarction and cardiac arrest. J Card Surg. 2012;27:327–330. doi: 10.1111/j.1540-8191.2011.01385.x. [DOI] [PubMed] [Google Scholar]
- 4.Bossert T., Diegeler A., Spyrantis N., Mohr F.W. Papillary fibroelastoma of the aortic valve with temporary occlusion of the left coronary ostium. J Heart Valve Dis. 2000;9:842–843. [PubMed] [Google Scholar]
- 5.Maestroni A., Zecca B., Triqqiani M. Cardiac papillary fibroelastoma presenting with acute coronary syndrome and syncope. Acta Cardiol. 2006;61:363–365. doi: 10.2143/AC.61.3.2014843. [DOI] [PubMed] [Google Scholar]
- 6.Tazelaar H.D., Locke T.J., McGregor C.G. Pathology of surgically excised primary cardiac tumors. Mayo Clin Proc. 1992;67:957–965. doi: 10.1016/s0025-6196(12)60926-4. [DOI] [PubMed] [Google Scholar]
- 7.Sun J.P., Asher C.R., Yang X.S., Cheng G.G., Scalia G.M., Massed A.G., Griffin B.P., Ratliff N.B., Stewart W.J., Thomas J.D. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001;103:2687–2693. doi: 10.1161/01.cir.103.22.2687. [DOI] [PubMed] [Google Scholar]
- 8.Aryal M.R., Badal M., Mainali N.R., Jalota L., Prahan R. Papillary fibroelastoma of the aortic valve: an unusual cause of angina. World J Cardiol. 2013;5:102–105. doi: 10.4330/wjc.v5.i4.102. [DOI] [PMC free article] [PubMed] [Google Scholar]