Abstract
Prostate cancer is a major cause of disease and mortality among men, and each year 1.6 million men are diagnosed with and 366,000 men die of prostate cancer. In this review, we discuss the state of evidence for specific genetic, lifestyle, and dietary factors associated with prostate cancer risk. Given the biological heterogeneity of this cancer, we focus on risk factors for advanced or fatal prostate cancer. First, we provide descriptive epidemiology statistics and patterns for prostate cancer incidence and mortality around the world. This includes discussion of the impact of prostate-specific antigen screening on prostate cancer epidemiology. Next, we summarize evidence for selected risk factors for which there is strong or probable evidence of an association: genetics, obesity and weight change, physical activity, smoking, lycopene and tomatoes, fish, vitamin D and calcium, and statins. Finally, we highlight future directions for prostate cancer epidemiology research.
DESCRIPTIVE EPIDEMIOLOGY OF PROSTATE CANCER
The global burden of prostate cancer is substantial, ranking among the top five cancers for both incidence and mortality (Ferlay et al. 2015). Prostate cancer is characterized by striking geographical variation in both incidence and mortality rates. An examination of patterns in prostate cancer incidence and mortality across populations and over time provides insights into the role of individual risk factors and population screening behaviors in the epidemiology of this disease.
Incidence
Globally, prostate cancer is the most commonly diagnosed cancer in men, with approximately 1.6 million incident cases in 2015 (Global Burden of Disease Cancer Collaboration 2016). Prostate cancer is particularly common in developed countries. The odds of prostate cancer diagnosis by age 79 years are one in 47 among countries with a low–middle sociodemographic index, compared with one in six among countries with a high sociodemographic index (Global Burden of Disease Cancer Collaboration 2016). In the United States, prostate cancer is the leading cause of incident cancer and it is estimated that 180,890 new cases were diagnosed in 2016 (Howlader et al. 2016).
Prostate cancer incidence is notable for its substantial global variation (Fig. 1). There is a 40-fold difference in age-adjusted incidence rates between men with the highest (African-American men in the United States) and lowest (Asian men living in their native countries) incidence (Ferlay et al. 2013; Howlader et al. 2016). In part, this variation in incidence rates across populations can be attributed to differences in diagnostic intensity arising from the practice of prostate-specific antigen (PSA) screening. However, evidence of geographic variation in prostate cancer incidence predating the introduction of PSA screening highlights a potential role of lifestyle factors in disease risk. Furthermore, evidence from migration studies provides support for a role of lifestyle factors. For example, prostate cancer incidence and mortality rates increase among men who migrate from low-risk (e.g., Asia) to high-risk (e.g., United States) countries compared with those in their native countries, although rates remain below the host countries’ rates (Shimizu et al. 1991; Yu et al. 1991).
Figure 1.
Age-adjusted prostate cancer incidence rates worldwide. Rates are age-adjusted for comparisons across countries and are presented per 100,000 in the population. Gray, no data available. (Figure based on data from Globocan 2012 [globocan.iarc.fr].)
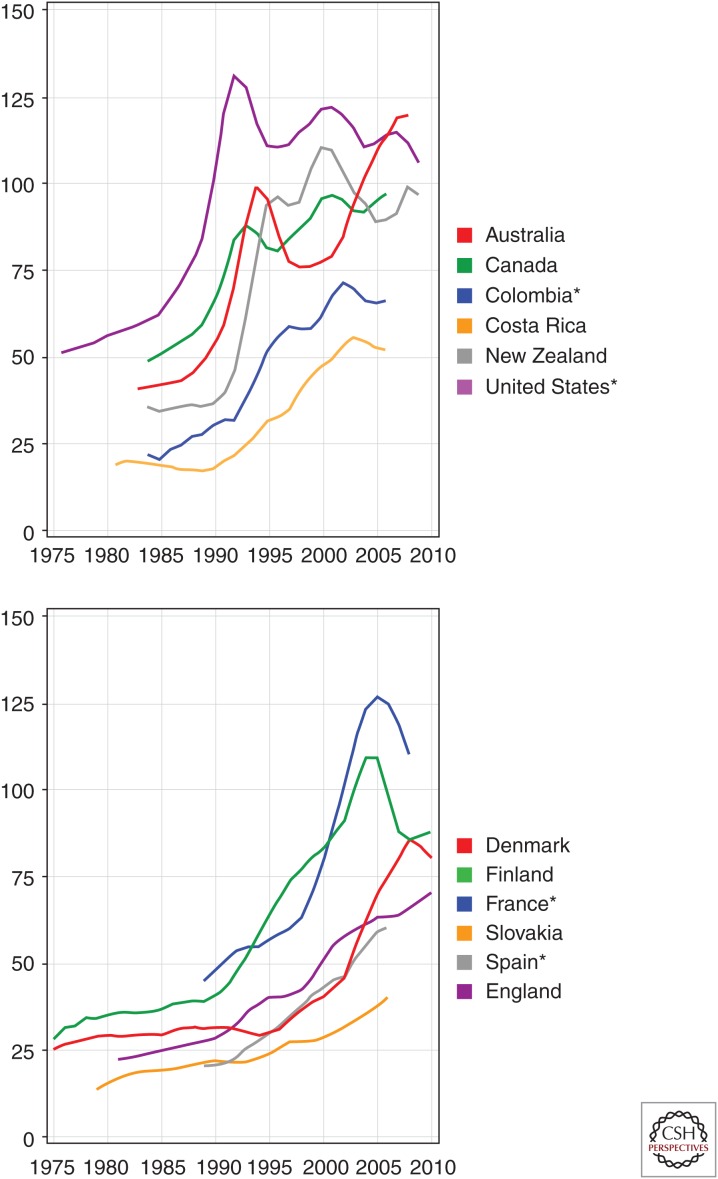
Global patterns of change in incidence rates over time show the impact of PSA screening on prostate cancer epidemiology. During the past 40 years, age-adjusted incidence rates have generally increased across the world (Fig. 2). Notably, this increasing trend has paralleled the uptake of PSA screening in certain regions, such as the United States, Europe, and Australia. In the United States, for example, a large peak in prostate cancer incidence is observed in the early 1990s when PSA screening is first introduced at the population level. The emergence of PSA screening has also led to a shift in the stage at diagnosis, with a higher proportion of men diagnosed with localized disease. Moreover, because of the lead time associated with prostate cancer, estimated to be 3 to 10 years, men are being diagnosed at an earlier age (Etzioni et al. 2008). A further consequence of PSA screening has been an increasing incidence of cancers considered to be overdiagnosis (i.e., cancers that would not have come to light clinically nor led to mortality in the absence of screening) (Etzioni et al. 2002; Ciatto et al. 2005). However, incidence rates have also increased in regions where PSA testing has not yet been widely used, such as in Japan and some other Asian and Eastern European countries (Jemal et al. 2010). The trend in these regions suggests that environmental or lifestyle factors, as discussed later in this chapter, may also influence prostate cancer incidence.
Figure 2.
Trends over time in age-adjusted prostate cancer incidence rates (per 100,000). *, Regional data. (Figure based on data from Globocan 2012 [globocan.iarc.fr].)
Mortality
Prostate cancer is the fifth most common cause of cancer death globally, accounting for an estimated 366,000 deaths and 6.3 million disability-adjusted life years in 2015 (Global Burden of Disease Cancer Collaboration 2016). Compared with prostate cancer incidence rates, there is less global variation in mortality rates, with an approximately 10-fold difference across countries (Fig. 3). Global mortality patterns differ from incidence also in that less-developed regions experience higher mortality caused by prostate cancer than more developed regions. The highest prostate cancer mortality rates are among populations in the Caribbean and in Middle and Southern Africa. In contrast, the lowest prostate cancer mortality rates are observed in Asia, particularly in Eastern and South-Central Asia. Prostate cancer is the second most common cause of cancer death among men in the United States, with 26,120 cancer deaths expected in 2016 (Howlader et al. 2016). There have been notable reductions in prostate cancer mortality across a number of Westernized countries including the United States. The reasons underlying this decrease are unclear. However, it is likely that part of the reduction can be explained through the identification of prostate cancer earlier through PSA screening and the resulting earlier treatment (Chu et al. 2003). It is notable that some countries with low or no screening are experiencing increased prostate mortality such as in Africa.
Figure 3.
Age-adjusted prostate cancer mortality rates worldwide. Rates are age-adjusted for comparisons across countries and are presented per 100,000 in the population. Gray, no data available. (Figure based on data from Globocan 2012 [globocan.iarc.fr].)
Changes in mortality are a result of both changes in the incidence of prostate cancer as well as survival among patients. There is considerable variability in the ratio of incidence to mortality, with the highest ratio in North America (10:1), lower in Australia (2:1), and almost equal in some countries in the Caribbean and parts of Africa (1.2:1). These differences may be explained in part by a larger proportion of slow-growing cancers diagnosed in countries with PSA screening (Johansson et al. 2004; Albertsen et al. 2005) and, conversely, by later presentation of disease in countries with lower diagnostic intensity. The magnitude of burden attributable to prostate cancer is reflected also in the high prevalence of this disease. As a consequence of its high incidence and long survival, prostate cancer has the highest 5-year prevalence of any cancer type, accounting for 25% of all prevalent cancers (Ferlay et al. 2013). More than four million men are prostate cancer survivors living with a cancer diagnosis around the world, of whom 2.7 million are in the United States (SEER Cancer Statistics Review 1975–2008 2011). This has important implications for the allocation of resources for men who are undergoing treatment or surveillance for this disease.
As shown, several important characteristics of prostate cancer epidemiology can be gleaned from examining incidence and mortality rates across regions and over time. The evidence for specific risk factors associated with prostate cancer will be discussed in detail in the following sections.
RISK FACTORS
Epidemiologic studies of prostate cancer have revealed numerous ways in which individual biology and lifestyle factors influence risk of developing prostate cancer and survival from this disease (Tables 1 and 2). Although many questions remain about the etiology of this common disease, our current understanding of risk factors points to ways to identify individuals at high risk and use behavior change to reduce the burden of disease. As discussed in the previous section, prostate cancer is a clinically heterogeneous disease. Whereas some men have an aggressive form of prostate cancer, most others have a slow-growing or indolent form of disease. This clinical heterogeneity is reflected also in the underlying etiology of this disease. As detailed in the sections below, several risk factors show different associations for indolent as compared with lethal disease (Jahn et al. 2015). Thus, it is imperative in prostate cancer epidemiology to differentiate between risk factors for total prostate cancer and for advanced or fatal disease.
Table 1.
Summary of evidence for selected risk factors of total prostate cancer
| Risk factor | Strength of evidence |
|---|---|
| Increased risk | |
| Older age | Strong |
| African descent | Strong |
| Family history | Strong |
| Genetic risk loci | Strong |
| Taller height | Probable |
Table 2.
Summary of evidence for selected risk factors of advanced or lethal prostate cancer
| Risk factor | Strength of evidence |
|---|---|
| Increased risk | |
| Taller height | Strong |
| Lipid levels | Possible |
| Obesity | Strong |
| Smoking | Strong |
| Dairy, calcium | Possible |
| Decreased risk | |
| Physical activity | Strong |
| Coffee | Limited |
| Tomatoes | Probable |
| Fish | Possible |
| Vitamin D | Possible |
| Statins | Probable |
To evaluate evidence for prostate cancer risk factors, the role of PSA screening in epidemiologic studies must also be considered given its potential to influence the observed associations between risk factors and prostate cancer. On the one hand, risk factors may influence prostate cancer across the pathogenesis of the disease, from cancer initiation to metastases to death. Thus, the association between a factor and prostate cancer risk may differ according to clinical features of the disease, such as stage or tumor grade (Giovannucci et al. 2007). Indeed, it seems biologically plausible that risk factors for prostate cancer overall would differ from those for more aggressive prostate cancer. Moreover, PSA testing can have a potentially confounding effect, because men who engage in regular screening tend to also be healthier in general, and independently with prostate cancer diagnosis. When one is evaluating epidemiological studies in prostate cancer, it is critical to investigate the extent to which a study integrates information on PSA screening.
Risk Factors for Total Prostate Cancer
Established risk factors for total prostate cancer incidence are limited to older age, African-American race, and positive family history of prostate cancer. More recently, genome-wide association studies (GWAS) have provided additional evidence of genetic predisposition to prostate cancer. In populations with ethnically diverse ancestry, more than 180 genetic risk loci have been confirmed (Eeles et al. 2013; Al Olama et al. 2014). Additionally, there is probable evidence that taller height increases risk of total prostate cancer (MacInnis and English 2006). Although these factors are not modifiable, they are illustrative of the possible mechanisms involved in prostate carcinogenesis and could be used to identify individuals at increased risk of developing this disease (risk stratification).
Age is strongly associated with risk of total prostate cancer. Prostate cancer is rare among men younger than 40 years of age. The incidence rate of prostate cancer increases dramatically after 55 years of age, following a similar trend as other epithelial cancers. This trend is evident in global prostate cancer rates, as well as in both low and highly developed regions (Ferlay et al. 2015). It is noteworthy that 10% of U.S. men diagnosed with prostate cancer in 2012 were less than 55 years of age, and early-onset prostate cancer may have a distinct etiology and clinical phenotype (Salinas et al. 2014). The practice of PSA screening results in a lead time of ∼10 years because of detection of prostate cancer before symptom onset. Following the implementation of PSA screening in the United States, the average age at prostate cancer diagnosis shifted earlier and is currently 66 years of age (Howlader et al. 2016).
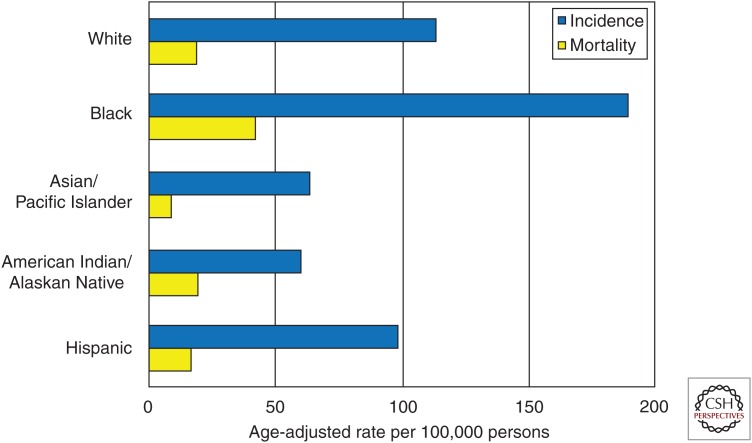
There are striking differences in prostate cancer incidence and mortality across racial and ethnic groups (Fig. 4). For example, there is a threefold difference in incidence rates of prostate cancer across race/ethnicity groups in the United States, with the highest incidence observed among black men. Moreover, deaths attributable to prostate cancer are 2.4 times greater among black compared to white men in the United States (Howlader et al. 2016). Prostate cancer incidence and mortality rates are lower among Asian/Pacific Islanders, American Indian/Alaskan Natives, and Hispanic men compared with non-Hispanic white men (Howlader et al. 2016). Further study is needed to explain the causes of these disparities. There is some evidence that differences in mortality may be a result, in part, of differences in access to care and stage at diagnosis (Taksler et al. 2012). Observed differences in the prevalence of multiple prostate cancer genetic risk loci across racial/ethnic groups (Haiman et al. 2011) suggests that genetic factors could account for some differences in incidence rates.
Figure 4.
Prostate cancer incidence and mortality rates (per 100,000) by race/ethnicity in the United States, 2010–2014. Rates are age-adjusted. (Figure based on SEER Registry data [seer.cancer.gov/csr/1975-2014/].)
There is strong evidence from family studies that family history of prostate cancer influences risk of prostate cancer. Compared with men without a positive family history, men with a father or brother diagnosed with prostate cancer are at a two- to threefold higher risk of being diagnosed, and the risk is nearly ninefold higher for men with both (Hemminki and Czene 2002). A similar association has been observed for risk of lethal prostate cancer. Men with a father or brother who died of prostate cancer have twofold higher risk of death from prostate cancer compared with men diagnosed with prostate cancer without a family history (Brandt et al. 2011). Further evidence from twin studies shows that much of familial aggregation of prostate cancer results from shared genetic factors, with a high heritability estimate of 57% (Lichtenstein et al. 2000; Mucci et al. 2016). The more than 105 prostate cancer risk loci confirmed across multiple studies explain about one-third of the heritability (Eeles et al. 2013; Hoffmann et al. 2015). The majority of identified germline risk loci are not strongly associated with lethal or nonlethal prostate cancer (Pomerantz and Freedman 2010; Shui et al. 2014), which suggests that inherited factors may be involved early in prostate carcinogenesis.
Risk Factors for Advanced and Fatal Prostate Cancer
Obesity and Weight Change
Obesity is a growing public health issue because the prevalence of obesity worldwide has more than doubled since 1980 (World Health Organization 2014). In 2014, there were an estimated 1.9 billion overweight adults, of whom 600 million qualified as obese (World Health Organization 2014). Among men worldwide, the prevalence of overweight and obesity is 39% and 11%, respectively. Obesity is implicated in the dysregulation of various hormonal pathways, leading to higher levels of insulin, estradiol, and inflammatory cytokines and lower levels of adiponectin, testosterone, and sex hormone binding globulin (Platz and Giovannucci 2004; De Marzo et al. 2007; Ma et al. 2008; Li et al. 2010).
The relationship between body size and prostate cancer incidence is complex and has been studied extensively (MacInnis and English 2006; Giovannucci et al. 2007; Ma et al. 2008; Robinson et al. 2008; Cao and Ma 2011; Discacciati et al. 2011). Obesity is associated with an increased risk of prostate cancer mortality and recurrence. In a meta-analysis of six postdiagnosis studies of men with prostate cancer, a 5 kg/m2 increase in body mass index (BMI) was associated with a 20% (RR 1.20, 95% CI: 0.99–1.46) higher risk of death from prostate cancer (Cao and Ma 2011). An association of similar magnitude was observed for risk of biochemical recurrence. Although it has been suggested that differences in screening may explain the association between obesity and worse prostate cancer outcomes, the association remains after accounting for stage and grade at diagnosis as well as PSA screening (Wright et al. 2007; Ma et al. 2008). Biomarkers have been used to identify possible mechanisms through which obesity may affect prostate cancer progression. In a prospective cohort with prediagnostic bloods, men who had high-circulating levels of C-peptide, a marker of insulin secretion, had an increased cancer-specific mortality (Ma et al. 2008). However, this association was not confirmed in two prospective studies for risk of aggressive disease (Ma et al. 2008; Stevens et al. 2014).
Waist circumference is often used as a measure of abdominal obesity and is thought to have more metabolically active adipose tissue. In a prospective study of 150,000 European men, waist circumference was positively associated with risk of advanced prostate cancer (Pischon et al. 2008). This finding was confirmed in the Melbourne Collaborative Cohort Study (MacInnis et al. 2003), but not in the Health Professionals Follow-up Study (HPFS) (Giovannucci et al. 1997; Möller et al. 2016).
Additional studies have investigated the effect of weight across the life course. There appears to be no association between weight gain from early adulthood (age 18 or 21) to midlife and prostate cancer risk (Nomura et al. 1985; Cerhan et al. 1997; Giovannucci et al. 1997; Putnam et al. 2000; Schuurman et al. 2000; Spitz et al. 2000; Jonsson et al. 2003; Friedenreich et al. 2004; Littman et al. 2007) except for one study in the multiethnic cohort (Hernandez et al. 2009). Among prostate cancer patients, men who lost weight in the period from shortly before to after prostate cancer diagnosis had a suggestively lower risk of recurrence, whereas weight gain was positively associated with recurrence after prostatectomy (Joshu et al. 2011b). Further investigation of the mechanisms underlying these associations of obesity and weight change is needed to inform strategies for prostate cancer prevention.
Height
There is probable evidence that taller height may increase risk of overall prostate cancer, and strong evidence for advanced disease. A meta-analysis of 58 studies found a relative risk of 1.09 (95% CI: 1.06–1.12) for prostate cancer overall and 1.12 (95% CI: 1.05–1.19) for advanced prostate cancer per 10 cm of height (Zuccolo et al. 2008). Results were similar when comparing before and during the PSA era. In the Health-Professionals Follow-up Study, taller height was associated with advanced and fatal prostate cancer but was not associated with total prostate cancer (Möller et al. 2016). However, a prospective study conducted in the multiethnic cohort found no association between height and risk of total or advanced prostate cancer (Hernandez et al. 2009). Although height is not considered a modifiable risk factor, its role in prostate cancer provides insight into the underlying biology of this disease. A potential explanation for this association is that height attained in adult life reflects early-life exposure to growth hormones such as insulin-like growth factor 1 (IGF-1). Birth size is not associated with prostate cancer risk, further suggesting that the etiologically relevant time period may be during puberty when the prostate undergoes maturation and rapid growth (Zuccolo et al. 2008).
Physical Activity
Evidence from prospective cohort studies has shown a moderate inverse association between physical activity and risk of advanced and fatal prostate cancer. Among men >65 years of age in the HPFS cohort, men in the highest quintile of vigorous activity had a 77% lower risk of advanced prostate cancer (Giovannucci et al. 2005). In the CPS-II cohort, men with the highest level of recreational physical activity had a 31% lower risk of aggressive prostate cancer compared with men who did not engage in recreational physical activity (Patel et al. 2005). In contrast, the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort showed an inverse association between occupational activity and risk of advanced prostate cancer but no association for leisure time activity (Johnsen et al. 2009). However, activity levels in the EPIC cohort were substantially higher, and the reference group included men with up to 25 metabolic equivalent (MET)-hours per week.
Among men diagnosed with prostate cancer, physical activity has been linked to improved survival and decreased prostate cancer progression. A study of 2705 men with prostate cancer found that those who exercised vigorously for at least 3 hours per week had a 61% lower risk of prostate cancer–specific mortality compared to those with less than 1 hour per week of vigorous activity (RR 0.4, 95% CI: 0.2–0.8) (Kenfield et al. 2011b). Although the association with prostate cancer–specific mortality was isolated to vigorous activity, both vigorous and nonvigorous activity were linked to lower risk of all-cause mortality in this population. A similar association was observed for brisk walking, with a lower risk of recurrence (RR 0.4, 95% CI: 0.2–0.9) for men engaging in brisk walking at least 3 hours per week compared with easy walking for less than 3 hours per week (Richman et al. 2011). The mechanism through which physical activity may alter prostate cancer risk is yet unclear but may act through changes in sex hormone levels, anti-inflammatory pathways, or the IGF axis (Gann et al. 1996).
Smoking
The role of smoking in cancers, including prostate cancer, is one of great public health significance. The latest 2014 report by the U.S. Surgeon General concluded there is “suggestive” evidence that smoking increases risk of death from prostate cancer, as well as risk of advanced-stage disease and less-well-differentiated cancer (U.S. Department of Health and Human Services 2014). The largest study to examine this question was conducted in HPFS, in which 5366 men diagnosed with prostate cancer were followed prospectively for 22 years and 524 prostate cancer deaths were observed. Compared with men who never smoked, current smokers had a 60% higher risk of prostate cancer mortality (HR 1.61; 95% CI: 1.11–2.32) after adjusting for potential confounders (Kenfield et al. 2011a). The association for current smoking remained elevated after further adjustment for prostate cancer stage and grade. However, attenuation of this association suggests that stage and grade may mediate the effect of smoking on prostate cancer mortality. Current smokers report less PSA testing than nonsmokers (Byrne et al. 2010), and this may contribute to later diagnosis and treatment of cancer among smokers. Another possible mechanism through which smoking may affect prostate cancer mortality is by influencing response to treatment. There have been consistent findings of worse outcomes among smokers compared to nonsmokers in prostate cancer patients treated by radiation, androgen-deprivation therapy (ADT), and radical prostatectomy (Oefelein and Resnick 2004; Pickles et al. 2004; Pantarotto et al. 2007; Moreira et al. 2010; Joshu et al. 2011a).
Epidemiologic evidence indicates that the effect of smoking on prostate cancer may depend on the time period of exposure. A prospective study in HPFS found that pack-years of smoking 10 years before prostate cancer diagnosis was positively associated with risk of lethal disease, whereas total lifetime smoking was not associated with risk (Giovannucci et al. 1999). A separate study showed that among former smokers who quit 10 or more years before diagnosis, the risk of prostate cancer mortality and recurrence was similar to that of never smokers (Kenfield et al. 2011a). The biological basis for the association between smoking and prostate cancer risk and mortality remains unclear. Several potential mechanisms have been proposed, including tumor promotion through carcinogens contained in tobacco smoke, changes in testosterone levels, and epigenetic and nicotine-induced effects (Kenfield et al. 2011a).
Lycopene and Tomato-Based Products
Oxidative stress may damage molecules, including proteins and DNA, and has been implicated in carcinogenesis. Lycopene is a carotenoid with powerful antioxidant properties and accumulates in high concentrations in prostate tissue. The primary food sources of lycopene include tomatoes and tomato-based products, pink grapefruit, and watermelon (Ilic et al. 2011). Antioxidants such as lycopene may limit the damaging effects of oxidation in animal tissues. The hypothesis that lycopene and lycopene-rich foods may have a protective role in prostate cancer risk has been extensively studied (Mills et al. 1989; Le Marchand et al. 1991; Giovannucci et al. 1995, 2002; Key et al. 1997, 2007; Meyer et al. 1997; Cerhan et al. 1998; Schuurman et al. 1998; Deneo-Pellegrini et al. 1999; Jain et al. 1999, 2007; Vogt et al. 2002; Huang et al. 2003; Bosetti et al. 2004; Kirsh et al. 2006; Peters et al. 2007; Pourmand et al. 2007; Beilby et al. 2010; Kristal et al. 2010, 2011). A 2004 meta-analysis found that high dietary intakes of tomato or tomato-based products were associated with a 10%–20% reduction in risk of incident prostate cancer (Etminan et al. 2004). A separate analysis of studies assessing lycopene in serum or plasma found a 25% lower prostate cancer risk associated with higher concentrations of lycopene. Findings of epidemiologic studies more recently have been inconsistent, with some finding an inverse association between lycopene and prostate cancer (Wu et al. 2004; Jian et al. 2005; Key et al. 2007; Pourmand et al. 2007), whereas others are null (Bosetti et al. 2004; Kirsh et al. 2006; Peters et al. 2007; Karppi et al. 2009; Kristal et al. 2010, 2011).
Epidemiologic studies have also focused on tomatoes as a specific source of lycopene with more consistent findings supporting a protective effect of higher intake of tomatoes on prostate cancer risk. The strongest benefit has been observed for cooked tomatoes, which contain higher levels of bioavailable lycopene than fresh tomatoes (Maiani et al. 2009). A meta-analysis was published in 2004 and included available prospective cohort and nested case control studies (Etminan et al. 2004). Men who consumed higher amounts of raw tomato (5th quintile of intake) had a lower risk of prostate cancer (RR 0.89, 95% CI 0.80–1.00). The association was somewhat stronger for higher intake of cooked tomato products; lycopene is lipophilic and thus cooking allows for more bioavailable sources of lycopene than fresh tomatoes (Maiani et al. 2009). Notably, the associations between tomato products are stronger for risk of advanced or lethal prostate cancer, compared with overall risk, suggesting that tomato products may play a role in disease progression. In a European study, prediagnostic plasma levels of lycopene were not associated with risk overall, although men who had the top quintile of plasma lycopene had lower risk of advanced disease (RR 0.40, 95% CI: 0.19–0.88) (Key et al. 2007). In the HPFS cohort, the relative risk for high lycopene was 0.91 (95% CI: 0.84–1.00) for prostate cancer risk overall but 0.72 (95% CI: 0.56–0.94) for lethal disease (Zu et al. 2014). Moreover, men who consumed high lycopene also had tumors indicating lower angiogenic potential.
Current epidemiologic evidence is not definitive but suggests that higher intake of tomato-based products is associated with reduced risk of prostate cancer and potentially lower risk of progression. Further study is required to determine whether the effect is because of lycopene or other components of tomatoes. Nonetheless, the association appears to be stronger for advanced prostate cancer than for indolent disease.
Calcium, Dairy Products, and Vitamin D
Calcium intake has been positively associated with prostate cancer in most epidemiological studies. A 2005 meta-analysis reported that men with the highest calcium intake had a relative risk of 1.39 (95% CI: 1.09–1.77), for extreme categories (Gao et al. 2005). Four prospective studies have been published subsequently, each suggesting an increased risk associated with high calcium (Kesse et al. 2006; Mitrou et al. 2007; Park et al. 2007; Allen et al. 2008), whereas five studies found no associations (Ahn et al. 2007; Park et al. 2007; Rohrmann et al. 2007; Kurahashi et al. 2008; Butler et al. 2010; Kristal et al. 2010). There was notable variability in total calcium intake across study populations, with the highest intake ranging from <1000 mg/day in three studies to >2000 mg/day in three other studies (Ahn et al. 2007; Mitrou et al. 2007; Park et al. 2007; Rohrmann et al. 2007; Kurahashi et al. 2008; Butler et al. 2010). In some, but not all, studies, higher calcium intake has been associated with more aggressive prostate cancer defined by high grade or advanced or lethal disease (Tseng et al. 2005; Giovannucci et al. 2006, 2007).
Serum levels of calcium and prostate cancer risk have been examined in several prospective studies. High-serum calcium was associated with a higher risk of fatal prostate cancer in National Health and Nutrition Examination Survey (NHANES) studies, with an RR of 2.68 (95% CI: 1.02–6.99) (Skinner and Schwartz 2008, 2009). Two nested case-control studies of Swedish men found no association with overall risk of prostate cancer (Halthur et al. 2009; Van Hemelrijck et al. 2012); in fact, there was a weak inverse association with overall risk in one study but no association with risk of fatal disease (Van Hemelrijck et al. 2012). The findings on serum calcium must be taken in context, as circulating levels are tightly regulated and are influenced by diet only at high levels of dietary consumption.
Dairy foods are a major source of calcium in the diet, and high intake of dairy has been positively associated with prostate cancer risk. In a meta-analysis, the per-serving RR for total dairy were 1.11 (95% CI: 1.03–1.19), for milk 1.06 (95% CI: 0.91–1.23), and for cheese 1.11 (95% CI: 0.99–1.25) (Gao et al. 2005). Whereas most studies (Rohrmann et al. 2007; Allen et al. 2008; Park et al. 2009) subsequently published also showed a positive association for higher milk or dairy and total prostate cancer (Koh et al. 2006; Park et al. 2007), results for advanced or lethal disease are mixed (Park et al. 2007). The correlation between dairy foods and calcium and other nutrients presents a challenge of distinguishing the independent effects of these compounds. The 2007 expert report from the World Cancer Research Fund on Diet and Cancer stated that calcium is a “probable” risk factor for prostate cancer, but the evidence for dairy was weak/inconclusive (Wiseman 2008). Since the expert report, the EPIC cohort reported a positive association between dairy calcium, but not nondairy calcium, with both total and high-grade prostate cancer (Allen et al. 2008).
A potential mechanism underlying the association with calcium is through suppressing circulating levels of dihydroxyvitamin D (1,25(OH)2D), the more bioactive vitamin D metabolite. Most vitamin D is from endogenous production in the skin as a result of sun exposure, whereas dietary sources are secondary. Although 1,25(OH)2D is the most biologically active form, 25(OH)D is found in higher concentrations and may be a strong indicator of sun and dietary exposure (Ali and Vaidya 2007). Dairy protein is also associated with increased circulating levels of IGF (Giovannucci et al. 2003), which has been linked with advanced prostate cancer (Chan et al. 2002).
Studies examining vitamin D through diet or supplemental sources have generally found no association for prostate cancer risk (Chan et al. 1998, 2000; Kristal et al. 2002). Studies using prediagnostic circulating vitamin D have been quite mixed for overall risk, finding no association (Corder et al. 1993; Braun et al. 1995; Gann et al. 1996; Nomura et al. 1998; Jacobs et al. 2004; Platz et al. 2004; Faupel-Badger et al. 2007; Li et al. 2007; Ahn et al. 2008; Travis et al. 2009; Barnett et al. 2010; Park et al. 2010; Gilbert et al. 2012), in addition to significant positive (Albanes et al. 2011), inverse (Meyer et al. 2013), and U-shaped (Kristal et al. 2014) associations. Other lines of evidence point to a role of vitamin D for prostate cancer progression. Inherited genetic variants in the vitamin D pathway, which may influence metabolism or uptake, have been associated with an increased risk of recurrence and prostate cancer mortality (Holt et al. 2010). Moreover, high-protein tumor expression of the vitamin D receptor has been inversely associated with lethal prostate cancer in a survival analysis (Hendrickson et al. 2011). Low pre-diagnostic 25(OH)D levels were associated with higher mortality among prostate cancer patients, with an RR of 1.59 (95% CI: 1.06–2.39) for the highest versus lowest quartiles (Fang et al. 2011).
Fish
Populations with diets rich in fish, such as Japanese and Alaskan Eskimos, tend to have lower incidence of prostate cancer compared to populations following a Western diet low in fish (Zhang et al. 1999; Dewailly et al. 2003). There is some epidemiologic evidence to support a role of fish intake with prostate cancer mortality. A meta-analysis of four cohort studies showed a 63% reduction in prostate cancer–specific mortality associated with higher total fish intake (Szymanski et al. 2010). Although one study found an increased risk of prostate cancer among men with higher blood levels of long-chain omega-3 fatty acids (Brasky et al. 2013), this is likely to have arisen because the cases represented primarily early-stage disease and men with higher fish intake are more likely to receive PSA screening. One study examining fish intake after prostate cancer diagnosis found a 17% reduction in risk of prostate cancer recurrence with two additional servings of fish per week (Chan et al. 2006). A second study of postdiagnostic fish intake found no association (Richman et al. 2010). Although the mechanism is unknown, fish contain long-chain marine omega-3 polyunsaturated fatty acids, which could lower risk of prostate cancer through anti-inflammatory pathways (Chan et al. 2005).
Coffee
Coffee has been studied extensively in prostate cancer epidemiology, although most prior studies focused on total prostate cancer and with generally null results. However, studies investigating risk of fatal or advanced disease support an inverse association (Cao et al. 2014; Discacciati et al. 2014; Lu et al. 2014; Zhong et al. 2014). In addition, meta-analysis by Discacciati et al. (2014) reported an RR of 0.89 (95% CI: 0.82–0.97) for total prostate per three cups/day of coffee and an inverse association with high-grade (Gleason 8–10) disease.
Coffee is composed of a diverse array of biologically active compounds that may underlie the association with prostate cancer progression. For example, coffee is linked with improved glucose metabolism and insulin secretion in observational and animal studies, and it is one of the most potent antioxidant dietary factors.
Statins
Several studies show that higher cholesterol levels are associated with increased risk of total or advanced prostate cancer (Platz et al. 2008, 2009; Kitahara et al. 2011; Shafique et al. 2012). Furthermore, there are several shared genetic risk loci for lipid levels and prostate cancer (Andreassen et al. 2014). The associations between the lipid-lowering class of drugs, statins, has been investigated and consistently suggest an inverse association for advanced disease. The first study was in HPFS, and found the RR of statin users versus nonusers was 0.39 (95% CI: 0.19–0.77) for advanced prostate cancer (Platz et al. 2006). A meta-analysis undertaken in 2012 reported a pooled RR for statin use of 0.93 (95% CI: 0.87–0.99) for total and 0.80 (95% CI: 0.70–0.90) for advanced prostate cancer (Bansal et al. 2012). Six epidemiological studies have been conducted subsequently, all supporting an inverse association between statin use and lethal prostate cancer (Nielsen et al. 2012; Geybels et al. 2013; Margel et al. 2013; Grytli et al. 2014). Among 11,000 prostate cancer patients in the United Kingdom, men who were on postdiagnostic statins had a 34% (95% CI: 0.66–0.88) lower risk of prostate cancer death (Yu et al. 2014). Moreover, the effect was also stronger among men using statins before diagnosis. A retrospective study showed that, during ADT, men using statins experienced longer time-to-progression compared with those not using statins (Harshman et al. 2015). Findings from an in vitro study show statins may act through pathways that decrease available androgen in the tumor (Harshman et al. 2015). Additional studies are needed to elucidate the relevant etiological window as well as identify mechanisms of association.
FUTURE RESEARCH
As discussed in the previous sections, several modifiable risk factors hold promise in reducing risk of aggressive prostate cancer or progression of the disease. To better understand disease heterogeneity, an emerging area of research is the molecular characterization of prostate cancer tumors. A study in The Cancer Genome Atlas showed that most prostate tumors could be classified into seven molecular subtypes based on presence of gene fusions or mutations (Abeshouse et al. 2015). Moreover, growing evidence suggests that prostate tumors defined by molecular features share unique etiology and risk factors (Pettersson et al. 2013). Differences in risk factor associations by prostate cancer phenotype—based on clinical, molecular, or genetic features—could have profound implications for prevention. Future studies should aim to expand and integrate this knowledge to develop targeted interventions that maximize benefit to prostate cancer patients. Furthermore, an improved understanding of underlying biological mechanisms may open the door to identification of biomarkers of susceptibility or early disease.
As highlighted in this review, epidemiologic studies of prostate cancer pose unique methodological challenges. Future studies of prostate cancer epidemiology should focus on clinically relevant prostate cancer, with particular consideration of high-grade and advanced-stage or fatal disease. Additionally, epidemiologic studies of prostate cancer should be designed with consideration of potential biases specific to this disease. For example, the ascertainment of information on PSA screening is required to provide adequate adjustment and avoid potential detection bias.
SUMMARY
Prostate cancer epidemiology is complex, in part because of the biological heterogeneity of the disease as well as PSA screening. Prevention of prostate cancer is challenging given that established risk factors, including age, race/ethnicity, family history, and genetic variants, are primarily nonmodifiable. Smoking cessation, regular exercise, and maintaining healthy weight are important public health targets for intervention. Importantly, several lifestyle modifications may lower risk of developing more aggressive cancer or offer survival benefits to prostate cancer patients. Future research has potential to improve efficacy of these prevention strategies through targeted interventions.
Footnotes
Editors: Michael M. Shen and Mark A. Rubin
Additional Perspectives on Prostate Cancer available at www.perspectivesinmedicine.org
REFERENCES
- Abeshouse A, Ahn J, Akbani R, Ally A, Amin S, Andry CD, Annala M, Aprikian A, Armenia J, Arora A, et al. 2015. The molecular taxonomy of primary prostate cancer. Cell 163: 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Albanes D, Peters U, Schatzkin A, Lim U, Freedman M, Chatterjee N, Andriole GL, Leitzmann MF, Hayes RB. 2007. Dairy products, calcium intake, and risk of prostate cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 16: 2623–2630. [DOI] [PubMed] [Google Scholar]
- Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, Horst RL, Hollis BW, Huang WY, Shikany JM, et al. 2008. Serum vitamin D concentration and prostate cancer risk: A nested case-control study. J Natl Cancer Inst 100: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanes D, Mondul AM, Yu K, Parisi D, Horst RL, Virtamo J, Weinstein SJ. 2011. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Biomarkers Prev 20: 1850–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsen PC, Hanley JA, Fine J. 2005. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 293: 2095–2101. [DOI] [PubMed] [Google Scholar]
- Ali MM, Vaidya V. 2007. Vitamin D and cancer. J Cancer Res Ther 3: 225–230. [DOI] [PubMed] [Google Scholar]
- Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Tjonneland A, Johnsen NF, Overvad K, Linseisen J, Rohrmann S, et al. 2008. Animal foods, protein, calcium and prostate cancer risk: The European prospective investigation into cancer and nutrition. Br J Cancer 98: 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, Benlloch S, Hazelett DJ, Wang Z, Saunders E, et al. 2014. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet 46: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Zuber V, Thompson WK, Schork AJ, Bettella F, Djurovic S, Desikan RS, Mills IG, Dale AM. 2014. Shared common variants in prostate cancer and blood lipids. Int J Epidemiol 43: 1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Undela K, D’Cruz S, Schifano F. 2012. Statin use and risk of prostate cancer: A meta-analysis of observational studies. PloS ONE 7: e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett CM, Nielson CM, Shannon J, Chan JM, Shikany JM, Bauer DC, Hoffman AR, Barrett-Connor E, Orwoll E, Beer TM. 2010. Serum 25-OH vitamin D levels and risk of developing prostate cancer in older men. Cancer Causes Control 21: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilby J, Ambrosini GL, Rossi E, de Klerk NH, Musk AW. 2010. Serum levels of folate, lycopene, beta-carotene, retinol and vitamin E and prostate cancer risk. Euro J Clin Nutr 64: 1235–1238. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Talamini R, Montella M, Negri E, Conti E, Franceschi S, La Vecchia C. 2004. Retinol, carotenoids and the risk of prostate cancer: A case-control study from Italy. Int J Cancer 112: 689–692. [DOI] [PubMed] [Google Scholar]
- Brandt A, Sundquist J, Hemminki K. 2011. Risk for incident and fatal prostate cancer in men with a family history of any incident and fatal cancer. Ann Oncol 23: 251–256. [DOI] [PubMed] [Google Scholar]
- Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, Meyskens FLJ, Goodman GE, Minasian LM, Parnes HL, et al. 2013. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst 105: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. 1995. Prostate cancer and prediagnostic levels of serum vitamin D metabolites (Maryland, United States). Cancer Causes Control 6: 235–239. [DOI] [PubMed] [Google Scholar]
- Butler LM, Wong AS, Koh WP, Wang R, Yuan JM, Yu MC. 2010. Calcium intake increases risk of prostate cancer among Singapore Chinese. Cancer Res 70: 4941–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne MM, Davila EP, Zhao W, Parker D, Hooper MW, Caban-Martinez A, Dietz N, Huang Y, Messiah A, Lee DJ. 2010. Cancer screening behaviors among smokers and non-smokers. Cancer Epidemiol 34: 611–617. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ma J. 2011. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prev Res (Phila) 4: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Liu L, Yin X, Wang Y, Liu J, Lu Z. 2014. Coffee consumption and risk of prostate cancer: A meta-analysis of prospective cohort studies. Carcinogenesis 35: 256–261. [DOI] [PubMed] [Google Scholar]
- Cerhan JR, Torner JC, Lynch CF, Rubenstein LM, Lemke JH, Cohen MB, Lubaroff DM, Wallace RB. 1997. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 8: 229–238. [DOI] [PubMed] [Google Scholar]
- Cerhan JR, Cantor KP, Williamson K, Lynch CF, Torner JC, Burmeister LF. 1998. Cancer mortality among Iowa farmers: Recent results, time trends, and lifestyle factors (United States). Cancer Causes Control 9: 311–319. [DOI] [PubMed] [Google Scholar]
- Chan JM, Giovannucci E, Andersson SO, Yuen J, Adami HO, Wolk A. 1998. Dairy products, calcium, phosphorous, vitamin D, and risk of prostate cancer (Sweden). Cancer Causes Control 9: 559–566. [DOI] [PubMed] [Google Scholar]
- Chan JM, Pietinen P, Virtanen M, Malila N, Tangrea J, Albanes D, Virtamo J. 2000. Diet and prostate cancer risk in a cohort of smokers, with a specific focus on calcium and phosphorus (Finland). Cancer Causes Control 11: 859–867. [DOI] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E. 2002. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst 94: 1099–1106. [DOI] [PubMed] [Google Scholar]
- Chan JM, Gann PH, Giovannucci EL. 2005. Role of diet in prostate cancer development and progression. J Clin Oncol 23: 8152–8160. [DOI] [PubMed] [Google Scholar]
- Chan JM, Holick CN, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Giovannucci EL. 2006. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control 17: 199–208. [DOI] [PubMed] [Google Scholar]
- Chu KC, Tarone RE, Freeman HP. 2003. Trends in prostate cancer mortality among black men and white men in the United States. Cancer 97: 1507–1516. [DOI] [PubMed] [Google Scholar]
- Ciatto S, Gervasi G, Bonardi R, Frullini P, Zendron P, Lombardi C, Crocetti E, Zappa M. 2005. Determining overdiagnosis by screening with DRE/TRUS or PSA (Florence pilot studies, 1991–1994). Eur J Cancer 41: 411–415. [DOI] [PubMed] [Google Scholar]
- Corder EH, Guess HA, Hulka BS, Friedman GD, Sadler M, Vollmer RT, Lobaugh B, Drezner MK, Vogelman JH, Orentreich N. 1993. Vitamin D and prostate cancer: A prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev 2: 467–472. [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. 2007. Inflammation in prostate carcinogenesis. Nat Rev Cancer 7: 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneo-Pellegrini H, De Stefani E, Ronco A, Mendilaharsu M. 1999. Foods, nutrients and prostate cancer: A case-control study in Uruguay. Br J Cancer 80: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Sloth Pedersen H, Hansen JC, Behrendt N, Hart Hansen JP. 2003. Inuit are protected against prostate cancer. Cancer Epidemiol Biomarkers Prev 12: 926–927. [PubMed] [Google Scholar]
- Discacciati A, Orsini N, Andersson SO, Andren O, Johansson JE, Wolk A. 2011. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: A population-based prospective study. Br J Cancer 105: 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discacciati A, Orsini N, Wolk A. 2014. Coffee consumption and risk of nonaggressive, aggressive and fatal prostate cancer—A dose-response meta-analysis. Ann Oncol 25: 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, et al. 2013. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet 45: 385–391, e391–e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan M, Takkouche B, Caamano-Isorna F. 2004. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev 13: 340–345. [PubMed] [Google Scholar]
- Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, Feuer EJ. 2002. Overdiagnosis due to prostate-specific antigen screening: Lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 94: 981–990. [DOI] [PubMed] [Google Scholar]
- Etzioni R, Gulati R, Falcon S, Penson DF. 2008. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: A surveillance modeling approach. Med Decis Making 28: 323–331. [DOI] [PubMed] [Google Scholar]
- Fang F, Kasperzyk JL, Shui I, Hendrickson W, Hollis BW, Fall K, Ma J, Gaziano JM, Stampfer MJ, Mucci LA, et al. 2011. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PloS ONE 6: e18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faupel-Badger JM, Diaw L, Albanes D, Virtamo J, Woodson K, Tangrea JA. 2007. Lack of association between serum levels of 25-hydroxyvitamin D and the subsequent risk of prostate cancer in Finnish men. Cancer Epidemiol Biomarkers Prev 16: 2784–2786. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2013. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer (globocan.iarc.fr). [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2015. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, McGregor SE, Courneya KS, Angyalfi SJ, Elliott FG. 2004. Case-control study of anthropometric measures and prostate cancer risk. Int J Cancer 110: 278–283. [DOI] [PubMed] [Google Scholar]
- Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. 1996. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst 88: 1118–1126. [DOI] [PubMed] [Google Scholar]
- Gao X, LaValley MP, Tucker KL. 2005. Prospective studies of dairy product and calcium intakes and prostate cancer risk: A meta-analysis. J Natl Cancer Inst 97: 1768–1777. [DOI] [PubMed] [Google Scholar]
- Geybels MS, Wright JL, Holt SK, Kolb S, Feng Z, Stanford JL. 2013. Statin use in relation to prostate cancer outcomes in a population-based patient cohort study. Prostate 73: 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, Lane JA, Martin RM. 2012. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer 131: 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. 1995. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 87: 1767–1776. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. 1997. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 6: 557. [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Ascherio A, Colditz GA, Spiegelman D, Stampfer MJ, Willett WC. 1999. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol Biomarkers Prev 8: 277–282. [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. 2002. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst 94: 391–398. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. 2003. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev 12: 84–89. [PubMed] [Google Scholar]
- Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. 2005. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med 165: 1005–1010. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Stampfer MJ, Willett WC. 2006. A prospective study of calcium intake and incident and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev 15: 203–210. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. 2007. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer 121: 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Cancer Collaboration. 2016. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 3: 524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grytli HH, Fagerland MW, Fossa SD, Tasken KA. 2014. Association between use of beta-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 65: 635–641. [DOI] [PubMed] [Google Scholar]
- Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, Rybicki BA, Isaacs WB, Ingles SA, Stanford JL, et al. 2011. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet 7: e1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halthur C, Johansson ALV, Almquist M, Malm J, Gronberg H, Manjer J, Dickman PW. 2009. Serum calcium and the risk of prostate cancer. Cancer Causes Control 20: 1205–1214. [DOI] [PubMed] [Google Scholar]
- Harshman LC, Wang X, Nakabayashi M, Xie W, Valenca L, Werner L, Yu Y, Kantoff AM, Sweeney CJ, Mucci LA, et al. 2015. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol 1: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K, Czene K. 2002. Attributable risks of familial cancer from the Family-Cancer Database. Cancer Epidemiol Biomarkers Prev 11: 1638–1644. [PubMed] [Google Scholar]
- Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R, Fiore C, Penney KL, Ma J, Kantoff PW, et al. 2011. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol 29: 2378–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez BY, Park SY, Wilkens LR, Henderson BE, Kolonel LN. 2009. Relationship of body mass, height, and weight gain to prostate cancer risk in the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 18: 2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TJ, Van Den Eeden SK, Sakoda LC, Jorgenson E, Habel LA, Graff RE, Passarelli MN, Cario CL, Emami NC, Chao CR, et al. 2015. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov 5: 878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SK, Kwon EM, Koopmeiners JS, Lin DW, Feng Z, Ostrander EA, Peters U, Stanford JL. 2010. Vitamin D pathway gene variants and prostate cancer prognosis. Prostate 70: 1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Altekruse S, Kosary C, Ruhl J, Tatalovich Z, Mariotto A, et al. 2016. SEER Cancer Statistics Review 1975–2013. National Cancer Institute, Bethesda, MD: (seer.cancer.gov/csr/1975_2013). [Google Scholar]
- Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. 2003. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol 157: 335–344. [DOI] [PubMed] [Google Scholar]
- Ilic D, Forbes KM, Hassed C. 2011. Lycopene for the prevention of prostate cancer. Cochrane Database Syst Rev CD008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ET, Giuliano AR, Martinez ME, Hollis BW, Reid ME, Marshall JR. 2004. Plasma levels of 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and the risk of prostate cancer. J Steroid Biochem Mol Biol 89–90: 533–537. [DOI] [PubMed] [Google Scholar]
- Jahn JL, Giovannucci EL, Stampfer MJ. 2015. The high prevalence of undiagnosed prostate cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era: High prostate cancer prevalence: Research implications in the PSA-ERA. Int J Cancer 137: 2795–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain MG, Hislop GT, Howe GR, Ghadirian P. 1999. Plant foods, antioxidants, and prostate cancer risk: Findings from case-control studies in Canada. Nutr Cancer 34: 173–184. [DOI] [PubMed] [Google Scholar]
- Jemal A, Center MM, DeSantis C, Ward EM. 2010. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 19: 1893–1907. [DOI] [PubMed] [Google Scholar]
- Jian L, Du CJ, Lee AH, Binns CW. 2005. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer 113: 1010–1014. [DOI] [PubMed] [Google Scholar]
- Jian L, Lee AH, Binns CW. 2007. Tea and lycopene protect against prostate cancer. Asia Pac J Clin Nutr 16: 453–457. [PubMed] [Google Scholar]
- Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, Adami HO. 2004. Natural history of early, localized prostate cancer. JAMA 291: 2713–2719. [DOI] [PubMed] [Google Scholar]
- Johnsen NF, Tjonneland A, Thomsen BL, Christensen J, Loft S, Friedenreich C, Key TJ, Allen NE, Lahmann PH, Mejlvig L, et al. 2009. Physical activity and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer 125: 902–908. [DOI] [PubMed] [Google Scholar]
- Jonsson F, Wolk A, Pedersen NL, Lichtenstein P, Terry P, Ahlbom A, Feychting M. 2003. Obesity and hormone-dependent tumors: Cohort and co-twin control studies based on the Swedish Twin Registry. Int J Cancer 106: 594–599. [DOI] [PubMed] [Google Scholar]
- Joshu CE, Mondul AM, Meinhold CL, Humphreys EB, Han M, Walsh PC, Platz EA. 2011a. Cigarette smoking and prostate cancer recurrence after prostatectomy. J Natl Cancer Inst 103: 835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshu CE, Mondul AM, Menke A, Meinhold C, Han M, Humphreys EB, Freedland SJ, Walsh PC, Platz EA. 2011b. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 4: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karppi J, Kurl S, Nurmi T, Rissanen TH, Pukkala E, Nyyssonen K. 2009. Serum lycopene and the risk of cancer: The Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Ann Epidemiol 19: 512–518. [DOI] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. 2011a. Smoking and prostate cancer survival and recurrence. JAMA 305: 2548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. 2011b. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol 29: 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse E, Bertrais S, Astorg P, Jaouen A, Arnault N, Galan P, Hercberg S. 2006. Dairy products, calcium and phosphorus intake, and the risk of prostate cancer: Results of the French prospective SU.VI.MAX (Supplementation en Vitamines et Mineraux Antioxydants) study. Br J Nutr 95: 539–545. [DOI] [PubMed] [Google Scholar]
- Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. 1997. A case-control study of diet and prostate cancer. Br J Cancer 76: 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Allen NE, Travis RC, Roddam AW, Jenab M, Egevad L, Tjonneland A, Johnsen NF, Overvad K, et al. 2007. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition Study. Am J Clin Nutr 86: 672–681. [DOI] [PubMed] [Google Scholar]
- Kirsh VA, Mayne ST, Peters U, Chatterjee N, Leitzmann MF, Dixon LB, Urban DA, Crawford ED, Hayes RB. 2006. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 15: 92–98. [DOI] [PubMed] [Google Scholar]
- Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. 2011. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 29: 1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KA, Sesso HD, Paffenbarger RSJ, Lee IM. 2006. Dairy products, calcium and prostate cancer risk. Br J Cancer 95: 1582–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Cohen JH, Qu P, Stanford JL. 2002. Associations of energy, fat, calcium, and vitamin D with prostate cancer risk. Cancer Epidemiol Biomarkers Prev 11: 719–725. [PubMed] [Google Scholar]
- Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA, Albanes D, Thompson IM. 2010. Diet, supplement use, and prostate cancer risk: Results from the prostate cancer prevention trial. Am J Epidemiol 172: 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Till C, Platz EA, Song X, King IB, Neuhouser ML, Ambrosone CB, Thompson IM. 2011. Serum lycopene concentration and prostate cancer risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 20: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, Schenk JM, Thompson IM, Meyskens FLJ, Goodman GE, et al. 2014. Plasma vitamin D and prostate cancer risk: Results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 23: 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS. 2008. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol Biomarkers Prev 17: 930–937. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Hankin JH, Kolonel LN, Wilkens LR. 1991. Vegetable and fruit consumption in relation to prostate cancer risk in Hawaii: A reevaluation of the effect of dietary β-carotene. Am J Epidemiol 133: 215–219. [DOI] [PubMed] [Google Scholar]
- Li H, Stampfer MJ, Hollis JBW, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. 2007. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med 4: e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, Ma J. 2010. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem 56: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. 2000. Environmental and heritable factors in the causation of cancer––Analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343: 78–85. [DOI] [PubMed] [Google Scholar]
- Littman AJ, White E, Kristal AR. 2007. Anthropometrics and prostate cancer risk. Am J Epidemiol 165: 1271–1279. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhai L, Zeng J, Peng Q, Wang J, Deng Y, Xie L, Mo C, Yang S, Li S, et al. 2014. Coffee consumption and prostate cancer risk: An updated meta-analysis. Cancer Causes Control 25: 591–604. [DOI] [PubMed] [Google Scholar]
- Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, Gaziano JM, Pollak M, Stampfer MJ. 2008. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: A long-term survival analysis. Lancet Oncol 9: 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis RJ, English DR. 2006. Body size and composition and prostate cancer risk: Systematic review and meta-regression analysis. Cancer Causes Control 17: 989–1003. [DOI] [PubMed] [Google Scholar]
- MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. 2003. Body size and composition and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 12: 1417. [PubMed] [Google Scholar]
- Maiani G, Caston MJP, Catasta G, Toti E, Cambrodon IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, et al. 2009. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res 53: S194–S218. [DOI] [PubMed] [Google Scholar]
- Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, Fleshner N. 2013. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol 31: 3069–3075. [DOI] [PubMed] [Google Scholar]
- Meyer F, Bairati I, Fradet Y, Moore L. 1997. Dietary energy and nutrients in relation to preclinical prostate cancer. Nutr Cancer 29: 120–126. [DOI] [PubMed] [Google Scholar]
- Meyer HE, Robsahm TE, Bjorge T, Brustad M, Blomhoff R. 2013. Vitamin D, season, and risk of prostate cancer: A nested case-control study within Norwegian health studies. Am J Clin Nutr 97: 147–154. [DOI] [PubMed] [Google Scholar]
- Mills PK, Beeson WL, Phillips RL, Fraser GE. 1989. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 64: 598–604. [DOI] [PubMed] [Google Scholar]
- Mitrou PN, Albanes D, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Leitzmann MF. 2007. A prospective study of dietary calcium, dairy products and prostate cancer risk (Finland). Int J Cancer 120: 2466–2473. [DOI] [PubMed] [Google Scholar]
- Möller E, Wilson KM, Batista JL, Mucci LA, Bälter K, Giovannucci E. 2016. Body size across the life course and prostate cancer in the Health Professionals Follow-up Study. Int J Cancer 138: 853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira DM, Antonelli JA, Presti JC Jr, Aronson WJ, Terris MK, Kane CJ, Amling CL, Freedland SJ. 2010. Association of cigarette smoking with interval to biochemical recurrence after radical prostatectomy: Results from the SEARCH database. Urology 76: 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, Graff RE, Holst K, Möller S, Unger RH, et al. 2016. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 315: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SF, Nordestgaard BG, Bojesen SE. 2012. Statin use and reduced cancer-related mortality. N Engl J Med 367: 1792–1802. [DOI] [PubMed] [Google Scholar]
- Nomura A, Heilbrun LK, Stemmermann GN. 1985. Body mass index as a predictor of cancer in men. J Natl Cancer Inst 74: 319–323. [PubMed] [Google Scholar]
- Nomura AM, Stemmermann GN, Lee J, Kolonel LN, Chen TC, Turner A, Holick MF. 1998. Serum vitamin D metabolite levels and the subsequent development of prostate cancer (Hawaii, United States). Cancer Causes Control 9: 425–432. [DOI] [PubMed] [Google Scholar]
- Oefelein MG, Resnick MI. 2004. Association of tobacco use with hormone refractory disease and survival of patients with prostate cancer. J Urol 171: 2281–2284. [DOI] [PubMed] [Google Scholar]
- Pantarotto J, Malone S, Dahrouge S, Gallant V, Eapen L. 2007. Smoking is associated with worse outcomes in patients with prostate cancer treated by radical radiotherapy. BJU Int 99: 564–569. [DOI] [PubMed] [Google Scholar]
- Park Y, Mitrou PN, Kipnis V, Hollenbeck A, Schatzkin A, Leitzmann MF. 2007. Calcium, dairy foods, and risk of incident and fatal prostate cancer: The NIH-AARP Diet and Health Study. Am J Epidemiol 166: 1270–1279. [DOI] [PubMed] [Google Scholar]
- Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. 2009. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med 169: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Cooney RV, Wilkens LR, Murphy SP, Henderson BE, Kolonel LN. 2010. Plasma 25-hydroxyvitamin D and prostate cancer risk: The multiethnic cohort. Eur J Cancer 46: 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AV, Rodriguez C, Jacobs EJ, Solomon L, Thun MJ, Calle EE. 2005. Recreational physical activity and risk of prostate cancer in a large cohort of U.S. men. Cancer Epidemiol Biomarkers Prev 14: 275–279. [PubMed] [Google Scholar]
- Peters U, Leitzmann MF, Chatterjee N, Wang Y, Albanes D, Gelmann EP, Friesen MD, Riboli E, Hayes RB. 2007. Serum lycopene, other carotenoids, and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 16: 962–968. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, Finn S, Graff RE, Penney KL, Rider JR, et al. 2013. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. J Natl Cancer Inst 105: 1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles T, Liu M, Berthelet E, Kim-Sing C, Kwan W, Tyldesley S. 2004. The effect of smoking on outcome following external radiation for localized prostate cancer. J Urol 171: 1543–1546. [DOI] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, Tjonneland A, Severinsen MT, Overvad K, Rohrmann S, et al. 2008. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 17: 3252–3261. [DOI] [PubMed] [Google Scholar]
- Platz EA, Giovannucci E. 2004. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol 92: 237–253. [DOI] [PubMed] [Google Scholar]
- Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. 2004. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control 15: 255–265. [DOI] [PubMed] [Google Scholar]
- Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. 2006. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst 98: 1819–1825. [DOI] [PubMed] [Google Scholar]
- Platz EA, Clinton SK, Giovannucci E. 2008. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer 123: 1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IMJ, Kristal AR. 2009. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 18: 2807–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz MM, Freedman ML. 2010. Genetics of prostate cancer risk. Mt Sinai J Med 77: 643–654. [DOI] [PubMed] [Google Scholar]
- Pourmand G, Salem S, Mehrsai A, Lotfi M, Amirzargar MA, Mazdak H, Roshani A, Kheirollahi A, Kalantar E, Baradaran N, et al. 2007. The risk factors of prostate cancer: A multicentric case-control study in Iran. Asian Pac J Cancer Prev 8: 422–428. [PubMed] [Google Scholar]
- Putnam SD, Cerhan JR, Parker AS, Bianchi GD, Wallace RB, Cantor KP, Lynch CF. 2000. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol 10: 361–369. [DOI] [PubMed] [Google Scholar]
- Richman EL, Stampfer MJ, Paciorek A, Broering JM, Carroll PR, Chan JM. 2010. Intakes of meat, fish, poultry, and eggs and risk of prostate cancer progression. Am J Clin Nutr 91: 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. 2011. Physical activity after diagnosis and risk of prostate cancer progression: Data from the cancer of the prostate strategic urologic research endeavor. Cancer Res 71: 3889–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WR, Poole C, Godley PA. 2008. Systematic review of prostate cancer’s association with body size in childhood and young adulthood. Cancer Causes Control 19: 793–803. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Platz EA, Kavanaugh CJ, Thuita L, Hoffman SC, Helzlsouer KJ. 2007. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control 18: 41–50. [DOI] [PubMed] [Google Scholar]
- Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. 2014. Prostate cancer in young men: An important clinical entity. Nat Rev Urol 11: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. 1998. Vegetable and fruit consumption and prostate cancer risk: A cohort study in The Netherlands. Cancer Epidemiol Biomarkers Prev 7: 673–680. [PubMed] [Google Scholar]
- Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. 2000. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol 151: 541–549. [DOI] [PubMed] [Google Scholar]
- SEER Cancer Statistics Review 1975–2008. 2011. National Cancer Institute; Bethesda, MD: (seer.cancer.gov/csr/1975_2008). [Google Scholar]
- Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. 2012. Cholesterol and the risk of grade-specific prostate cancer incidence: Evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer 12: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. 1991. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 63: 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui IM, Lindstrom S, Kibel AS, Berndt SI, Campa D, Gerke T, Penney KL, Albanes D, Berg C, Bueno-de-Mesquita HB, et al. 2014. Prostate cancer (PCa) risk variants and risk of fatal PCa in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Eur Urol 65: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HG, Schwartz GG. 2008. Serum calcium and incident and fatal prostate cancer in the National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev 17: 2302–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HG, Schwartz GG. 2009. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev 18: 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz MR, Strom SS, Yamamura Y, Troncoso P, Babaian RJ, Scardino PT, Wheeler T, Amos CI, von Eschenbach A, Kagan J. 2000. Epidemiologic determinants of clinically relevant prostate cancer. Int J Cancer 89: 259–264. [DOI] [PubMed] [Google Scholar]
- Stevens VL, Jacobs EJ, Sun J, Gapstur SM. 2014. No association of plasma levels of adiponectin and c-peptide with risk of aggressive prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 23: 890–892. [DOI] [PubMed] [Google Scholar]
- Szymanski KM, Wheeler DC, Mucci LA. 2010. Fish consumption and prostate cancer risk: A review and meta-analysis. Am J Clin Nutr 92: 1223–1233. [DOI] [PubMed] [Google Scholar]
- Taksler GB, Keating NL, Cutler DM. 2012. Explaining racial differences in prostate cancer mortality. Cancer 118: 4280–4289. [DOI] [PubMed] [Google Scholar]
- Travis RC, Crowe FL, Allen NE, Appleby PN, Roddam AW, Tjonneland A, Olsen A, Linseisen J, Kaaks R, Boeing H, et al. 2009. Serum vitamin D and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Epidemiol 169: 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng M, Breslow RA, Graubard BI, Ziegler RG. 2005. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr 81: 1147–1154. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. 2014. The health consequences of smoking—50 years of progress: A report of the Surgeon General (www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html). [Google Scholar]
- Van Hemelrijck M, Hermans R, Michaelsson K, Melvin J, Garmo H, Hammar N, Jungner I, Walldius G, Holmberg L. 2012. Serum calcium and incident and fatal prostate cancer in the Swedish AMORIS study. Cancer Causes Control 23: 1349–1358. [DOI] [PubMed] [Google Scholar]
- Vogt TM, Mayne ST, Graubard BI, Swanson CA, Sowell AL, Schoenberg JB, Swanson GM, Greenberg RS, Hoover RN, Hayes RB, et al. 2002. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US Blacks and Whites. Am J Epidemiol 155: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Wiseman M. 2008. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc Nutr Soc 67: 253–256. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2014. Global status report on noncommunicable diseases 2014 (www.who.int/nmh/publications/ncd-status-report-2014/en). [Google Scholar]
- Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. 2007. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109: 675–684. [DOI] [PubMed] [Google Scholar]
- Wu K, Erdman JWJ, Schwartz SJ, Platz EA, Leitzmann M, Clinton SK, DeGroff V, Willett WC, Giovannucci E. 2004. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case-control study. Cancer Epidemiol Biomarkers Prev 13: 260–269. [DOI] [PubMed] [Google Scholar]
- Yu H, Harris RE, Gao YT, Gao R, Wynder EL. 1991. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol 20: 76–81. [DOI] [PubMed] [Google Scholar]
- Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, Azoulay L. 2014. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol 32: 5–11. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sasaki S, Amano K, Kesteloot H. 1999. Fish consumption and mortality from all causes, ischemic heart disease, and stroke: An ecological study. Prev Med 28: 520–529. [DOI] [PubMed] [Google Scholar]
- Zhong S, Chen W, Yu X, Chen Z, Hu Q, Zhao J. 2014. Coffee consumption and risk of prostate cancer: An up-to-date meta-analysis. Eur J Clin Nutr 68: 330–337. [DOI] [PubMed] [Google Scholar]
- Zu K, Mucci L, Rosner BA, et al. 2014. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J Natl Cancer Inst 106: djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccolo L, Harris R, Gunnell D, Oliver S, Lane JA, Davis M, Donovan J, Neal D, Hamdy F, Beynon R, et al. 2008. Height and prostate cancer risk: A large nested case-control study (ProtecT) and meta-analysis. Cancer Epidemiol Biomarkers Prev 17: 2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]