Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease with a complex and heterogeneous pathophysiology. The number of people living with AD is predicted to increase; however, there are no disease-modifying therapies currently available and none have been successful in late-stage clinical trials. Fluid biomarkers measured in cerebrospinal fluid (CSF) or blood hold promise for enabling more effective drug development and establishing a more personalized medicine approach for AD diagnosis and treatment. Biomarkers used in drug development programmes should be qualified for a specific context of use (COU). These COUs include, but are not limited to, subject/patient selection, assessment of disease state and/or prognosis, assessment of mechanism of action, dose optimization, drug response monitoring, efficacy maximization, and toxicity/adverse reactions identification and minimization. The core AD CSF biomarkers Aβ42, t-tau, and p-tau are recognized by research guidelines for their diagnostic utility and are being considered for qualification for subject selection in clinical trials. However, there is a need to better understand their potential for other COUs, as well as identify additional fluid biomarkers reflecting other aspects of AD pathophysiology. Several novel fluid biomarkers have been proposed, but their role in AD pathology and their use as AD biomarkers have yet to be validated. In this review, we summarize some of the pathological mechanisms implicated in the sporadic AD and highlight the data for several established and novel fluid biomarkers (including BACE1, TREM2, YKL-40, IP-10, neurogranin, SNAP-25, synaptotagmin, α-synuclein, TDP-43, ferritin, VILIP-1, and NF-L) associated with each mechanism. We discuss the potential COUs for each biomarker.
Keywords: Alzheimer’s disease, Amyloid, Biomarker, Blood, Cerebrospinal fluid, Tau
Introduction
Worldwide, approximately 50 million people are living with dementia, with Alzheimer’s disease (AD) comprising 60–70% of cases [391]. AD is a progressive, neurodegenerative disease characterized clinically by cognitive decline and behavioural disturbances and pathologically by the accumulation of amyloid beta (Aβ) plaques and neurofibrillary tangles formed by tau fibrils, together with degeneration of neurons and their synapses, glial activation, and neuroinflammation [37, 149, 314]. The incidence of AD increases with age, and the prevalence is growing as a result of the ageing of the population [6]. To date, no disease-modifying therapy (DMT) has been successful [18]. This lack of success may be partly explained by AD having a complex aetiology and considerable heterogeneity in its pathophysiology, and by limitations in past clinical trial designs, which have generally enrolled participants later in the course of the disease (e.g. mild-to-moderate AD), and which did not enrich for Aβ-positive individuals, resulting in substantial misclassification (i.e. inclusion of participants without Aβ pathology) [12, 241, 317].
Biomarkers hold promise for enabling more effective drug development in AD and establishing a more personalized medicine approach [126, 127, 314]; they may soon become essential in staging, tracking, and providing a more quantitative categorization of the disease, as well as for documenting the effect of potential therapeutics. These points are underscored in the 2018 draft guidance documents issued by both the US Food and Drug Administration (FDA) (Early Alzheimer’s disease: developing drugs for treatment; draft guidance for industry) [96] and the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) (Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease) [78]. Fluid biomarkers have the potential to be easy to implement in clinical trials, and several biomarkers reflecting different pathophysiological mechanisms can be analyzed in the same sample. Furthermore, cerebrospinal fluid (CSF) or blood may provide a window for detection of some biomarkers that cannot be identified via brain imaging [125].
CSF represents a logical source for developing viable biomarkers in AD given its direct interaction with the extracellular space in the brain, thus potentially reflecting the associated pathophysiological alterations [32]. The overall safety record of lumbar puncture is strongly supported by extensive meta-analyses [76, 262]. However, fluid biomarkers are unable to reflect brain regional pathogeographies, which may be particularly important during early AD [47, 281]. Other limitations of CSF include the relative invasiveness of CSF collection by lumbar puncture, limited access and acceptability in some countries, the inability to collect samples from large populations especially if serial measures are needed, concerns over slowing for subject recruitment into clinical trials, educational gaps on the safety of lumbar puncture, development and validation of CSF assays, and clinical utility. Some of the limitations of CSF have prompted research efforts into the development and validation of diagnostic or prognostic AD biomarkers in blood [215, 216]. Indeed, the Biofluid Based Biomarkers Professional Interest Area [of the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART)], an international working group of leading AD scientists, has been established to scrutinize potential blood-based biomarkers and to provide standards for the collection of biofluids [128, 140, 268, 269].
The ideal fluid biomarker for AD would be reliable, reproducible, non-invasive, simple to measure, and inexpensive [360], as well as easy to implement into large populations such as clinical trials and the primary care setting. Importantly, biomarkers used in drug-development programmes should be qualified for a specific context of use (COU); these include (but are not limited to) patient/clinical trial diagnosis and subject selection, assessment of disease state and/or prognosis, assessment of mechanism of action, dose optimization, drug–response monitoring, efficacy maximization, and toxicity/adverse reaction identification and minimization [95]. For successful AD drug development, it is critical to ensure that subjects enrolled into clinical trials are those who have AD pathology and are most likely to progress along the disease continuum. Fluid biomarkers could have an important role in clinical trial subject selection (including subject enrichment or stratification) [123, 126, 127], and could be useful for measuring target engagement of the drug and the impact of the drug on the pathogenic mechanisms [184, 279]. Additionally, fluid biomarkers, especially blood biomarkers, could be used in early screening in primary care to identify potential clinical trial subjects and patients at risk of AD, thereby improving early diagnosis and enabling longitudinal tracking of various disease indicators over extended periods of time [22, 128].
Currently, three core AD CSF biomarkers are included in research guidelines for AD and are being increasingly used in clinical trials as inclusion criteria and/or outcome measures: CSF amyloid beta 42 (Aβ42), total tau (t-tau), and tau phosphorylated at threonine 181 (p-tau) [75, 214, 217, 247, 298]. These biomarkers have been validated as core CSF biomarkers of AD pathophysiology [33, 99, 122, 124]. Qualification opinions have also been published for CSF Aβ42 and t-tau by the EMA, supporting their use as patient-selection tools [153]. Although these core biomarkers are now recognized for their diagnostic utility, there is a need to identify additional fluid biomarkers for other COUs such as subject enrichment, risk stratification, prognosis, and (eventually) drug–response monitoring, and to better understand the complex heterogeneity of AD pathology [78, 96]. Several novel biomarkers have been proposed; some have been extensively investigated, but they have yet to be validated and integrated into guidelines for use in clinical practice and drug development [99, 206].
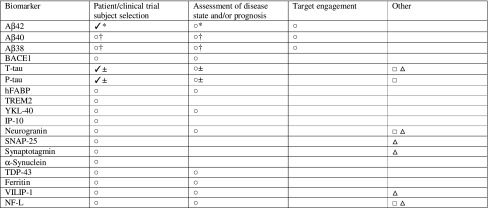
This review summarizes some of the pathological mechanisms implicated in sporadic AD (Fig. 1) and highlights several established and novel fluid biomarkers associated with each mechanism. For each biomarker, a summary of published studies, the stage of assay development (Table 1), and the potential COU (Table 2) are discussed. Most of the fluid biomarkers examined in this review are CSF biomarkers, owing to the limited number of published studies on blood-based biomarkers. It should be noted that unselected biomarker combinations (“panels”) identified through “omics” technologies are not included; this topic has been recently reviewed elsewhere [46, 219, 222, 260].
Fig. 1.
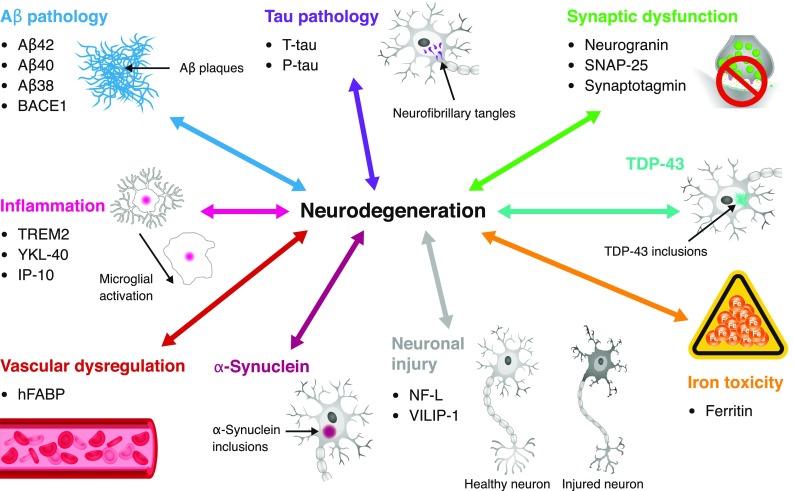
Pathological mechanisms implicated in AD and associated fluid biomarkers. In this figure, the arrows reflect hypothetical relationships, not direct causal links between the pathological mechanisms and neurodegeneration. Only select pathological mechanisms (and associated biomarkers) of AD are represented. Aβ38 amyloid beta 38, Aβ40 amyloid beta 40, Aβ42 amyloid beta 42, AD Alzheimer’s disease, BACE1 β-site amyloid precursor protein cleaving enzyme 1, hFABP heart-type fatty acid-binding protein, IP-10 interferon-γ-induced protein 10, NF-L neurofilament light, P-tau phosphorylated tau, SNAP-25 synaptosome-associated protein 25, TDP-43 transactive response DNA-binding protein 43, TREM2 triggering receptor expressed on myeloid cells 2, T-tau total tau, VILIP-1 visinin-like protein 1
Table 1.
Summary of selected candidate AD fluid biomarkers
| Biomarker | Stage of clinical validation | Levels in AD vs healthy controls | Stage of assay development | |
|---|---|---|---|---|
| CSF | Plasma/serum | |||
| Aβ42 | CSF Aβ42 is accepted as part of research criteria [75, 247] IWG-2 criteria recommend using CSF Aβ42 in combination with CSF t-tau or p-tau [75] Many studies on plasma but inconsistent results [160, 227, 271, 273, 274, 377] |
Consistently decreased in CSF Inconsistent results in plasma, although recent studies have shown a decrease |
Commercially available assays, including fully automated (IVDs in Europe) | Commercially available assays |
| Aβ40 | Many studies on CSF and plasma Inconsistent results for Aβ40 alone [73, 83, 160, 243, 248, 271, 338] Consistent results for ratio of CSF Aβ42/Aβ40 [161, 202, 205, 275, 276, 294] Consistent results for ratio of plasma Aβ42/Aβ40 [31, 87, 179, 261, 273, 371, 373, 377, 383] |
Aβ40 alone: inconsistent results in CSF and plasma Ratio of Aβ42/Aβ40: consistently decreased in CSF and plasma |
Commercially available assays (IVDs in Europe) | Commercially available assays |
| Aβ38 | Several studies on CSF Aβ38 alone Inconsistent results for Aβ38 alone [161, 243, 271] Very few studies on CSF Aβ42/Aβ38 [161, 259] One study on plasma Aβ38 [273] |
Aβ38 alone: inconsistent results in CSF and plasma Ratio of Aβ42/Aβ38: decreased in CSF but data are limited |
Commercially available assays | Commercially available assays |
| BACE1 | Several studies on CSF Inconsistent results [79, 81, 258, 283, 311, 392, 401, 407] Very few studies on plasma [330, 393] |
Inconsistent results in CSF but most studies showed increased levels/activity Increased activity in plasma but data are limited |
Commercially available assays | Commercially available assays |
| T-tau | CSF t-tau is accepted as part of research criteria [75, 247] IWG-2 criteria recommend using CSF t-tau in combination with CSF Aβ42 [75] Several studies on plasma Consistent results [61, 68, 245, 251, 271, 404] |
Consistently increased in CSF Consistently increased in plasma |
Commercially available assays, including fully automated (IVDs in Europe) | Commercially available assays |
| P-tau | CSF p-tau is accepted as part of research criteria [75, 247] IWG-2 criteria recommend using CSF p-tau in combination with CSF Aβ42 [75] Few studies on plasma or serum Consistent results [329, 358, 395] |
Consistently increased in CSF Increased in plasma and serum but data are limited |
Commercially available assays, including fully automated (IVDs in Europe) | In-house assays |
| hFABP | Several studies on CSF Consistent results [53, 67, 119, 146, 154, 201, 271] Very few studies on plasma or serum [254, 271] |
Consistently increased in CSF No change in plasma or serum but data are limited |
Commercially available assays | Commercially available assays |
| TREM2 | Few studies on CSF Inconsistent results [38, 107, 139, 141, 287, 344, 346] Few studies on blood [145, 255, 352] |
Inconsistent results in CSF but most studies showed an increase No change in plasma levels but data are limited; increased mRNA and protein levels in blood cells but data are limited |
Commercially available assays | Commercially available assays |
| YKL-40 | Several studies on CSF Inconsistent results [2–4, 11, 23, 57, 106, 138, 176, 244, 271, 303, 347, 384] Very few studies on plasma [54, 57] |
Inconsistent results in CSF but most studies showed an increase Increased in plasma but data are limited |
Commercially available assays | Commercially available assays |
| IP-10 | Few studies on CSF Inconsistent results [29, 101, 384] Very few studies on plasma or serum [102, 154] |
Inconsistent results in CSF Inconsistent results in plasma or serum |
Commercially available assays | Commercially available assays |
| Neurogranin | Many studies on CSF Inconsistent results [63, 64, 138, 175, 189, 190, 221, 242, 282, 291, 310, 347, 354, 361] Few studies on plasma [63, 110, 190, 389] |
Inconsistent results in CSF but most studies showed an increase No change in plasma but studies are limited; decreased in plasma neuronally derived exosomes but data are limited |
Commercially available assays | Commercially available assays |
| SNAP-25 | Two studies on CSF [36, 347] No studies on plasma |
Increased in CSF but data are limited Unknown for plasma |
Commercially available assays | Commercially available assays |
| Synaptotagmin | One study on CSF [270] One study on plasma [110] |
Increased in CSF but data are limited Decreased in plasma neuronally derived exosomes but data are limited |
Commercially available assays | Commercially available assays |
| α-Synuclein | Few studies on CSF Inconsistent results [27, 170, 172, 183, 232, 339, 366] Very few studies on plasma [49, 331] |
Inconsistent results in CSF but most studies showed an increase No change in plasma but data are limited |
Commercially available assays | Commercially available assays |
| TDP-43 | No studies on CSF Very few studies on plasma [97, 387] |
Unknown for CSF Increased in plasma but data are limited |
Commercially available assays | Commercially available assays |
| Ferritin | Very few studies on CSF [13–15] Very few studies on plasma [15, 112] |
No change in CSF but data are limited; increased CSF levels are associated with cognitive decline but data are limited No change in plasma but data are limited; plasma levels are associated with PET Aβ but data are limited |
Commercially available assays | Commercially available assays |
| VILIP-1 | Several studies on CSF Inconsistent results [17, 176, 194, 230, 257, 271, 347, 355–357] One study on plasma [355] |
Inconsistent results in CSF but most studies showed an increase Increased in plasma but data are limited |
Commercially available assays | Commercially available assays |
| NF-L | Several studies on CSF Consistent results [4, 220, 271, 282, 288, 334, 335, 403] Few studies on plasma or serum [240, 385, 408] |
Consistently increased in CSF Increased in plasma but data are limited |
Commercially available assays (IVDs in Europe) | Commercially available assays |
Aβ38 amyloid beta 38, Aβ40 amyloid beta 40, Aβ42 amyloid beta 42, AD Alzheimer’s disease, BACE1 β-site amyloid precursor protein cleaving enzyme 1, CSF cerebrospinal fluid, hFABP heart-type fatty acid-binding protein, IP-10 interferon-γ-induced protein 10, IVD in vitro diagnostic, IWG-2 International Working Group 2, NF-L neurofilament light, P-tau phosphorylated tau, SNAP-25 synaptosome-associated protein 25, TDP-43 transactive response DNA-binding protein 43, TREM2 triggering receptor expressed on myeloid cells 2, T-tau total tau, VILIP-1 visinin-like protein 1
Table 2.
Potential uses for selected candidate AD fluid biomarkers
Aβ38 amyloid beta 38, Aβ40 amyloid beta 40, Aβ42 amyloid beta 42, AD Alzheimer’s disease, BACE1 β-site amyloid precursor protein cleaving enzyme 1, hFABP heart-type fatty acid-binding protein, IP-10 interferon-γ-induced protein 10, NF-L neurofilament light, P-tau phosphorylated tau, SNAP-25 synaptosome-associated protein 25, TDP-43 transactive response DNA-binding protein 43, TREM2 triggering receptor expressed on myeloid cells 2, T-tau total tau, VILIP-1 visinin-like protein 1
✓Accepted (validated) use
○Potential use, supportive data available
□Speculative use for drug response monitoring, no supportive data available
△Speculative use for toxicity/adverse reactions minimization, no supportive data available
*Alone or when measured as a ratio with tau
†When measured as a ratio with Aβ42
±Alone or when measured as a ratio with Aβ42
Alzheimer’s disease pathological mechanisms
Extracellular plaque deposits of Aβ peptides and intraneuronal tau-containing neurofibrillary tangles (NFTs) and neuropil threads (NTs), are the defining neuropathological features of AD brains [238, 314]. Aβ-plaque deposition is an insidious process that occurs over decades, well before symptoms emerge [156, 379]. Approximately one-third of people over the age of 65 years who are cognitively normal have Aβ-plaque deposition equivalent to that of someone with AD [305]; the significance of this finding is a topic of intensive evaluation.
In AD, comorbid pathology is often present and contributes to the clinical symptoms. For example, there is an increasing burden of cerebrovascular pathology as a function of age, and approximately 30% of AD patients have concomitant cerebrovascular disease [365]. In addition to plaques and tangles, more than half of AD patients also show widespread cortical Lewy bodies (LBs) and Lewy neurites formed by misfolded α-synuclein like those found in patients with Parkinson’s disease dementia (PDD) or dementia with Lewy bodies (DLB) [121, 207]. Conversely, approximately 40% of DLB patients have AD pathology as determined by their CSF-biomarker profile [199]. Furthermore, up to half of AD patients harbour transactive response DNA-binding protein 43 (TDP-43) inclusions that are characteristic of frontotemporal lobar degeneration (FTLD) and sporadic amyotrophic lateral sclerosis (ALS) [7, 50, 159]. Amylin deposits, which are found in the pancreas of most patients with type 2 diabetes mellitus, have also been found in AD (and type 2 diabetes) brains [157]. Thus, although AD is typically characterized by Aβ plaques and NFTs, most AD patients have multiple pathologies and different types of brain proteinopathies [21]. In the Center for Neurodegenerative Disease Research (CNDR) Brain Bank at the University of Pennsylvania, only 35% of 247 autopsy-confirmed AD brains examined for the presence of tau, Aβ, α-synuclein, and TDP-43 deposits had only plaques and tangles as the underlying cause of dementia, while 22% had all four of these pathologies [300]. This finding emphasizes the urgent need for biomarkers that can indicate the presence of multiple pathologies in AD patients, with the methodological attributes of being reliable, accessible, and cost-efficient.
Aβ pathology
The “amyloid cascade hypothesis”, initially proposed in 1992 [134], but essentially embedded in the reports of the initial discovery of the partial Aβ sequence in cerebral vessels [108, 109] and the whole 4 kDa Aβ peptide in plaques [239], is supported by genetic and biochemical data, and has been the dominant model of AD pathogenesis. The model is based on the gradual deposition of fibrillar Aβ as diffuse plaques, which triggers an inflammatory response, altered ion homeostasis, oxidative stress, and altered kinase/phosphatase activity, leading to the formation of NFTs and to widespread synaptic dysfunction and neuronal loss [320]. Notably, recent experimental evidence suggests that the Aβ plaque environment can accelerate the templated spread of tau pathology [136].
Genetic data strongly implicate Aβ in AD pathogenesis [315, 390]. Whether monomeric or aggregated forms are more relevant to the neurodegenerative process remains unknown. Recent reports indicate that soluble Aβ oligomers may be more toxic than Aβ neuritic plaques [320, 321], suggesting that other forms of Aβ may be more relevant to measure. Phase 3 clinical studies of solanezumab, a monoclonal antibody (mAb) that targets monomeric Aβ, resulted in very modest slowing of clinical decline [72], whereas a Phase 1b study of aducanumab, a mAb that targets soluble and insoluble forms of aggregated Aβ, demonstrated robust plaque reduction and a slowing of clinical decline [42, 323].
Fluid biomarkers of Aβ metabolism and aggregation
Aβ peptides
Aβ is generated as the result of the sequential cleavage of amyloid precursor protein (APP) by β-site amyloid precursor protein cleaving enzyme 1 (BACE1) and γ-secretase [45, 394]. The cleavage position of the γ-secretase in the transmembrane domain of APP is imprecise, resulting in the production of Aβ peptides of variable length [166, 289]. Changes in some of these Aβ species have been associated with AD, as discussed below, but little is known about the changes over time in relation to clinical presentation.
Aβ42
The 42-amino acid form of Aβ, Aβ42, is a minor component of Aβ peptides in the CSF [290] and plasma [277], but in AD brains, Aβ42 is the principal Aβ peptide in plaques [113, 155]. Decreases in CSF Aβ42 levels in AD patients were first reported by Motter et al. [256]. Several subsequent studies have consistently shown that CSF levels of Aβ42 correlate inversely with plaque load as observed in autopsies and in vivo with positron emission tomography (PET) [82, 114, 158, 343, 353]. CSF Aβ43 is also reported to decrease in AD, but it has similar diagnostic accuracy to CSF Aβ42 [39, 193] so research has focused on the latter.
CSF Aβ42, together with t-tau and p-tau, are biomarkers accepted as supportive of an AD diagnosis [75, 247] (Table 2), and evidence suggests they may be prognostic of disease progression in both cognitively normal individuals [84, 209] and those with mild cognitive impairment (MCI) [5, 9, 91, 133]. CSF Aβ42 has the potential to discriminate AD from FTLD but shows significant overlap with other non-AD dementias [80].
The development of automated assays to measure CSF Aβ42 will reduce variability among samples and laboratories and make it easier to interpret results and implement this biomarker into routine clinical practice [295]. However, several unresolved issues remain when using CSF Aβ42 in clinical trials. First, there needs to be a better understanding of how to interpret changes in CSF Aβ42 levels in AD in response to DMTs, since this is likely to vary with the mechanism of action of the DMT and with the duration of treatment. It seems logical to measure Aβ42 to help determine target engagement of drugs designed to reduce Aβ pathology [88, 208]; however, some trials have reported changes in CSF Aβ42 but no improvement in clinical endpoints [298]. Furthermore, truncated, post-translationally modified fragments of Aβ (e.g. pyroglutamate Aβ42) may be more prone to pathogenic aggregation [25, 117], and consideration needs to be given to what forms of Aβ42 are being measured. In addition, CSF Aβ42 remains relatively stable over time in patients with AD dementia and may have limited utility for monitoring disease progression in this group [34, 41, 367, 402]. Finally, CSF Aβ42 measures are influenced by pre-analytical factors such as the type of collection tube and number of freeze/thaw cycles [198, 284, 368], so it is essential to develop harmonized standard operating procedures for sample collection and handling, as established for biomarker studies in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [326, 327].
There has been great interest in developing new techniques to measure Aβ42 in blood. Although most studies have failed to show an association between plasma Aβ42 alone and risk of AD or association with PET Aβ [227, 271], recent studies using ultrasensitive analytical assays as well as fully automated immunoassays suggest that plasma Aβ could be a useful screening biomarker. Using an ultrasensitive immunoassay technique (Simoa platform), levels of Aβ42 and the ratio of Aβ42/Aβ40 in plasma were shown to correlate with CSF levels and with Aβ deposition measured by PET [160, 377], and plasma Aβ42/Aβ40 associated with risk of progression to MCI or dementia in cognitively normal individuals with subjective cognitive decline [377]. Ovod et al. used mass spectrometry to demonstrate lower levels of plasma Aβ42 and Aβ42/Aβ40 in subjects with an Aβ-positive PET [273]. In addition to the use of novel technologies, plasma samples could be chemically treated to reduce degradation of Aβ and improve the accuracy of plasma Aβ measurements [278]. Recently, a fully automated immunoassay has been shown to detect plasma Aβ42 and Aβ40 and accurately predict Aβ-positivity (using the CSF Aβ42/40 ratio as reference standard) in cognitively normal, subjective cognitive decline, MCI and AD dementia patients [274].
In summary, CSF Aβ42 is widely accepted and used as an AD biomarker, and both CSF and plasma Aβ42 continue to be intensively studied (Tables 1 and 2). CSF Aβ42 is recognized as a core biomarker for AD diagnosis and is currently being considered by the FDA for qualification for subject selection in clinical trials (Table 2). It shows great promise as a biomarker for prognosis and has the potential to be used during drug trials to help assess target engagement. Plasma Aβ42 may prove to be useful for subject/patient selection (screening) and research is ongoing. There are several commercially available assays, and for CSF Aβ42, there are in vitro diagnostic (IVD) assays in Europe and fully automated assays.
Aβ40
Aβ40 is the predominant form of Aβ peptide in the brain [322, 348, 349], CSF [290, 322], and plasma [277], but it does not appear to be as pathogenic as Aβ42 [246]. Aβ40 may have protective effects against Aβ plaque formation [180] but it is the relative amount of Aβ40 to Aβ42 that may be more important than the absolute amounts of either peptide [188].
Research on CSF Aβ40 and its correlation with AD dementia has shown inconsistent results [73, 248, 338], and a meta-analysis by Olsson et al. (data from 25 AD cohorts and 24 control cohorts) found only a minor association [271]. One study demonstrated an association between PET Aβ and CSF Aβ40 levels (as well as CSF Aβ38 and a combination of Aβ40 and Aβ38), although the association was stronger in individuals who were not carriers of the apolipoprotein-E (APOE) ε4 allele (a major genetic risk factor for AD) compared with APOE ε4-positive individuals [243]. CSF Aβ40 may be useful (together with other biomarkers) in assessing target engagement of drugs such as BACE1 inhibitors, which selectively decrease Aβ40 and Aβ42 [173].
Although CSF Aβ40 shows no consistent change in AD across studies, the ratio of CSF Aβ42/Aβ40 has been shown to be a better predictor of Aβ-positive PET than CSF Aβ42 alone [161, 202, 205, 276], and comparable to the ratios of t-tau/Aβ42 and p-tau/Aβ42 [275, 294]. The Aβ42/Aβ40 ratio also appears to be better than CSF Aβ42 alone at distinguishing AD from non-AD dementias [73, 161]. Assessment of the CSF Aβ42/Aβ40 ratio (together with CSF tau levels), instead of absolute levels of Aβ42, may reduce misdiagnosis of cognitively normal individuals who are low Aβ producers and AD patients who are high Aβ producers [388], and to correct for inter-individual differences in CSF dynamics. The use of the Aβ42/Aβ40 ratio can also help to reduce the impact of pre-analytical factors affecting Aβ42 (and Aβ40) levels [104].
Studies on plasma Aβ40 have had mixed results [160, 248], but the meta-analysis by Olsson et al. (data from 21 AD cohorts and 19 control cohorts) found no difference between AD and controls [271]. In a separate study, plasma Aβ40 did not correlate with Aβ-positive PET in cognitively normal elderly individuals [83]. However, as with CSF, the ratio of Aβ42/Aβ40 in plasma may be more useful than Aβ40 alone; plasma Aβ42/Aβ40 appears to be associated with an increased risk of progression to AD dementia [373, 377], and has shown promise in detecting Aβ-positivity [87, 261, 273, 274, 371, 377] and supporting the diagnosis of AD [31, 179, 383].
Overall, the data suggest that CSF or plasma Aβ40 alone has limited utility as a biomarker for AD diagnosis but could be used to confirm target engagement of certain drugs (Tables 1 and 2). The ratio of Aβ42/Aβ40 in CSF and plasma appears to be useful for subject/patient selection and may be superior to Aβ42 alone (Table 2). Additionally, fluid-based Aβ42/Aβ40 may be useful for prognosis but the data are limited. Commercial assays are available for fluid-based Aβ40 (and Aβ42), and IVD assays are available for CSF Aβ40 (and Aβ42) in Europe. Ongoing research may lead to the availability of validated assays for blood-based Aβ peptides in the future.
Shorter Aβ peptides
Aβ peptides shorter than 40 residues have been evaluated for potential utility as AD biomarkers. CSF Aβ38 was included in the meta-analysis by Olsson et al. (eight studies were analyzed) but there was no difference between AD patients and control subjects [271]. However, CSF Aβ38 has been found to correlate with PET Aβ [243] and the ratio of CSF Aβ42/Aβ38 is better at predicting Aβ-positive PET than CSF Aβ42 alone (and comparable to CSF Aβ42/Aβ40) [161]. Furthermore, CSF Aβ42/Aβ38 may be useful for differentiating between AD and DLB [259] and other non-AD dementias [161].
Another possible use for shorter Aβ peptides is to demonstrate target engagement of drugs designed to affect Aβ processing. For example, treatment with γ-secretase modulators is associated with a selective decrease in CSF Aβ42 and Aβ40 and an increase in Aβ38 and Aβ37 [272], so these biomarkers can be used to monitor patients receiving these drugs [341].
In summary, the evidence is limited for fluid-based Aβ peptides < 40 residues (Table 1) but commercial assays are available for CSF Aβ38 and this biomarker has the potential to be used for subject/patient selection (in combination with Aβ42) and to help demonstrate target engagement of γ-secretase modulators (Table 2).
Aβ oligomers
Aβ oligomers may play a key role in AD pathogenesis so the accurate detection and quantification of these species in CSF or blood could prove useful. Different technologies have been investigated and some have shown promise; examples include ELISA-based methods in CSF [143, 312, 396] and in plasma [381], single-molecule fluorescence microscopy in CSF [144], and a protein misfolding cyclic amplification assay method in CSF [308]. However, the overall findings have been inconsistent or unsatisfactory (reviewed by Schuster and Funke [318]). A number of methodological issues complicate measurement of Aβ oligomers, including the fact that the oligomeric state of these proteins varies and is affected by numerous factors.
BACE1
BACE1 has been shown to have several physiological functions in addition to APP processing [376, 394]. It is believed to be a major protease for cell surface proteolysis, contributing to ~ 19% of identified shed proteins [186], including neuregulin, which has important functions in myelination [94]. Therefore, monitoring of BACE1 activity may be helpful in subjects receiving investigational BACE1 inhibitors.
CSF BACE1 activity and/or protein levels have been reported to be higher in subjects with MCI compared with AD patients or controls [407], and higher in AD patients versus controls [79, 258, 401]. Furthermore, the APOE ε4 allele has been associated with increased CSF BACE1 activity in both AD and MCI subjects [81]. CSF BACE1 activity has also been shown to be higher in subjects with MCI who progressed to AD compared with those with stable MCI [401]. However, some studies have found no differences in BACE1 activity among AD, MCI, and control groups [283, 311], and one study found a decline in age-adjusted CSF BACE1 activity in AD patients compared with controls [392]. A recent study of elderly healthy subjects, who received chronic treatment with a BACE1 inhibitor, reported no change in CSF BACE1 levels after BACE1 inhibition, but did find strong correlations between levels of CSF BACE1 and its downstream markers including CSF Aβ42 [362].
Plasma BACE1 has also been studied and has been shown to differentiate AD patients from controls [330, 393]. In addition, plasma BACE1 activity was found to be higher in subjects with MCI who progressed to AD compared with those with stable MCI or AD [330].
Overall, studies of BACE1 have given mixed results, and the association between BACE1 and AD remains unclear (Table 1). Recent research on BACE1 activity in plasma shows potential for subject/patient selection and prognosis (Table 2) but further studies are needed to validate the initial findings. Commercial assays are available to measure both BACE1 protein levels and BACE1 activity.
Tau pathology
Tau is a microtubule-associated protein comprised of six human isoforms predominantly located in the axon of neurons [177]. Neuronal and/or glial inclusions of tau can be detected in several neurodegenerative diseases, or “tauopathies”, including AD [152], which may be characterized, to some extent, by their tau isoform profile [252]. The NFTs characteristic of AD are composed primarily of hyperphosphorylated tau [19, 196].
The abnormal phosphorylation of tau in AD has been hypothesized to be driven by Aβ pathology [19, 177], although transgenic mice genetically engineered to develop Aβ plaques do not develop tau tangles [197], except after intracerebral injections of AD brain-derived tau [136].
Hyperphosphorylation of tau has several pathogenic effects. It reduces tau’s affinity for microtubules, and increases its likelihood to aggregate and fibrillize [309]. This leads to destabilization of microtubules with subsequent axonal transport failure and neurodegeneration, which can be offset or corrected by microtubule-stabilizing drugs [24, 40, 405]. Hyperphosphorylation of tau is thought to cause its mislocalization to somatodendritic compartments, where it interacts with Aβ to cause synaptotoxicity through the excessive activation of the N-methyl-D-aspartate (NMDA) receptors [177]. In addition, hyperphosphorylation of tau is implicated in Aβ-induced cell death [200], possibly via a toxic gain of function mechanism [89].
Studies have shown that the density of neocortical NFTs has a stronger correlation than Aβ plaques with ante-mortem cognitive status (reviewed by Nelson et al.) [263]. This finding, together with the involvement of tau in neurodegenerative processes, has led to increasing interest in tau as a therapeutic target for AD, with several compounds now in the early stages of clinical development [19, 60].
Fluid biomarkers of tau pathology
T-tau and p-tau
CSF t-tau and p-tau (tau phosphorylated at threonine 181), together with CSF Aβ42, are considered core biomarkers to support AD diagnosis [75, 247] (Table 2). Both CSF t-tau and p-tau differentiate AD from controls, and given that CSF p-tau levels are normal in most other dementias, this biomarker is also important for differential diagnosis [33]. In Creutzfeldt–Jakob disease (CJD), CSF t-tau levels are very high (around 20 times higher than in AD), whereas p-tau is close to normal [297, 336]. As with CSF Aβ42, CSF tau has the potential to predict disease progression in cognitively unimpaired individuals [301] and in those with MCI [91, 285]. CSF t-tau has been shown to predict more aggressive disease progression in patients with MCI due to AD or in mild-to-moderate AD [65].
Although CSF t-tau and p-tau are well-established AD biomarkers, their utility for diagnosis of AD is markedly improved when measured in combination with Aβ42 [75]. Hulstaert et al. found that the combination of CSF tau and Aβ42 was better than the individual biomarkers at discriminating AD patients from controls or subjects with other neurological disorders [148]. In the initial CSF study of the ADNI cohort, a logistic regression model combining Aβ42, t-tau, and the APOE ε4 allele count showed a stronger association with mild AD than Aβ42, t-tau, p-tau, or tau/Aβ42 alone [326]. Both CSF t-tau/Aβ42 and p-tau/Aβ42 ratios have been shown to outperform any of the individual biomarkers for distinguishing individuals with an Aβ-positive PET [85]. In a study of the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort, CSF t-tau/Aβ40 and p-tau/Aβ42 were the best discriminators of autopsy-confirmed AD from controls [319]. The combination of CSF tau and Aβ42, in particular, p-tau/Aβ42, has also shown promise for differentiating AD from other dementias [299, 319].
The combination of tau and Aβ markers has also demonstrated their utility for predicting disease progression. CSF t-tau/Aβ42 and p-tau/Aβ42 have been shown to predict cognitive decline in cognitively normal individuals [84], and the combination or ratios of tau (t-tau or p-tau) and Aβ42 have been shown to be better at predicting progression from MCI to AD than the individual biomarkers [91, 374]. Furthermore, the EMA approved CSF Aβ42/t-tau for use as an enrichment biomarker in a study of a γ-secretase inhibitor [77]. More recently, the synergistic interaction between CSF p-tau and Aβ imaging was found to be associated with the progression from MCI to AD dementia [280].
CSF t-tau and p-tau are frequently measured in clinical trials but, as with CSF Aβ42, the relationship between clinical endpoints or therapeutic drug (interventional) response and these biomarkers is unclear [298], owing to their small longitudinal variation [34, 41] and the lack of DMTs that precludes testing their performance. Other challenges include variability in measured values due to pre-analytical and analytical factors, and the lack of consensus on cut-off values [98]. However, it is feasible that CSF tau could be used to assess target engagement of tau-targeted drugs [174, 363].
In addition to the CSF tau biomarkers, plasma tau has also been evaluated and has shown potential for clinical utility. The meta-analysis by Olsson et al. (data from six AD cohorts and five control cohorts) found an association between plasma t-tau and AD [271]; however, no significant difference in plasma t-tau has been reported between MCI subjects and controls [61, 245, 404]. Elevated plasma t-tau is associated with lower grey matter density but the brain atrophy pattern associated with plasma t-tau is different from that of CSF t-tau [68]. Longitudinally, higher levels of plasma t-tau have been associated with greater cognitive decline and risk of MCI [245, 251]. Notably, the relationship between plasma t-tau and cognition was independent of elevated brain Aβ [251]. These findings suggest that plasma t-tau could be useful as a screening tool or a prognostic marker for non-specific cognitive decline in cases where acute central nervous system (CNS) injury has been ruled out. Blood-based p-tau has also been measured in a few recent studies and found to be elevated in AD patients [329, 358, 395] and MCI subjects [329, 395] compared with controls. In addition, platelet-derived tau has been explored, and preliminary studies suggest that the ratio of high molecular weight to low molecular weight tau is higher in AD than in controls [264, 337]. However, the role of platelet-derived tau is not clear; it could either be a confounder or help to clarify the relationship between central and peripheral compartment tau measurements.
Although most tau biomarker research has focused on t-tau and p-tau, studies suggest that a variety of other tau peptides and fragments can be detected in CSF [250] and in serum [150], and some may have potential as AD biomarkers. For example, one study found that 11 (of 47) different tau phosphopeptides were upregulated in AD patients relative to controls [306], while another study found that non-phosphorylated tau also has potential as a diagnostic biomarker [204]. These initial studies may lead to further research into novel tau biomarkers, which may be especially helpful in the development of tau-directed therapies.
In summary, CSF t-tau and p-tau are widely accepted and used in AD research (Tables 1 and 2). They are recognized as core biomarkers to support the diagnosis of AD and are currently being considered for qualification by the FDA for subject selection in clinical trials (Table 2). CSF tau also shows promise as a biomarker for prognosis and target engagement. Commercial assays are available for both CSF t-tau and p-tau, including IVD assays in Europe and fully automated assays. There has been recent renewed interest in plasma t-tau, which shows potential for subject/patient selection (screening) and for prognosis (Table 2). In addition, research into other tau biomarkers (blood-based p-tau, platelet-derived tau, tau peptides/fragments) is ongoing but is still in its early stages.
Vascular dysregulation
Concurrent cerebrovascular disease is more common in AD than in most other neurodegenerative disorders [365] and vascular dysregulation as a contributing factor to AD has been a long-standing hypothesis for AD pathogenesis [296, 340]. The time sequence of the impact of vascular dysregulation has been debated, but recent work supports the possibility that these changes may be an early pathological event that precedes Aβ pathology. The spatiotemporal changes in various neuroimaging, plasma, and CSF biomarkers from the ADNI cohort, suggest that vascular dysregulation is the earliest and strongest pathological factor associated with late-onset AD, followed by Aβ deposition, glucose metabolism dysregulation, functional impairment, and grey matter atrophy [154].
Vascular dysregulation reduces oxygen and nutrient supply to the brain, causing cell damage and dysfunction of the blood–brain barrier, which lead in turn to neurotoxic effects such as oxidative stress and inflammation [69]. The hypoxic conditions are thought to increase the accumulation of Aβ peptides through the activation of BACE1 and γ-secretase [307]. Additionally, disruption of the blood–brain barrier has been suggested to impair clearance of Aβ peptides from the brain [48].
Fluid biomarkers associated with the vascular system
hFABP
Heart-type fatty acid-binding protein (hFABP), which has been proposed as a biomarker of myocardial infarction [1], was the CSF analyte with the highest degree of abnormalities in the spatiotemporal analysis of the ADNI cohort [154]. It was also identified as a potential AD biomarker in an independent cohort [146]. FABP showed associations with CSF Aβ42 levels but not with cognitive impairment [201]. In the meta-analysis by Olsson et al., CSF hFABP had a moderate association with AD (data from five AD and control cohorts), with a lower degree of change in AD versus controls than seen for t-tau [271]. CSF hFABP has also been shown to predict progression from MCI to AD [119], correlate with brain atrophy among individuals with low CSF Aβ42 [67], differentiate AD and DLB from Parkinson’s disease (PD) and other neurological diseases [53], and correlate with cognitive impairment in a cohort of patients with different neurodegenerative diseases [53]. The source of hFABP in CSF is uncertain but it is highly expressed in the brain where hFABP levels are second only to levels in muscle tissues (https://www.proteinatlas.org/ENSG00000121769-FABP3/tissue).
Serum hFABP was included in the meta-analysis by Olsson et al. and showed no association with AD (data from two AD and control cohorts) [271]; one study suggested it may be useful for differentiating between AD and DLB when measured as a ratio with CSF tau [254].
In summary, recent data for hFABP suggest that it may play a more important role in AD than previously thought (Table 1). CSF hFABP could be useful for both subject/patient selection and prognosis (Table 2) but further studies are needed to confirm these hypotheses. Commercial assays are available for CSF and serum hFABP.
Inflammation/glial activation
Inflammation has been proposed as a contributor to AD pathogenesis [37, 44]. Aβ plaques and NFTs induce an immune response in the brain, which is characterized by activated glial cells [37]. Microglia and astrocytes are the two main types of glial cells implicated in the pathogenesis of AD [37]. Microglia, the resident immune effector cells of the CNS, are important for brain homeostasis as well as immune responses [52]. Astrocytes are the most abundant type of glial cell in the CNS. They have important roles in homeostasis, synaptogenesis, signal transmission, and synaptic plasticity, and provide trophic and metabolic support to neurons [342].
The activation of glial cells serves to protect the brain; however, uncontrolled and prolonged activation can lead to detrimental effects that override the beneficial effects [37]. In this condition, glial cells lose some of their homeostatic functions and acquire a pro-inflammatory phenotype. The release of pro-inflammatory molecules, reactive oxygen species, and nitric oxide contribute to neuronal cell death. In addition, pro-inflammatory molecules increase Aβ synthesis as well as tau hyperphosphorylation [37].
Fluid biomarkers of inflammation/glial activation
TREM2
Triggering receptor expressed on myeloid cells 2 (TREM2) is expressed by many cells of the myeloid lineage, including microglial cells in the CNS, and has several physiological functions including the regulation of myeloid cell number, phagocytosis, and inflammation [162]. TREM2 expression is upregulated in AD brains, where it may have a protective effect in the early stages, through the phagocytic clearance of Aβ, but a pathogenic effect in the later stages, through activation of the inflammatory response [162]. Rare TREM2 gene variants have been associated with an increased risk of developing AD [59, 116, 304, 332]. TREM2 haplodeficiency in mice and humans has been associated with increased axonal dystrophy and p-tau accumulation around Aβ plaques [400].
A soluble variant, sTREM2, can be detected in CSF and has the potential to be used as a biomarker for AD. One study found that CSF sTREM2 levels were increased in autosomal dominant AD mutation carriers 5 years before expected symptom onset but after initial Aβ deposition (as measured by PET) and changes in CSF Aβ42 and t-tau [344]. Some studies have found slightly higher CSF sTREM2 levels in AD [38, 141, 287, 346] and MCI groups [38] compared with controls, and in subjects with MCI due to AD compared with other AD groups (preclinical AD or AD dementia) [346]. However, one study found no difference between patients with AD or MCI and cognitively normal controls [139]. In patients with MCI, elevated CSF sTREM2 levels correlated with increased grey matter volume and reduced diffusivity, suggesting a role for TREM2 in the regulation of the neuroinflammatory response in early AD [107].
Levels of TREM2 mRNA in peripheral blood mononuclear cells and TREM2 protein expression on monocytes have been reported to be higher in patients with AD than in controls, and inversely correlated with cognitive performance [145]. In the same study, there was also a trend for upregulation of TREM2 protein on granulocytes and in plasma but this was not statistically significant [145]. Subsequent studies by other groups also found increased peripheral TREM2 mRNA expression in AD compared with controls [255, 352].
In summary, a few studies have observed increased levels of CSF sTREM2 and peripheral TREM2 expression in AD (Table 1), suggesting possible use in subject/patient selection (Table 2) but additional research is required to validate these findings. Commercial assays are available for the measurement of TREM2 protein.
YKL-40
YKL-40 (or chitinase-3-like protein 1) is upregulated in a variety of inflammatory conditions and cancers, and may have a role in promoting inflammation and angiogenesis [211]. In AD, YKL-40 is expressed in astrocytes near Aβ plaques [57] and correlates positively with tau pathology [293], suggesting a role for YKL-40 in the inflammatory response in AD and other tauopathies.
Several studies have shown that CSF YKL-40 levels are higher in AD patients compared with controls [4, 11, 23, 57, 176, 303, 384], and in the late preclinical AD stages compared with early preclinical stages [2]. The meta-analysis by Olsson et al. found that the degree of increase is modest (data from six AD cohorts and five control cohorts) compared with the change in neuronal proteins such as t-tau and neurofilament light (NF-L) [271]. However, a recent study of the ADNI cohort found no significant difference between the AD and cognitively normal groups, although levels were higher in AD versus MCI Aβ-negative (based on CSF Aβ42) subjects [347]. Longitudinally, all groups showed an increase in CSF YKL-40 over time, but the change was statistically significant only in the MCI Aβ-positive group (mean follow-up was 4 years) [347]. CSF YKL-40 levels have been shown to correlate with neuroimaging parameters, including cortical thickness in AD-vulnerable areas in subjects who were Aβ42-positive (by CSF) [3] and grey matter volume in APOE ε4 carriers [106].
Higher levels of CSF YKL-40 and YKL-40/Aβ42 ratio have been associated with increased risk of progression from normal cognition to MCI [57]. Levels of CSF YKL-40 have been found to predict progression from MCI to AD and increase longitudinally in MCI and AD patients but not in cognitively normal individuals [176]. CSF YKL-40 has also been shown to differentiate AD from DLB, PD [384], FTLD [23], and non-AD MCI [138], although one early study found no differences among diagnostic groups [244].
Plasma YKL-40 has also been assessed as an AD biomarker, and elevated levels have been reported in patients with mild AD [57] and early AD [54] compared with controls. However, plasma YKL-40 did not demonstrate utility for predicting cognitive decline [57].
In summary, the available evidence supports a role for CSF YKL-40 as a biomarker of neuroinflammation or astrogliosis in AD and other neurodegenerative diseases (reviewed by Baldacci et al. [20]), with the potential to aid subject/patient selection and prognosis (Tables 1 and 2). Plasma YKL-40 could also be useful for subject/patient selection, but further studies are needed. Commercial assays are available.
Other inflammatory markers
Interferon-γ-induced protein 10 (IP-10), which has roles in angiogenesis as well as inflammation and is secreted by a variety of cells [10, 223], has been reported to be increased in the CSF of patients with MCI and mild AD but not in severe AD [101]. However, in another study, IP-10 levels were not increased in the AD group [384]. In a recent study in asymptomatic older adults, increased levels of CSF IP-10 were associated with increased levels of CSF t-tau and p-tau [29].
IP-10 was the plasma analyte with the highest degree of abnormalities in a spatiotemporal analysis of biomarkers from the ADNI cohort [154]. However, a previous study found no association between serum IP-10 and AD [102].
Overall, very few studies have investigated IP-10 in AD and the results have been mixed (Table 1). Potentially, CSF or blood-based IP-10 could support subject/patient selection (Table 2), but further research is warranted to clarify the role of IP-10 in AD. Commercial assays are available.
Many other inflammatory markers have been investigated for their potential use as biomarkers for AD, but results have been inconsistent [142, 350]. In a meta-analysis of 40 studies on blood and 14 on CSF, AD patients had higher levels of interleukin (IL)-6, tumour necrosis factor (TNF)-α, IL-1β, transforming growth factor-β (TGF-β), IL-12, and IL-18 in blood, and higher levels of TGF-β in CSF, compared with controls [350]. In a more recent meta-analysis of 175 studies on blood, increased IL-1β, IL-2, IL-6, IL-18, interferon-γ, homocysteine, high-sensitivity C reactive protein, C-X-C motif chemokine-10, epidermal growth factor, vascular cell adhesion molecule-1, TNF-α converting enzyme, soluble TNF receptors 1 and 2, α1-antichymotrypsin and decreased IL-1 receptor antagonist and leptin were found in patients with AD compared with controls [191]. These findings strengthen the evidence that AD is accompanied by inflammatory responses, although the effects of age and sex and the precise roles of different inflammatory mediators are still to be established. A more systematic, within- and between-subject, rigorous longitudinal evaluation may improve the utility of inflammatory markers in AD and other neurodegenerative diseases.
Synaptic dysfunction
Synaptic dysfunction and synapse loss are early events in AD pathogenesis [167, 218, 359]. Notably, hippocampal synapse loss and impaired synaptic function were detected in 3-month-old tau transgenic mice, when pathological tau was detectable biochemically but before microscopically visible neurofibrillary tau tangles emerged [397]. The level of synaptic loss in post-mortem brains has been found to correlate with pre-mortem cognitive function in individuals with MCI or early AD [62, 313]. The synaptic pathology in AD is found throughout the neuropil, without any clear accentuation in relation to plaques [30, 235]. Importantly, the synaptic loss in AD is more severe than the neuronal loss in the same cortical region [137, 237]. A PET tracer has recently been developed that binds to synaptic vesicle glycoprotein 2A (SV2A) and can be used to quantify synaptic density in vivo; this could be used to complement existing AD imaging tools in the future [93].
Evidence suggests that NMDA receptors are central to the synaptic dysfunction observed in AD. Overstimulation of NMDA receptors triggers an excessive influx of calcium, which in turn can lead to a series of downstream events that culminate in synaptic dysfunction and apoptosis [167, 369]. Aβ oligomers are thought to contribute to NMDA activation, possibly by causing an aberrant rise in extrasynaptic glutamate levels [369].
Fluid biomarkers of synaptic dysfunction
Neurogranin
Neurogranin is predominantly expressed in dendritic spines and is involved in post-synaptic signalling pathways through the regulation of the calcium-binding protein calmodulin [70]. Animal models and genetic studies have linked neurogranin to cognitive function and synaptic plasticity [70]. Notably, CSF neurogranin has been proposed as a marker of synaptic degeneration [361] and, together with other synaptic proteins, holds promise to serve as a novel candidate marker for AD [218].
CSF neurogranin levels are higher in AD [63, 175, 190, 221, 242, 291, 310, 347, 354, 361] or MCI patients [291, 347] compared with controls or non-AD dementia patients [354]. Higher levels of CSF neurogranin have been reported in AD compared with MCI [138, 291], although there was no significant difference between AD and MCI Aβ-positive (based on CSF Aβ42) groups in a recent study of the ADNI cohort [347]. Also in the ADNI study, CSF neurogranin levels decreased longitudinally in the AD group (mean follow-up was 4 years) but there was no significant longitudinal change in any other group [347]. Neurogranin is processed to a series of C-terminal peptides before release into the CSF [190], but the relevance of the individual peptides is unknown. However, one study that used an assay specific for C-terminally truncated neurogranin observed increased levels in MCI patients but no significant difference between AD patients and controls [64]. CSF neurogranin has been shown to predict disease progression in several studies [175, 189, 291, 354] including future cognitive impairment in cognitively normal controls [354]. In addition, CSF neurogranin levels have been found to correlate with brain atrophy but only in individuals with Aβ pathology [282].
To date, no significant differences have been reported in plasma levels of neurogranin between patients with AD and controls [63, 190]. However, levels of neurogranin in neuronally derived exosomes in plasma have been found to be lower in AD patients compared with controls [110, 389], as well as in MCI subjects who progressed to AD compared with stable MCI subjects [389].
Overall, the available data indicate that CSF neurogranin (and potentially, plasma neuronally derived exosomes) could be useful as an AD biomarker for subject/patient selection and prognosis (Tables 1 and 2), although results may vary depending on the neurogranin fragment being measured (full-length vs C-terminal peptides and C-terminus intact vs truncated). Commercial assays are available.
SNAP-25 and synaptotagmin
The exocytosis of synaptic vesicles for neurotransmitter release is a complex process, mediated by several proteins including the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex and the calcium sensor protein synaptotagmin [131]. Post-mortem studies on AD brains have shown altered levels of several synaptic proteins, including synaptosome-associated protein 25 (SNAP-25), a component of the SNARE complex [74], and synaptotagmin [236, 351].
CSF levels of SNAP-25 [36, 347] and synaptotagmin [270] have been assessed and found to be elevated in patients with AD or MCI compared with controls. In a study of the ADNI cohort, baseline CSF SNAP-25 levels were higher in AD and MCI Aβ-positive (based on CSF Aβ42) groups than the cognitively normal (Aβ-positive or -negative) and MCI Aβ-negative groups [347]. CSF SNAP-25 levels decreased longitudinally in the AD group (mean follow-up was 4 years) but there was no significant longitudinal change in any other group [347]. No studies have been published to date on blood-based SNAP-25, but synaptotagmin levels in plasma neuronally derived exosomes have been reported to be lower in AD patients than in controls [110]. The data are limited but suggest there could be a role for the synaptic proteins, SNAP-25 and synaptotagmin, as AD biomarkers for subject/patient selection (Tables 1 and 2). Commercial assays are available for both SNAP-25 and synaptotagmin.
α-Synuclein pathology
α-Synuclein is an abundant neuronal protein, predominantly localized in the presynaptic terminals, and involved in vesicle fusion and neurotransmitter release [181]. Aggregates of α-synuclein are the main component of LBs, which are intracellular inclusions characteristic of certain neurodegenerative diseases termed α-synucleinopathies [181]. Primary α-synucleinopathies include PD, PDD, DLB, and multiple system atrophy [181]; however, α-synuclein aggregates are also found in approximately half of sporadic AD brains [121] and Down’s syndrome brains with concomitant AD pathology [213], and in almost all cases of familial AD with PSEN 1 mutations [203].
α-Synuclein oligomers have been shown to have multiple toxic effects including inflammation, synaptic dysfunction, compromised cell membrane integrity, and impaired intracellular protein degradation [151, 406]. Furthermore, there is growing evidence that α-synuclein may act in a prion-like manner such that misfolded α-synuclein can be propagated from cell to cell [35, 118, 164, 370], even in wild-type non-transgenic mice [229]. The relationship between AD pathology and α-synuclein is unclear, although studies suggest that α-synuclein can act synergistically with both tau [105] and Aβ [234] to promote their aggregation and accumulation.
Fluid biomarkers of α-synuclein pathology
α-Synuclein
Although CSF α-synuclein was developed as a candidate biomarker for PD, levels of CSF α-synuclein have been found to be higher in patients with MCI [183] and AD [183, 232, 339] compared with controls. However, in one study, no differences were reported between diagnostic groups except for higher levels in rapid progressors (MCI patients who developed AD during the 2-year study and had a short duration of symptoms before the study) [27]. CSF α-synuclein shows a strong correlation with CSF t-tau and a weaker correlation with p-tau in AD, but a subset of patients in the ADNI cohort had a mismatch—high p-tau accompanied by low α-synuclein levels—it was hypothesized that this CSF signature could represent concomitant LB pathology in AD patients [366].
CSF α-synuclein has been assessed as a biomarker in PD and other neurodegenerative diseases [66, 171, 253] and is a major focus area (together with tau and Aβ) of the Parkinson’s Progression Marker Initiative (PPMI) [168, 169]. α-Synuclein in plasma [212], and even in salivary secretions [380], has been investigated in PD.
CSF α-synuclein levels have been reported to be slightly lower in PD compared with AD [253] or controls [66, 168, 169, 253]. CSF α-synuclein levels were lower in DLB patients compared with AD patients in some studies [172, 253, 339], most often with a large overlap between the diagnostic groups, but the opposite was observed in one study [170]. Importantly, CSF α-synuclein levels are many-fold higher in CJD than in PD [171, 253]. Commercial assays are available for total α-synuclein and one has been clinically validated for the diagnosis of sporadic CJD [225].
Most currently available assays for α-synuclein have been designed to measure total amounts of the protein and not LB-specific fragments, although phosphorylated α-synuclein has been detected in CSF of PD patients [382]. There are reports of increased CSF concentrations of α-synuclein oligomers in CSF of PD patients [132, 233, 364], and recent publications on sensitive assays that appear to detect the minute amounts of putative seeds of α-synuclein oligomers in CSF [86, 325].
Plasma levels of α-synuclein have been reported to be elevated in patients with PD compared with controls [195] and correlate with cognitive decline [212]. No differences in plasma have been found between AD and controls [49, 331].
In summary, although fluid-based α-synuclein has promise as a diagnostic and prognostic biomarker for PD and CJD, studies in AD have been relatively limited and its potential role as a biomarker is unknown (Table 1). Nevertheless, α-synuclein may prove to be useful for identifying LB pathology among AD patients, therefore, could support subject/patient selection (Table 2).
TDP-43 pathology
TDP-43 binds both DNA and RNA and is involved in transcription and splicing. Under pathophysiological conditions, TDP-43 accumulates in the cytoplasm and is hyperphosphorylated and/or ubiquitinated, and this is characteristic of the cytoplasmic inclusions observed in ALS and in many cases of FTLD [51, 265]. TDP-43 pathology is also detected in 20–50% of AD patients [7, 50, 159], and appears to be associated with greater brain atrophy, memory loss, and cognitive impairment [50, 163]. Studies suggest that TDP-43 pathology can be triggered by Aβ peptides, and that TDP-43 contributes to neuroinflammation and may have a role in mitochondrial and neural dysfunction [50].
Fluid biomarkers of TDP-43 pathology
TDP-43
A few studies have reported on CSF and plasma TDP-43 in ALS and FTLD [165, 187, 345], but research has been hampered by difficulties with detecting the protein (candidate antibodies have been reviewed by Goossens et al.) [111]. Furthermore, CSF TDP-43 appears to be mainly blood-derived, although it may be possible to enrich for brain-specific fractions of TDP-43 from exosomes in CSF [90].
One study reported elevated plasma TDP-43 in a greater proportion of AD patients compared with controls [97]. Another small study found that plasma levels of disease-related TDP-43 variants were increased in the pre-MCI stage in subjects who subsequently progressed to AD dementia [387].
Overall, research to date suggests that blood-based TDP-43 may have a role as an AD biomarker for subject/patient selection and prognosis and could be more useful than CSF TDP-43 (Tables 1 and 2). Commercial assays are available.
Iron toxicity
Iron is important for normal functioning of the brain, but when present in excess it is known to cause neurodegeneration, for example in the genetic disorders classified as neurodegeneration with brain iron accumulation (NBIA) [135]. Studies have shown elevated iron in AD [55, 226] and MCI [372] brains, which is also replicated in animal models [210]. Iron is a redox-active biometal that has been shown to bind Aβ in vitro and cause its aggregation, while releasing hydrogen peroxide [147]. Intracellular iron can influence APP processing and bind to hyperphosphorylated tau and induce its aggregation [58]. In a recent magnetic resonance imaging study, brain iron measured by quantitative susceptibility mapping was shown to be moderately elevated in people with PET-confirmed Aβ, but highly predictive of cognitive decline over 6 years only in subjects with Aβ, suggesting that iron accelerates the clinical manifestation of the underlying pathology [16].
Fluid biomarkers associated with iron metabolism
Ferritin
Ferritin is the major intracellular iron storage protein in the body and has an important role in brain iron homeostasis [333]. Inherited ferritinopathies are associated with motor and cognitive dysfunction [333], and ferritin levels are increased in AD brain tissue [58]. CSF levels of ferritin have been shown to be higher in APOE ε4 carriers than in non-carriers, but there was no difference in levels among subjects with AD or MCI and controls [15]. Increased CSF ferritin levels were associated with cognitive decline and predicted progression from MCI to AD, regardless of APOE genotype [15]. In a subsequent analysis, CSF ferritin was associated with cognitive decline in cognitively normal subjects, but the association was strongest in APOE ε4 carriers [14]. In the same cohort, high CSF ferritin was associated with accelerated depreciation of CSF Aβ42 in subjects with a high tau/Aβ42 ratio [13]. Plasma ferritin levels showed a modest correlation with CSF levels, but unlike CSF ferritin, there was no difference in plasma ferritin between APOE ε4 carriers and non-carriers [15]. In another study, plasma ferritin levels were elevated in cognitively normal subjects with Aβ pathology identified by PET when adjusted for covariates (age, sex, APOE ε4 status, and levels of C-reactive protein), although ferritin alone had a relatively minor effect compared with the base model (derived from logistic regression of the same covariates) [112].
In summary, the data are limited but a small number of studies suggest that both CSF and plasma ferritin may be useful as AD biomarkers (Table 1). CSF ferritin may have a role as a prognostic biomarker, whereas plasma ferritin could be used for subject/patient selection (screening) to help identify preclinical AD (Table 2); however, further studies by independent groups are needed to validate the initial findings. Commercial assays are available.
Other neuronal proteins
VILIP-1
Visinin-like protein 1 (VILIP-1, or VLP-1) is a neuronal calcium sensor protein involved in signalling pathways related to synaptic plasticity [115]. CSF VILIP-1 was identified through mouse gene array analyses as being abundantly produced in the brain [192]. It was subsequently associated with AD and found to correlate with CSF t-tau and p-tau [194], supporting its use as a neurodegeneration marker.
CSF VILIP-1 levels have been shown to be higher in patients with AD compared with controls in several studies [194, 230, 257, 347, 355], although one study found no significant difference [176]. The meta-analysis by Olsson et al. found VILIP-1 to have a moderate association with AD (data from four AD and control cohorts) with around 50% higher levels in AD than in controls [271]. AD patients had higher levels than MCI subjects in one study [257] but no difference was noted in a later study [17]. In a recent study of the ADNI cohort, baseline CSF VILIP-1 levels were higher in Aβ-positive (based on CSF Aβ42) MCI and AD subjects compared with both the Aβ-negative MCI and Aβ-negative cognitively normal groups [347]. No significant differences were found between any of the Aβ-positive subsets of the cognitively normal, MCI, and AD groups. CSF VILIP-1 levels decreased longitudinally in the AD group (mean follow-up was 4 years) but there were no significant longitudinal changes in any other group [347]. CSF VILIP-1 may be prognostic of future cognitive decline [355], rates of cognitive decline [357], rates of brain atrophy [356], and progression from MCI to AD [176]. In addition, studies suggest that CSF VILIP-1 can diagnostically differentiate AD from other dementias [17, 230, 355].
Data on plasma VILIP-1 are limited; plasma levels were found to be higher in patients with mild AD compared with controls in one study, although the difference was more significant in CSF than in plasma [355].
Overall, the data for VILIP-1 suggest a possible role in subject/patient selection and prognosis (Tables 1 and 2) but study results have varied so further research is warranted. Commercial assays are available.
NF-L
Neurofilaments are intermediate filaments expressed in neurons and are particularly abundant in axons [398]. They are composed of four subunits—neurofilament light (NF-L), neurofilament middle (NF-M), neurofilament heavy (NF-H), and α-internexin in the CNS, and NF-L, NF-M, NF-H, and peripherin in the peripheral nervous system [398]. Neurofilaments are essential for the radial growth of axons during development, structural support, and the transmission of electrical impulses [398]. Recent research suggests that they are also important for normal synaptic function [399]. Abnormal aggregation and other alterations of neurofilaments are evident in several neurological diseases including AD [378, 398, 399] and in the LBs of PD [316].
CSF levels of the NF-L subunit are known to be increased in several neurodegenerative diseases, supporting its role as a marker of axonal injury [231, 286, 334]. CSF NF-L levels have been shown to be higher in AD [4, 220, 288, 334, 403] and MCI patients [403] compared with controls, and correlate with cognitive impairment and short survival time in patients with dementia [335]. The meta-analysis by Olsson et al. found CSF NF-L to have a large effect size for differentiating between AD patients and controls (data from nine AD cohorts and eight control cohorts) [271]. CSF NF-L correlates with brain atrophy [282, 403], but appears not to be specific for AD since levels are elevated in other neurodegenerative diseases, likely reflecting non-specific axonal injury [28, 103, 282]. In multiple sclerosis (MS), CSF NF-L has been shown to correlate with clinical and radiological outcomes, making it potentially useful for monitoring response to therapy [182, 249, 266, 267].
Recently, there has been great interest in the potential utility of NF-L in blood as a biomarker for several neurodegenerative diseases including AD, MS, progressive supranuclear palsy (PSP), ALS, and Huntington’s disease [43, 71, 228, 240, 302, 385, 408], as well as a marker of traumatic brain injury [224, 324]. In AD, plasma or serum levels of NF-L have been shown to be elevated compared with controls in presymptomatic individuals known to be carriers of AD-causing gene mutations [385] and subjects with MCI or AD [240, 408]. Furthermore, blood-based NF-L appears to correlate with poor cognition and brain atrophy [240, 385]. In MS, serum NF-L has demonstrated potential as a biomarker for monitoring response to DMTs and predicting relapse [71], and in PSP, plasma NF-L has been shown to predict disease progression [302].
Taken together, these findings indicate that both CSF and plasma NF-L are promising biomarkers, although the specific COU has not been determined given that changes are observed in various neurodegenerative diseases, not just AD (Tables 1 and 2). Potentially, CSF NF-L could be useful as a non-specific marker of axonal injury and for prognosis, and recent research gives hope that plasma NF-L could be used as a non-invasive biomarker for subject/patient selection (screening) and prognosis (Table 2). Commercial assays are available and IVD assays are available for CSF NF-L in Europe.
Discussion
In addition to the established core CSF biomarkers, Aβ42, t-tau, and p-tau, several candidate fluid biomarkers show potential for clinical use in AD, particularly to support diagnosis (and clinical trial subject selection) and prognosis (or assessment of disease state) (Table 2). Of all the biomarkers reviewed, CSF Aβ42, t-tau, p-tau, and the ratio of tau/Aβ42 are already accepted for use as diagnostic biomarkers, while several other biomarkers hold promise for future use (Table 1). Further studies are needed for the validation and regulatory qualification of all these biomarkers. In addition, the relationship between the biomarkers and clinical presentation (i.e. cognitive measures), as well as the effects of patient variables (e.g. sex, APOE ε4 status) on biomarker changes need to be investigated.
It should be noted that only a selection of promising biomarkers has been included in this review, and many other candidates are being studied at present. As well as other protein/peptide markers and panels [206], non-protein analytes such as lipids [8], amino acids [56], and microRNAs [120, 328] are being explored. Advances in technologies such as mass spectrometry enable the precise measurement of analytes, helping to identify new candidate biomarkers [26] as well as supporting harmonization efforts for the core biomarkers [292].
Of all the possible biomarker COUs, there appears to be an unmet need for validated fluid biomarkers for drug development, especially for monitoring response to therapy and adverse reactions (Table 2). This is not surprising given the current absence of approved DMTs but highlights the need for fluid-based surrogate biomarkers of drug efficacy and safety. The important role of biomarkers in AD drug development has been highlighted in the FDA draft guidance for industry [96].
Further development of candidate biomarkers, as well as identification of new ones, would benefit greatly from a unified and coordinated approach [100, 124, 178, 269]. There is a need to reach a consensus on the areas that require the most focus and to implement effective strategies to advance the field. This effort requires collaboration among academia, industry, laboratory managers, and clinicians, at an international level.
An ever-increasing number of biomarkers are being researched, and studies have a considerable degree of heterogeneity (biomarker collection/methodology, disease diagnosis/stage of disease, and characterization of comorbid CNS diseases, especially neurodegenerative diseases), making it difficult to interpret results and establish how the biomarkers fit within the stages of AD pathogenesis. Publication bias may be a barrier in this step, as “negative” studies may be under-published. To fast-track data dissemination, a centralized database would be useful to share individual patient-level biomarker data. The Coalition Against Major Diseases (CAMD), one of 12 consortia of the Critical Path Institute (C-Path), aims to include CSF biomarker data in a central repository as part of their on-going initiative to advance regulatory drug development tools [12].
Once the data gaps are identified, studies can be designed to address the specific unmet needs. Careful planning of study design, subjects, and methodology is critical, to ensure that data gaps are appropriately addressed and that outcomes are reliable and representative of a wider population. The COU should be decided from the outset, and this will influence the subject inclusion criteria and study design. For example, studies on biomarkers for preclinical AD should enroll cognitively normal subjects with evidence of AD pathology and include longitudinal follow-ups and biomarker measurements over 5 years or more.
For studies to provide meaningful and comparable data, a concerted effort needs to be made to reduce heterogeneity in study methodologies. The development and/or update of consensus recommendations and guidelines should help in this regard, for example, by standardizing diagnostic criteria for different stages of the AD continuum, pre-analytical variables, assays, threshold values, and study designs and populations used for any given COU. The National Institute on Aging–Alzheimer’s Association (NIA–AA) is currently updating a research framework for AD, which will help to harmonize subject selection and disease staging in future studies. There have been longstanding efforts to better understand and control for pre-analytical sources of variability in CSF AD biomarkers, and consensus conferences have defined these [375]. A CSF pre-analytics consortium, sponsored by the Alzheimer’s Association is working to develop a consensus regarding remaining pre-analytical factors such as tube plastic type and other collection parameters that can be implemented into routine clinical practice. Factors that had been recognized but incompletely understood, such as the effect of tube type and CSF volume involved in transfer steps, were recently described and will help to clarify the potential impact of such factors on CSF AD biomarker measurements [386]. The International Federation of Clinical Chemistry Working Group for CSF proteins (IFCC WG-CSF) is an international joint effort to develop reference measurement procedures (RMP) and certified reference materials (CRM) with the aim of standardizing CSF biomarkers and harmonizing read-outs between assay formats [185]. To date, two Joint Committee for Traceability in Laboratory Medicine (JCTLM) -approved RMPs and three CRMs for CSF Aβ42 are available, and work on Aβ40 and tau proteins is ongoing. In parallel with the recent drive to standardize CSF pre-analytics, guidelines have also been proposed by the Biofluid Based Biomarkers Professional Interest Area (of ISTAART) for the pre-analytical processing of blood-based AD biomarkers [268]. Furthermore, “Appropriate use criteria for CSF in clinical practice” are also being developed by the Alzheimer’s Association, which will help define the use of AD CSF biomarkers by clinicians for assessment of cognitive decline and impairment.
Although biomarkers are routinely included in drug studies for understanding target engagement and for patient enrichment, the hurdles are high for biomarker adoption to inform standard of care in daily clinical practice. Health agencies have recognized the importance of biomarkers, and both the FDA and EMA have developed pathways to accelerate biomarker qualification for clinical trials [12]. To achieve biomarker qualification, evidence is needed that the biomarker can reliably support a specified manner of interpretation and application in drug development for a specifically stated COU [12]. Therefore, the aforementioned hurdles, such as data sharing and standardization of study methods, are important to address at the earliest stages of biomarker research. Once a biomarker has been shown to be useful for a specific COU in the clinical trial setting, measures can be taken to further develop it as a companion biomarker useful to practitioners.
The ultimate goal in AD is to follow the approach developed in the more advanced research field of oncology and deliver precision medicine to all patients, in such a way that diagnosis, treatment, and prevention are “tailored” to the characteristics of the individual according to the precision medicine paradigm [46, 92, 126, 127, 129, 130]. In this context, the precision medicine strategy enables a paradigm shift from the traditional “one treatment fits all” approach in drug discovery towards biomarker-guided “tailored” therapies, i.e. targeted interventions adapted to the biological profile of the individual patient. In this regard, the US Precision Medicine Initiative (PMI) (https://obamawhitehouse.archives.gov/precision-medicine) and the US All of Us Research Program (https://allofus.nih.gov/)—formerly known as and evolved from the US PMI Cohort Program (PMI–CP)—have been inaugurated recently. As is the case in most fields of medicine, important progress in detecting, treating, and preventing AD is anticipated from the implementation of a systematic precision medicine strategy. Therefore, after more than a decade of failed therapy trials and one of the lowest success rates in drug development in medicine, the time has come to launch an international Alzheimer PMI (APMI) and associate it with the US PMI and other related worldwide initiatives [92, 126, 127, 129, 130].
Acknowledgements
The development of this review manuscript was financially supported by Roche Diagnostics International. Medical writing was provided by Papia Das of Elements Communications Ltd and funded by Roche Diagnostics International. HH is supported by the AXA Research Fund, the “Fondation partenariale Sorbonne Université” and the “Fondation pour la Recherche sur Alzheimer”, Paris, France. Ce travail a bénéficié d’une aide de l’Etat “Investissements d’avenir” ANR-10-IAIHU-06. The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06 (Agence Nationale de la Recherche-10-IA Agence Institut Hospitalo-Universitaire-6).
Compliance with ethical standards
Conflict of interest
JLM is Principal Investigator of trials funded by Roche, Merck, Novartis, and Janssen and has received speaker or consultant fees from Roche, Roche Diagnostics, Biogen, Merck, Novartis, Oryzon, IBL, Axovant, Lundbeck, and Lilly. SA receives current research support from the National Health and Medical Research Council, Australia (GNT1147442, GNT1123625, GNT1124356, GNT1100441, GNT1101533, GNT1100458) and several non-profit organizations. SA is a co-inventor on biomarker patents related to ferritin. RB is a full-time employee of Roche Diagnostics International. MMB is a full-time employee of Takeda Pharmaceuticals International Co. TB is a full-time employee of Genentech, a member of the Roche Group. JC has provided consultation to Acadia, Accera, Actinogen, ADAMAS, Alkahest, Allergan, Alzheon, Avanir, Axovant, Axsome, BiOasis Technologies, Biogen, Boehinger-Ingelheim, Eisai, Genentech, Grifols, Kyowa, Lilly, Lundbeck, Merck, Nutricia, Otsuka, QR Pharma, Resverlogix, Roche, Samus, Servier, Suven, Takeda, Toyoma, and United Neuroscience companies. JC receives support from Keep Memory Alize (KMA), COBRE grant #P20GM109025; TRC-PAD #R01AG053798; DIAGNOSE CTE #U01NS093334. AMF has served as a consultant or on advisory boards for AbbVie, Araclon/Grifols, DiamiR LLC, Eli Lilly, Genentech, IBL International, and Roche Diagnostics, and has received research support from Biogen, Fujirebio, and Roche Diagnostics. HH serves as Senior Associate Editor for the Journal Alzheimer’s and Dementia; he received lecture fees from Biogen and Roche, research grants from Pfizer, Avid, and MSD Avenir (paid to the institution), travel funding from Functional Neuromodulation, Axovant, Eli Lilly and company, Takeda and Zinfandel, GE-Healthcare and Oryzon Genomics, consultancy fees from Jung Diagnostics, Cytox Ltd., Axovant, Anavex, Takeda and Zinfandel, GE Healthcare Oryzon Genomics, and Functional Neuromodulation, and participated in scientific advisory boards of Functional Neuromodulation, Axovant, Eli Lilly and company, Cytox Ltd., GE Healthcare, Takeda and Zinfandel, Oryzon Genomics and Roche Diagnostics. HH is co-inventor in the following patents as a scientific expert and has received no royalties: (1) In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Patent Number: 8916388. (2) In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Patent Number: 8298784. (3) Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20120196300. (4) In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100062463. (5) In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100035286. (6) In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Publication Number: 20090263822. (7) In Vitro Method for The Diagnosis of Neurodegenerative Diseases Patent Number: 7547553. (8) CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases Publication Number: 20080206797. (9) In Vitro Method for The Diagnosis of Neurodegenerative Diseases Publication Number: 20080199966. (10) Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20080131921. MMM served as a consultant to Eli Lilly and Lysosomal Therapeutics, Inc., and receives research support from the National Institutes of Health (R01 AG49704, P50 AG44170, U01 AG06786 RF1 AG55151), Department of Defense (W81XWH-15-1), and unrestricted research grants from Biogen, Roche, and Lundbeck. AM was an employee of Biogen when this manuscript was started. SO has served as a consultant to Roche Diagnostics and has multiple patents on blood biomarkers related to neurodegenerative disease. He is a founder of Cx Precision Medicine and owns stock. He receives research support for the NIH (R01AG058252, R01AG051848, R01AG054073) and the opinions discussed here do not reflect those of the NIA. PS has acquired grant support (for the institution) from Danone Research, Piramal and Merck. In the past 2 years he has received consultancy/speaker fees (paid to the institution) from Lilly, GE Healthcare, Novartis, Sanofi, Probiodrug, Biogen, Roche and EIP Pharma. JS was an employee of Roche when this manuscript was started. LMS receives research support from the National Institutes of Health (U19 AG024904, P30 AG-10124 for Biomarker Core), Eli Lilly, Hoffman LaRoche, MJFox Foundation for Parkinson’s Research for Biofind study and has served as consultant and/or advisory boards for Roche Diagnostics and Eli Lilly. HS is a full-time employee of AbbVie Inc. GT is a full-time employee of Lundbeck Pharmaceuticals LLC. JQT receives research support from the National Institutes of Health (P30 AG-10124-27, P01 AG-17586-18 and P50 NS-053488-11), Eli Lilly, Janssen, Biogen, and several non-profit organizations. JQT has received revenue from the sale of Avid to Eli Lily as co-inventor on imaging related patents submitted by the University of Pennsylvania, and may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-Inventor. HZ has served at advisory boards for Roche Diagnostics and Eli Lilly and has received travel support from Teva. KB has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics.
References
- 1.Agnello L, Bivona G, Novo G, Scazzone C, Muratore R, Levantino P, et al. Heart-type fatty acid binding protein is a sensitive biomarker for early AMI detection in troponin negative patients: a pilot study. Scand J Clin Lab Invest. 2017;77:428–432. doi: 10.1080/00365513.2017.1335880. [DOI] [PubMed] [Google Scholar]
- 2.Alcolea D, Martínez-Lage P, Sánchez-Juan P, Olazáran J, Antúnez C, Izagirre A, et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology. 2015;85:626–633. doi: 10.1212/wnl.0000000000001859. [DOI] [PubMed] [Google Scholar]
- 3.Alcolea D, Vilaplana E, Pegueroles J, Montal V, Sánchez-Juan P, González-Suárez A, et al. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer’s disease. Neurobiol Aging. 2015;36:2018–2023. doi: 10.1016/j.neurobiolaging.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Alcolea D, Vilaplana E, Suárez-Calvet M, Illán-Gala I, Blesa R, Clarimón J, et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology. 2017;89:178–188. doi: 10.1212/WNL.0000000000004088. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos P, Werle L, Roesler J, Thierjung N, Gleixner LS, Yakushev I, et al. Conflicting cerebrospinal fluid biomarkers and progression to dementia due to Alzheimer’s disease. Alzheimers Res Ther. 2016;8:51. doi: 10.1186/s13195-016-0220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association Alzheimer’s. Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand S, Barnes JM, Young SA, Garcia DM, Tolley HD, Kauwe JSK, et al. Discovery and confirmation of diagnostic serum lipid biomarkers for Alzheimer’s disease using direct infusion mass spectrometry. J Alzheimers Dis. 2017;59:277–290. doi: 10.3233/JAD-170035. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen N, Minthon L, Vanmechelen E, Vanderstichele H, Davidsson P, Winblad B, et al. Cerebrospinal fluid tau and Aβ42 as predictors of development of Alzheimer’s disease in patients with mild cognitive impairment. Neurosci Lett. 1999;273:5–8. doi: 10.1016/S0304-3940(99)00617-5. [DOI] [PubMed] [Google Scholar]
- 10.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonell A, Mansilla A, Rami L, Lladó A, Iranzo A, Olives J, et al. Cerebrospinal fluid level of YKL-40 protein in preclinical and prodromal Alzheimer’s disease. J Alzheimers Dis. 2014;42:901–908. doi: 10.3233/jad-140624. [DOI] [PubMed] [Google Scholar]
- 12.Arnerić SP, Batrla-Utermann R, Beckett L, Bittner T, Blennow K, Carter L, et al. Cerebrospinal fluid biomarkers for Alzheimer’s disease: a view of the regulatory science qualification landscape from the Coalition Against Major Diseases CSF Biomarker Team. J Alzheimers Dis. 2017;55:19–35. doi: 10.3233/JAD-160573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayton S, Diouf I, Bush AI, Alzheimer’s Disease Neuroimaging Initiative Evidence that iron accelerates Alzheimer’s pathology: a CSF biomarker study. J Neurol Neurosurg Psychiatry. 2017;89:456–460. doi: 10.1136/jnnp-2017-316551. [DOI] [PubMed] [Google Scholar]
- 14.Ayton S, Faux NG, Bush AI. Association of cerebrospinal fluid ferritin level with preclinical cognitive decline in APOE-ɛ4 carriers. JAMA Neurol. 2017;74:122–125. doi: 10.1001/jamaneurol.2016.4406. [DOI] [PubMed] [Google Scholar]
- 15.Ayton S, Faux NG, Bush AI, Alzheimer’s Disease Neuroimaging Initiative Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY, et al. Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain. 2017;140:2112–2119. doi: 10.1093/brain/awx137. [DOI] [PubMed] [Google Scholar]
- 17.Babić Leko M, Borovečki F, Dejanović N, Hof PR, Ŝimić G. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J Alzheimers Dis. 2016;50:765–778. doi: 10.3233/jad-150705. [DOI] [PubMed] [Google Scholar]
- 18.Bachurin SO, Bovina EV, Ustyugov AA. Drugs in clinical trials for Alzheimer’s disease: the major trends. Med Res Rev. 2017;37:1186–1225. doi: 10.1002/med.21434. [DOI] [PubMed] [Google Scholar]
- 19.Bakota L, Brandt R. Tau biology and tau-directed therapies for Alzheimer’s disease. Drugs. 2016;76:301–313. doi: 10.1007/s40265-015-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldacci F, Lista S, Cavedo E, Bonuccelli U, Hampel H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Rev Proteom. 2017;14:285–299. doi: 10.1080/14789450.2017.1304217. [DOI] [PubMed] [Google Scholar]
- 21.Baldacci F, Lista S, Garaci F, Bonuccelli U, Toschi N, Hampel H. Biomarker-guided classification scheme of neurodegenerative diseases. J Sport Health Sci. 2016;5:383–387. doi: 10.1016/j.jshs.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldacci F, Lista S, O’Bryant SE, Ceravolo R, Toschi N, Hampel H, et al. Blood-based biomarker screening with agnostic biological definitions for an accurate diagnosis within the dimensional spectrum of neurodegenerative diseases. Methods Mol Biol. 2018;1750:139–155. doi: 10.1007/978-1-4939-7704-8_9. [DOI] [PubMed] [Google Scholar]
- 23.Baldacci F, Toschi N, Lista S, Zetterberg H, Blennow K, Kilimann I, et al. Two-level diagnostic classification using cerebrospinal fluid YKL-40 in Alzheimer’s disease. Alzheimers Dement. 2017;13:993–1003. doi: 10.1016/j.jalz.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Ballatore C, Brunden KR, Trojanowski JQ, Lee VM, Smith AB., 3rd Non-naturally occurring small molecule microtubule-stabilizing agents: a potential tactic for CNS-directed therapies. ACS Chem Neurosci. 2017;8:5–7. doi: 10.1021/acschemneuro.6b00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayer TA, Wirths O. Focusing the amyloid cascade hypothesis on N-truncated Abeta peptides as drug targets against Alzheimer’s disease. Acta Neuropathol. 2014;127:787–801. doi: 10.1007/s00401-014-1287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begcevic I, Brinc D, Brown M, Martinez-Morillo E, Goldhardt O, Grimmer T, et al. Brain-related proteins as potential CSF biomarkers of Alzheimer’s disease: a targeted mass spectrometry approach. J Proteom. 2018;182:12–20. doi: 10.1016/j.jprot.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Berge G, Sando SB, Albrektsen G, Lauridsen C, Møller I, Grøntvedt GR, et al. Alpha-synuclein measured in cerebrospinal fluid from patients with Alzheimer’s disease, mild cognitive impairment, or healthy controls: a two year follow-up study. BMC Neurol. 2016;16:180. doi: 10.1186/s12883-016-0706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergman J, Dring A, Zetterberg H, Blennow K, Norgren N, Gilthorpe J, et al. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol Neuroimmunol Neuroinflamm. 2016;3:e271. doi: 10.1212/NXI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettcher BM, Johnson SC, Fitch R, Casaletto KB, Heffernan KS, Asthana S, et al. Cerebrospinal fluid and plasma levels of inflammation differentially relate to CNS markers of Alzheimer’s disease pathology and neuronal damage. J Alzheimers Dis. 2018;62:385–397. doi: 10.3233/JAD-170602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blennow K, Bogdanovic N, Alafuzoff I, Ekman R, Davidsson P. Synaptic pathology in Alzheimer’s disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the ApoE4 allele. J Neural Transm (Vienna) 1996;103:603–618. doi: 10.1007/BF01273157. [DOI] [PubMed] [Google Scholar]
- 31.Blennow K, De Meyer G, Hansson O, Minthon L, Wallin A, Zetterberg H, et al. Evolution of Abeta42 and Abeta40 levels and Abeta42/Abeta40 ratio in plasma during progression of Alzheimer’s disease: a multicenter assessment. J Nutr Health Aging. 2009;13:205–208. doi: 10.1007/s12603-009-0059-0. [DOI] [PubMed] [Google Scholar]
- 32.Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 34.Blennow K, Zetterberg H, Minthon L, Lannfelt L, Strid S, Annas P, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419:18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 35.Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 2015;16:109–120. doi: 10.1038/nrn3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinkmalm A, Brinkmalm G, Honer WG, Frölich L, Hausner L, Minthon L, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener. 2014;9:53. doi: 10.1186/1750-1326-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bronzuoli MR, Iacomino A, Steardo L, Scuderi C. Targeting neuroinflammation in Alzheimer’s disease. J Inflamm Res. 2016;9:199–208. doi: 10.2147/JIR.S86958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brosseron F, Traschutz A, Widmann CN, Kummer MP, Tacik P, Santarelli F, et al. Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res Ther. 2018;10:25. doi: 10.1186/s13195-018-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruggink KA, Kuiperij HB, Claassen JA, Verbeek MM. The diagnostic value of CSF amyloid-beta(43) in differentiation of dementia syndromes. Curr Alzheimer Res. 2013;10:1034–1040. doi: 10.2174/15672050113106660168. [DOI] [PubMed] [Google Scholar]
- 40.Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010;30:13861–13866. doi: 10.1523/jneurosci.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchhave P, Blennow K, Zetterberg H, Stomrud E, Londos E, Andreasen N, Minthon L, et al. Longitudinal study of CSF biomarkers in patients with Alzheimer’s disease. PLoS One. 2009;4:e6294. doi: 10.1371/journal.pone.0006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Budd Haeberlein S, O’Gorman J, Chiao P, Bussiere T, von Rosenstiel P, Tian Y, et al. Clinical development of aducanumab, an anti-Abeta human monoclonal antibody being investigated for the treatment of early Alzheimer’s disease. J Prev Alzheimers Dis. 2017;4:255–263. doi: 10.14283/jpad.2017.39. [DOI] [PubMed] [Google Scholar]
- 43.Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RAC, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol. 2017;16:601–609. doi: 10.1016/s1474-4422(17)30124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Carroll CM, Li YM. Physiological and pathological roles of the γ-secretase complex. Brain Res Bull. 2016;126:199–206. doi: 10.1016/j.brainresbull.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castrillo JI, Lista S, Hampel H, Ritchie CW. Systems biology methods for Alzheimer’s disease research toward molecular signatures, subtypes, and stages and precision medicine: application in cohort studies and trials. Methods Mol Biol. 2018;1750:31–66. doi: 10.1007/978-1-4939-7704-8_3. [DOI] [PubMed] [Google Scholar]
- 47.Catafau AM, Bullich S. Non-amyloid PET imaging biomarkers for neurodegeneration: focus on tau, alpha-synuclein and neuroinflammation. Curr Alzheimer Res. 2017;14:169–177. doi: 10.2174/1567205013666160620111408. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty A, de Wit NM, van der Flier WM, de Vries HE. The blood brain barrier in Alzheimer’s disease. Vascul Pharmacol. 2017;89:12–18. doi: 10.1016/j.vph.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Chang KA, Shin KY, Nam E, Lee YB, Moon C, Suh YH, et al. Plasma soluble neuregulin-1 as a diagnostic biomarker for Alzheimer’s disease. Neurochem Int. 2016;97:1–7. doi: 10.1016/j.neuint.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Chang XL, Tan MS, Tan L, Yu JT. The role of TDP-43 in Alzheimer’s disease. Mol Neurobiol. 2016;53:3349–3359. doi: 10.1007/s12035-015-9264-5. [DOI] [PubMed] [Google Scholar]
- 51.Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z, Trapp BD. Microglia and neuroprotection. J Neurochem. 2016;136(Suppl 1):10–17. doi: 10.1111/jnc.13062. [DOI] [PubMed] [Google Scholar]
- 53.Chiasserini D, Biscetti L, Eusebi P, Salvadori N, Frattini G, Simoni S, et al. Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimers Res Ther. 2017;9:52. doi: 10.1186/s13195-017-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi J, Lee HW, Suk K. Plasma level of chitinase 3-like 1 protein increases in patients with early Alzheimer’s disease. J Neurol. 2011;258:2181–2185. doi: 10.1007/s00415-011-6087-9. [DOI] [PubMed] [Google Scholar]
- 55.Cornett CR, Markesbery WR, Ehmann WD. Imbalances of trace elements related to oxidative damage in Alzheimer’s disease brain. Neurotoxicology. 1998;19:339–345. [PubMed] [Google Scholar]
- 56.Corso G, Cristofano A, Sapere N, la Marca G, Angiolillo A, Vitale M, et al. Serum amino acid profiles in normal subjects and in patients with or at risk of Alzheimer dementia. Dement Geriatr Cogn Dis Extra. 2017;7:143–159. doi: 10.1159/000466688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, et al. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cristóvão JS, Santos R, Gomes CM. Metals and neuronal metal binding proteins implicated in Alzheimer’s disease. Oxid Med Cell Longev. 2016;2016:9812178. doi: 10.1155/2016/9812178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cruchaga C, Kauwe JS, Harari O, Jin SC, Cai Y, Karch CM, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron. 2013;78:256–268. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2017. Alzheimers Dement (N Y) 2017;3:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dage JL, Wennberg AM, Airey DC, Hagen CE, Knopman DS, Machulda MM, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12:1226–1234. doi: 10.1016/j.jalz.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davidsson P, Blennow K. Neurochemical dissection of synaptic pathology in Alzheimer’s disease. Int Psychogeriatr. 1998;10:11–23. doi: 10.1017/S1041610298005110. [DOI] [PubMed] [Google Scholar]
- 63.De Vos A, Jacobs D, Struyfs H, Fransen E, Andersson K, Portelius E, et al. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer’s disease. Alzheimers Dement. 2015;11:1461–1469. doi: 10.1016/j.jalz.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 64.De Vos A, Struyfs H, Jacobs D, Fransen E, Klewansky T, De Roeck E, et al. The cerebrospinal fluid neurogranin/BACE1 ratio is a potential correlate of cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2016;53:1523–1538. doi: 10.3233/jad-160227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Degerman Gunnarsson M, Ingelsson M, Blennow K, Basun H, Lannfelt L, Kilander L. High tau levels in cerebrospinal fluid predict nursing home placement and rapid progression in Alzheimer’s disease. Alzheimers Res Ther. 2016;8:22. doi: 10.1186/s13195-016-0191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delgado-Alvarado M, Gago B, Gorostidi A, Jiménez-Urbieta H, Dacosta-Aguayo R, Navalpotro-Gómez I, et al. Tau/alpha-synuclein ratio and inflammatory proteins in Parkinson’s disease: an exploratory study. Mov Disord. 2017;32:1066–1073. doi: 10.1002/mds.27001. [DOI] [PubMed] [Google Scholar]
- 67.Desikan RS, Thompson WK, Holland D, Hess CP, Brewer JB, Zetterberg H, et al. Heart fatty acid binding protein and Aβ-associated Alzheimer’s neurodegeneration. Mol Neurodegener. 2013;8:39. doi: 10.1186/1750-1326-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deters KD, Risacher SL, Kim S, Nho K, West JD, Blennow K, et al. Plasma tau association with brain atrophy in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2017;58:1245–1254. doi: 10.3233/JAD-161114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis. 2015;82:593–606. doi: 10.1016/j.nbd.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 70.Díez-Guerra FJ. Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life. 2010;62:597–606. doi: 10.1002/iub.357. [DOI] [PubMed] [Google Scholar]
- 71.Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81:857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 73.Dorey A, Perret-Liaudet A, Tholance Y, Fourier A, Quadrio I. Cerebrospinal fluid Aβ40 improves the interpretation of Aβ42 concentration for diagnosing Alzheimer’s disease. Front Neurol. 2015;6:247. doi: 10.3389/fneur.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Downes EC, Robson J, Grailly E, Abdel-All Z, Xuereb J, Brayne C, et al. Loss of synaptophysin and synaptosomal-associated protein 25-kDa (SNAP-25) in elderly down syndrome individuals. Neuropathol Appl Neurobiol. 2008;34:12–22. doi: 10.1111/j.1365-2990.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 75.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/s1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 76.Engelborghs S, Niemantsverdriet E, Struyfs H, Blennow K, Brouns R, Comabella M, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst) 2017;8:111–126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.European Medicines Agency, Committee for Medicinal Products for Human Use (2011) Qualification opinion of Alzheimer’s disease novel methodologies/biomarkers for the use of CSF amyloid beta 1-42 and t-tau signature and/or PET-amyloid imaging (positive/negative) as biomarkers for enrichment, for use in regulatory clinical trials in mild and moderate Alzheimer’s disease. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2011/12/WC500118365.pdf
- 78.European Medicines Agency, Committee for Medicinal Products for Human Use (2018) Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2018/02/WC500244609.pdf
- 79.Ewers M, Cheng X, Zhong Z, Nural HF, Walsh C, Meindl T, et al. Increased CSF-BACE1 activity associated with decreased hippocampus volume in Alzheimer’s disease. J Alzheimers Dis. 2011;25:373–381. doi: 10.3233/jad-2011-091153. [DOI] [PubMed] [Google Scholar]
- 80.Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, et al. CSF biomarkers for the differential diagnosis of Alzheimer’s disease: a large-scale international multicenter study. Alzheimers Dement. 2015;11:1306–1315. doi: 10.1016/j.jalz.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Ewers M, Zhong Z, Bürger K, Wallin A, Blennow K, Teipel SJ, et al. Increased CSF-BACE 1 activity is associated with ApoE-ε4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain. 2008;131:1252–1258. doi: 10.1093/brain/awn034. [DOI] [PubMed] [Google Scholar]
- 82.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 83.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 85.Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, et al. Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–1144. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016;3:812–818. doi: 10.1002/acn3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fandos N, Pérez-Grijalba V, Pesini P, Olmos S, Bossa M, Villemagne VL, et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst) 2017;8:179–187. doi: 10.1016/j.dadm.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 89.Fath T, Eidenmuller J, Brandt R. Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer’s disease. J Neurosci. 2002;22:9733–9741. doi: 10.1523/JNEUROSCI.22-22-09733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feneberg E, Steinacker P, Lehnert S, Schneider A, Walther P, Thal DR, et al. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:351–356. doi: 10.3109/21678421.2014.905606. [DOI] [PubMed] [Google Scholar]
- 91.Ferreira D, Rivero-Santana A, Perestelo-Pérez L, Westman E, Wahlund LO, Sarría A, et al. Improving CSF biomarkers’ performance for predicting progression from mild cognitive impairment to Alzheimer’s disease by considering different confounding factors: a meta-analysis. Front Aging Neurosci. 2014;6:287. doi: 10.3389/fnagi.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferretti M, Lulita M, Cavedo E, Chiesa P, Schumacher Dimech A, Chadha Santuccione A, et al. Sex-specific phenotypes of Alzheimer’s disease: the gateway to precision neurology. Nat Rev Neurol. 2018;14:457–469. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- 93.Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra396. doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- 94.Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci. 2013;33:7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Food and Drug Administration (2011) Guidance for industry—E16 biomarkers related to drug or biotechnology product development: context, structure, and format of qualification submissions. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm267449.pdf [PubMed]
- 96.Food and Drug Administration (2018) Early Alzheimer’s disease: developing drugs for treatment; draft guidance for industry. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf
- 97.Foulds P, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, et al. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol. 2008;116:141–146. doi: 10.1007/s00401-008-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fourier A, Portelius E, Zetterberg H, Blennow K, Quadrio I, Perret-Liaudet A. Pre-analytical and analytical factors influencing Alzheimer’s disease cerebrospinal fluid biomarker variability. Clin Chim Acta. 2015;449:9–15. doi: 10.1016/j.cca.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 99.Frank RA, Galasko D, Hampel H, Hardy J, de Leon MJ, Mehta PD et al (2003) Biological markers for therapeutic trials in Alzheimer’s disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer’s disease. Neurobiol Aging 24:521–536 [DOI] [PubMed]
- 100.Frisoni GB, Boccardi M, Barkhof F, Blennow K, Cappa S, Chiotis K, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol. 2017;16:661–676. doi: 10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
- 101.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 102.Galimberti D, Venturelli E, Fenoglio C, Lovati C, Guidi I, Scalabrini D, et al. IP-10 serum levels are not increased in mild cognitive impairment and Alzheimer’s disease. Eur J Neurol. 2007;14:e3–e4. doi: 10.1111/j.1468-1331.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- 103.Gendron TF, C9ORF72 Neurofilament Study Group, Daughrity LM, Heckman MG, Diehl NN, Wuu J et al (2017) Phosphorylated neurofilament heavy chain: a biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann Neurol 82:139–146. 10.1002/ana.24980 [DOI] [PMC free article] [PubMed]
- 104.Gervaise-Henry C, Watfa G, Albuisson E, Kolodziej A, Dousset B, Olivier JL, et al. Cerebrospinal fluid Aβeta42/Aβeta40 as a means to limiting tube- and storage-dependent pre-analytical variability in clinical setting. J Alzheimers Dis. 2017;57:437–445. doi: 10.3233/jad-160865. [DOI] [PubMed] [Google Scholar]
- 105.Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 106.Gispert JD, Monte GC, Suárez-Calvet M, Falcon C, Tucholka A, Rojas S, et al. The APOE ε4 genotype modulates CSF YKL-40 levels and their structural brain correlates in the continuum of Alzheimer’s disease but not those of sTREM2. Alzheimers Dement (Amst) 2017;6:50–59. doi: 10.1016/j.dadm.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gispert JD, Suárez-Calvet M, Monté GC, Tucholka A, Falcon C, Rojas S, et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimers Dement. 2016;12:1259–1272. doi: 10.1016/j.jalz.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291X(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 109.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 110.Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016;30:4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goossens J, Vanmechelen E, Trojanowski JQ, Lee VM, Van Broeckhoven C, van der Zee J, et al. TDP-43 as a possible biomarker for frontotemporal lobar degeneration: a systematic review of existing antibodies. Acta Neuropathol Commun. 2015;3:15. doi: 10.1186/s40478-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goozee K, Chatterjee P, James I, Shen K, Sohrabi HR, Asih PR, et al. Elevated plasma ferritin in elderly individuals with high neocortical amyloid-beta load. Mol Psychiatry. 2018;23:1807–1812. doi: 10.1038/mp.2017.146. [DOI] [PubMed] [Google Scholar]
- 113.Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Jr, Younkin LH, et al. Amyloid beta protein (A beta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43) J Biol Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- 114.Grimmer T, Riemenschneider M, Förstl H, Henriksen G, Klunk WE, Mathis CA, et al. Beta amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groblewska M, Muszynski P, Wojtulewska-Supron A, Kulczynska-Przybik A, Mroczko B. The role of visinin-like protein-1 in the pathophysiology of Alzheimer’s disease. J Alzheimers Dis. 2015;47:17–32. doi: 10.3233/jad-150060. [DOI] [PubMed] [Google Scholar]
- 116.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gunn AP, Masters CL, Cherny RA. Pyroglutamate-Aβ: role in the natural history of Alzheimer’s disease. Int J Biochem Cell Biol. 2010;42:1915–1918. doi: 10.1016/j.biocel.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 118.Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo LH, Alexopoulos P, Perneczky R. Heart-type fatty acid binding protein and vascular endothelial growth factor: cerebrospinal fluid biomarker candidates for Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2013;263:553–560. doi: 10.1007/s00406-013-0405-4. [DOI] [PubMed] [Google Scholar]
- 120.Guo R, Fan G, Zhang J, Wu C, Du Y, Ye H, et al. A 9-microRNA signature in serum serves as a noninvasive biomarker in early diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2017;60:1365–1377. doi: 10.3233/JAD-170343. [DOI] [PubMed] [Google Scholar]
- 121.Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hampel H, Bürger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 123.Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 124.Hampel H, Lista S, Khachaturian ZS. Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 125.Hampel H, Lista S, Teipel SJ, Garaci F, Nisticó R, Blennow K, et al. Perspective on future role of biological markers in clinical therapy trials of Alzheimer’s disease: a long-range point of view beyond 2020. Biochem Pharmacol. 2014;88:426–449. doi: 10.1016/j.bcp.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 126.Hampel H, O’Bryant SE, Castrillo JI, Ritchie C, Rojkova K, Broich K, et al. PRECISION MEDICINE—the Golden Gate for detection, treatment and prevention of Alzheimer’s disease. J Prev Alzheimers Dis. 2016;3:243–259. doi: 10.14283/jpad.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hampel H, O’Bryant SE, Durrleman S, Younesi E, Rojkova K, Escott-Price V, et al. A precision medicine initiative for Alzheimer’s disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric. 2017;20:107–118. doi: 10.1080/13697137.2017.1287866. [DOI] [PubMed] [Google Scholar]
- 128.Hampel H, O’Bryant SE, Molinuevo JL, Zetterberg H, Masters CL, Lista S, et al. Blood-based biomarkers for Alzheimer’s disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14:639–652. doi: 10.1038/s41582-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hampel H, Toschi N, Babiloni C, Baldacci F, Black KL, Bokde ALW, et al. Revolution of Alzheimer precision neurology. Passageway of systems biology and neurophysiology. J Alzheimers Dis. 2018;64(Suppl 1):S47–S105. doi: 10.3233/JAD-179932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hampel H, Vergallo A, Aguilar LF, Benda N, Broich K, Cuello AC, et al. Precision pharmacology for Alzheimer’s disease. Pharmacol Res. 2018;130:331–365. doi: 10.1016/j.phrs.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Han J, Pluhackova K, Böckmann RA. The multifaceted role of SNARE proteins in membrane fusion. Front Physiol. 2017;8:5. doi: 10.3389/fphys.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hansson O, Hall S, Ohrfelt A, Zetterberg H, Blennow K, Minthon L, et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Ther. 2014;6:25. doi: 10.1186/alzrt255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 134.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 135.Hare D, Ayton S, Bush A, Lei P. A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci. 2013;5:34. doi: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He Z, Guo JL, McBride JD, Narasimhan S, Kim H, Changolkar L, et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med. 2018;24:29–38. doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heinonen O, Soininen H, Sorvari H, Kosunen O, Paljärvi L, Koivisto E, et al. Loss of synaptophysin-like immunoreactivity in the hippocampal formation is an early phenomenon in Alzheimer’s disease. Neuroscience. 1995;64:375–384. doi: 10.1016/0306-4522(94)00422-2. [DOI] [PubMed] [Google Scholar]
- 138.Hellwig K, Kvartsberg H, Portelius E, Andreasson U, Oberstein TJ, Lewczuk P, et al. Neurogranin and YKL-40: independent markers of synaptic degeneration and neuroinflammation in Alzheimer’s disease. Alzheimers Res Ther. 2015;7:74. doi: 10.1186/s13195-015-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Henjum K, Almdahl IS, Årskog V, Minthon L, Hansson O, Fladby T, et al. Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimers Res Ther. 2016;8:17. doi: 10.1186/s13195-016-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Henriksen K, O’Bryant SE, Hampel H, Trojanowski JQ, Montine TJ, Jeromin A, et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement. 2014;10:115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener. 2016;11:3. doi: 10.1186/s13024-016-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hesse R, Wahler A, Gummert P, Kirschmer S, Otto M, Tumani H, et al. Decreased IL-8 levels in CSF and serum of AD patients and negative correlation of MMSE and IL-1β. BMC Neurol. 2016;16:185. doi: 10.1186/s12883-016-0707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hölttä M, Hansson O, Andreasson U, Hertze J, Minthon L, Nägga K, et al. Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS One. 2013;8:e66381. doi: 10.1371/journal.pone.0066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Horrocks MH, Lee SF, Gandhi S, Magdalinou NK, Chen SW, Devine MJ, et al. Single-molecule imaging of individual amyloid protein aggregates in human biofluids. ACS Chem Neurosci. 2016;7:399–406. doi: 10.1021/acschemneuro.5b00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu N, Tan MS, Yu JT, Sun L, Tan L, Wang YL, et al. Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. J Alzheimers Dis. 2014;38:497–501. doi: 10.3233/jad-130854. [DOI] [PubMed] [Google Scholar]
- 146.Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, Shaw LM, et al. Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol. 2010;119:669–678. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 148.Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, et al. Improved discrimination of AD patients using β-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52:1555–1562. doi: 10.1212/WNL.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 149.Huynh RA, Mohan C. Alzheimer’s disease: biomarkers in the genome, blood, and cerebrospinal fluid. Front Neurol. 2017;8:102. doi: 10.3389/fneur.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Inekci D, Henriksen K, Linemann T, Karsdal MA, Habib A, Bisgaard C, et al. Serum fragments of tau for the differential diagnosis of Alzheimer’s disease. Curr Alzheimer Res. 2015;12:829–836. doi: 10.2174/1567205012666150710111211. [DOI] [PubMed] [Google Scholar]
- 151.Ingelsson M. Alpha-synuclein oligomers-neurotoxic molecules in Parkinson’s disease and other Lewy body disorders. Front Neurosci. 2016;10:408. doi: 10.3389/fnins.2016.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Irwin DJ. Tauopathies as clinicopathological entities. Parkinsonism Relat Disord. 2016;22(Suppl 1):S29–S33. doi: 10.1016/j.parkreldis.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Isaac M, Vamvakas S, Abadie E, Jonsson B, Gispen C, Pani L. Qualification opinion of novel methodologies in the predementia stage of Alzheimer’s disease: cerebro-spinal-fluid related biomarkers for drugs affecting amyloid burden—regulatory considerations by European Medicines Agency focusing in improving benefit/risk in regulatory trials. Eur Neuropsychopharmacol. 2011;21:781–788. doi: 10.1016/j.euroneuro.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 154.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, Alzheimer’s Disease Neuroimaging Initiative Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 156.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: a second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139:2983–2993. doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Janelidze S, Stomrud E, Palmqvist S, Zetterberg H, van Westen D, Jeromin A, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6:26801. doi: 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Janelidze S, Zetterberg H, Mattsson N, Palmqvist S, Vanderstichele H, Lindberg O, et al. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3:154–165. doi: 10.1002/acn3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jay TR, von Saucken VE, Landreth GE. TREM2 in neurodegenerative diseases. Mol Neurodegener. 2017;12:56. doi: 10.1186/s13024-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Junttila A, Kuvaja M, Hartikainen P, Siloaho M, Helisalmi S, Moilanen V, et al. Cerebrospinal fluid TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis patients with and without the C9ORF72 hexanucleotide expansion. Dement Geriatr Cogn Dis Extra. 2016;6:142–149. doi: 10.1159/000444788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kakuda N, Miyasaka T, Iwasaki N, Nirasawa T, Wada-Kakuda S, Takahashi-Fujigasaki J, et al. Distinct deposition of amyloid-beta species in brains with Alzheimer’s disease pathology visualized with MALDI imaging mass spectrometry. Acta Neuropathol Commun. 2017;5:73. doi: 10.1186/s40478-017-0477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, et al. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol. 2016;53:648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, et al. Association of cerebrospinal fluid β-amyloid 1-42, t-tau, p-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013;70:1277–1287. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kang JH, Mollenhauer B, Coffey CS, Toledo JB, Weintraub D, Galasko DR, et al. CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s Progression Markers Initiative study. Acta Neuropathol. 2016;131:935–949. doi: 10.1007/s00401-016-1552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kapaki E, Paraskevas GP, Emmanouilidou E, Vekrellis K. The diagnostic value of CSF α-synuclein in the differential diagnosis of dementia with Lewy bodies vs. normal subjects and patients with Alzheimer’s disease. PLoS One. 2013;8:e81654. doi: 10.1371/journal.pone.0081654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kasai T, Tokuda T, Ishii R, Ishigami N, Tsuboi Y, Nakagawa M, et al. Increased α-synuclein levels in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. J Neurol. 2014;261:1203–1209. doi: 10.1007/s00415-014-7334-7. [DOI] [PubMed] [Google Scholar]
- 172.Kasuga K, Tokutake T, Ishikawa A, Uchiyama T, Tokuda T, Onodera O, et al. Differential levels of α-synuclein, β-amyloid42 and tau in CSF between patients with dementia with Lewy bodies and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2010;81:608–610. doi: 10.1136/jnnp.2009.197483. [DOI] [PubMed] [Google Scholar]
- 173.Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med. 2016;8:363ra150. doi: 10.1126/scitranslmed.aad9704. [DOI] [PubMed] [Google Scholar]
- 174.Kerchner G, Ayalon G, Blendstrup M, Brunstein F, Chandra P, Datwani A et al (2017) Targeting tau with RO7105705: Phase I results and design of a Phase II study in prodromal-to-mild AD. Abstract presented at the 10th Clinical Trials on Alzheimer’s Disease (CTAD). Boston (1–4 November 2017)
- 175.Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 2015;72:1275–1280. doi: 10.1001/jamaneurol.2015.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kester MI, Teunissen CE, Sutphen C, Herries EM, Ladenson JH, Xiong C, et al. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimers Res Ther. 2015;7:59. doi: 10.1186/s13195-015-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khan SS, Bloom GS. Tau: the center of a signaling nexus in Alzheimer’s disease. Front Neurosci. 2016;10:31. doi: 10.3389/fnins.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim D, Kim YS, Shin DW, Park CS, Kang JH. Harnessing cerebrospinal fluid biomarkers in clinical trials for treating Alzheimer’s and Parkinson’s diseases: potential and challenges. J Clin Neurol. 2016;12:381–392. doi: 10.3988/jcn.2016.12.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim HJ, Park KW, Kim TE, Im JY, Shin HS, Kim S, et al. Elevation of the plasma Aβ40/Aβ42 ratio as a diagnostic marker of sporadic early-onset Alzheimer’s disease. J Alzheimers Dis. 2015;48:1043–1050. doi: 10.3233/JAD-143018. [DOI] [PubMed] [Google Scholar]
- 180.Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, et al. Aβ40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/jneurosci.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim WS, Kågedal K, Halliday GM. Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther. 2014;6:73. doi: 10.1186/s13195-014-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Komori M, Kosa P, Stein J, Zhao V, Blake A, Cherup J, et al. Pharmacodynamic effects of daclizumab in the intrathecal compartment. Ann Clin Transl Neurol. 2017;4:478–490. doi: 10.1002/acn3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Korff A, Liu C, Ginghina C, Shi M, Zhang J, Initiative Alzheimer’s Disease Neuroimaging. α-Synuclein in cerebrospinal fluid of Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2013;36:679–688. doi: 10.3233/jad-130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kuhle J, Nourbakhsh B, Grant D, Morant S, Barro C, Yaldizli O, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88:826–831. doi: 10.1212/wnl.0000000000003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kuhlmann J, Andreasson U, Pannee J, Bjerke M, Portelius E, Leinenbach A, et al. CSF Abeta1-42—an excellent but complicated Alzheimer’s biomarker—a route to standardisation. Clin Chim Acta. 2017;467:27–33. doi: 10.1016/j.cca.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 186.Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J. 2012;31:3157–3168. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuiperij HB, Versleijen AA, Beenes M, Verwey NA, Benussi L, Paterlini A, et al. Tau rather than TDP-43 proteins are potential cerebrospinal fluid biomarkers for frontotemporal lobar degeneration subtypes: a pilot study. J Alzheimers Dis. 2017;55:585–595. doi: 10.3233/jad-160386. [DOI] [PubMed] [Google Scholar]
- 188.Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kvartsberg H, Duits FH, Ingelsson M, Andreasen N, Öhrfelt A, Andersson K, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11:1180–1190. doi: 10.1016/j.jalz.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 190.Kvartsberg H, Portelius E, Andreasson U, Brinkmalm G, Hellwig K, Lelental N, et al. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer’s disease patients and healthy controls. Alzheimers Res Ther. 2015;7:40. doi: 10.1186/s13195-015-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lai KSP, Liu CS, Rau A, Lanctot KL, Kohler CA, Pakosh M, et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017;88:876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- 192.Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, et al. Identification of novel brain biomarkers. Clin Chem. 2006;52:1713–1721. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- 193.Lauridsen C, Sando SB, Moller I, Berge G, Pomary PK, Grontvedt GR, et al. Cerebrospinal fluid Abeta43 Is reduced in early-onset compared to late-onset Alzheimer’s Disease, but has similar diagnostic accuracy to Abeta42. Front Aging Neurosci. 2017;9:210. doi: 10.3389/fnagi.2017.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee JM, Blennow K, Andreasen N, Laterza O, Modur V, Olander J, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54:1617–1623. doi: 10.1373/clinchem.2008.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee PH, Lee G, Park HJ, Bang OY, Joo IS, Huh K. The plasma alpha-synuclein levels in patients with Parkinson’s disease and multiple system atrophy. J Neural Transm (Vienna) 2006;113:1435–1439. doi: 10.1007/s00702-005-0427-9. [DOI] [PubMed] [Google Scholar]
- 196.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 197.Lee VM, Trojanowski JQ. Transgenic mouse models of tauopathies: prospects for animal models of Pick’s disease. Neurology. 2001;56:S26–S30. doi: 10.1212/WNL.56.suppl_4.S26. [DOI] [PubMed] [Google Scholar]
- 198.Leitão MJ, Baldeiras I, Herukka SK, Pikkarainen M, Leinonen V, Simonsen AH, et al. Chasing the effects of pre-analytical confounders—a multicenter study on CSF-AD biomarkers. Front Neurol. 2015;6:153. doi: 10.3389/fneur.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lemstra AW, de Beer MH, Teunissen CE, Schreuder C, Scheltens P, van der Flier WM, et al. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2017;88:113–118. doi: 10.1136/jnnp-2016-313775. [DOI] [PubMed] [Google Scholar]
- 200.Leschik J, Welzel A, Weissmann C, Eckert A, Brandt R. Inverse and distinct modulation of tau-dependent neurodegeneration by presenilin 1 and amyloid-β in cultured cortical neurons: evidence that tau phosphorylation is the limiting factor in amyloid-β-induced cell death. J Neurochem. 2007;101:1303–1315. doi: 10.1111/j.1471-4159.2006.04435.x. [DOI] [PubMed] [Google Scholar]
- 201.Leung YY, Toledo JB, Nefedov A, Polikar R, Raghavan N, Xie SX, et al. Identifying amyloid pathology-related cerebrospinal fluid biomarkers for Alzheimer’s disease in a multicohort study. Alzheimers Dement (Amst) 2015;1:339–348. doi: 10.1016/j.dadm.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leuzy A, Chiotis K, Hasselbalch SG, Rinne JO, de Mendonça A, Otto M, et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain. 2016;139:2540–2553. doi: 10.1093/brain/aww160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leverenz JB, Fishel MA, Peskind ER, Montine TJ, Nochlin D, Steinbart E, et al. Lewy body pathology in familial Alzheimer disease: evidence for disease- and mutation-specific pathologic phenotype. Arch Neurol. 2006;63:370–376. doi: 10.1001/archneur.63.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lewczuk P, Lelental N, Lachmann I, Holzer M, Flach K, Brandner S, et al. Non-phosphorylated tau as a potential biomarker of Alzheimer’s disease: analytical and diagnostic characterization. J Alzheimers Dis. 2017;55:159–170. doi: 10.3233/JAD-160448. [DOI] [PubMed] [Google Scholar]
- 205.Lewczuk P, Matzen A, Blennow K, Parnetti L, Molinuevo JL, Eusebi P, et al. Cerebrospinal fluid Aβ42/40 corresponds better than Aβ42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis. 2017;55:813–822. doi: 10.3233/jad-160722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lewczuk P, Riederer P, O’Bryant SE, Verbeek MM, Dubois B, Visser PJ et al (2017) Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry. 10.1080/15622975.2017.1375556 [DOI] [PMC free article] [PubMed]
- 207.Lewis KA, Su Y, Jou O, Ritchie C, Foong C, Hynan LS, et al. Abnormal neurites containing C-terminally truncated alpha-synuclein are present in Alzheimer’s disease without conventional Lewy body pathology. Am J Pathol. 2010;177:3037–3050. doi: 10.2353/ajpath.2010.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Leyhe T, Andreasen N, Simeoni M, Reich A, von Arnim CA, Tong X, et al. Modulation of beta-amyloid by a single dose of GSK933776 in patients with mild Alzheimer’s disease: a phase I study. Alzheimers Res Ther. 2014;6:19. doi: 10.1186/alzrt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 210.Li X, Lei P, Tuo Q, Ayton S, Li QX, Moon S, et al. Enduring elevations of hippocampal amyloid precursor protein and iron are features of β-amyloid toxicity and are mediated by tau. Neurotherapeutics. 2015;12:862–873. doi: 10.1007/s13311-015-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Libreros S, Iragavarapu-Charyulu V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J Leukoc Biol. 2015;98:931–936. doi: 10.1189/jlb.3VMR0415-142R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lin CH, Yang SY, Horng HE, Yang CC, Chieh JJ, Chen HH, et al. Plasma α-synuclein predicts cognitive decline in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88:818–824. doi: 10.1136/jnnp-2016-314857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol. 1999;45:353–357. doi: 10.1002/1531-8249(199903)45:3<353::AID-ANA11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 214.Lista S, Emanuele E. Role of amyloid beta1-42 and neuroimaging biomarkers in Alzheimer’s disease. Biomark Med. 2011;5:411–413. doi: 10.2217/bmm.11.50. [DOI] [PubMed] [Google Scholar]
- 215.Lista S, Faltraco F, Hampel H. Biological and methodical challenges of blood-based proteomics in the field of neurological research. Prog Neurobiol. 2013;101–102:18–34. doi: 10.1016/j.pneurobio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 216.Lista S, Faltraco F, Prvulovic D, Hampel H. Blood and plasma-based proteomic biomarker research in Alzheimer’s disease. Prog Neurobiol. 2013;101–102:1–17. doi: 10.1016/j.pneurobio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 217.Lista S, Garaci FG, Ewers M, Teipel S, Zetterberg H, Blennow K, et al. CSF Abeta1-42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer’s disease. Alzheimers Dement. 2014;10:381–392. doi: 10.1016/j.jalz.2013.04.506. [DOI] [PubMed] [Google Scholar]
- 218.Lista S, Hampel H. Synaptic degeneration and neurogranin in the pathophysiology of Alzheimer’s disease. Expert Rev Neurother. 2017;17:47–57. doi: 10.1080/14737175.2016.1204234. [DOI] [PubMed] [Google Scholar]
- 219.Lista S, Khachaturian ZS, Rujescu D, Garaci F, Dubois B, Hampel H. Application of systems theory in longitudinal studies on the origin and progression of Alzheimer’s disease. Methods Mol Biol. 2016;1303:49–67. doi: 10.1007/978-1-4939-2627-5_2. [DOI] [PubMed] [Google Scholar]
- 220.Lista S, Toschi N, Baldacci F, Zetterberg H, Blennow K, Kilimann I, et al. Diagnostic accuracy of CSF neurofilament light chain protein in the biomarker-guided classification system for Alzheimer’s disease. Neurochem Int. 2017;108:355–360. doi: 10.1016/j.neuint.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 221.Lista S, Toschi N, Baldacci F, Zetterberg H, Blennow K, Kilimann I, et al. Cerebrospinal fluid neurogranin as a biomarker of neurodegenerative diseases: a cross-sectional study. J Alzheimers Dis. 2017;59:1327–1334. doi: 10.3233/jad-170368. [DOI] [PubMed] [Google Scholar]
- 222.Lista S, Zetterberg H, O’Bryant SE, Blennow K, Hampel H. Evolving relevance of neuroproteomics in Alzheimer’s disease. Methods Mol Biol. 2017;1598:101–115. doi: 10.1007/978-1-4939-6952-4_5. [DOI] [PubMed] [Google Scholar]
- 223.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22:121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ljungqvist J, Zetterberg H, Mitsis M, Blennow K, Skoglund T. Serum neurofilament light protein as a marker for diffuse axonal injury: results from a case series study. J Neurotrauma. 2017;34:1124–1127. doi: 10.1089/neu.2016.4496. [DOI] [PubMed] [Google Scholar]
- 225.Llorens F, Kruse N, Karch A, Schmitz M, Zafar S, Gotzmann N, et al. Validation of α-synuclein as a CSF biomarker for sporadic Creutzfeldt-Jakob disease. Mol Neurobiol. 2017;55:2249–2257. doi: 10.1007/s12035-017-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Loeffler DA, Connor JR, Juneau PL, Snyder BS, Kanaley L, DeMaggio AJ, et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem. 1995;65:710–724. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- 227.Lövheim H, Elgh F, Johansson A, Zetterberg H, Blennow K, Hallmans G, et al. Plasma concentrations of free amyloid β cannot predict the development of Alzheimer’s disease. Alzheimers Dement. 2017;13:778–782. doi: 10.1016/j.jalz.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 228.Lu CH, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Luo X, Hou L, Shi H, Zhong X, Zhang Y, Zheng D, et al. CSF levels of the neuronal injury biomarker visinin-like protein-1 in Alzheimer’s disease and dementia with Lewy bodies. J Neurochem. 2013;127:681–690. doi: 10.1111/jnc.12331. [DOI] [PubMed] [Google Scholar]
- 231.Lycke JN, Karlsson JE, Andersen O, Rosengren LE. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64:402–404. doi: 10.1136/jnnp.64.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Majbour NK, Chiasserini D, Vaikath NN, Eusebi P, Tokuda T, van de Berg W, et al. Increased levels of CSF total but not oligomeric or phosphorylated forms of alpha-synuclein in patients diagnosed with probable Alzheimer’s disease. Sci Rep. 2017;7:40263. doi: 10.1038/srep40263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Majbour NK, Vaikath NN, van Dijk KD, Ardah MT, Varghese S, Vesterager LB, et al. Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson’s disease. Mol Neurodegener. 2016;11:7. doi: 10.1186/s13024-016-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marsh SE, Blurton-Jones M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimers Res Ther. 2012;4:11. doi: 10.1186/alzrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Masliah E, Hansen L, Albright T, Mallory M, Terry RD. Immunoelectron microscopic study of synaptic pathology in Alzheimer’s disease. Acta Neuropathol. 1991;81:428–433. doi: 10.1007/BF00293464. [DOI] [PubMed] [Google Scholar]
- 236.Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127–129. doi: 10.1212/WNL.56.1.127. [DOI] [PubMed] [Google Scholar]
- 237.Masliah E, Terry RD, Alford M, DeTeresa R, Hansen LA. Cortical and subcortical patterns of synaptophysinlike immunoreactivity in Alzheimer’s disease. Am J Pathol. 1991;138:235–246. [PMC free article] [PubMed] [Google Scholar]
- 238.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 239.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mattsson N, Carrillo MC, Dean RA, Devous MD, Sr, Nikolcheva T, Pesini P, et al. Revolutionizing Alzheimer’s disease and clinical trials through biomarkers. Alzheimers Dement (Amst) 2015;1:412–419. doi: 10.1016/j.dadm.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol Med. 2016;8:1184–1196. doi: 10.15252/emmm.201606540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mattsson N, Insel PS, Palmqvist S, Stomrud E, van Westen D, Minthon L, et al. Increased amyloidogenic APP processing in APOE ɛ4-negative individuals with cerebral β-amyloidosis. Nat Commun. 2016;7:10918. doi: 10.1038/ncomms10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mattsson N, Tabatabaei S, Johansson P, Hansson O, Andreasson U, Månsson JE, et al. Cerebrospinal fluid microglial markers in Alzheimer’s disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromol Med. 2011;13:151–159. doi: 10.1007/s12017-011-8147-9. [DOI] [PubMed] [Google Scholar]
- 245.Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–1835. doi: 10.1212/WNL.0000000000003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 249.Mellergård J, Tisell A, Blystad I, Grönqvist A, Blennow K, Olsson B, et al. Cerebrospinal fluid levels of neurofilament and tau correlate with brain atrophy in natalizumab-treated multiple sclerosis. Eur J Neurol. 2017;24:112–121. doi: 10.1111/ene.13162. [DOI] [PubMed] [Google Scholar]
- 250.Meredith JE, Jr, Sankaranarayanan S, Guss V, Lanzetti AJ, Berisha F, Neely RJ, et al. Characterization of novel CSF tau and ptau biomarkers for Alzheimer’s disease. PLoS One. 2013;8:e76523. doi: 10.1371/journal.pone.0076523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017;74:1073–1080. doi: 10.1001/jamaneurol.2017.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mohorko N, Bresjanac M. Tau protein and human tauopathies: an overview. Zdrav Vestn. 2008;77:II-35-41. [Google Scholar]
- 253.Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, et al. Direct quantification of CSF α-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213:315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 254.Mollenhauer B, Steinacker P, Bahn E, Bibl M, Brechlin P, Schlossmacher MG, et al. Serum heart-type fatty acid-binding protein and cerebrospinal fluid tau: marker candidates for dementia with Lewy bodies. Neurodegener Dis. 2007;4:366–375. doi: 10.1159/000105157. [DOI] [PubMed] [Google Scholar]
- 255.Mori Y, Yoshino Y, Ochi S, Yamazaki K, Kawabe K, Abe M, et al. TREM2 mRNA expression in leukocytes is increased in Alzheimer’s disease and schizophrenia. PLoS One. 2015;10:e0136835. doi: 10.1371/journal.pone.0136835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 257.Mroczko B, Groblewska M, Zboch M, Muszynski P, Zajkowska A, Borawska R, et al. Evaluation of visinin-like protein 1 concentrations in the cerebrospinal fluid of patients with mild cognitive impairment as a dynamic biomarker of Alzheimer’s disease. J Alzheimers Dis. 2015;43:1031–1037. doi: 10.3233/jad-141050. [DOI] [PubMed] [Google Scholar]
- 258.Mulder SD, van der Flier WM, Verheijen JH, Mulder C, Scheltens P, Blankenstein MA, et al. BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis. 2010;20:253–260. doi: 10.3233/jad-2010-1367. [DOI] [PubMed] [Google Scholar]
- 259.Mulugeta E, Londos E, Ballard C, Alves G, Zetterberg H, Blennow K, et al. CSF amyloid β38 as a novel diagnostic marker for dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2011;82:160–164. doi: 10.1136/jnnp.2009.199398. [DOI] [PubMed] [Google Scholar]
- 260.Naj AC, Schellenberg GD, Alzheimer’s Disease Genetics Consortium (2017) Genomic variants, genes, and pathways of Alzheimer’s disease: an overview. Am J Med Genet B Neuropsychiatr Genet 174:5–26. 10.1002/ajmg.b.32499 [DOI] [PMC free article] [PubMed]
- 261.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554:249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 262.Nath S, Koziarz A, Badhiwala JH, Alhazzani W, Jaeschke R, Sharma S, et al. Atraumatic versus conventional lumbar puncture needles: a systematic review and meta-analysis. Lancet. 2017;391:1197–1204. doi: 10.1016/S0140-6736(17)32451-0. [DOI] [PubMed] [Google Scholar]
- 263.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Neumann K, Farías G, Slachevsky A, Perez P, Maccioni RB. Human platelets tau: a potential peripheral marker for Alzheimer’s disease. J Alzheimers Dis. 2011;25:103–109. doi: 10.3233/JAD-2011-101641. [DOI] [PubMed] [Google Scholar]
- 265.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 266.Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmeström C, et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J Neurochem. 2017;141:296–304. doi: 10.1111/jnc.13881. [DOI] [PubMed] [Google Scholar]
- 267.Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmeström C, et al. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler. 2017;23:62–71. doi: 10.1177/1352458516639384. [DOI] [PubMed] [Google Scholar]
- 268.O’Bryant SE, Gupta V, Henriksen K, Edwards M, Jeromin A, Lista S, et al. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer’s disease research. Alzheimers Dement. 2015;11:549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13:45–58. doi: 10.1016/j.jalz.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Öhrfelt A, Brinkmalm A, Dumurgier J, Brinkmalm G, Hansson O, Zetterberg H, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res Ther. 2016;8:41. doi: 10.1186/s13195-016-0208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/s1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 272.Olsson F, Schmidt S, Althoff V, Munter LM, Jin S, Rosqvist S, et al. Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions. J Biol Chem. 2014;289:1540–1550. doi: 10.1074/jbc.M113.498246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–849. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Palmqvist S, Janelidze S, Stromrud E, Zetterberg H, Karl J, Mattsson N et al (2018) Detecting brain amyloid status using fully automated plasma Aβ biomarker assays. Abstract presented at the Alzheimer’s Association International Conference (AAIC) 2018. Chicago (22–26 July 2018)
- 275.Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Alzheimer’s Disease Neuroimaging Initiative, Minthon L et al (2015) Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 85:1240–1249. 10.1212/wnl.0000000000001991 [DOI] [PMC free article] [PubMed]
- 276.Pannee J, Portelius E, Minthon L, Gobom J, Andreasson U, Zetterberg H, et al. Reference measurement procedure for CSF amyloid beta (Aβ)1-42 and the CSF Aβ1-42/Aβ1-40 ratio—a cross-validation study against amyloid PET. J Neurochem. 2016;139:651–658. doi: 10.1111/jnc.13838. [DOI] [PubMed] [Google Scholar]
- 277.Pannee J, Törnqvist U, Westerlund A, Ingelsson M, Lannfelt L, Brinkmalm G, et al. The amyloid-β degradation pattern in plasma—a possible tool for clinical trials in Alzheimer’s disease. Neurosci Lett. 2014;573:7–12. doi: 10.1016/j.neulet.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 278.Park JC, Han SH, Cho HJ, Byun MS, Yi D, Choe YM, et al. Chemically treated plasma Aβ is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Ther. 2017;9:20. doi: 10.1186/s13195-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Parnetti L, Eusebi P, Lleó A. Cerebrospinal fluid biomarkers for target engagement and efficacy in clinical trials for Alzheimer’s and Parkinson’s diseases. Front Neurol Neurosci. 2016;39:117–123. doi: 10.1159/000445452. [DOI] [PubMed] [Google Scholar]
- 280.Pascoal TA, Mathotaarachchi S, Shin M, Benedet AL, Mohades S, Wang S, et al. Synergistic interaction between amyloid and tau predicts the progression to dementia. Alzheimers Dement. 2017;13:644–653. doi: 10.1016/j.jalz.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 281.Pereira JB, Strandberg TO, Palmqvist S, Volpe G, van Westen D, Westman E, et al. Amyloid network topology characterizes the progression of Alzheimer’s disease during the predementia stages. Cereb Cortex. 2018;28:340–349. doi: 10.1093/cercor/bhx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pereira JB, Westman E, Hansson O, Alzheimer’s Disease Neuroimaging Initiative Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol Aging. 2017;58:14–29. doi: 10.1016/j.neurobiolaging.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 283.Perneczky R, Alexopoulos P, Alzheimer’s Disease Neuroimaging Initiative (2014) Cerebrospinal fluid BACE1 activity and markers of amyloid precursor protein metabolism and axonal degeneration in Alzheimer’s disease. Alzheimers Dement 10:S425–S429 e421. 10.1016/j.jalz.2013.09.006 [DOI] [PMC free article] [PubMed]
- 284.Perret-Liaudet A, Pelpel M, Tholance Y, Dumont B, Vanderstichele H, Zorzi W, et al. Risk of Alzheimer’s disease biological misdiagnosis linked to cerebrospinal collection tubes. J Alzheimers Dis. 2012;31:13–20. doi: 10.3233/JAD-2012-120361. [DOI] [PubMed] [Google Scholar]
- 285.Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik RJ, Knopman DS, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol. 2013;74:199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Petzold A, Keir G, Warren J, Fox N, Rossor MN. A systematic review and meta-analysis of CSF neurofilament protein levels as biomarkers in dementia. Neurodegener Dis. 2007;4:185–194. doi: 10.1159/000101843. [DOI] [PubMed] [Google Scholar]
- 287.Piccio L, Deming Y, Del-Águila JL, Ghezzi L, Holtzman DM, Fagan AM, et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131:925–933. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pijnenburg YA, Janssen JC, Schoonenboom NS, Petzold A, Mulder C, Stigbrand T, et al. CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer’s disease and controls. Dement Geriatr Cogn Disord. 2007;23:225–230. doi: 10.1159/000099473. [DOI] [PubMed] [Google Scholar]
- 289.Portelius E, Price E, Brinkmalm G, Stiteler M, Olsson M, Persson R, et al. A novel pathway for amyloid precursor protein processing. Neurobiol Aging. 2011;32:1090–1098. doi: 10.1016/j.neurobiolaging.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 290.Portelius E, Westman-Brinkmalm A, Zetterberg H, Blennow K. Determination of β-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. J Proteome Res. 2006;5:1010–1016. doi: 10.1021/pr050475v. [DOI] [PubMed] [Google Scholar]
- 291.Portelius E, Zetterberg H, Skillback T, Tornqvist U, Andreasson U, Trojanowski JQ, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer’s disease. Brain. 2015;138:3373–3385. doi: 10.1093/brain/awv267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pottiez G, Yang L, Stewart T, Song N, Aro P, Galasko DR, et al. Mass-spectrometry-based method to quantify in parallel tau and amyloid beta 1-42 in CSF for the diagnosis of Alzheimer’s disease. J Proteome Res. 2017;16:1228–1238. doi: 10.1021/acs.jproteome.6b00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, San Martín-Paniello C, Clarimon J, Belbin O, et al. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J Neuroinflammation. 2017;14:118. doi: 10.1186/s12974-017-0893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Racine AM, Koscik RL, Nicholas CR, Clark LR, Okonkwo OC, Oh JM, et al. Cerebrospinal fluid ratios with Aβ42 predict preclinical brain β-amyloid accumulation. Alzheimers Dement (Amst) 2016;2:27–38. doi: 10.1016/j.dadm.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Reiman EM. Alzheimer disease in 2016: putting AD treatments and biomarkers to the test. Nat Rev Neurol. 2017;13:74–76. doi: 10.1038/nrneurol.2017.1. [DOI] [PubMed] [Google Scholar]
- 296.Rhodin JA, Thomas T. A vascular connection to Alzheimer’s disease. Microcirculation. 2001;8:207–220. doi: 10.1038/sj/mn/7800086. [DOI] [PubMed] [Google Scholar]
- 297.Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, et al. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8:343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 298.Ritter A, Cummings J. Fluid biomarkers in clinical trials of Alzheimer’s disease therapeutics. Front Neurol. 2015;6:186. doi: 10.3389/fneur.2015.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rivero-Santana A, Ferreira D, Perestelo-Pérez L, Westman E, Wahlund LO, Sarría A, et al. Cerebrospinal fluid biomarkers for the differential diagnosis between Alzheimer’s disease and frontotemporal lobar degeneration: systematic review, HSROC analysis, and confounding factors. J Alzheimers Dis. 2017;55:625–644. doi: 10.3233/JAD-160366. [DOI] [PubMed] [Google Scholar]
- 300.Robinson J, Lee E, Xie S, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. doi: 10.1093/brain/awy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Rojas JC, Karydas A, Bang J, Tsai RM, Blennow K, Liman V, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. 2016;3:216–225. doi: 10.1002/acn3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rosén C, Andersson CH, Andreasson U, Molinuevo JL, Bjerke M, Rami L, et al. Increased levels of chitotriosidase and YKL-40 in cerebrospinal fluid from patients with Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2014;4:297–304. doi: 10.1159/000362164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Roussos P, Katsel P, Fam P, Tan W, Purohit DP, Haroutunian V. The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer’s dementia. Alzheimers Dement. 2015;11:1163–1170. doi: 10.1016/j.jalz.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R, et al. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian Imaging, Biomarkers, and Lifestyle Study of Ageing. Ann Neurol. 2013;74:905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 306.Russell CL, Mitra V, Hansson K, Blennow K, Gobom J, Zetterberg H, et al. Comprehensive quantitative profiling of tau and phosphorylated tau peptides in cerebrospinal fluid by mass spectrometry provides new biomarker candidates. J Alzheimers Dis. 2017;55:303–313. doi: 10.3233/JAD-160633. [DOI] [PubMed] [Google Scholar]
- 307.Salminen A, Kauppinen A, Kaarniranta K. Hypoxia/ischemia activate processing of amyloid precursor protein: impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J Neurochem. 2017;140:536–549. doi: 10.1111/jnc.13932. [DOI] [PubMed] [Google Scholar]
- 308.Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F, Soto C. Detection of misfolded Aβ oligomers for sensitive biochemical diagnosis of Alzheimer’s disease. Cell Rep. 2014;7:261–268. doi: 10.1016/j.celrep.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 309.Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C. Molecular pathogenesis of Alzheimer’s disease: an update. Ann Neurosci. 2017;24:46–54. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sanfilippo C, Forlenza O, Zetterberg H, Blennow K. Increased neurogranin concentrations in cerebrospinal fluid of Alzheimer’s disease and in mild cognitive impairment due to AD. J Neural Transm (Vienna) 2016;123:1443–1447. doi: 10.1007/s00702-016-1597-3. [DOI] [PubMed] [Google Scholar]
- 311.Savage MJ, Holder DJ, Wu G, Kaplow J, Siuciak JA, Potter WZ. Soluble BACE-1 activity and sAβPPβ concentrations in Alzheimer’s disease and age-matched healthy control cerebrospinal fluid from the Alzheimer’s Disease Neuroimaging Initiative-1 baseline cohort. J Alzheimers Dis. 2015;46:431–440. doi: 10.3233/jad-142778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Savage MJ, Kalinina J, Wolfe A, Tugusheva K, Korn R, Cash-Mason T, et al. A sensitive aβ oligomer assay discriminates Alzheimer’s and aged control cerebrospinal fluid. J Neurosci. 2014;34:2884–2897. doi: 10.1523/JNEUROSCI.1675-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 314.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/s0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 315.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 316.Schmidt ML, Murray J, Lee VM, Hill WD, Wertkin A, Trojanowski JQ. Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol. 1991;139:53–65. [PMC free article] [PubMed] [Google Scholar]
- 317.Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Schuster J, Funke SA. Methods for the specific detection and quantitation of amyloid-β oligomers in cerebrospinal fluid. J Alzheimers Dis. 2016;53:53–67. doi: 10.3233/JAD-151029. [DOI] [PubMed] [Google Scholar]
- 319.Seeburger JL, Holder DJ, Combrinck M, Joachim C, Laterza O, Tanen M, et al. Cerebrospinal fluid biomarkers distinguish postmortem-confirmed Alzheimer’s disease from other dementias and healthy controls in the OPTIMA cohort. J Alzheimers Dis. 2015;44:525–539. doi: 10.3233/JAD-141725. [DOI] [PubMed] [Google Scholar]
- 320.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sengupta U, Nilson AN, Kayed R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, et al. Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 323.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 324.Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88:1788–1794. doi: 10.1212/wnl.0000000000003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C, et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 2017;74:163–172. doi: 10.1001/jamaneurol.2016.4547. [DOI] [PubMed] [Google Scholar]
- 326.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s Disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sheinerman KS, Toledo JB, Tsivinsky VG, Irwin D, Grossman M, Weintraub D, et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimers Res Ther. 2017;9:89. doi: 10.1186/s13195-017-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shekhar S, Kumar R, Rai N, Kumar V, Singh K, Upadhyay AD, et al. Estimation of tau and phosphorylated tau181 in serum of Alzheimer’s disease and mild cognitive impairment patients. PLoS One. 2016;11:e0159099. doi: 10.1371/journal.pone.0159099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Shen Y, Wang H, Sun Q, Yao H, Keegan AP, Mullan M, et al. Increased plasma beta-secretase 1 may predict conversion to Alzheimer’s disease dementia in individuals with mild cognitive impairment. Biol Psychiatry. 2018;83:447–455. doi: 10.1016/j.biopsych.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Shi M, Zabetian CP, Hancock AM, Ginghina C, Hong Z, Yearout D, et al. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosci Lett. 2010;480:78–82. doi: 10.1016/j.neulet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Singh N, Haldar S, Tripathi AK, Horback K, Wong J, Sharma D, et al. Brain iron homeostasis: from molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid Redox Signal. 2014;20:1324–1363. doi: 10.1089/ars.2012.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sjögren M, Blomberg M, Jonsson M, Wahlund LO, Edman A, Lind K, et al. Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J Neurosci Res. 2001;66:510–516. doi: 10.1002/jnr.1242. [DOI] [PubMed] [Google Scholar]
- 335.Skillbäck T, Farahmand B, Bartlett JW, Rosén C, Mattsson N, Nägga K, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83:1945–1953. doi: 10.1212/wnl.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 336.Skillbäck T, Rosén C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol. 2014;71:476–483. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- 337.Slachevsky A, Guzmán-Martínez L, Delgado C, Reyes P, Farías GA, Muñoz-Neira C, et al. Tau platelets correlate with regional brain atrophy in patients with Alzheimer’s disease. J Alzheimers Dis. 2017;55:1595–1603. doi: 10.3233/JAD-160652. [DOI] [PubMed] [Google Scholar]
- 338.Slaets S, Le Bastard N, Martin JJ, Sleegers K, Van Broeckhoven C, De Deyn PP, et al. Cerebrospinal fluid Aβ1-40 improves differential dementia diagnosis in patients with intermediate p-tau181 levels. J Alzheimers Dis. 2013;36:759–767. doi: 10.3233/jad-130107. [DOI] [PubMed] [Google Scholar]
- 339.Slaets S, Vanmechelen E, Le Bastard N, Decraemer H, Vandijck M, Martin JJ, et al. Increased CSF α-synuclein levels in Alzheimer’s disease: correlation with tau levels. Alzheimers Dement. 2014;10:S290–S298. doi: 10.1016/j.jalz.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 340.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Soares HD, Gasior M, Toyn JH, Wang JS, Hong Q, Berisha F, et al. The γ-secretase modulator, BMS-932481, modulates Aβ peptides in the plasma and cerebrospinal fluid of healthy volunteers. J Pharmacol Exp Ther. 2016;358:138–150. doi: 10.1124/jpet.116.232256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Steardo L, Jr, Bronzuoli MR, Iacomino A, Esposito G, Steardo L, Scuderi C. Does neuroinflammation turn on the flame in Alzheimer’s disease? Focus on astrocytes. Front Neurosci. 2015;9:259. doi: 10.3389/fnins.2015.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.WNL.0000046581.81650.D0. [DOI] [PubMed] [Google Scholar]
- 344.Suárez-Calvet M, Araque Caballero MÁ, Kleinberger G, Bateman RJ, Fagan AM, Morris JC, et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci Transl Med. 2016;8:369ra178. doi: 10.1126/scitranslmed.aag1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Suárez-Calvet M, Dols-Icardo O, Lladó A, Sánchez-Valle R, Hernández I, Amer G, et al. Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation. J Neurol Neurosurg Psychiatry. 2014;85:684–691. doi: 10.1136/jnnp-2013-305972. [DOI] [PubMed] [Google Scholar]
- 346.Suárez-Calvet M, Kleinberger G, Araque Caballero MÁ, Brendel M, Rominger A, Alcolea D, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med. 2016;8:466–476. doi: 10.15252/emmm.201506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sutphen CL, McCue L, Herries EM, Xiong C, Ladenson JH, Holtzman DM, et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimers Dement. 2018;14:869–879. doi: 10.1016/j.jalz.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 349.Suzuki N, Iwatsubo T, Odaka A, Ishibashi Y, Kitada C, Ihara Y. High tissue content of soluble beta 1-40 is linked to cerebral amyloid angiopathy. Am J Pathol. 1994;145:452–460. [PMC free article] [PubMed] [Google Scholar]
- 350.Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 351.Sze CI, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer’s disease brains. J Neurol Sci. 2000;175:81–90. doi: 10.1016/S0022-510X(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 352.Tan YJ, Ng ASL, Vipin A, Lim JKW, Chander RJ, Ji F, et al. Higher peripheral TREM2 mRNA levels relate to cognitive deficits and hippocampal atrophy in Alzheimer’s disease and amnestic mild cognitive impairment. J Alzheimers Dis. 2017;58:413–423. doi: 10.3233/JAD-161277. [DOI] [PubMed] [Google Scholar]
- 353.Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 354.Tarawneh R, D’Angelo G, Crimmins D, Herries E, Griest T, Fagan AM, et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 2016;73:561–571. doi: 10.1001/jamaneurol.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Tarawneh R, D’Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, et al. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70:274–285. doi: 10.1002/ana.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Tarawneh R, Head D, Allison S, Buckles V, Fagan AM, Ladenson JH, et al. Cerebrospinal fluid markers of neurodegeneration and rates of brain atrophy in early Alzheimer disease. JAMA Neurol. 2015;72:656–665. doi: 10.1001/jamaneurol.2015.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Tarawneh R, Lee JM, Ladenson JH, Morris JC, Holtzman DM. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology. 2012;78:709–719. doi: 10.1212/WNL.0b013e318248e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener. 2017;12:63. doi: 10.1186/s13024-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 360.The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group (1998) Consensus report of the working group on: “Molecular and biochemical markers of Alzheimer’s disease”. Neurobiol Aging 19:109–116 [PubMed]
- 361.Thorsell A, Bjerke M, Gobom J, Brunhage E, Vanmechelen E, Andreasen N, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res. 2010;1362:13–22. doi: 10.1016/j.brainres.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 362.Timmers M, Barão S, Van Broeck B, Tesseur I, Slemmon J, De Waepenaert K, et al. BACE1 dynamics upon inhibition with a BACE inhibitor and correlation to downstream Alzheimer’s disease markers in elderly healthy participants. J Alzheimers Dis. 2017;56:1437–1449. doi: 10.3233/JAD-160829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Tirucherai G, Ahlijanian M, Crowell J, Kolaitis G, Skudalski S, Medlock M (2016) A single ascending dose study of the tau-directed monoclonal antibody BMS-986168. Abstract presented at the 20th International Congress of Parkinson’s Disease and Movement Disorders. Berlin (19–23 June 2016)
- 364.Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, et al. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75:1766–1772. doi: 10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- 365.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF alpha-synuclein improves diagnostic and prognostic performance of CSF tau and Abeta in Alzheimer’s disease. Acta Neuropathol. 2013;126:683–697. doi: 10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013;126:659–670. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Toombs J, Paterson RW, Lunn MP, Nicholas JM, Fox NC, Chapman MD, et al. Identification of an important potential confound in CSF AD studies: aliquot volume. Clin Chem Lab Med. 2013;51:2311–2317. doi: 10.1515/cclm-2013-0293. [DOI] [PubMed] [Google Scholar]
- 369.Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Tyson T, Steiner JA, Brundin P. Sorting out release, uptake and processing of alpha-synuclein during prion-like spread of pathology. J Neurochem. 2016;139(Suppl 1):275–289. doi: 10.1111/jnc.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tzen KY, Yang SY, Chen TF, Cheng TW, Horng HE, Wen HP, et al. Plasma Aβ but not tau is related to brain PiB retention in early Alzheimer’s disease. ACS Chem Neurosci. 2014;5:830–836. doi: 10.1021/cn500101j. [DOI] [PubMed] [Google Scholar]
- 372.van Bergen JM, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC, et al. Colocalization of cerebral iron with amyloid beta in mild cognitive impairment. Sci Rep. 2016;6:35514. doi: 10.1038/srep35514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Aβ(1-40) and Aβ(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 374.van Rossum IA, Vos S, Handels R, Visser PJ. Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: implications for trial design. J Alzheimers Dis. 2010;20:881–891. doi: 10.3233/JAD-2010-091606. [DOI] [PubMed] [Google Scholar]
- 375.Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 376.Vassar R, Kuhn PH, Haass C, Kennedy ME, Rajendran L, Wong PC, et al. Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J Neurochem. 2014;130:4–28. doi: 10.1111/jnc.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM et al (2018) Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 10.1002/ana.25334 [DOI] [PMC free article] [PubMed]
- 378.Vickers JC, Riederer BM, Marugg RA, Buee-Scherrer V, Buee L, Delacourte A, et al. Alterations in neurofilament protein immunoreactivity in human hippocampal neurons related to normal aging and Alzheimer’s disease. Neuroscience. 1994;62:1–13. doi: 10.1016/0306-4522(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 379.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 380.Vivacqua G, Latorre A, Suppa A, Nardi M, Pietracupa S, Mancinelli R, et al. Abnormal salivary total and oligomeric alpha-synuclein in Parkinson’s disease. PLoS One. 2016;11:e0151156. doi: 10.1371/journal.pone.0151156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wang MJ, Yi S, Han JY, Park SY, Jang JW, Chun IK, et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimers Res Ther. 2017;9:98. doi: 10.1186/s13195-017-0324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, et al. Phosphorylated α-synuclein in Parkinson’s disease. Sci Transl Med. 2012;4:121ra120. doi: 10.1126/scitranslmed.3002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Waragai M, Yoshida M, Mizoi M, Saiki R, Kashiwagi K, Takagi K, et al. Increased protein-conjugated acrolein and amyloid-β40/42 ratio in plasma of patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2012;32:33–41. doi: 10.3233/JAD-2012-120253. [DOI] [PubMed] [Google Scholar]
- 384.Wennström M, Surova Y, Hall S, Nilsson C, Minthon L, Hansson O, et al. The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or dementia with Lewy bodies. PLoS One. 2015;10:e0135458. doi: 10.1371/journal.pone.0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Weston PSJ, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology. 2017;89(21):2167–2175. doi: 10.1212/WNL.0000000000004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Willemse E, van Uffelen K, Brix B, Engelborghs S, Vanderstichele H, Teunissen C. How to handle adsorption of cerebrospinal fluid amyloid β (1-42) in laboratory practice? Identifying problematic handlings and resolving the issue by use of the Aβ42/Aβ40 ratio. Alzheimers Dement. 2017;13:885–892. doi: 10.1016/j.jalz.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 387.Williams SM, Schulz P, Rosenberry TL, Caselli RJ, Sierks MR. Blood-based oligomeric and other protein variant biomarkers to facilitate pre-symptomatic diagnosis and staging of Alzheimer’s disease. J Alzheimers Dis. 2017;58:23–35. doi: 10.3233/jad-161116. [DOI] [PubMed] [Google Scholar]
- 388.Wiltfang J, Esselmann H, Bibl M, Hüll M, Hampel H, Kessler H, et al. Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-tau in patients with low- and high-CSF Aβ40 load. J Neurochem. 2007;101:1053–1059. doi: 10.1111/j.1471-4159.2006.04404.x. [DOI] [PubMed] [Google Scholar]
- 389.Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 2016;3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Tybulewicz VL, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16:564–574. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.World Health Organization (2017) Dementia factsheet. http://www.who.int/mediacentre/factsheets/fs362/en/. Accessed August 2017
- 392.Wu G, Sankaranarayanan S, Tugusheva K, Kahana J, Seabrook G, Shi XP, et al. Decrease in age-adjusted cerebrospinal fluid β-secretase activity in Alzheimer’s subjects. Clin Biochem. 2008;41:986–996. doi: 10.1016/j.clinbiochem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 393.Wu G, Sankaranarayanan S, Wong J, Tugusheva K, Michener MS, Shi X, et al. Characterization of plasma β-secretase (BACE1) activity and soluble amyloid precursor proteins as potential biomarkers for Alzheimer’s disease. J Neurosci Res. 2012;90:2247–2258. doi: 10.1002/jnr.23122. [DOI] [PubMed] [Google Scholar]
- 394.Yan R. Physiological functions of the β-site amyloid precursor protein cleaving enzyme 1 and 2. Front Mol Neurosci. 2017;10:97. doi: 10.3389/fnmol.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Yang CC, Chiu MJ, Chen TF, Chang HL, Liu BH, Yang SY. Assay of plasma phosphorylated tau protein (threonine 181) and total tau protein in early-stage Alzheimer’s disease. J Alzheimers Dis. 2018;61:1323–1332. doi: 10.3233/JAD-170810. [DOI] [PubMed] [Google Scholar]
- 396.Yang T, O’Malley TT, Kanmert D, Jerecic J, Zieske LR, Zetterberg H, et al. A highly sensitive novel immunoassay specifically detects low levels of soluble Abeta oligomers in human cerebrospinal fluid. Alzheimers Res Ther. 2015;7:14. doi: 10.1186/s13195-015-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 398.Yuan A, Rao MV, Veeranna Nixon RA. Neurofilaments at a glance. J Cell Sci. 2012;125:3257–3263. doi: 10.1242/jcs.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Yuan A, Rao MV, Veeranna Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9:a018309. doi: 10.1101/cshperspect.a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Yuan P, Condello C, Keene CD, Wang Y, Bird TD, Paul SM, et al. TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron. 2016;92:252–264. doi: 10.1016/j.neuron.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 401.Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol. 2008;65:1102–1107. doi: 10.1001/archneur.65.8.1102. [DOI] [PubMed] [Google Scholar]
- 402.Zetterberg H, Pedersen M, Lind K, Svensson M, Rolstad S, Eckerström C, et al. Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis. 2007;12:255–260. doi: 10.3233/JAD-2007-12307. [DOI] [PubMed] [Google Scholar]
- 403.Zetterberg H, Skillbäck T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73:60–67. doi: 10.1001/jamaneurol.2015.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32:3601–3611. doi: 10.1523/jneurosci.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Zhang QS, Heng Y, Yuan YH, Chen NH. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol Lett. 2017;265:30–37. doi: 10.1016/j.toxlet.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 407.Zhong Z, Ewers M, Teipel S, Bürger K, Wallin A, Blennow K, et al. Levels of β-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry. 2007;64:718–726. doi: 10.1001/archpsyc.64.6.718. [DOI] [PubMed] [Google Scholar]
- 408.Zhou W, Zhang J, Ye F, Xu G, Su H, Su Y, et al. Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci Lett. 2017;650:60–64. doi: 10.1016/j.neulet.2017.04.027. [DOI] [PubMed] [Google Scholar]