Introduction
Traditionally, cryotherapy has been used in dermatology to treat skin lesions, such as actinic keratoses, viral warts, seborrheic keratoses, and select keratinocyte carcinomas. Whole-body cryotherapy (WBC) is a new and trendy treatment of which practitioners should be aware because of potential adverse events. WBC is a popular technique used for purported improvements in muscle recovery, back pain, muscle stiffness, energy, sleep, and skin health among other benefits. This technique was originally given to patients with multiple sclerosis or rheumatoid arthritis because of claimed beneficial effects, such as analgesia and decreased inflammation.1 It has since become accepted by athletes and more accessible through various spas, wellness centers, and cryotherapy facilities.
Case report
A 71-year-old man presented with a cold burn injury. One day prior, he was undergoing WBC at a cryotherapy facility for back pain and arthritis. However, it was suspected that a nozzle likely malfunctioned, as the liquid nitrogen sprayed directly on his back for a prolonged period that was believed to be less than 1 minute in duration. He experienced stinging and pain at the site, which subsequently turned red and blistered (Fig 1). No first aid was administered by the facility, and the patient applied topical emollient with a bandage at home. He previously had completed 4 WBC sessions without any adverse events.
Fig 1.
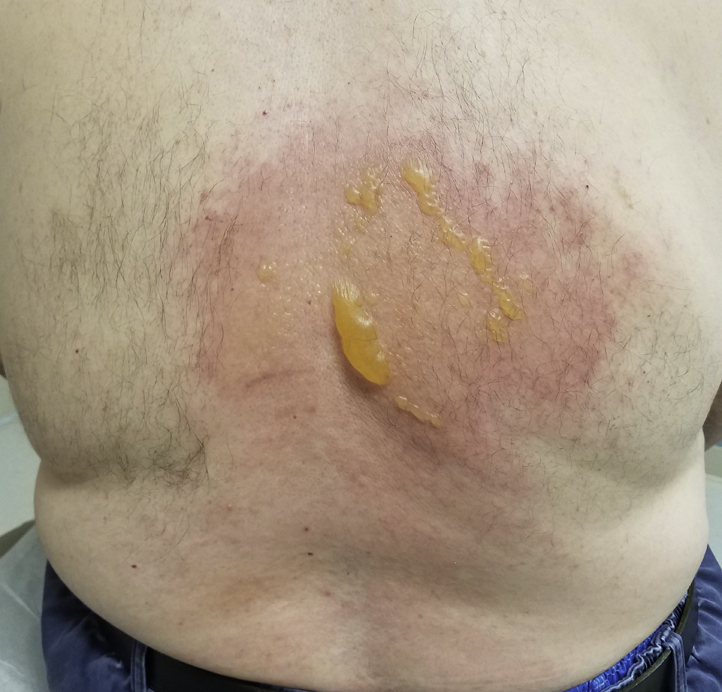
Cold burn injury after WBC.
Discussion
Spas, wellness centers, and cryotherapy facilities have opened to the public in recent years and begun offering WBC treatments to anyone interested. The cryotherapy chamber is approximately the size of a phototherapy light box. While standing in the chamber, the head of the patient is above the top and outside of the chamber. The remainder of the body is engulfed in a liquid nitrogen mist, which cools the chamber to –100 to –140°C for about 2 to 5 minutes and can be completed several times per week.1
It has been suggested that WBC offers advantages in enhanced muscle recovery after strenuous activity because of the extreme nature of the cold air administration compared with traditional cooling methods, such as ice packs and cold water immersion.1 However, the US Food and Drug Administration has yet to approve any WBC chambers and warns about potential harmful effects, such as asphyxiation, frostbite, burns, and eye injury.2 Additionally, a 2015 Cochrane review found insufficient evidence to determine whether it improved muscle recovery in active young adult males, whereas no data exists for females or elite athletes.3 The review also found a lack of evidence on adverse events.
Several documented adverse events associated with WBC exist in the literature. For example, Cámara-Lemarroy et al4 reported on a 56-year-old physically active male who suffered an abdominal aortic dissection after multiple WBC sessions. Additionally, Greenwald et al5 observed an episode of cold panniculitis in a 47-year-old man after 8 WBC treatments.
Our patient suffered a cold burn injury caused by what was presumed to be a malfunctioning nozzle in the cryotherapy chamber. Cold burns are much less common than their thermal counterparts. Freezing temperatures can cause intracellular water crystallization with damage to proteins and membranes. Cold-induced vasoconstriction can lead to hypoperfusion, and endothelial injury can cause decreased vascular integrity.
Treatment of a cold burn injury is dependent on severity. In our patient with a relatively focal and superficial cryotherapy burn, we offered systemic steroids, topical corticosteroids, ibuprofen, and silver sulfadiazine cream. Although controversial in the treatment of burns, steroids may offer improvements in inflammation, pain, and healing processes.6 Ibuprofen can also help with pain control while counteracting the vasoconstriction caused by local release of inflammatory mediators. Silver sulfadiazine offers a protective barrier to denuded skin with its painless application and broad-spectrum antibacterial activity.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Bleakley C.M., Bieuzen F., Davison G.W. Whole-body cryotherapy: empirical evidence and theoretical perspectives. Open Access J Sports Med. 2014;5:25–36. doi: 10.2147/OAJSM.S41655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whole body cryotherapy (WBC): a “cool” trend that lacks evidence, poses risks [U.S. Food and Drug Administration Web site]. July 5, 2016. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm508739.htm Available at:
- 3.Costello J.T., Baker P.R., Minett G.M. Cochrane review: Whole-body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. Cochrane Database Syst Rev. 2015;9:CD010789. doi: 10.1002/14651858.CD010789.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cámara-Lemarroy C.R., Azpiri-López J.R., Vázquez-Díaz L.A. Abdominal aortic dissection and cold-intolerance after whole-body cryotherapy: a case report. Clin J Sport Med. 2017;27(5):e67–e68. doi: 10.1097/JSM.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald E., Christman M., Penn L. Cold panniculitis: adverse cutaneous effect of whole-body cryotherapy. JAAD Case Rep. 2018;4(4):344–345. doi: 10.1016/j.jdcr.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowan M.P., Cancio L.C., Elster E.A. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19:243. doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


