Abstract
Background
Myocardial external efficiency (MEE) is defined as the ratio of kinetic energy associated with cardiac work [forward cardiac output (FCO)*mean systemic pressure] and the chemical energy from oxygen consumed (MVO2) by the left ventricular mass (LVM). We developed a fully automated method for estimating MEE based on a single 11C-acetate PET scan without ECG-gating.
Methods and Results
Ten healthy controls, 34 patients with aortic valve stenosis (AVS), and 20 patients with mitral valve regurgitation (MVR) were recruited in a dual-center study. MVO2 was calculated using washout of 11C -acetate activity. FCO and LVM were calculated automatically using dynamic PET and parametric image formation. FCO and LVM were also obtained using cardiac magnetic resonance (CMR) in all subjects. The correlation between MEEPET-CMR and MEEPET was high (r = 0.85, P < 0.001) without significant bias. MEEPET was 23.6 ± 4.2% for controls and was lowered in AVS (17.2 ± 4.3%, P < 0.001) and in MVR (18.0 ± 5.2%, P = 0.004). MEEPET was strongly associated with both NYHA class (P < 0.001) and the magnitude of valvular dysfunction (mean aortic gradient: P < 0.001, regurgitant fraction: P = 0.009).
Conclusion
A single 11C-acetate PET yields accurate and automated MEE results on different scanners. MEE might provide an unbiased measurement of the phenotypic response to valvular disease.
Electronic supplementary material
The online version of this article (10.1007/s12350-018-1338-0) contains supplementary material, which is available to authorized users.
Keywords: Myocardial efficiency, myocardial energetics, positron emission tomography, 11C-acetate
Introduction
A common feature in most cardiomyopathies is a reduction in myocardial external efficiency (MEE)1 i.e., an imbalance between cardiac work and total energy consumption by the left ventricle (LV). MEE reflects both mechanical performance and metabolic integrity. A reduction in MEE may result from ischemia, increased wall stress or filling pressures and leads to a further deterioration of LV function. Preserving or restoring MEE is associated with better prognosis and a reduction of symptoms in patients with left ventricular systolic dysfunction,2 dilated cardiomyopathy,3,4 aortic valve stenosis,5 and hypertrophic cardiomyopathy6 and was thus suggested as a therapeutic target. In addition, MEE may serve as an early marker of cardiac performance and can potentially be used as a more sensitive marker of interventions.7,8
The gold standard for measuring MEE makes use of pressure-volume loops and the Fick-principle to estimate cardiac work and myocardial oxygen consumption (MVO2), respectively. However, the requirement for dual-sided catheterization has ruled out this method in a clinical setting. Accurate non-invasive alternatives are available,9 requiring positron emission tomography (PET) with either a combination of 15O-labeled PET tracers or, more commonly, 11C-acetate washout10–12 to assess MVO2, combined with cardiac magnetic resonance imaging (CMR) or echocardiography to assess Cardiac Output (CO) and LV mass (LVM).
Since MEE integrates measurements of LV mass, function, and oxidative metabolism, a near-simultaneous acquisition of all measurements is preferred. This could be accomplished using hybrid PET/CMR or ECG-gated PET,13 but neither can be applied to all potential patient groups and both require significant post-processing of data, which would introduce observer bias. An optimal solution would be to extract CO and LVM directly from the dynamic PET data in an automated and scanner-independent fashion. Recently, automated methods of obtaining forward cardiac output (FCO)14 and LVM15 have become available, using only a dynamic 11C-acetate PET scan. Therefore, the aim of this study was to evaluate the accuracy of MEE derived from a single dynamic 11C-acetate PET scan. In addition, obtained MEE values for patients with aortic valve stenosis (AVS) and mitral valve regurgitation (MVR) are compared with those of healthy controls.
Materials and Methods
Patient Population
This study consists of a retrospective analysis of three groups of subjects undergoing efficiency measures in research studies from two different research sites. The first group consisted of 34 patients (69.0 ± 8.4y, 24 men) with AVS and varying degrees of heart failure (22 asymptomatic, 8 NYHA class II and 4 NYHA class III patients). All patients had sinus rhythm, no signs of myocardial ischemia and aortic valve area ≤ 1.2 cm2 and/or transaortic maximal velocity of 3.0–5.0 m s−1 based on echocardiography. The second group consisted of 20 patients (56.4 ± 15.6y, 19 men) with significant mitral regurgitation (regurgitant fraction > 30% on echocardiography) and with no or mild symptoms of heart failure (15 and five NYHA class I and II, respectively). The final group consisted of 10 healthy controls (62.5 ± 4.4y, 7 men) with no signs or history of cardiac disease. AVS patients and controls were scanned at the Aarhus University Hospital, Aarhus, Denmark whilst MVR patients were scanned at the Uppsala University Hospital, Uppsala, Sweden. The study was approved by the respective local ethical committees and all subjects gave written informed consent prior to inclusion in this study.
Image Acquisition
PET
11C-acetate synthesis was done according to Pike16 with minor in-house modifications. After a fasting period of > 4 h, AVS patients and controls underwent 11C-acetate PET scan on a Siemens Biograph TruePoint TrueV 64 PET/CT scanner. Following a scout CT scan, a low-dose CT scan (120 kV, 30 mAs) was performed. After this, a 27-minute list mode emission scan was performed, starting simultaneously with automated injection of 407 ± 30 MBq 11C-acetate as a 5–10 mL bolus (1 mL·s−1) in a peripheral vein, followed by a 35-mL saline flush (2.0 mL·s−1). List mode emission data were rebinned into a dynamic series consisting of 29 time frames using all data. Dynamic images were reconstructed using the TrueX algorithm, applying all appropriate corrections as supplied by the vendor.
MVR patients were scanned on a GE discovery ST with an acquisition protocol identical to that of AVS patients and controls. Data were reconstructed using the 3D IR algorithm with all appropriate corrections as supplied by the vendor.
CMR
AVS patients and controls were scanned on an Ingenia 1.5 T whole body scanner (Philips Healthcare, Best, The Netherlands) as described in14 and.15 MVR patients were scanned on an Ingenia 3 T whole body scanner (Philips Healthcare, Best, The Netherlands) with an 80 mT·m−1 gradient system, using similar imaging protocols. Details on exact settings can be found in the supplemental files.
Calculation of Myocardial External Efficiency
Myocardial external efficiency (MEE) was calculated using the methods as described in9,17 and the workflow used in this study is summarized in the supplemental file. MEE was defined as
| 1 |
In which EW is the effective external work performed by the heart (J); TE is the total energy use (J); MAP is the mean arterial pressure (mmHg); FCO is the forward cardiac output (mL·min−1); MVO2 is the myocardial oxygen consumption (mL·g−1·min−1); LVM is the mass of the left ventricle (LV, g), and 1.33 · 10−4 and 20 are the conversion factors from 1 mmHg mL to J and from 1 mL of O2 to J, respectively. The numerator, output energy (Eout) is similar to cardiac work, expressed in Joules, and represents the area enclosed within a pressure-volume loop. The denominator represents input energy (Ein), the total energy consumed by the LV.
HR and MAP were measured 1 minute before and 1 and 5 minutes after injection and averaged for calculation of MEE. MVO2 was derived from 11C-acetate PET data, whereas both FSV and LVM were derived from either CMR (FSVCMR and LVMCMR) or 11C-acetate PET data (FSVPET and LVMPET). Supplemental Figure 1 summarizes the steps required for calculation of MEE, which are outlined below. An example of a dynamic time-series and all relevant intermediate steps of the analysis for each of the patient categories is shown in Supplemental Figures 2, 3, and 4.
MVO2
PET scans were analyzed using aQuant18 (available at no cost for collaborative, non-commercial research purposes via https://aquantsoft.com/go/aquantresearch). Arterial (CA(t)) and right-ventricular (CRV(t)) blood concentrations were obtained automatically using cluster analysis.14,18 Arterial blood concentrations were converted into arterial plasma input functions (CP(t)) by applying the average plasma metabolite correction as presented by Sun et al.19 Finally, CP(t) was used for calculation of washout rate k2 using a standard single compartment model20 with all appropriate corrections for spillover from the blood and blood volume fractions:
| 2 |
In which CPET(t) represents the myocardial time-activity curve, and CP(t), CA(t), and CRV(t) are the aforementioned blood time-activity curves. VA represents arterial blood volume fraction (dimensionless), K1 the uptake rate of 11C-acetate in tissue (mL·g−1·min−1), k2 the washout rate of 11C-acetate (in min−1), VRV is the right-ventricular spillover fraction (dimensionless).
Parametric images were generated using basis function methods18 and used for automatic segmentation of the LV, as described in detail elsewhere.15 In brief, for each short-axis slice the ventricular mid-point is defined using the center of gravity of VA, from which radial profiles are generated every 10°. Then, for each profile, the first and last point above 2/3rd of the maximum of each profile were considered to represent the endo- and epicardial borders. This process was repeated for each profile and each short-axis slice until the entire LV was segmented.
After LV segmentation, activity concentrations in the LV were extracted and used as CPET(t) in Eq. (2), yielding average k2 of the entire LV. This global k2 was converted into MVO2 using the empirically derived conversion factors of Sun et al.19:
| 3 |
Identical values of MVO2 were used for both MEEPET-CMR and MEEPET.
Forward cardiac output
For AVS patients and controls, CMR-based forward stroke volume (FSVCMR) was calculated from phase contrast velocity measurement in the LV outflow tract. Flow analysis was performed using the freely available software Segment (version 1.9 R3746).21 As AVS regularly results in turbulent flow patterns, phase contrast velocity was imaged at the level of the LV outflow tract where flow velocity was laminar. For MVR patients, FSVCMR was calculated from phase contrast velocity measurement in the ascending aorta and flow analyses were performed on a ViewForum workstation (Philips, Best, the Netherlands). FSVCMR was multiplied with HR during PET to obtain FCO
FSV based on PET (FSVPET) was calculated using indicator-dilution techniques using the methods described in 14 correcting for scanner-dependent differences between FSVPET-CMR and FSVPET as presented in that study. In brief, the peak of the first-pass of the 11C-acetate bolus through the arterial blood, obtained for calculation of MVO2 as described above, was isolated automatically from CA(t). Using this peak, forward cardiac output was estimated using
| 4 |
In which FCOPET is forward cardiac output (mL·min−1); I is the injected dose of 11C-acetate (Bq) and ∫CA(t) is the area under the curve of the isolated peak (Bq·mL−1·min).
LVM
LVMCMR was derived by manually tracing the endo- and epicardium in end-diastole on short-axis cine images tracing using the software Segment v1.9 R285421 for AVS patients and ViewForum (Philips) for MVR patients. LVMPET was defined using the volume of interest of the LV used to obtain CPET(t). For both LVMPET-CMR and LVMPET, a density of myocardial tissue of 1.05 g cm3 was assumed.
Statistical Analysis
Data are presented as mean ± SD. Correlation and agreement were assessed using linear regression and Bland Altman plots. Paired t tests were used to evaluate systematic differences. Repeatability coefficient (RPC) was defined as 2 times the standard deviation of the difference. Differences between patient groups were assessed using One-way ANOVA followed by student’s t tests. The independent capacity of MEEPET and MEEPET-CMR for prediction of NYHA class was assessed by ordinal logistic fitting.
Results
Data of one AVS patient showed significant motion during PET, and this patient was excluded from further analysis.
Patient Characteristics
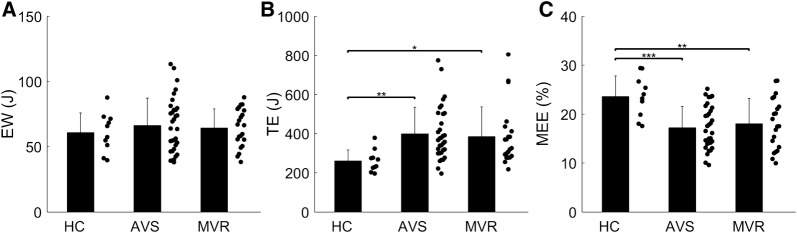
Table 1 shows relevant hemodynamic parameters and all parameters used in calculation of MEEPET for all three groups. Compared to controls, systolic blood pressure was significantly higher for AVS (P = 0.015) but not for MVR (P = 0.09). Neither MAP (P = 0.55 and 0.30) nor FCI (P = 0.27 and 0.17) were significantly different from controls, resulting in similar values for EW (P = 0.47 and 0.56 for AVS and MVR). MVO2 in patients did not differ from that of controls (P=0.26 and 0.92 for AVS and MVR) but LVM was larger (P = 0.003 and < 0.001), resulting in a significantly increased TE (P = 0.003 and 0.019).
Table 1.
Mean ± standard deviation of all hemodynamic or PET-derived parameters involved in calculation of myocardial external efficiency (MEE) for healthy controls, aortic valve stenosis (AVS), and mitral regurgitation (MVR) patients
| Controls (n = 10) | AVS (n = 33) | MVR (n = 20) | |
|---|---|---|---|
| SBP (mmHg) | 124 ± 11 | 139 ± 18* | 134 ± 15 |
| DBP (mmHg) | 79 ± 6 | 79 ± 11 | 71 ± 13† |
| HR (min−1) | 62 ± 8 | 65 ± 11 | 60 ± 12 |
| MAP (mmHg) | 97 ± 9 | 99 ± 12 | 92 ± 12† |
| MVO2 (mLO2·g−1·min−1) | 0.10 ± 0.02 | 0.12 ± 0.04 | 0.11 ± 0.03 |
| FCI (L·min−1·m−2) | 2.4 ± 0.4 | 2.6 ± 0.6 | 2.6 ± 0.4 |
| LVMI (g·m−2) | 63.8 ± 8.4 | 89.3 ± 25.0** | 90.5 ± 17.2*** |
| EW (J) | 60.9 ± 15.0 | 66.2 ± 21.0 | 64.3 ± 14.6 |
| TE (J) | 260 ± 57 | 399 ± 137** | 385 ± 152* |
| MEE (%) | 23.6 ± 4.2 | 17.2 ± 4.3*** | 18.0 ± 5.2** |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; MVO2, rate of oxygen consumption; FCI, forward cardiac index; LVMI, left ventricular mass corrected for body-surface area; EW, external work; TE, total energy usage
*, ** and ***P < 0.05, < 0.01 and < 0.001 vs healthy controls
†P < 0.05 vs AVS
Validation vs CMR
Correlation between PET and CMR was good for both FCO and LVM (r = 0.83 and r = 0.94, respectively, Figure 1). Correlation between PET- and CMR-derived measures was high for both EW and TE (r = 0.89, and 0.97, respectively, Figure 2). Bland Altman analysis revealed no significant differences for EW (− 1.2 ± 8.5 J, P = 0.445) or TE (3.4 ± 33.3 J, P = 0.564). Finally, correlation of MEEPET and MEEPET-CMR was high (r = 0.85, Figure 3) without significant bias (absolute difference of − 0.4 ± 2.8%, P = 0.511).
Figure 1.
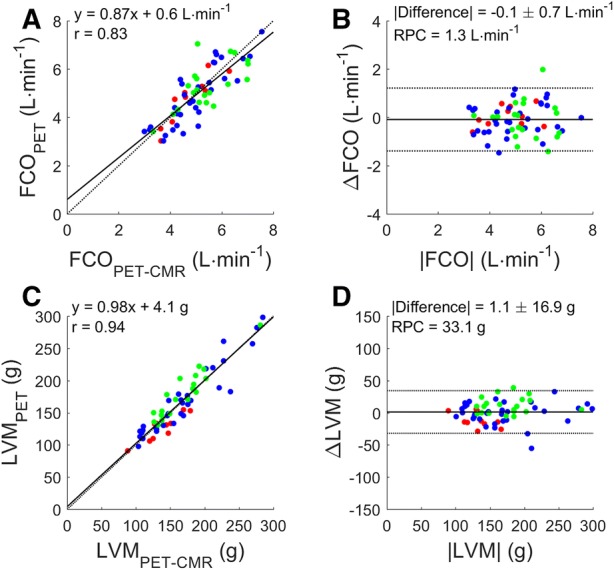
Correlation (A, C) and Bland Altman plot (B, D) of forward cardiac output (A, B) and left ventricular mass (C, D) based on CMR (FCOPET-CMR and LVMPET-CMR) and PET (FCOPET and LVMPET). Black and gray lines indicate the line of identity and the linear fit in (A, C) and the mean difference and the 95% confidence interval in (B, D). RPC: repeatability coefficient. Red: healthy controls (n = 10), blue: AVS patients (n = 33), green: MVR patients (n = 20)
Figure 2.
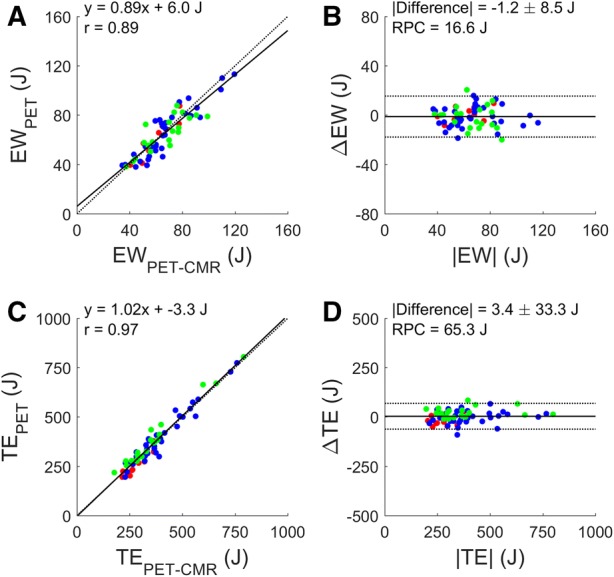
Correlation (A, C) and Bland Altman plot (B, D) of external work (A, B) and total energy use (C, D) based on CMR (EWPET-CMR and TEPET-CMR) and PET (EWPET and TEPET). Black and gray lines indicate the line of identity and the linear fit in (A, C) and the mean difference and the 95% confidence interval in (B, D). RPC, repeatability coefficient. Red: healthy controls (n = 10), blue: AVS patients (n = 33), green: MVR patients (n = 20)
Figure 3.
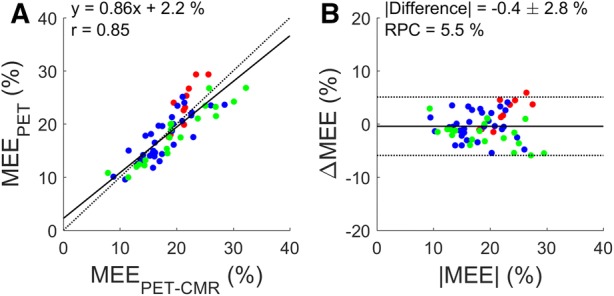
Correlation (A) and Bland Altman plot (B) of myocardial external efficiency based on a combined PET-CMR protocol (MEEPET-CMR) and PET-only (MEEPET). Black and gray lines indicate the line of identity and the linear fit in (A) and the mean difference and the 95% confidence interval in (B). RPC: repeatability coefficient. Red: healthy controls (n = 10), blue: AVS patients (n = 33), green: MVR patients (n = 20)
EWPET and EWPET-CMR were not significantly different for any patient group. On the other hand, TEPET was significantly higher than TEPET-CMR for MVR (22.9 ± 26.0 J, P = 0.001) and significantly lower for controls (− 28.1 ± 19.0 J, P = 0.001). Finally, MEEPET was significantly different as compared to MEEPET-CMR for controls (2.8 ± 3.0%, P = 0.02) and MVR (− 1.8 ± 2.3%, P = 0.011) but not for AVS (− 0.2 ± 2.6%, P = 0.62). Residual analysis identified the difference in FCO as the main source of difference in MEE in all groups (Controls: r = 0.58, P = 0.02; AVS: r = 0.79, P < 0.001; MVR: r = 0.58, P = 0.01).
Clinical Characteristics of MEE
When comparing patient groups, MEEPET was significantly lower for both AVS (P < 0.001) and MVR (P = 0.006) as compared to controls (Figure 4), whilst MEEPET-CMR was significantly lower for AVS (P = 0.009) but not MVR (P = 0.49). Mean aortic gradient in AVS was correlated to MEEPET-CMR (r = − 0.40, P = 0.023) and to MEEPET (r = − 0.62, P < 0.001, Figure 5), of which the correlation to MEEPET was significantly higher (P = 0.01). In addition, regurgitant fraction was correlated to both MEEPET (r = − 0.61, P = 0.009) and to MEEPET-CMR (r = − 0.49, P = 0.045, Figure 6) with no significant difference between correlations (P = 0.10). Finally, MEEPET was strongly associated with NYHA class (ANOVA P < 0.001) and significantly separated most groups (Figure 7), while the association was less clear for MEEPET-CMR (ANOVA P = 0.03). Using Ordinal Logistic fitting only MEEPET was independently associated with NYHA class (χ2 14.2, P < 0.0002), compared to MEEPET-CMR (χ2 3.8, P = 0.052).
Figure 4.
Mean ± SD and individual values for EWPET (A), TEPET (B) and MEEPET (C) for all three groups. *, **, *** indicate significance level of < 0.05, < 0.01, and < 0.001, respectively
Figure 5.
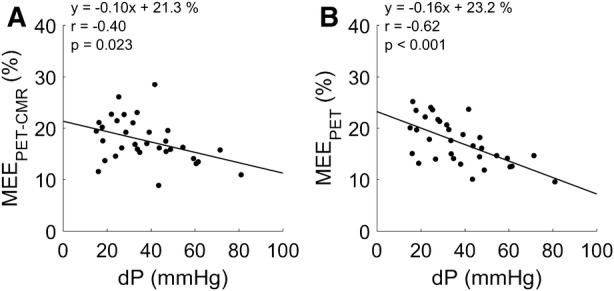
Correlation of mean pressure gradient over de aortic valve and MEE derived using PET-CMR (A) and PET-only (B) for AVS patients
Figure 6.
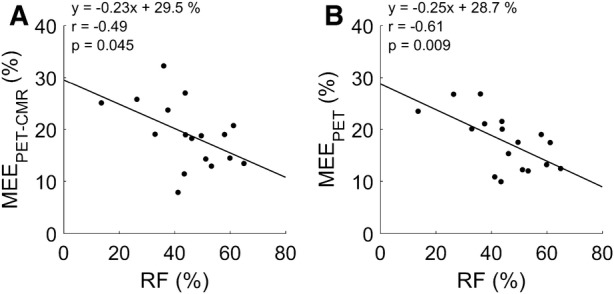
Correlation of CMR-derived regurgitant fractions and MEE derived using PET-CMR (A) and PET-only (B) for MVR patients. RF, regurgitant fraction
Figure 7.
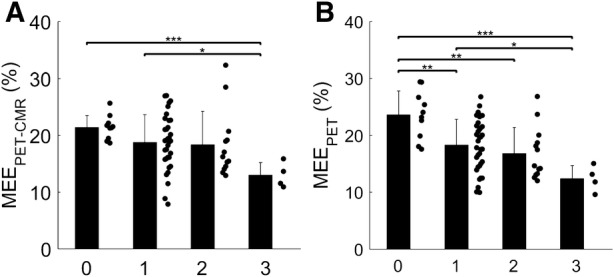
Mean ± standard deviation of MEEPET-CMR (A) and MEEPET (B) per NYHA heart failure class. *, **, and *** denote a P value below 0.05, 0.01, and 0.001, respectively
Discussion
This study presents a fully automated method of calculating myocardial external efficiency (MEE) solely from a dynamic 11C-acetate PET/CT scan without the use of ECG-gating. This method eliminates the need for separate measurements of cardiac output and LV mass, reducing protocol duration, cost and analysis time. MEE is a sensitive marker of cardiac performance and has been used as surrogate end-point in several interventional studies,7,8 potentially lowering the sample size as compared to traditional markers such as ejection fraction or outcomes.
Both FCO and LVM have been validated vs CMR before14,15 in a subset of the subjects included in this study. LVM measured by PET without ECG-gating in particular appears to perform well across different scanners, compared to CMR. FCO requires a scanner-dependent correction factor, as previously observed.14 The difference in FCO was identified as the only significant source of MEE deviation between modalities. Since associations towards clinical parameters were stronger for MEEPET, the difference in FCO points to a significant change in LV loading conditions between the CMR and PET scans. This is encouraging in terms of using MEE from PET alone as an end-point in multicenter trials, but including other scanner models requires further validation against CMR to establish correction factors.
In a test-retest study, we show that repeatability of MEEPET-CMR and MEEPET were high and not significantly different (coefficient of variation of 6.3% and 9.5%, respectively, P = 0.25).22 The range of control values was narrower for MEEPET-CMR as compared to MEEPET, as can also be appreciated in Figure 7, suggesting a higher sensitivity and the need for smaller groups when using MEEPET-CMR as marker for efficiency. However, Figure 7 also shows that the difference between controls and patients with valvular diseases was higher for MEEPET, suggesting that the (non-significantly) increased test-retest variability for MEEPET is largely off-set by an increased effect size. The exact benefit of using either MEEPET and MEEPET-CMR must be studied in larger clinical studies. Finally, the use of a combined PET-echocardiography protocol was not recommended from a reproducibility point of view.22
MEE calculated with both approaches resulted in control values ranging from 17 to 30%, which is in line with previously published invasive measurements.9 Patients were consecutively recruited from on-going larger studies and two-thirds were asymptomatic. Half of the patients had MEE lower than any control subject, suggesting that attenuated efficiency at rest is common in valvular diseases. MEEPET was significantly correlated to the echocardiographically derived mean pressure gradient of the aortic valve in AVS patients (r = − 0.62, P < 0.001) and to the regurgitant fraction obtained from CMR in MVR patients (r = − 0.61, P = 0.009). In addition, MEE corresponded to the subjective level of disease burden defined by the NYHA class. This indicates that MEE reflects the phenotypic response to the causative disease process in valvular disease. To what extent MEE can be used to define the optimal time point for valvuloplasty requires larger outcome studies, for which the current study suggests that a PET-alone approach might perform better than serial multimodality imaging.
Care has to be taken when comparing different values for MEE in literature. In some studies, pressure gradient over the aortic valve is used in Eq. (1) (replacing MAP by MAP+∆P), which is likely to minimize differences in MEE. Similarly, when the total stroke volume including the blood that regurgitates over either valve is used instead of FSV, differences between controls and MVR are expected to be smaller and it becomes clear that there are conceptual differences between MEE obtained in either case. MEE as calculated using Eq. (1) represents the energetic cost of the entire LV required to pump a certain amount of blood into the systemic circulation, ignoring any pathological pressures in the LV cavity and excluding any regurgitating volume. This could be considered the net efficiency of the whole heart as a pump or global LV efficiency and reflects both the metabolic and mechanical state of the heart. The result of this study suggests that global LV efficiency is a sensitive marker of generic cardiac performance. If, on the other hand, regurgitation or elevated LV pressures are taken into account, MEE represents the energetic cost of displacing blood in any direction which can be considered the efficiency of the cardiomyocytes, reflecting the metabolic state of the heart specifically i.e., the metabolic efficiency. Noteworthy, global LV and metabolic efficiency deviate only in the case of valvular dysfunction. When pressure gradients are essential, echocardiography can be performed during a PET examination,23 although echocardiography is limited by the acoustic window and the presence of significant operator differences. Similarly, total stroke volume can be obtained using gated PET but accuracy of that method is so far suboptimal.13 However, the present study shows that it is feasible to obtain MEE according to either definition during a single scan session.
This study has several limitations that need to be acknowledged. The PET acquisition protocols were aligned between both participating sites, but since differences between PET scanners were observed widespread implementation of this all-in-one approach in multicenter studies requires further validation. CMR equipment and protocols differed between sites, which is likely to induce bias.
Secondly, this study mainly included subjects with valvular abnormalities which typically show discrepancies in pressure-volume loops. The assumption that EW, formally defined as the area encompassed in a patient’s pressure-volume loop, can be approximated by the product of MAP and SV is often incorrect in these patients.9 Errors in EW estimates can be reduced by utilizing forward instead of total stroke volume and/or adding the mean pressure gradient over the aortic valve, although the latter increases complexity of the method. As discussed above, care has to be taken when considering the use of mean pressure gradients or whether to use the forward or total stroke volume. In this study, we chose to use forward stroke volume and exclude mean pressure gradient to obtain a ‘net’ or global LV efficiency, equally affected by mechanical abnormalities of the heart and valves and by any potential metabolic alterations.
To conclude, myocardial efficiency can be measured accurately using a single 11C-acetate PET/CT scan, without the need for additional imaging modalities. Because of the more generic, highly automated, and less logistically demanding approach, this novel technique might widen the applicability of MEE to more patient groups.
New Knowledge Gained
The work presented in this study enables a simplified, faster, and more automated assessment of myocardial external efficiency using a single 11C-acetate scan. Using a single scan protocol instead of a combined PET-CMR protocol leads to lower potential errors due to differences in loading conditions. When applying this method to a cohort of controls and patients with valvular diseases, MEE based on PET-only correlated more closely to the underlying disease state and to NYHA class.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported financially by the Lundbeck Foundation, Arvids Nilssons Foundation, Karen Elise Jensens Foundation, the Snedkermester Sophus Jacobsen & Hustru Astrid Jacobsens Foundation, the Health Research Fund of Central Denmark Region and the Swedish Heart-Lung Foundation.
Disclosure
None of the authors have any conflicts of interest to declare.
Abbreviations
- MEE
Myocardial external efficiency
- TE
Total energy use
- EW
External work
- LVM
Left ventricular mass
- FCO
Forward cardiac output
- MVO2
Myocardial oxygen consumption
- CMR
Cardiac magnetic resonance imaging
- PET
Positron emission tomography
- AVS
Aortic valve stenosis
- MVR
Mitral valve regurgitation
Contributor Information
Hendrik J. Harms, Phone: +45 784 56238, Email: hans.harms@clin.au.dk.
Jens Sörensen, Email: jens.sorensen@pet.uu.se.
References
- 1.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 2.Beanlands RS, Nahmias C, Gordon E, et al. The effects of beta(1)-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction: a double-blind, placebo-controlled, positron-emission tomography study. Circulation. 2000;102:2070–2075. doi: 10.1161/01.CIR.102.17.2070. [DOI] [PubMed] [Google Scholar]
- 3.Beanlands RS, Bach DS, Raylman R, et al. Acute effects of dobutamine on myocardial oxygen consumption and cardiac efficiency measured using carbon-11 acetate kinetics in patients with dilated cardiomyopathy. J Am Coll Cardiol. 1993;22:1389–1398. doi: 10.1016/0735-1097(93)90548-F. [DOI] [PubMed] [Google Scholar]
- 4.Sugiki T, Naya M, Manabe O, et al. Effects of surgical ventricular reconstruction and mitral complex reconstruction on cardiac oxidative metabolism and efficiency in nonischemic and ischemic dilated cardiomyopathy. JACC Cardiovasc Imaging. 2011;4:762–770. doi: 10.1016/j.jcmg.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Güçlü A, Knaapen P, Harms HJ, et al. Myocardial efficiency is an important determinant of functional improvement after aortic valve replacement in aortic valve stenosis patients: a combined PET and CMR study. Eur Heart J Cardiovasc Imaging. 2015;16:882–889. doi: 10.1093/ehjci/jev009. [DOI] [PubMed] [Google Scholar]
- 6.Timmer SAJ, Knaapen P. Coronary microvascular function, myocardial metabolism, and energetics in hypertrophic cardiomyopathy: insights from positron emission tomography. Eur Heart J Cardiovasc Imaging. 2013;14:95–101. doi: 10.1093/ehjci/jes242. [DOI] [PubMed] [Google Scholar]
- 7.Chen WJY, Diamant M, de Boer K, et al. Effects of exenatide on cardiac function, perfusion, and energetics in type 2 diabetic patients with cardiomyopathy: a randomized controlled trial against insulin glargine. Cardiovasc Diabetol. 2017;16:67. doi: 10.1186/s12933-017-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson NH, Sörensen J, Harms HJ, et al. Metoprolol reduces hemodynamic and metabolic overload in asymptomatic aortic valve stenosis patients: a randomized trial. Circ Cardiovasc Imaging. 2017;10:e006557. doi: 10.1161/CIRCIMAGING.117.006557. [DOI] [PubMed] [Google Scholar]
- 9.Knaapen P, Germans T, Knuuti J, et al. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation. 2007;115:918–927. doi: 10.1161/CIRCULATIONAHA.106.660639. [DOI] [PubMed] [Google Scholar]
- 10.Brown M, Marshall DR, Sobel BE, Bergmann SR. Delineation of myocardial oxygen utilization with carbon-11-labeled acetate. Circulation. 1987;76:687–696. doi: 10.1161/01.CIR.76.3.687. [DOI] [PubMed] [Google Scholar]
- 11.Buxton DB, Schwaiger M, Nguyen A, et al. Radiolabeled acetate as a tracer of myocardial tricarboxylic acid cycle flux. Circ Res. 1988;63:628–634. doi: 10.1161/01.RES.63.3.628. [DOI] [PubMed] [Google Scholar]
- 12.Armbrecht JJ, Buxton DB, Schelbert HR. Validation of [1-11C]acetate as a tracer for noninvasive assessment of oxidative metabolism with positron emission tomography in normal, ischemic, postischemic, and hyperemic canine myocardium. Circulation. 1990;81:1594–1605. doi: 10.1161/01.CIR.81.5.1594. [DOI] [PubMed] [Google Scholar]
- 13.Hansson NH, Tolbod L, Harms HJ, et al. Evaluation of ECG-gated [11C]acetate PET for measuring left ventricular volumes, mass, and myocardial external efficiency. J Nucl Cardiol. 2016;23:670–679. doi: 10.1007/s12350-015-0331-0. [DOI] [PubMed] [Google Scholar]
- 14.Harms HJ, Tolbod LP, Hansson NHS, et al. Automatic extraction of forward stroke volume using dynamic PET/CT: a dual-tracer and dual-scanner validation in patients with heart valve disease. EJNMMI Phys. 2015;2:25. doi: 10.1186/s40658-015-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms HJ, Stubkjær Hansson NH, Tolbod LP, et al. Automatic extraction of myocardial mass and volume using parametric images from dynamic nongated PET. J Nucl Med. 2016;57:1382–1387. doi: 10.2967/jnumed.115.170613. [DOI] [PubMed] [Google Scholar]
- 16.Pike VW, Eakins MN, Allan RM, Selwyn AP. Preparation of [1-11C]acetate–an agent for the study of myocardial metabolism by positron emission tomography. Int J Appl Radiat Isot. 1982;33:505–512. doi: 10.1016/0020-708X(82)90003-5. [DOI] [PubMed] [Google Scholar]
- 17.Porenta G, Cherry S, Czernin J, et al. Noninvasive determination of myocardial blood flow, oxygen consumption and efficiency in normal humans by carbon-11 acetate positron emission tomography imaging. Eur J Nucl Med. 1999;26:1465–1474. doi: 10.1007/s002590050480. [DOI] [PubMed] [Google Scholar]
- 18.Harms HJ, Knaapen P, De Haan S, et al. Automatic generation of absolute myocardial blood flow images using [(15)O]H (2)O and a clinical PET/CT scanner. Eur J Nucl Med Mol Imaging. 2011;38:930–939. doi: 10.1007/s00259-011-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun KT, Yeatman LA, Buxton DB, et al. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon-11]acetate. J Nucl Med. 1998;39:272–280. [PubMed] [Google Scholar]
- 20.Timmer SA, Lubberink M, van Rossum AC, et al. Reappraisal of a single-tissue compartment model for estimation of myocardial oxygen consumption by [11C]acetate PET: an alternative to conventional monoexponential curve fitting. Nucl Med Commun. 2011;32:59–62. doi: 10.1097/MNM.0b013e32834113b0. [DOI] [PubMed] [Google Scholar]
- 21.Heiberg E, Sjögren J, Ugander M, et al. Design and validation of Segment–freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. doi: 10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansson NH, Harms HJ, Kim WY, et al. Test-retest repeatability of myocardial oxidative metabolism and efficiency using standalone dynamic 11C-acetate PET and multimodality approaches in healthy controls. J Nucl Cardiol. 2018 doi: 10.1007/s12350-018-1302-z. [DOI] [PubMed] [Google Scholar]
- 23.Ukkonen H, Saraste M, Akkila J, et al. Myocardial efficiency during calcium sensitization with levosimendan: a noninvasive study with positron emission tomography and echocardiography in healthy volunteers. Clin Pharmacol Ther. 1997;61:596–607. doi: 10.1016/S0009-9236(97)90139-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.