Abstract
Multiple sclerosis (MS) is a genetically mediated autoimmune disease of the central nervous system. Allelic variants lead to lower thresholds of T-cell activation resulting in activation of autoreactive T cells. Environmental factors, including, among others, diet, vitamin D, and smoking, in combination with genetic predispositions, play a substantial role in disease development and activation of autoreactive T cells. FoxP3+ regulatory T cells (Tregs) have emerged as central in the control of autoreactive T cells. A consistent finding in patients with MS is defects in Treg cell function with reduced suppression of effector T cells and production of proinflammatory cytokines. Emerging data suggests that functional Tregs become effector-like T cells with loss of function associated with T-bet expression and interferon γ (IFN-γ) secretion.
DISCOVERY OF REGULATORY T CELLS (Tregs) IN MOUSE AND HUMAN
Since the discovery of Tregs by Sakaguchi and coworkers (1995) two decades ago, our understanding of the CD4+ T helper (Th) cell subtype, first characterized by the expression of the interleukin (IL)-2 receptor α-chain (CD25), has vastly expanded. Another breakthrough discovery in the Treg field was the identification of FoxP3 as the main transcription factor driving and maintaining Treg phenotype and function (Fontenot et al. 2003; Hori et al. 2003; Khattri et al. 2003). Patients with the IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy X-linked syndrome), a severe autoimmune disorder that develops early in life, carry mutations in the FoxP3 gene locus. FoxP3 mutations lead to dysfunctional FoxP3 protein expression; patients harboring FoxP3 mutations do not develop functional Tregs (Bennett et al. 2001). A similar phenotype is observed in scurfy mice, which lack functional FoxP3 (Bennett et al. 2001). FoxP3+ CD25+ Tregs can be broadly subdivided into naturally arising Tregs and peripherally induced Tregs. Naturally arising Tregs develop in the thymus. In animal models, it was first noted that those receiving postnatal thymectomy developed severe autoimmunity. Furthermore, disease development could be prevented by the transfer of CD4+ T cells (Sakaguchi et al. 2006). Moreover, the depletion of CD25+ cells from thymocytes or peripheral T cells could not prevent autoimmunity in cotransfer experiments in immune-deficient animals. This led to the terminology of “naturally arising” or “natural” Treg cells (Sakaguchi et al. 2006). Thymic development of natural Tregs is strictly related to the stable induction of FoxP3, and requires high-affinity binding of major histocompatibility complex (MHC)–self-peptide complexes from thymic antigen-presenting cells (APCs) to the T-cell receptor (TCR). As thymic Tregs are reactive against self-peptides, they are likely to be predominantly involved in controlling autoimmune reactions. Additionally, thymic Treg development requires certain costimulatory signals and cytokine environments (in particular IL-2), different from conventional effector T cells, which leads to the generation of stable FoxP3-expressing Treg cells in the periphery (Klein and Jovanovic 2011; Hsieh et al. 2012). Fate-mapping and thymic selection studies of Tregs so far have only been conducted in mouse models and it remains to be seen whether the same processes apply to human Treg development.
Stable expression of FoxP3 is essential for Treg function and is maintained through epigenetic modifications both in the FoxP3 gene locus and Treg-specific demethylated region (TSDR) (Floess et al. 2007; Huehn et al. 2009). Naïve murine FoxP3− CD4+ T cells can express FoxP3 in the presence of transforming growth factor β (TGF-β) or retinoic acid, which gives rise to peripherally induced Tregs (iTregs). As iTregs arise from conventional CD4+ T cells, they are considered to play a more pronounced role in general immune regulation (de Lafaille and Lafaille 2009). Although there are phenotypic and functional overlaps to natural Tregs, iTregs show distinctive differences in stability and gene expression (de Lafaille and Lafaille 2009; Sakaguchi et al. 2010). For instance, the TSDR region of iTregs is not fully demethylated, whereas natural Treg TSDR is fully demethylated (Floess et al. 2007).
Although the term “regulatory T cell” conventionally is used to describe FoxP3+ CD4+ T cells, it has to be noted that mouse models helped to identify subtypes of Tregs that lack the expression of FoxP3. IL-10-producing Tr1 (Treg type 1) cells, and TGF-β-producing Th3 cells are the well-established FoxP3− Treg populations that can exert suppressive function on effector T cells (Chen et al. 1994; Bach 2001; Roncarolo et al. 2001).
We and others were able to identify human CD4 cells expressing very high levels of CD25, which are analogous in function in vitro to mouse Tregs (Baecher-Allan et al. 2001; Stephens et al. 2001). These populations of CD25high CD4+ cells were subsequently found to express high levels of FoxP3. Although FoxP3 is essential for development and function of Treg cells (Fontenot et al. 2003), we now understand that FoxP3 can be expressed by other cell populations, and thus cannot be described as a lineage-defining transcription factor for Tregs. Specifically, as T-cell subpopulations develop in the thymus, not all CD4+ FoxP3+ T cells are homogenous in gene expression, phenotype, and suppressive function. Further, effector T cells have the potential to express the FoxP3 marker on activation. Thus, human Tregs cannot be reliably identified by FoxP3 expression alone. At present, the surface expression of CD25 and CD127 is the most consistent marker, with high levels of CD25 expression and low/negative expression of CD127 being indicative of the Treg population (Liu et al. 2006; Seddiki et al. 2006).
Treg HETEROGENEITY: ONE FAMILY, DIVERSE APPEARANCES
Parallel to the discovery of diversity among T cells is the identification of diversity within the CD4+ CD25+ FoxP3+ Treg population. A widely used classification of human Tregs is based on the expression of CD45RA+ (naïve-like or resting Tregs) and CD45RO+ (activated effector or memory-like Tregs) (Miyara et al. 2009). Additionally, surface expression of HLA-DR can further divide Tregs into DR+ and DR− Tregs, in which expression is correlated with higher suppressive activity (Baecher-Allan et al. 2006, 2011). The subset of CCR4+ memory Tregs has also been isolated as a highly suppressive population. Additionally, subsets can be defined based on chemokine receptor expression. Thus, subset-specific markers can be used in the laboratory to isolate several subpopulations of the heterogeneous Treg population for study.
The expression of CD25 is not restricted to human Tregs, as it is also up-regulated on activated conventional T cells, which can be best identified by the surface expression of CD25 in combination with CD127, the α-chain of the IL-7 receptor. This is contrary to Tregs, which display a high expression of CD25 while they are predominantly negative for CD127 (CD25high CD127low/neg) (Liu et al. 2006; Seddiki et al. 2006). Another method to isolate human Tregs is the combination of CD49d (the α-chain of VLA-4) and CD127. We have shown that the majority of human Tregs only express low levels of CD49d and that by depleting both CD49+ and CD127+ cells, the isolation of Tregs is achievable without the use of CD25. Importantly, because most of the cytokine-expressing activated CD25+ effector cells express higher levels of CD49d, this method removes contaminating cytokine-expressing cells from human Treg preparations (Kleinewietfeld et al. 2009). FoxP3+ Tregs further express a set of characteristic markers and molecules, of which some are direct targets of FoxP3. Tregs express, for example, high levels of cytotoxic T-lymphocyte antigen 4 (CTLA-4) and glucocorticoid-induced tumor necrosis factor (TNF) receptor–related protein (GITR) (Sakaguchi et al. 2010). Subsets of both murine and human Tregs express the TNF receptor 2 (TNFR2) (Chen et al. 2008, 2010).
TNF-α stimulation induces, synergistically with IL-2, the expression of other members of the TNFR superfamily, such as 4-1BB and OX40 (Hamano et al. 2011). Interestingly, OX40 (CD134, TNFRSF4) has recently been shown to induce Treg activation and suppressive function (Piconese et al. 2014b). Furthermore, in vitro experiments have shown an induction of human Treg proliferation and promotion of Treg stability on OX40 engagement via APC-mediated activation and stimulation with OX40 ligand (OX40L). TNF-α stimulation further increases OX40 expression and promotes human Treg-suppressive function (Piconese et al. 2014a,b).
HETEROGENOUS Tregs EXERT DIVERSE MODES OF ACTION
Cytolysis mediated by the secretion of granzymes, the method typically used by natural killer (NK) cells and CD8+ cytotoxic T cells, is now considered a possible mechanism of suppression by Tregs. Additionally, cell–cell contact-dependent suppression plays an important role for the cell death receptor CD95, which mediates killing of CD8+ T cells via CD95L interaction (Strauss et al. 2009). Mechanisms that mediate the metabolic disruption of the effector T-cell target suggest that Treg cells induce apoptosis via IL-2 deprivation (Pandiyan et al. 2007). Tregs may also modulate the function of dendritic cells (DCs), which are required for the activation of effector T cells (Bluestone and Tang 2005). However, the complexity of suppressive function by Tregs and the great heterogeneity of the Treg population continue to challenge our understanding of the molecular mechanisms and physiological context of human Tregs.
Tregs control peripheral tolerance and have the ability to suppress the activation, proliferation, and effector functions (cytokine production) of several immune cells, including CD4+ and CD8+ T cells, NK and NK T (NKT) cells, B cells, and APCs (Roncarolo et al. 2006; Sakaguchi et al. 2008). As Tregs can control a variety of cell types under differential inflammatory conditions, investigating the mode of action and mediation of suppressive function has been a main target of research. In general, human CD4+ CD25+ FoxP3+ Tregs can exert suppressive function in four distinct ways: release of inhibitory cytokines, mediation of cytolysis, metabolic disruption of the target cell, and modulation of APCs (Vignali et al. 2008). Some mechanisms are specific to certain Treg subpopulations and are highly dependent on the microenvironment and type of immune reaction (Vignali et al. 2008; Sakaguchi et al. 2010). Most of the suppressive mechanisms have been described in in vitro suppression assays, leaving room for speculation about whether the mechanisms are similar in vivo. In 1998, Thornton and Shevach showed that murine Tregs can inhibit T effector cell activation through cell–cell contact (Thornton and Shevach 1998).
Studies over the following years discovered that Tregs, mostly linked to ICOS costimulation, release IL-10 and TGF-β to suppress effector T cells and APCs (Li et al. 2006; Ito et al. 2008; Vignali et al. 2008). Along this line, we have shown that subsets of human Tregs can secrete IL-10 during in vitro suppression assays to suppress effector T-cell proliferation (Baecher-Allan et al. 2006). FoxP3+ Tregs express high levels of CTLA-4, GITR, and other costimulatory receptors (Sakaguchi et al. 2010). Through CTLA-4 ligation of CD80 and CD86, FoxP3+ Tregs can modulate DC function, thereby inhibiting DC costimulation of effector T cells (Wing et al. 2008). This mechanism might work, at least in part, by trans-endocytosis, as it was shown that CTLA-4 of human Tregs was able to capture CD86 from interacting DCs (Qureshi et al. 2011). Another Treg-intrinsic strategy to modulate APC function is the induction of IDO. Induction and stabilization of this enzyme by T-cell CTLA-4 and IL-10 initiates the tryptophan catabolism, depriving the microenvironment of tryptophan. The lack of tryptophan causes production of inhibitory kynurenines, which block proliferation of effector T cells in the proximity of the DC (Fallarino et al. 2003; Mellor and Munn 2004; Munn et al. 2004a,b). Because human IDO+ DCs express CCR6 (Munn et al. 2002), the IL-10-producing CCR6+ effector memory-like Treg subset may amplify suppression by this mechanism while being attracted to similar sites through CCL20 gradients in vivo (Kleinewietfeld et al. 2005). Cytolysis mediated by granzyme A (GZMA) and perforin is another mechanism to suppress ongoing immune reactions (Grossman et al. 2004a,b). Metabolic disruption by cytokine deprivation is another mode of action Tregs can use to facilitate suppression of effector T-cell proliferation. Murine CD25+ Tregs can deprive the microenvironment of IL-2 by their high-affinity IL-2 receptor and thereby blocking effector T-cell growth (de la Rosa et al. 2004; Busse et al. 2010). However, it remains to be seen whether human Tregs can use a similar mechanism. The discovery that the ectonucleotidase CD39/ENTPD1 is highly expressed on a subset of human Tregs led to the identification of an additional and distinct pathway, namely, the hydrolysis of extracellular adenosine triphosphate (ATP) and the generation of immune-suppressive adenosine (Borsellino et al. 2007; Sakaguchi et al. 2010). Treg activation increases enzymatic activity of CD39, which facilitates the hydrolysis of extracellular ATP. As ATP is a classical “danger signal,” removal of ATP from the microenvironment can extend cell survival of Tregs, prevent APC activation, and generate antiproliferative adenosine through a CD39/CD73-induced signaling cascade (Borsellino et al. 2007; Deaglio et al. 2007). Joint expression of both CD39 and CD73 has only been shown on murine Tregs (Borsellino et al. 2007; Deaglio et al. 2007), so an involvement of the CD39/CD73 signaling cascade may only work in inflammatory settings with CD73 up-regulation on effector T cells (Dieckmann et al. 2002; Doherty et al. 2012). Finally, human Tregs can indirectly confer suppression by the induction of other immune-suppressive cells, a mechanism termed “infectious tolerance.” Along this line, human Tregs have the potential to induce IL-10-expressing Tr1-like cells (Dieckmann et al. 2002) through a mechanism partly mediated by TGF-β (Jonuleit et al. 2002).
REGULATORY T-CELL PLASTICITY: MIMICKING EFFECTOR T-CELL PHENOTYPES FOR SPECIFIC IMMUNE REGULATIONS
An emerging body of literature recently described the capacity of Tregs acquiring effector T-cell phenotypes to specifically regulate Th-effector T-cell subtypes in mice. Tregs can induce expression of Th-specific transcription factors T-bet, GATA3 and IRF4, RORγt and STAT3, or Bcl6 to control Th1, Th2, Th17, or T follicular helper (Tfh) effector T-cell responses, respectively (Chaudhry and Rudensky 2013; Vahedi et al. 2013). Along this line, we and others showed that human Tregs display a great magnitude of plasticity as well and, under certain inflammatory circumstances, can convert into Th1-like or Th17-like Tregs that show pathogenic phenotypes characterized by loss of suppressive function and release of proinflammatory cytokines (Kleinewietfeld and Hafler 2013).
Recent studies have been aimed at identifying underlying mechanisms. For Th17-like Tregs, it has been shown that in vitro stimulation by dectin-1-activated DCs leads to up-regulation of RORγt and IL-17 in Tregs (Osorio et al. 2008). Human Th17-like Tregs can be induced in vitro by stimulation in the presence of IL-6 and IL-1β, and could have pathogenic potential (Beriou et al. 2009; Voo et al. 2009). Nevertheless, human Th17-like Tregs keep their suppressive phenotype despite IL-17 production (Beriou et al. 2009; Voo et al. 2009). Further, Th17-like Tregs have been observed in humans and mice in the context of a variety of pathological conditions such as colon cancer (Kryczek et al. 2011; Blatner et al. 2012), inflammatory bowel disease (Hovhannisyan et al. 2011), skin lesions of psoriasis patients (Bovenschen et al. 2011), as well as arthritis (Komatsu and Takayanagi 2015).
Th1-like Tregs have been observed in both mice and human diseases and are characterized by the expression of the Th1 signature transcription factor T-bet as well as the type I cytokine interferon γ (IFN-γ) (Dominguez-Villar et al. 2011). Interestingly, studies performed in mice identified two distinct Th1-Treg subtypes: Th1-supressing Tregs and Th1-like Tregs, with the latter losing suppressive function and acquiring a pathogenic phenotype. During type I immune responses, NK cells and Th1 effector T cells release the proinflammatory cytokine IFN-γ. IFN-γ signaling, in turn, increases T-bet and IL12RB2 expression through STAT4, a mechanism used both by murine and human Tregs (Koch et al. 2012; Piconese et al. 2014b). Up-regulation of IL12RB2 renders Tregs susceptible to IL-12 signaling (Koch et al. 2009). We and others observed that culturing Tregs with IL-12 in vitro renders them dysfunctional, and induces a pathogenic phenotype characterized by IFN-γ release and up-regulation of typical Th1 markers, including T-bet and CXCR3 (Wei et al. 2009; Lu et al. 2010; Dominguez-Villar et al. 2011). Previous studies were unable to detect IL-12RB2 expression in Tregs ex vivo, leading to the assumption that a preexposure to IFN-γ is necessary for Tregs to become susceptible to IL-12. However, we and others showed that exposure of ex vivo Tregs to IL-12 alone is sufficient to increase IFN-γ expression in mouse and human Tregs (Oldenhove et al. 2009; Wei et al. 2009; Dominguez-Villar et al. 2011; McClymont et al. 2011). IL-12-induced IFN-γ-producing Th1-like Tregs show defects in proliferation, suppression, and reduced expression of IL-2RA (Zheng et al. 2009; Dominguez-Villar et al. 2011; McClymont et al. 2011). Supporting this observation, Bhairavabhotla and coworkers were able to detect IL12RB2 expression at the RNA level in the steady state of human Tregs ex vivo (Bhairavabhotla et al. 2016).
LOSS OF REGULATORY T-CELL FUNCTION IN AUTOIMMUNE DISEASE
The loss of dominant peripheral tolerance, which is normally controlled by Tregs, can lead to spontaneous autoimmune disease, immunopathology, metabolic disease, allergy, and loss of fetal–maternal tolerance during pregnancy. Therefore, defective Treg cells have a crucial role in regulating autoimmune responses. Dysfunctional Th1-like Tregs have been observed in a variety of autoimmune settings, including multiple sclerosis (MS) (Dominguez-Villar et al. 2011; Kitz et al. 2016), type 1 diabetes (T1D) (McClymont et al. 2011), de novo autoimmune hepatitis in patients with liver transplant (Arterbery et al. 2016), and in inflammatory bowel disease (IBD) (Holmen et al. 2006; Feng et al. 2011; Cao et al. 2014).
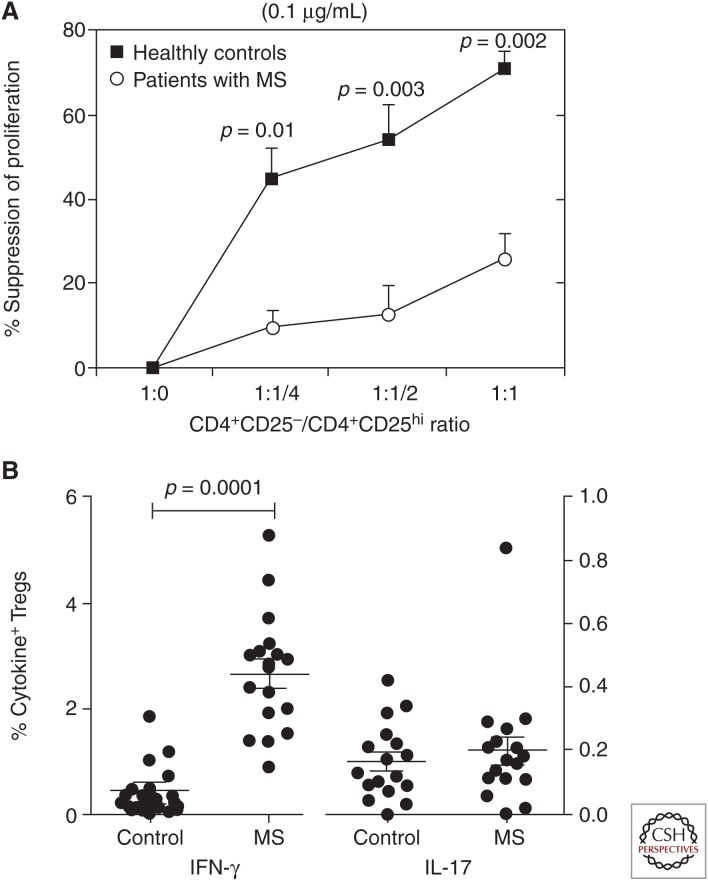
We examined the frequency and function of Tregs in a series of patients with MS using in vitro suppression assays, and observed a marked decrease in function (Fig. 1A), which has been widely replicated (Viglietta et al. 2004; Haas et al. 2005; Huan et al. 2005; Kumar et al. 2006; Feger et al. 2007; Venken et al. 2008a,b; Frisullo et al. 2009), whereas there was no significant difference in the frequency of Tregs in peripheral blood when compared with healthy control subjects (Putheti et al. 2003; Viglietta et al. 2004). However, MS patients did show an enrichment of Tregs with effector-memory-like phenotypes in their cerebrospinal fluid (CSF) compared with peripheral blood (Feger et al. 2007; Venken et al. 2008b).
Figure 1.
T regulatory cells (Tregs) from multiple sclerosis (MS) patients are less suppressive and produce more proinflammatory interferon (IFN)-γ. (A) Tregs from MS patients are significantly less suppressive compared with healthy controls. (From Viglietta et al. 2004; modified, with permission, from the authors.) (B) Tregs isolated from MS patients produce significantly increased level of IFN-γ but not interleukin (IL)-17A compared with healthy controls (Dominguez-Villar et al. 2011).
Although a proinflammatory cytokine expression in human CD25+ Treg cells was noticed from early on (Dieckmann et al. 2001), these data were complicated owing to the limitation of valid Treg markers and, thus, by potential contamination with effector T cells in Treg preparations. The presence of IFN-γ-producing cells was also observed in FoxP3-expressing cells after PMA/ionomycin stimulation (Kleinewietfeld et al. 2009) or after prolonged in vitro expansion of FoxP3+ Treg cells (Hoffmann et al. 2006, 2009). However, because FoxP3 can also be transiently up-regulated on human effector T cells (Gavin et al. 2006), it is unclear whether the IFN-γ-producing cells derived from Tregs or contaminated effector T cells.
We first discovered that untreated relapsing-remitting MS (RRMS) patients harbor an increased frequency of Th1-like Tregs in peripheral blood compared with healthy controls (Dominguez-Villar et al. 2011). Focusing on the Treg phenotype, Th1-like Tregs from MS patients showed increased expression of the classical Th1 markers CXCR3, T-bet, CCR5, and IFN-γ (Fig. 1B), whereas the expression level of TGF-β and CTLA-4 are decreased. The distinct phenotype translates into defects in function, with decreased proliferation and reduced suppressive capacity. Further, IFN-γ-producing Tregs displayed an almost fully demethylated TSDR region in the FoxP3 locus, similar to IFN-γ-negative Tregs, which was indicative of functional natural Tregs (Baron et al. 2007). IFN-γ-producing Tregs strongly contribute to the observed Treg dysfunction in RRMS patients, and blocking IFN-γ in coculture-suppression assays with RRMS-derived Tregs restored their suppressive capability (Dominguez-Villar et al. 2011).
Interestingly, an increased frequency of Th1-like Tregs could also be observed in humans with T1D (McClymont et al. 2011), indicating that Th1-like Tregs are associated with several autoimmune diseases in humans. It remains to be seen, however, whether the Th1-Treg plasticity plays a role for disease pathogenesis. Moreover, the question of whether this Treg subset has a physiological role under nonautoimmune conditions remains unanswered. In the context of intestinal inflammation, increased Treg frequencies, accompanied by increase ratios of potentially pathogenic IFN-γ and IL17-producing Tregs, have been observed in animal models and IBD patients (Holmen et al. 2006; Feng et al. 2011). Several animal models of colitis reported a potentially protective role of IFN-γ-producing Th1-Tregs, which suggests the presence of a Th1-supressing Treg subtype in this particular setting (Holmen et al. 2006; Feng et al. 2011).
Several observations provide possible mechanisms leading to the loss of Treg-suppressive capacity, and even a trend toward their aberrant proinflammatory activity in MS patients. Loss of suppression correlates with a decrease in FoxP3 and CTLA-4 expression (Huan et al. 2005; Venken et al. 2008b; Sellebjerg et al. 2012). Additionally, it has been seen that a lower thymic output and decreased CD58/CD2-dependent costimulation contributes to Treg loss of function (De Jager et al. 2009; Baecher-Allan et al. 2011). An altered distribution of distinct Treg subsets was also seen in RRMS patients (Haas et al. 2007; Venken et al. 2008a), and we specifically observed a lower frequency of CD39+ Tregs in these patients (Borsellino et al. 2007). The CD39+ Treg population is of particular importance for the suppression of Th17 cells, but is functionally impaired in MS patients (Fletcher et al. 2009).
The critical involvement of regulatory CD4+ CD25+ T cells in MS was initially indicated by studies in experimental autoimmune encephalomyelitis (EAE), a murine animal model of MS. The most significant findings show that the adoptive transfer of CD4+ CD25+ Tregs was sufficient to ameliorate disease, whereas the depletion of Tregs worsened disease in models of EAE (Randolph and Fathman 2006; Lowther and Hafler 2012). Interestingly, Th1-like Tregs have been observed in the central nervous system (CNS) of mice after EAE induction using myelin oligodendrocyte glycoprotein (MOG) (Korn et al. 2007). MOG-specific Tregs isolated from mice at different time points during EAE could not suppress MOG-specific effector T cells neither in vivo nor in vitro, and produced significant levels of IFN-γ at different disease stages (Korn et al. 2007).
It is interesting to note that FoxP3+ Tregs and IL-17-producing Th17 cells are reciprocally related; whereas Tregs are anti-inflammatory, Th17 cells are proinflammatory. The balance between these two cell populations is critical in the development of human autoimmunity (Sakaguchi et al. 2008; Korn et al. 2009), and TGF-β is a key determinant of this balance between Th17 and FoxP3+ Treg differentiation. Whereas TGF-β induces the differentiation of Treg cells, the combination of TGF-β with IL-6 or IL-21 induces Th17 cells while simultaneously blocking Treg differentiation (Korn et al. 2009). At a molecular level, FoxP3 can physically bind to Rorγt and Rorα to antagonize each other’s function (Du et al. 2008; Zhou et al. 2008). Furthermore, in the presence of retinoic acid, which enhances TGF-β signaling and blocks expression of the IL-6 receptor, Tregs are preferentially induced over Th17 cells (Mucida et al. 2007).
The notion that Tregs play a critical role in MS is further supported by the genetic changes seen in conventional FoxP3+ Tregs of MS patients. Disease susceptibility is highly associated with genes that are strongly implicated in Treg function. Of importance are the IL2RA and IL7RA loci (Hafler et al. 2007; Nylander and Hafler 2012). Genome-wide association studies (GWAS) on MS patients uncovered an association with the CD58 gene encoding for the costimulatory molecule lymphocyte function–associated antigen 3 (LFA-3). As previously described, CD58/CD2 costimulation is important in normal Treg function. After analyzing this variant in more detail, we saw that the allelic variant of CD58 leads to increased CD58 expression, which could potentially lead to FoxP3 up-regulation and enhanced Treg function through CD2 engagement. Interestingly, MS patients in clinical remission showed increased messenger RNA (mRNA) expression of CD58, suggesting restored Treg function, potentially because of increased CD58/CD2 costimulation (De Jager et al. 2009). Additionally, the transcription factor BTB and CNC homology 2 (BACH2), which is essential for Treg cell development and function in mice, was recently discovered as a new risk variant for MS, and is also associated with type 1 diabetes, asthma, Crohn’s disease, celiac disease, and vitiligo (Sawcer et al. 2011; Roychoudhuri et al. 2013).
Tr1 REGULATORY T CELLS
In 1990, Bachetta and coworkers described a suppressive CD4+ T cell type that was not dependent on FOXP3 expression, termed Tr1 cells. Tr1 Tregs are induced in the periphery and differ from Foxp3+ Tregs, namely, releasing IL-10, TGF-β, IL-5, granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, and low levels of IL-4, IL-17, and IL-2 (Bacchetta et al. 1990, 1994; Groux et al. 1997). Interestingly, IL-10 secretion is a rapid process in Tr1 cells already starting 4 h after activation and reaching a maximum release 12–24 h after activation (Bacchetta et al. 1994). Tr1 cells were also found to express coinhibitory molecules like CTLA4 (Bacchetta et al. 2002; Akdis 2008), PD-1 (Akdis 2008), and ICOS (Haeringer et al. 2009), as well as CD39 and CD73, indicating that suppressive function might be reached through generation of adenosine and disrupting the metabolic state of effector T cells (Bergmann et al. 2007). On stimulation, FoxP3− Tr1 cells start to up-regulate FoxP3 expression, but the level of expression remains lower than in naturally arising or peripherally induced FOXP3+ Tregs (Levings et al. 2005; Brun et al. 2009, 2011).
Similar to FoxP3+ Tregs, IL-10-secreting Tr1 cells can be induced by CD58/CD2 costimulation (Wakkach et al. 2001), indicating that MS-associated protective allelic variant of CD58 is related to Tr1 cells as well. Higher expression levels of CD58 could potentially trigger TR1 cell induction, and subsequently impact IL-10 secretion in humans. Along this line, increased CD58 mRNA levels in MS patients during remission might be correlated with increased IL-10 levels, as well as enhanced Tr1 cell frequency and function (De Jager et al. 2009).
However, when comparing MS patients with healthy control subjects, we observed a remarkable reduction in IL-10 secretion and suppressive function in Tr1 cells (Astier et al. 2006). Notably, this observation only held true in Tr1 cells induced by anti-CD46 costimulation, but not under anti-CD28 stimulatory conditions; this only impacted IL-10 secretion but not CD46 surface expression, proliferation, or IFN-γ production (Astier et al. 2006). To investigate the effect of IFN-β treatment on Tr1 cells, we analyzed CD46-induced IL-10 secretion in RRMS patients with IFN-β therapy. Although IFN-β therapy was reported to increase IL-10 levels associated with remissions in RRMS patients, we did not observe restored Tr1 cell function in IFN-β-treated patients (Astier and Hafler 2007), although another study could identify slight changes in a subset of patients receiving IFN-β (Chiarini et al. 2012). Focusing on CD46, we were able to correlate the impaired Tr1 function in RRMS patients with altered isoform expressions of CD46. RRMS patients showed increased expression of the isoform Cyt2 (CD46.2), which carries a cytoplasmic tail that leads to proinflammatory responses and blocks IL-10 secretion (Marie et al. 2002; Astier et al. 2006; Choileain et al. 2011). The defective Tr1 response in RRMS was replicated in a larger cohort of patients, linking the reduced IL-10 induction to abnormal IL-10 signaling in MS patients (Martinez-Forero et al. 2008). In line with the functionally impaired Tr1 population in RRMS patients, similar findings were observed in a model of MS in cynomolgus monkeys (Ma et al. 2009). A recent study indicated that the defective CD46-triggered Tr1 response of RRMS patients could be partly restored in vitro in the presence of vitamin D3 (Kickler et al. 2012).
Th1-LIKE TREGS: MANY PATHS LEAD TO IFN-γ SECRETION
In terms of Treg functionality, we have observed several stimuli and pathways rendering Tregs dysfunctional, promoting IFN-γ secretion, and destabilizing Treg phenotype through Foxo1 phosphorylation. Although IFN-γ is clearly involved in decreased Treg function, suppressive capacity can be restored by blocking of IFN-γ (Dominguez-Villar et al. 2011), and different stimuli use distinct pathways to induce IFN-γ release.
We observed Th1-like Tregs in RRMS patients, as discussed above, but also observed this in vitro through IL-12 induction (Fig. 2A,B), and in culture conditions with sodium chloride (Fig. 2C), as well as in GBM (Sarbassov et al. 2005; Gonzalez and McGraw 2009; Kerdiles et al. 2010; Ouyang et al. 2010, 2012; Dominguez-Villar et al. 2011; Hernandez et al. 2015; Huynh et al. 2015; Lowther et al. 2016).
Figure 2.
In vitro induction of defective T helper (Th)1-like T regulatory cells (Tregs) from healthy donors using interleukin (IL)-12 or salt. (A) IL-12 induces interferon γ (IFN-γ) release in Tregs from healthy controls and multiple sclerosis (MS) patients, and (B) decreases suppressive function (Dominguez-Villar et al. 2011). (C) 40-mm sodium chloride significantly reduces Treg-suppressive function in Tregs isolated from healthy donors (Hernandez et al. 2015).
Th1-like Tregs generated through IL-12 stimulation, as well as in RRMS patients, are highly correlated with activation of the PI3K/AKT/Foxo1/3 pathway (Kitz et al. 2016). Activation of this pathway starts with different stimuli that trigger a variety of receptors such as the TCR (Ward et al. 1992), costimulatory molecules such as CD28 (Di Cristofano et al. 1999), cytokine receptors (Migone et al. 1998), G protein–coupled receptors (GCPRs) (Schwindinger and Robishaw 2001), and insulin (Knight et al. 2006), among others. The AKT family is highly multifunctional and the PI3K/AKT pathway is involved in various cell functions. PI3K has recently been shown to be crucial in regulating Treg homeostasis and stability (Huynh et al. 2015). The AKT family further consists of three protein isoforms, AKT1, AKT2, and AKT3, each mediating differential downstream signaling events (Gonzalez and McGraw 2009). After the initial stimulation, PI3K is activated through phosphorylation, causing it to catalyze the formation of the second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3) (Okkenhaug 2013). PIP3 binds to PDK1 and AKT, allowing PDK1 to phosphorylate the Thr 308 residue on AKT (Stokoe et al. 1997). For full activation, AKT also has to be phosphorylated at Ser 473 by the mTROC2 complex (Sarbassov et al. 2005). Activated AKT then has the ability to further phosphorylate different downstream targets involved in a variety of cell functions, including the transcription factors Foxo1 and Foxo3 (Brunet et al. 1999), which are involved in Treg development and phenotype stabilization (Kerdiles et al. 2010; Ouyang et al. 2010, 2012). On activation, these transcription factors are excluded from the nucleus and thus can no longer regulate their transcription targets (Kitz et al. 2016). Treg-specific deletion of Foxo1 in the nucleus of mice resulted in a loss of Treg function and ectopic expression of IFN-γ (Ouyang et al. 2012). Consistent with these results, we show that the PI3K/AKT/Foxo1/3 pathway plays a pivotal role in obtaining a Th1-like phenotype in human Tregs, and, interestingly, the AKT1 isoform specifically contributes to Th1 polarization (Kitz et al. 2016).
The critical result of the activation of this major pathway is the induction of IFN-γ secretion. This is directly correlated with reduced suppressive capacity of Tregs. In other words, increased frequency of IFN-γ+ FoxP3+ Tregs leads to severe impairment of suppressive capacity (Kitz et al. 2016). IFN-γ secretion by Tregs is a signature defect in immune regulation observed in patients with multiple sclerosis. After blockade of this major pathway in Tregs from MS patients, IFN-γ secretion was inhibited and the suppressive ability of these Tregs was restored. Thus, this experiment suggests that the PI3K/AKT/Foxo1/3 pathway was activated in vivo in RRMS patients. On PI3K or AKT1 inhibition, we saw inhibited IFN-γ secretion in RRMS Tregs, but no effect on Tregs from healthy controls (Kitz et al. 2016). This further supports that the Th1-Treg phenotype observed in Tregs from RRMS is, at least in part, the result of an in vivo activation of this major pathway, which can be corrected by PI3K or AKT1 blockade.
Dietary salt has recently been linked to a variety of diseases and is discussed as an environmental factor contributing to the increase and clinical manifestation of multiple sclerosis (Farez et al. 2015; Hucke et al. 2016). The increase of dietary salt intake has been linked to increased sodium accumulation in skin, muscle, and lymphoid tissues (Go et al. 2004; Kopp et al. 2013; Wiig et al. 2013). The impact of increased tissue sodium levels on immune cells has been the focus of research. In recent studies, we and others showed that physiologic sodium concentrations directly favor the induction of Th17 T cells and initiate an inflammatory signature in human T cells in vitro (Kleinewietfeld et al. 2013), as well as in EAE in vivo where a high salt diet induced aggravated disease pathogenesis (Wu et al. 2013). Both human salt-induced Th17 T cells and Th17 T cells obtained from mice with EAE showed elevated expression of the serum- and glucocorticoid-regulated kinase-1 (SGK1), a serine/threonine protein kinase involved in the regulation of the natrium channel ENaC (Kuntzsch et al. 2012; Lang and Shumilina 2013). We then investigated the effect of sodium chloride treatment on Tregs and discovered that sodium chloride renders Tregs dysfunctional (Fig. 2C) and drives them toward a Th1-like phenotype both in vitro and in a mouse model of GvHD (Hernandez et al. 2015). Sodium-treated FoxP3+ Tregs up-regulated T-bet expression, released higher amounts of IFN-γ, and displayed higher levels of Foxo1 phosphorylation. Fitting our observations in salt-induced Th17 effector T cells, sodium-treated FoxP3+ Tregs expressed elevated levels of SGK1, emphasizing its role in salt-induced Treg dysfunction. Interference of the SGK1-IFN-γ axis by pharmacological inhibitors, or by lentiviral transmitted short hairpin RNA (shRNA) against either SGK1 or IFN-γ, could, in turn, restore Treg-suppressive function (Go et al. 2004; Kuntzsch et al. 2012; Kopp et al. 2013; Lang and Shumilina 2013; Wiig et al. 2013; Farez et al. 2015; Hucke et al. 2016; Jorg et al. 2016).
In cancer therapy, immunotherapies targeting T-cell surface markers have recently shown promising results. Monoclonal antibodies targeting CTLA-4 (Leach et al. 1996; Phan et al. 2003) and PD-1 (Brahmer et al. 2012; Topalian et al. 2012) are successful in modulating tumor immune responses in malignant tumors and improve clinical outcomes (Sznol and Chen 2013). PD-1 is expressed on a subset of tumor-infiltrating Tregs in the context of cancers in lung (Zhang et al. 2010) and brain (Jacobs et al. 2009). However, the function of these Tregs is largely unpredicted, as PD-1 can be involved both in Tregs activation and exhaustion. We were recently able to further characterize tumor-infiltrating Tregs in the context of glioblastoma multiforme (GBM) and observed that PD-1+ FoxP3+ Tregs from the circulation of both GBM patients and healthy controls show a reduction in suppressive capacity (Lowther et al. 2016) and release higher levels of IFN-γ (Fig. 3A,B). As we have seen in other circumstances, dysfunctional PD-1+ FoxP3+ Tregs produced elevated levels of IFN-γ (Fig. 3C,D), and blocking of IFN-γ could partially restore suppressive function. Additionally, PD-1+ FoxP3+ Tregs showed increased phosphorylation of Foxo1, indicating an increased activity of the PI3K/AKT pathway and subsequent destabilization of the Treg phenotype. On the other hand, circulating PD-1+ FoxP3+ Tregs expressed elevated level of exhaustion markers. Further, transcriptional profiling of tumor-infiltrating PD-1+ FoxP3+ Tregs identified even higher mRNA levels of exhaustion markers and coexpression of IFN-γ. PD-1 is usually a suppressor of the PI3K/AKT pathway so that increased PD-1 expression and exhaustion may occur to regulate and stabilize the PI3K/AKT pathways. Additionally, Foxo1 phosphorylation was recently shown to be associated with migration of Tregs into tissues, arguing that tumor-infiltrating PD-1+ FoxP3+ Tregs recirculate from the tumor tissue (Luo et al. 2016).
Figure 3.
Dysfunctional T helper (Th)1-like T regulatory cells (Tregs) in tumors. (A) Tregs isolated from tumor biopsies release high levels of interferon γ (IFN-γ), and (B) are less suppressive (Lowther et al. 2016). (C) PD-1hi Tregs isolated from healthy controls produce higher levels of IFN-γ, which correlates with (D) lower suppressive capacity (Lowther et al. 2016).
CONCLUDING REMARKS
In summary, there is clear evidence that CD4+ CD25+ Treg cells play a crucial role in autoimmune disease, specifically MS. The genetic factors related to MS are directly or indirectly linked to Treg cell phenotype and function. Plasticity of Tregs toward Th1 and Th2 phenotypes is a significant factor in MS pathogenesis. During Th1-Treg reprogramming, the PI3K/AKT/Foxo1/3 pathway is activated, triggering IFN-γ secretion. This consequentially reduces the suppressive capacity of Tregs. Environmental factors can also contribute to defective immune function. Specifically, we have seen that salt can enhance proinflammatory Th17 activity (Fig. 4) (Kleinewietfeld et al. 2013), while disrupting Treg-suppressive activity (Hernandez et al. 2015). Factors that regulate plasticity in these cell populations will be critical for developing immunotherapies for autoimmune diseases and, reciprocally, for cancer.
Figure 4.
The balance between tolerance and autoimmunity. Differentiation of regulatory T cells (Tregs) into dysfunctional T helper (Th)1-like Tregs enables autoimmunity. Treg cells express coinhibitory molecules glucocorticoid-induced tumor necrosis factor (TNF) receptor–related protein (GITR), LAG3, and cytotoxic T-lymphocyte antigen 4 (CTLA4) and release, among others, interleukin (IL)-10 to regulate effector T cells and maintain peripheral tolerance. Environmental factors (i.e., salt or the proinflammatory cytokine IL-12) can trigger Treg differentiation into IFN-γ-producing Th1-like Tregs, which up-regulate other Th1 effector markers like CXCR3, T-bet, and ICOS and lose capacity to suppress autoreactive effector T cells, leading to autoimmunity.
The available data on Treg cells in MS is complex, indicating that several mechanisms and subset populations could contribute to the observed effects of defective Treg function and its association to the disease. There is an exponential combination of genetic and environmental factors that can interact to contribute to disease development. The role of the gut microbiota in the induction, maintenance, and function of Treg cells is under preliminary investigation. It was shown that both Bacteroides fragilis and Clostridial species, which can be found in the microbiome, produce short-chain fatty acids, inducing Treg responses (Round and Mazmanian 2010; Rudensky and Chervonsky 2011). Additionally, future research to study the Treg-cell phenotype and function in the CNS or CSF would be necessary for further clarity on the role of CD4+ CD25+ Treg cells in MS.
Footnotes
Editors: Howard L. Weiner and Vijay K. Kuchroo
Additional Perspectives on Multiple Sclerosis available at www.perspectivesinmedicine.org
REFERENCES
- Akdis M. 2008. T cell tolerance to inhaled allergens: Mechanisms and therapeutic approaches. Expert Opin Biol Ther 8: 769–777. [DOI] [PubMed] [Google Scholar]
- Arterbery AS, Osafo-Addo A, Avitzur Y, Ciarleglio M, Deng Y, Lobritto SJ, Martinez M, Hafler DA, Kleinewietfeld M, Ekong UD. 2016. Production of proinflammatory cytokines by monocytes in liver-transplanted recipients with de novo autoimmune hepatitis is enhanced and induces TH1-like regulatory T cells. J Immunol 196: 4040–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Hafler DA. 2007. Abnormal Tr1 differentiation in multiple sclerosis. J Neuroimmunol 191: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. 2006. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest 116: 3252–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Malefijt RD, Yssel H, Abrams J, Devries JE, Spits H, Roncarolo MG. 1990. Host-reactive CD4+ and CD8+ T-cell clones isolated from a human chimera produce IL-5, IL-2, IFN-γ- and granulocyte macrophage-colony-stimulating factor but not IL-4. J Immunol 144: 902–908. [PubMed] [Google Scholar]
- Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, Malefyt RD, Devries JE, Roncarolo MG. 1994. High-levels of interleukin-10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem-cells. J Exp Med 179: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Sartirana C, Levings MK, Bordignon C, Narula S, Roncarolo MG. 2002. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur J Immunol 32: 2237–2245. [DOI] [PubMed] [Google Scholar]
- Bach JF. 2001. Non-Th2 regulatory T-cell control of Th1 autoimmunity. Scand J Immunol 54: 21–29. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. 2001. CD4+CD25high regulatory cells in human peripheral blood. J Immunol 167: 1245–1253. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Wolf E, Haller DA. 2006. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol 176: 4622–4631. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan CM, Costantino CM, Cvetanovich GL, Ashley CW, Beriou G, Dominguez-Villar M, Hafler DA. 2011. CD2 costimulation reveals defective activity by human CD4+CD25hi regulatory cells in patients with multiple sclerosis. J Immunol 186: 3317–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, et al. 2007. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol 37: 2378–2389. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–21. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. 2007. Expansion and characteristics of human T regulatory type 1 cells in co-cultures simulating tumor microenvironment. Cancer Immunol Immunother 56: 1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. 2009. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 113: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhairavabhotla R, Kim YC, Glass DD, Escobar TM, Patel MC, Zahr R, Nguyen CK, Kilaru GK, Muljo SA, Shevach EM. 2016. Transcriptome profiling of human FoxP3+ regulatory T cells. Hum Immunol 77: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, et al. 2012. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med 4: 164ra159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Tang Q. 2005. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol 17: 638–642. [DOI] [PubMed] [Google Scholar]
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hoepner S, Centonze D, Bernardi G, Dell’Acqua ML, et al. 2007. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 110: 1225–1232. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJPM. 2011. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol 131: 1853–1860. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun V, Bastian H, Neveu V, Foussat A. 2009. Clinical grade production of IL-10 producing regulatory Tr1 lymphocytes for cell therapy of chronic inflammatory diseases. Int Immunopharmacol 9: 609–613. [DOI] [PubMed] [Google Scholar]
- Brun V, Neveu V, Pers YM, Fabre S, Quatannens B, Bastian H, Clerget-Chossat N, Jorgensen C, Foussat A. 2011. Isolation of functional autologous collagen-II specific IL-10 producing Tr1 cell clones from rheumatoid arthritis blood. Int Immunopharmacol 11: 1074–1078. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. 1999. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96: 857–868. [DOI] [PubMed] [Google Scholar]
- Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A, Hoefer T. 2010. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci 107: 3058–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao AT, Yao S, Stefka AT, Liu Z, Qin H, Liu H, Evans-Marin HL, Elson CO, Nagler CR, Cong Y. 2014. TLR4 regulates IFN-γ and IL-17 production by both thymic and induced Foxp3+ Tregs during intestinal inflammation. J Leukoc Biol 96: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudensky AY. 2013. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest 123: 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. 1994. Regulatory T-cell clones induced by oral tolerance. Suppression of autoimmune encephalomyelitis. Science 265: 1237–1240. [DOI] [PubMed] [Google Scholar]
- Chen X, Subleski JJ, Kopf H, Howard OMZ, Mannel DN, Oppenheim JJ. 2008. Expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: Applicability to tumor-infiltrating T regulatory cells. J Immunol 180: 6467–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Subleski JJ, Hamano R, Howard OMZ, Wiltrout RH, Oppenheim JJ. 2010. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol 40: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini M, Serana F, Zanotti C, Capra R, Rasia S, Rottoli M, Rovaris M, Caputo D, Cavaletti G, Frigo M, et al. 2012. Modulation of the central memory and Tr1-like regulatory T cells in multiple sclerosis patients responsive to interferon-β therapy. Mult Scler J 18: 788–798. [DOI] [PubMed] [Google Scholar]
- Choileain SN, Weyand NJ, Neumann C, Thomas J, So M, Astier AL. 2011. The dynamic processing of CD46 intracellular domains provides a molecular rheostat for T cell activation. Plos ONE 6: e16287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Baecher-Allan C, Maier LM, Arthur AT, Ottoboni L, Barcellos L, McCauley JL, Sawcer S, Goris A, Saarela J, et al. 2009. The role of the CD58 locus in multiple sclerosis. Proc Natl Acad Sci 106: 5264–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafaille MAC, Lafaille JJ. 2009. Natural and adaptive Foxp3+ regulatory T cells: More of the same or a division of labor? Immunity 30: 626–635. [DOI] [PubMed] [Google Scholar]
- de la Rosa M, Rutz S, Dorninger H, Scheffold A. 2004. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 34: 2480–2488. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. 1999. Impaired Fas response and autoimmunity in Pten+/− mice. Science 285: 2122–2125. [DOI] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med 193: 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. 2002. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 1-producing, contact-independent type 1-like regulatory T cells. J Exp Med 196: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GA, Bai A, Hanidziar D, Longhi MS, Lawlor GO, Putheti P, Csizmadia E, Nowak M, Cheifetz AS, Moss AC, et al. 2012. CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur J Immunol 42: 3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Villar M, Baecher-Allan CM, Hafler DA. 2011. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med 17: 673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Huang C, Zhou B, Ziegler SF. 2008. Isoform-specific inhibition of ROR α-mediated transcriptional activation by human FOXP3. J Immunol 180: 4785–4792. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 4: 1206–1212. [DOI] [PubMed] [Google Scholar]
- Farez MF, Fiol MP, Gaitan MI, Quintana FJ, Correale J. 2015. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 86: 26–31. [DOI] [PubMed] [Google Scholar]
- Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. 2007. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol 147: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Cao AT, Weaver CT, Elson CO, Cong YZ. 2011. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis. Gastroenterology 140: 2031–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KHG. 2009. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 183: 7602–7610. [DOI] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang H-D, Bopp T, Schmitt E, et al. 2007. Epigenetic control of the foxp3 locus in regulatory T cells. Plos Biol 5: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336. [DOI] [PubMed] [Google Scholar]
- Frisullo G, Nociti V, Iorio R, Patanella AK, Caggiula M, Marti A, Sancricca C, Angelucci F, Mirabella M, Tonali PA, et al. 2009. Regulatory T cells fail to suppress CD4+ T-bet+ T cells in relapsing multiple sclerosis patients. Immunology 127: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. 2006. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci 103: 6659–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go WY, Liu XB, Roti MA, Liu F, Ho SN. 2004. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci 101: 10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. 2009. The Akt kinases isoform specificity in metabolism and cancer. Cell Cycle 8: 2502–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. 2004a. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21: 589–601. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. 2004b. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 104: 2840–2848. [DOI] [PubMed] [Google Scholar]
- Groux H, Ogarra A, Bigler M, Rouleau M, Antonenko S, deVries JE, Roncarolo MG. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389: 737–742. [DOI] [PubMed] [Google Scholar]
- Haas J, Hug A, Viehover A, Fritzsching B, Falk CS, Filser A, Vetter T, Milkova L, Korporal M, Fritz B, et al. 2005. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol 35: 3343–3352. [DOI] [PubMed] [Google Scholar]
- Haas J, Fritzsching B, Truebswetter P, Korporal M, Milkova L, Fritz B, Vobis D, Krammer PH, Suri-Payer E, Wildemann B. 2007. Prevalence of newly generated naïve regulatory T cells (T-reg) is critical for T-reg suppressive function and determines T-reg dysfunction in multiple sclerosis. J Immunol 179: 1322–1330. [DOI] [PubMed] [Google Scholar]
- Haeringer B, Lozza L, Steckel B, Geginat J. 2009. Identification and characterization of IL-10/IFN-γ-producing effector-like T cells with regulatory function in human blood. J Exp Med 206: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PIW, Gabriel SB, Mirel DB, Ivinson AJ, et al. 2007. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357: 851–862. [DOI] [PubMed] [Google Scholar]
- Hamano R, Huang JQ, Yoshimura T, Oppenheim JJ, Chen X. 2011. TNF optimally activatives regulatory T cells by inducing TNF receptor superfamily members TNFR2, 4–1BB and OX40. Eur J Immunol 41: 2010–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, et al. 2015. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 125: 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. 2006. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 108: 4260–4267. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. 2009. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol 39: 1088–1097. [DOI] [PubMed] [Google Scholar]
- Holmen N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjovall H, Ohman L. 2006. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis 12: 447–456. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Hovhannisyan Z, Treatman J, Littman DR, Mayer L. 2011. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology 140: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Lee HM, Lio CWJ. 2012. Selection of regulatory T cells in the thymus. Nat Rev Immunol 12: 157–167. [DOI] [PubMed] [Google Scholar]
- Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA. 2005. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res 81: 45–52. [DOI] [PubMed] [Google Scholar]
- Hucke S, Wiendl H, Klotz L. 2016. Implications of dietary salt intake for multiple sclerosis pathogenesis. Mult Scler J 22: 133–139. [DOI] [PubMed] [Google Scholar]
- Huehn J, Polansky JK, Hamann A. 2009. Epigenetic control of FOXP3 expression: The key to a stable regulatory T-cell lineage? Nat Rev Immunol 9: 83–89. [DOI] [PubMed] [Google Scholar]
- Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, et al. 2015. Control of PI(3) kinase in T-reg cells maintains homeostasis and lineage stability. Nat Immunol 16: 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Hanabuchi S, Wang Y-H, Park WR, Arima K, Bover L, Qin FX-F, Gilliet M, Liu Y-J. 2008. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28: 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JFM, Idema AJ, Bol KF, Nierkens S, Grauer OM, Wesseling P, Grotenhuis JA, Hoogerbrugge PM, de Vries IJM, Adema GJ. 2009. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol 11: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. 2002. Infectious tolerance: Human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J Exp Med 196: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorg S, Kissel J, Manzel A, Kleinewietfeld M, Haghikia A, Gold R, Muller DN, Linker RA. 2016. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol 279: 212–222. [DOI] [PubMed] [Google Scholar]
- Kerdiles YM, Stone EL, Beisner DL, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. 2010. Foxo transcription factors control regulatory T cell development and function. Immunity 33: 890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 4: 337–342. [DOI] [PubMed] [Google Scholar]
- Kickler K, Choileain SN, Williams A, Richards A, Astier AL. 2012. Calcitriol modulates the CD46 pathway in T cells. Plos ONE 7: e48486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitz A, de Marcken M, Gautron AS, Mitrovic M, Hafler DA, Dominguez-Villar M. 2016. AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease. EMBO Rep 17: 1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Jovanovic K. 2011. Regulatory T cell lineage commitment in the thymus. Semin Immunol 23: 401–409. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Hafler DA. 2013. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol 25: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. 2005. CCR6 expression defines regulatory effector/memory-like cells within the CD25+CD4+ T-cell subset. Blood 105: 2877–2886. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Roetzschke O, Falk K. 2009. CD49d provides access to “untouched” human Foxp3+ Treg free of contaminating effector cells. Blood 113: 827–836. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. 2013. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, et al. 2006. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 125: 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard Gs, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. 2009. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. 2012. T-bet+ treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β 2. Immunity 37: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Takayanagi H. 2015. Arthritogenic T cells in autoimmune arthritis. Int J Biochem Cell Biol 58: 92–96. [DOI] [PubMed] [Google Scholar]
- Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, et al. 2013. Na-23 magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61: 635. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao WD, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, et al. 2007. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 13: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 cells. Ann Rev Immunol 27: 485–517. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, et al. 2011. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 186: 4388–4395. [DOI] [PubMed] [Google Scholar]
- Kumar M, Putzki N, Limmroth V, Remus R, Lindemann M, Knop D, Mueller N, Hardt C, Kreuzfelder E, Grosse-Wilde H. 2006. CD4+CD25+FoxP3+ T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J Neuroimmunol 180: 178–184. [DOI] [PubMed] [Google Scholar]
- Kuntzsch D, Bergann T, Dames P, Fromm A, Fromm M, Davis RA, Melzig MF, Schulzke JD. 2012. The plant-derived glucocorticoid receptor agonist Endiandrin A acts as co-stimulator of colonic epithelial sodium channels (ENaC) via SGK-1 and MAPKs. Plos ONE 7: e49426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Shumilina E. 2013. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J 27: 3–12. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. 1996. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. 2005. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood 105: 1162–1169. [DOI] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. 2006. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25: 455–471. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther DE, Hafler DA. 2012. Regulatory T cells in the central nervous system. Immunol Rev 248: 156–169. [DOI] [PubMed] [Google Scholar]
- Lowther DE, Goods BA, Lucca LE, Lerner BA, Raddassi K, van Dijk D, Hernandez AL, Duan XG, Gunel M, Coric V, et al. 2016. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight 1: e85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. 2010. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142: 914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CT, Liao W, Dadi S, Toure A, Li MO. 2016. Graded Foxo1 activity in T-reg cells differentiates tumour immunity from spontaneous autoimmunity. Nature 529: 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Xiong Z, Hu Y, Qi S, Song L, Dun H, Zhang L, Lou D, Yang P, Zhao Z, et al. 2009. Dysfunction of IL-10-producing type 1 regulatory T cells and CD4+CD25+ regulatory T cells in a mimic model of human multiple sclerosis in cynomolgus monkeys. Int Immunopharmacol 9: 599–608. [DOI] [PubMed] [Google Scholar]
- Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. 2002. Linking innate and acquired immunity: Divergent role of CD46 cytoplasmic domains in T cell-induced inflammation. Nat Immunol 3: 659–666. [DOI] [PubMed] [Google Scholar]
- Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, Inoges S, de Cerio ALD, Palacios R, Sepulcre J, Moreno B, Gonzalez Z, Fernandez-Diez B, et al. 2008. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol 38: 576–586. [DOI] [PubMed] [Google Scholar]
- McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmuller U, Baron U, Olek S, Bluestone JA, et al. 2011. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 186: 3918–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. 2004. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol 4: 762–774. [DOI] [PubMed] [Google Scholar]
- Migone TS, Rodig S, Cacalano NA, Berg M, Schreiber RD, Leonard WJ. 1998. Functional cooperation of the interleukin-2 receptor β chain and Jak1 in phosphatidylinositol 3-kinase recruitment and phosphorylation. Mol Cell Biol 18: 6416–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30: 899–911. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, et al. 2002. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297: 1867–1870. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. 2004a. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 114: 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Mellor AL. 2004b. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 172: 4100–4110. [DOI] [PubMed] [Google Scholar]
- Nylander A, Hafler DA. 2012. Multiple sclerosis. J Clin Invest 122: 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K. 2013. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol 31: 675–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, et al. 2009. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31: 772–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. 2008. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol 38: 3274–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma QA, Paik JH, DePinho RA, Li MO. 2010. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol 11: U618–U691. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, et al. 2012. Novel Foxo1-dependent transcriptional programs control T-reg cell function. Nature 491: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8: 1353–1362. [DOI] [PubMed] [Google Scholar]
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. 2003. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci 100: 8372–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconese S, Timperi E, Barnaba V. 2014a. “Hardcore” OX40+ immunosuppressive regulatory T cells in hepatic cirrhosis and cancer. Oncoimmunology 3: e29257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconese S, Timperi E, Pacella I, Schinzari V, Tripodo C, Rossi M, Guglielmo N, Mennini G, Grazi GL, Di Filippo S, et al. 2014b. Human OX40 tunes the function of regulatory T cells in tumor and nontumor areas of hepatitis C virus-infected liver tissue. Hepatology 60: 1494–1507. [DOI] [PubMed] [Google Scholar]
- Putheti P, Soderstrom M, Link H, Huang YM. 2003. Effect of glatiramer acetate (Copaxone) on CD4+CD25high3 T regulatory cells and their IL-10 production in multiple sclerosis. J Neuroimmunol 144: 125–131. [DOI] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. 2011. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 332: 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph DA, Fathman CG. 2006. CD4+CD25+ regulatory T cells and their therapeutic potential. Annu Rev Med 57: 381–402. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. 2001. Type 1 T regulatory cells. Immunol Rev 182: 68–79. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. 2006. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 212: 28–50. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci 107: 12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, Bonelli M, Sciume G, Zare H, Vahedi G, Dema B, et al. 2013. BACH2 represses effector programs to stabilize T-reg-mediated immune homeostasis. Nature 498: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AY, Chervonsky AV. 2011. A narrow circle of mutual friends. Immunity 34: 697–699. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. 1995. Immunological self-tolerance maintained by activated T-cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune-diseases. J Immunol 155: 1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. 2006. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212: 8–27. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133: 775–787. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. 2010. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10: 490–500. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101. [DOI] [PubMed] [Google Scholar]
- Sawcer S, Hellenthal G, Pirinen M, Spencer CCA, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, et al. 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindinger WF, Robishaw JD. 2001. Heterotrimeric G-protein βγ-dimers in growth and differentiation. Oncogene 20: 1653–1660. [DOI] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellebjerg F, Krakauer M, Khademi M, Olsson T, Sorensen PS. 2012. FOXP3, CBLB and ITCH gene expression and cytotoxic T lymphocyte antigen 4 expression on CD4+CD25high T cells in multiple sclerosis. Clin Exp Immunol 170: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LA, Mottet C, Mason C, Powrie F. 2001. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol 31: 1247–1254. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PRJ, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277: 567–570. [DOI] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Whiteside TL. 2009. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol 182: 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznol M, Chen L. 2013. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 19: 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi G, Poholek AC, Hand TW, Laurence A, Kanno Y, O’Shea JJ, Hirahara K. 2013. Helper T-cell identity and evolution of differential transcriptomes and epigenomes. Immunol Rev 252: 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. 2008a. Natural naïve CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: Recovery of memory treg homeostasis during disease progression. J Immunol 180: 6411–6420. [DOI] [PubMed] [Google Scholar]
- Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens J-L, Medaer R, Hupperts R, Stinissen P. 2008b. Compromised CD4+ CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 123: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. 2004. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 199: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DAA, Collison LW, Workman CJ. 2008. How regulatory T cells work. Nat Rev Immunol 8: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, et al. 2009. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci 106: 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakkach A, Cottrez F, Groux H. 2001. Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J Immunol 167: 3107–3113. [DOI] [PubMed] [Google Scholar]
- Ward SG, Ley SC, Macphee C, Cantrell DA. 1992. Regulation of D-3 phosphoinositides during T-cell activation via the T-cell antigen receptor CD3 complex and CD2 antigens. Eur J Immunol 22: 45–49. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. 2009. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30: 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Schroeder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, et al. 2013. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123: 2803–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322: 271–275. [DOI] [PubMed] [Google Scholar]
- Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. 2013. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang S, Gong D, Qin Y, Shen Q. 2010. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol 7: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. 2009. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. 2008. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]